Abstract
This report describes a case of ductal carcinoma in situ (DCIS) that regressed after treatment with acupuncture, Chinese herbs, and other complementary and alternative medicine (CAM). The natural history of DCIS remains to be elucidated, and it is unclear whether all DCIS cases progress to invasive breast cancer. Surgery plus radiation therapy or mastectomy is recommended for women in whom this potentially nonprogressive cancer is detected. This case supports the developing trend toward active surveillance in lieu of breast-disfiguring surgery and offers evidence that CAM therapies may be of value in preventing progression of DCIS to invasive breast cancer.
Introduction
Ductal carcinoma in situ (DCIS) is a noninvasive form of breast cancer that accounts for one fifth of all breast cancer diagnoses in the United States.1 The neoplasm is confined to the milk ducts of the breast, and by definition (in situ) is nonmetastatic and noninvasive. Most DCIS lesions are asymptomatic and nonpalpable; they can be detected only by screening mammography,2 where they appear as microcalcifications. Incidence of DCIS has increased dramatically since screening mammography became the standard of care in 1983. Before 1975, the incidence was 1.87 per 100,000 adult women; as of 2004 that number had grown to 32.5 per 100,000.3 Approximately 50,000 women will be diagnosed with DCIS this year.
The uncertainties facing women with a DCIS diagnosis are profound. Informed decision-making relies on understanding the risk for progression to invasive breast cancer. However, because most DCIS lesions are excised, the percentage of DCIS cases that progress to invasive breast cancer (IBC) is unknown;4 few data on the natural history of DCIS exist on which to base an objective decision. Research to stratify women by risk for progression to IBC based on tumor markers and other variables5,6 is still in its nascency, and no conclusive recommendations have yet been adopted.
Research has clearly demonstrated that DCIS conveys risk for development of IBC.7–9 It is associated with risk for both locally invasive IBC and distant development of IBC.4 As little as 5%10 and much as 39%–59%11 of DCIS cases are actually found to be IBC at the time of excision, and 5%–19% of DCIS recurs after excision.12 Of these, roughly half are invasive at recurrence.
For these reasons, recommendations for treatment of DCIS are as aggressive as those for IBC. In the case of DCIS, treatment consists primarily of breast-conserving surgery (also known as segmental excision or lumpectomy), with or without radiation and chemotherapy, or mastectomy. Approximately 45% of women diagnosed with DCIS undergo lumpectomy alone, 35% undergo lumpectomy followed by radiation and/or chemotherapy, and 15% have mastectomy.13 Numerous studies demonstrate that radiation reduces cancer recurrence after breast-conserving surgery.12,14–16 Although research consistently shows a decline in recurrence of DCIS or invasive breast cancer after treatment,14–17 data fail to show any increase in long-term survival associated with treatment. In fact, long-term survival in women diagnosed with DCIS is excellent; in the United States, only 1.6% of women diagnosed with DCIS die of breast cancer within 10 years.17 Yet fewer than 5% of women diagnosed with DCIS elect to forgo treatment altogether.13 On the contrary, despite the excellent prognosis of DCIS, fear of invasive breast cancer has led to an alarming increase in prophylactic mastectomy of the contralateral breast.18 Even women who have undergone bilateral mastectomy as treatment for DCIS continue to have unrealistic fear of recurrence,19,20 and many believe they continue to be at significant risk for development of IBC, when in fact the risk for IBC after DCIS excision is extremely low (2% after 5 years and 4% after 8 years).21
Several studies support the theory that not all DCIS cases necessarily progress to IBC. Some DCIS lesions invade and metastasize and others do not.17 In a retrospective postmortem study, approximately 11% of women demonstrated DCIS lesions unrelated to the cause of death on autopsy.22 At least one case of regression of a breast neoplasm has been shown on radiologic imaging.23 Other studies on DCIS missed at initial biopsy demonstrated a rate of progression to IBC of 11%–50% over two decades of observation,7–9 and subsequent recurrence was unrelated to grade of the lesion. Clearly, ways of stratifying risk for progression are sorely needed.
Research is moving toward a stratification of risk among women with DCIS in order to prevent overtreatment.21,24 Characteristics such as histologic grade, tumor size, margin status, and comedo necrosis influence likelihood of recurrence of DCIS3,12 but are inadequate prognostic factors alone. Identification of modifiable risk factors that are associated with a reduced risk for progression to IBC is needed. Observations that estrogen receptor–positive IBC tumors regressed when treated presurgically with estrogen receptor blockers25 prompted Esserman and others at the University of California, San Francisco, to study prophylactic use of tamoxifen26,27 to prevent progression of DCIS and reduce overtreatment. These researchers are attempting to stratify risk for progression based on DCIS tumor markers.5,21 These stratification methods are not available to most women outside of research studies, and none of them represent modifiable risk factors.
Gradually, a move toward identifying women at low risk for progression and recommending noninvasive conservative therapy is developing.27 Noninvasive breast-conserving therapy avoids surgery altogether in favor of active surveillance with magnetic resonance imaging (MRI) plus mammography and detailed physical examination, including clinical breast examination. As this recommendation develops, it would be helpful for women who fall into low-risk categories to know what lifestyle choices they could make to improve their outcomes and inhibit progression from DCIS to IBC. These same risk-reduction strategies may also prove useful to extend time to progression for women with IBC.
The purpose of this report is to describe a case of biopsy- and MRI-confirmed DCIS treated with acupuncture and Traditional Chinese Medicine (TCM) that regressed over a period of 3 years.
Chinese Medicine Perspective
TCM views most cancer as an accumulation of phlegm and toxins.28 Phlegm, in TCM, is a complex pathologic concept that includes visible and invisible forms. Cancer is considered a form of visible phlegm. Phlegm always obstructs the movement of qi (life force and breath) and usually represents a combination of a fluid component with stagnation, which causes the fluid to congeal. Because the breast falls in line with the stomach meridian, and the stomach pertains to yang ming (“greater brightness”), this can be considered accumulation in the yang ming organs, which include the large intestine. The stomach meridian, together with the spleen, correlates to the Earth element, and both pertain to absorption and distribution of nutrients from foods harvested from the Earth. So, cancers in the breast can be a result of improper digestion, through poor nutrient choices, obstruction in the yang ming (either stomach or large intestine) or weakness of function of the stomach or spleen.
Etiologically, numerous factors negatively influence the yang ming and spleen meridians. Stagnation of food ranks near the top of these factors. Food stagnation may be the result of liver dysfunction, improper diet, a lack of exercise, or eating under stressful circumstances not conducive to good digestion. In biomedical parlance, when the parasympathetic nervous system operated by the neurotransmitter acetylcholine is overridden by the sympathetic fight or flight, epinephrine-operated system, blood is shunted away from the splanchnic circulation toward the heart and skeletal muscle. This occurs under stressful conditions, as when an individual is angry or fearful. Such conditions prohibit efficient function of the digestive organs, delay gastric emptying time, slow peristalsis, and inhibit the absorption of nutrients across the brush border, provoking food stagnation. Although Western biomedicine balks at identifying constipation per se as a risk factor for the development of cancer,29–33 in TCM, constipation serves as an etiologic agent in the development of stagnation, which induces the formation of phlegm.
Stagnation may be more figurative than literal in TCM. If that is the case, then the etiologic agents were negative emotions, such as anger, fear, resentment, or anger turned inward as depression. In TCM, these emotions impede the functions of the liver to “smooth” the flow of qi in the body, thus inhibiting its role in regulating digestive functions, imbalancing the spleen and leading to stagnation and, often, dampness. Stagnation and dampness together induce phlegm.34
Case Report
The patient is a 52-year-old perimenopausal woman with no history of hormone replacement therapy who underwent mammography, ultrasonography, and aspiration of her right breast in March 2008 to investigate a palpable abnormality and unilateral bloody nipple discharge. Findings were inconclusive, but apocrine metaplastic epithelial cells and histiocytes were identified in the aspirate taken from a cyst found at the 4 o'clock position of her breast. These were reported as a “probably benign finding,” and follow-up at 6 months was recommended. Mammography and ultrasonography repeated in November 2008 showed benign results.
In August 2009, the discharge from her right nipple recurred. On October 8, she underwent mammography and ultrasonography again, followed by a second ductography on October 14. The ductogram demonstrated innumerable filling defects at the 8 o'clock position consistent with papillomatosis. However, a neoplasm could not be ruled out, and microcalcifications were present, so the patient was referred for excisional biopsy. On November 13, 2009, excisional biopsy was performed on the lower, outer quadrant of the breast. Results demonstrated extensive chronic inflammation, fat necrosis, and DCIS in seven of 11 blocks of tissue, with a total estimated extent of at least 6 cm. The DCIS demonstrated comedo, cribriform, and papillary histologic patterns with nuclear grade III (high). To differentiate between ductal hyperplasia and DCIS, as well as the presence of possible invasive carcinoma, the cells were stained with pancytokeratin and smooth muscle eosin.35,36 Myoepithelial cells were found at the periphery. DCIS was found within 1 mm of the superior and lateral surgical margins.
Diagnostic MRI performed in early December 2009 confirmed this finding. There was a large wedge-shaped area of abnormal contrast with a nodular patchy appearance involving the lateral aspect of the right breast, overall measuring 9.3×3.0×3.5 cm, suspicious for additional involvement of DCIS.
Surgery was recommended to excise cleaner margins, but the patient declined. She then initiated a course of therapy including acupuncture and Chinese herbal formulas. This report details the current status of the patient, 2 years after DCIS diagnosis, and the treatments provided to maintain well-being.
Treatment
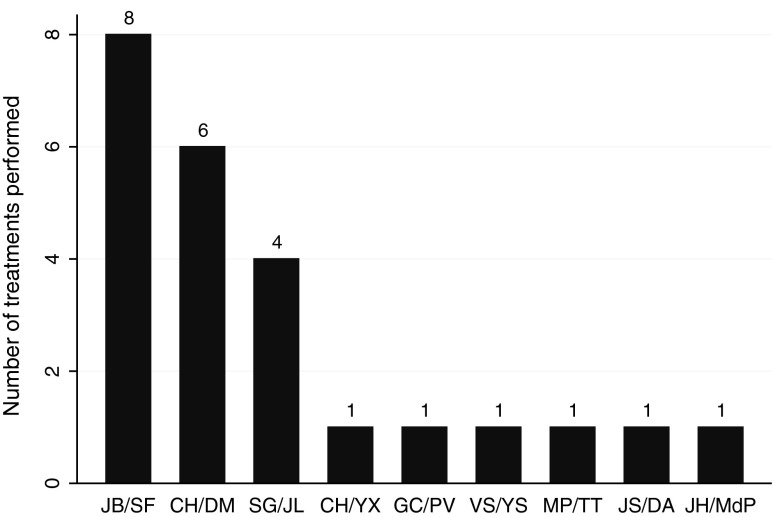
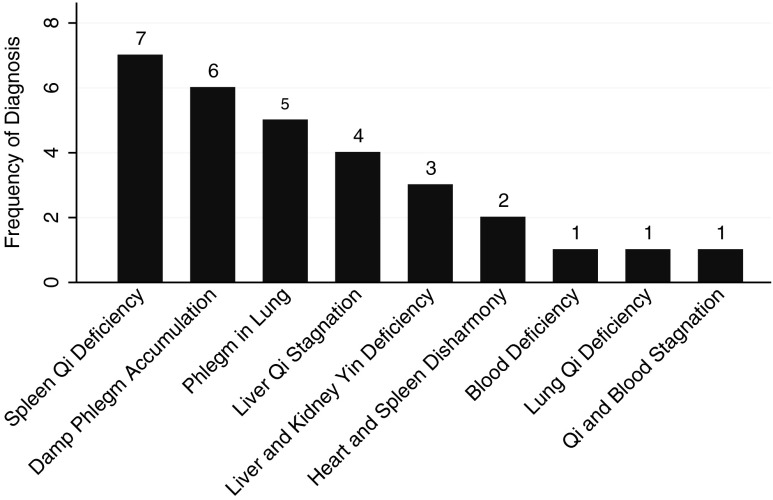
Although wider excisional surgery was recommended, the patient elected to continue active surveillance combined with CAM care. Between September 2010 and November 2011, the patient received 24 acupuncture and TCM treatments from eight separate interns under the supervision of nine discrete supervisors (Fig. 1). Three interns provided 19 treatments. Five interns provided one treatment each. The differential diagnoses and treatment principles varied, but not widely. Nine different diagnoses were made over the course of the 24 treatments, and it was not uncommon to have dual diagnoses, accounting for 30 diagnoses total (Fig. 2). The most common single diagnosis made was spleen qi deficiency (7 of 30 [23%]), but in truth, some form of phlegm was diagnosed more often than anything else (11 times out of 30 [37%]).
FIG. 1.
Number of treatments performed per intern. Bars represent number of treatments performed on the patient by each intern/supervisor pair in the outpatient clinic. Intern/supervisor initials are given. Eighteen of 24 treatments were given by three interns.
FIG. 2.
Traditional Chinese Medicine (TCM) differential diagnoses for patient with ductal carcinoma in situ (DCIS). Bars represent the frequency with which various TCM diagnoses were given preceding treatment. There are nine instances of spleen qi deficiency, either alone (seven instances) or as heart blood and spleen qi deficiency (two instances).
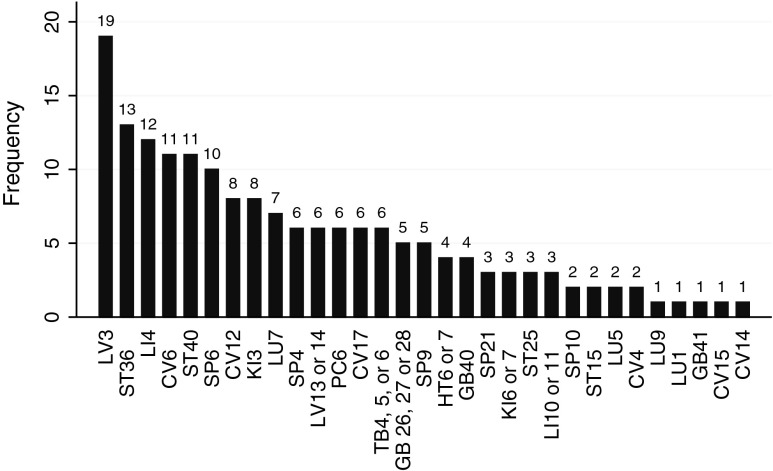
Treatment principles were to move qi and blood, stop pain, resolve stagnation, and transform phlegm and dampness. The interns and supervisors recommended numerous point combinations. Several points stand out as having been used more frequently than others (Fig. 3). The single most commonly used point was LV 3 (19 times), followed by ST 36, LI 4, CV 6, ST 40, and SP 6.
FIG. 3.
Frequency of acupuncture points used to treat ductal carcinoma in situ (DCIS). Point prescriptions varied, but the bar graph indicates the number of times each point was prescribed during the 24 office visits. Treatments focused on sedating wood, tonifying earth, building qi, and resolving phlegm.
Only four formulas were prescribed, all as granules that the patient took intermittently over the course of treatments. She received xiao yao wan (“free and easy wanderer” decoction, 5 g twice daily) for liver qi stagnation. She also had gui pi tang (“restore the spleen” decoction), modified with the addition of ren shen (Panax ginseng) and Cordyceps sinensis (3 g three times daily), to tonify spleen deficiency. Later, she was prescribed shen ling bai zhu san (ginseng, poria, and atractylodis) for spleen qi deficiency, dampness, and phlegm. Most recently, she was given lui wei di huang wan (“six flavor tea,” 5 g twice daily) to diminish hot flashes and irritability caused by kidney and liver yin deficiency.
The patient changed her diet by cutting out all refined sugar and white flour. She reduced her caffeine intake and consumed raw green juices, including 10 ounces of wheat grass juice, daily. She reduced meat, increased fish, sourcing as much as possible organically and locally. Organic dairy was consumed only minimally. She added supplemental vitamins E (400 IU daily), A (10,000 IU daily), D (1200 IU daily), and C (1500 mg daily); calcium (600 mg daily); and magnesium (250 mg daily). She took digestive enzymes, essential fatty acids (fish source, 2000 mg daily), and turmeric capsules (325 mg) with her meals.
The patient also practiced mind–body meditation techniques for emotional healing.
Outcomes
MRI followed by ultrasonography performed in July 2010 demonstrated a large area of stable hypervascular tissue similar in appearance and character to those seen on the previous studies. Six-month follow-up was recommended. In September 2011, repeat MRI performed by the same radiology consultants demonstrated regression of the DCIS. The report states:
On the last exam, multinodular rapid enhancement was present with measurements of 5.7×3.8×8.1 cm. On the current study, the area of abnormal contrast enhancement is markedly reduced in extent. There is still abnormal nodular appearing enhancement, but the overall volume is markedly decreased. There is now patchy non-mass-like enhancement…The greatest diameter measurement of 5.6…× 2×3.9 cm is estimated. Whereas before there was fairly confluent intense enhancement over the whole area, now there is intense enhancement at the inferior periphery and more patchy areas of enhancement in the remaining volume (Michael Hanslits, MD, Salem Radiology Consultants, September 8, 2011).
Of two other areas of focal enhancement seen on the previous MRI, only one could still be seen on this MRI. The areas persisting were still suspicious for residual hypervascularized neoplastic disease; however, there was a considerable, 75% reduction in extent and volume. The radiologist went on to ask, “Has the patient undergone anti-estrogen therapy or chemotherapy to account for the change in the MRI appearance?” She had not.
Discussion
This report describes a case of DCIS in a perimenopausal woman, treated by excisional biopsy followed only by CAM care without the use of chemotherapeutic agents or further surgery. The patient experienced a 75% reduction in volume of her DCIS between diagnosis in 2009 and November 2011, concomitant with the use of alternative therapies. To be sure, the remaining disease is still suspicious for neoplasm, and biopsy would be necessary to confirm this. However, this case represents evidence that DCIS may regress without chemotherapy or radiation, especially in perimenopausal women whose estrogen and progesterone levels are falling. This is not the first report of its kind. Burnside et al.23 reported similar findings in a case of DCIS that remitted when hormone replacement therapy was discontinued. It is important to note that in the Burnside case, the tumor was found to be invasive when it was finally excised.
Just as all cases of DCIS are not equally likely to progress to IBC, all cases of DCIS are not equally amenable to active surveillance with adjuvant CAM care. Unfortunately, predicting which DCIS lesions are likely to progress is not possible with the current state of clinical research. Women diagnosed with DCIS must apprise themselves of as much information about their particular form of DCIS as possible, including method of diagnosis, margin status, nuclear grade, histologic type, and cellular and genetic markers, before deciding on a course of therapy. Only by being well informed about the risks and benefits of all their treatment options, from active surveillance to mastectomy, and engaging their physicians in educated discussions can they come to a decision that is right for them.
Acknowledgments
This work was supported by a faculty scholarship grant from the Oregon College of Oriental Medicine. The author also wishes to thank the patient for encouraging the publication of this case study.
Disclosure Statement
No competing financial interests exist.
References
- 1.Allegra CJ. Aberle DR. Ganschow P, et al. National Institutes of Health State-of-the-Science Conference statement:Diagnosis and management of ductal carcinoma in situ September 22–24, 2009. J Natl Cancer Inst. 2010;102:161–169. doi: 10.1093/jnci/djp485. [DOI] [PubMed] [Google Scholar]
- 2.Evans AJ. Pinder SE. Ellis IO. Wilson AR. Screen detected ductal carcinoma in situ (DCIS): overdiagnosis or an obligate precursor of invasive disease? J Med Screen. 2001;8:149–151. doi: 10.1136/jms.8.3.149. [DOI] [PubMed] [Google Scholar]
- 3.Virnig BA. Tuttle TM. Shamliyan T. Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102:170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 4.Welch HG. Woloshin S. Schwartz LM. The sea of uncertainty surrounding ductal carcinoma in situ—the price of screening mammography. J Natl Cancer Inst. 2008;100:228–229. doi: 10.1093/jnci/djn013. [DOI] [PubMed] [Google Scholar]
- 5.Nelson JN. DCIS prognostic markers: a few new candidates emerge. J Natl Cancer Inst. 2010;102:588–590. doi: 10.1093/jnci/djq161. [DOI] [PubMed] [Google Scholar]
- 6.Lari SA. Kuerer HM. Biological markers in DCIS and risk of breast recurrence: a systematic review. J Cancer. 2011;2:232–261. doi: 10.7150/jca.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins LC. Tamimi RM. Baer HJ, et al. Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the Nurses' Health Study. Cancer. 2005;103:1778–1784. doi: 10.1002/cncr.20979. [DOI] [PubMed] [Google Scholar]
- 8.Page DL. Dupont WD. Rogers LW. Jensen RA. Schuyler PA. Continued local recurrence of carcinoma 15–25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy. Cancer. 1995;76:1197–2000. doi: 10.1002/1097-0142(19951001)76:7<1197::aid-cncr2820760715>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Eusebi V. Foschini MP. Cook MG. Berrino F. Azzopardi JG. Long-term follow-up of in situ carcinoma of the breast with special emphasis on clinging carcinoma. Semin Diagn Pathol. 1989;6:165–173. [PubMed] [Google Scholar]
- 10.Esserman L. Shieh Y. Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302:1685–1692. doi: 10.1001/jama.2009.1498. [DOI] [PubMed] [Google Scholar]
- 11.Dillion MF. McDermott EW. Quinn CM, et al. Predictors of invasive disease when core biopsy demonstrates DCIS only. J Surg Oncol. 2006;93:559–563. doi: 10.1002/jso.20445. [DOI] [PubMed] [Google Scholar]
- 12.Lagios MD. Margolin FR. Westdhal PR. Rose MR. Mammographically detected duct carcinoma in situ. Frequency of local recurrence following tylectomy and prognositic effect of nuclear grade on local recurrence. Cancer. 1989;63:618–624. doi: 10.1002/1097-0142(19890215)63:4<618::aid-cncr2820630403>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 13.Virnig BA. Shamilyan T. Tuttle TM, et al. Bethesda, Maryland: Agency for Healthcare Research and Quality; 2009. Diagnosis and management of ductal carcinoma in situ (DCIS). Evidence Report/Technology Assessment No. 185. AHRQ Publication No. 09-E018. [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher B. Land S. Mamounas E, et al. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28:400–418. doi: 10.1016/s0093-7754(01)90133-2. [DOI] [PubMed] [Google Scholar]
- 15.Solin LJ. McCormick B. Recht A, et al. Mammographically detected, clinically occult ductal carcinoma in situ treated with breast-conserving surgery and definitive breast irradiation. Cancer J Sci Am. 1996;2:158–165. [PubMed] [Google Scholar]
- 16.Fisher B. Anderson S. Redmond CK, et al. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995;333:1456–1461. doi: 10.1056/NEJM199511303332203. [DOI] [PubMed] [Google Scholar]
- 17.Silverstein MJ. Ductal carcinoma in situ of the breast: controversial issues. Oncologist. 1998;3:94–103. [PubMed] [Google Scholar]
- 18.Alvarado R. Lari SA. Roses RE, et al. Biology, treatment, and outcome in very young and older women with DCIS. Ann Surg Oncol. 2012;19:3777–3784. doi: 10.1245/s10434-012-2413-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Partridge A. Adloff K. Blood E, et al. Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: longitudinal results from a cohort study. J Natl Cancer Inst. 2008;100:243–251. doi: 10.1093/jnci/djn010. [DOI] [PubMed] [Google Scholar]
- 20.Lauzier S. Maunsell E. Levesque P, et al. Psychological distress and physical health in the year after diagnosis of DCIS or invasive breast cancer. Breast Cancer Res Treat. 2010;120:685–691. doi: 10.1007/s10549-009-0477-z. [DOI] [PubMed] [Google Scholar]
- 21.Kerlikowske K. Molinaro AM. Gauthier ML, et al. Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst. 2010;102:627–637. doi: 10.1093/jnci/djq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welch HG. Black WC. Using autopsy series to estimate the disease “reservoir” for ductal carcinoma in situ of the breast: how much more breast cancer can we find? Ann Intern Med. 1997;127:1023–1028. doi: 10.7326/0003-4819-127-11-199712010-00014. [DOI] [PubMed] [Google Scholar]
- 23.Burnside E. Trentham-Dietz A. Kelcz F. Collins J. An example of breast cancer regression on imaging. Radiol Case Reports [Online] 2006;1:4. doi: 10.2484/rcr.v1i2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamimi RM. Baer HJ. Marotti J, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 2008;10:R67. doi: 10.1186/bcr2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolmark N. Wang J. Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 26.Chen YY. DeVries S. Anderson J, et al. Pathologic and biologic response to preoperative endocrine therapy in patients with ER-positive ductal carcinoma in situ. BMC Cancer. 2009;9:285. doi: 10.1186/1471-2407-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyerson AF. Lessing JN. Itakura K, et al. Outcome of long term active surveillance for estrogen receptor-positive ductal carcinoma in situ. Breast. 2011;20:529–533. doi: 10.1016/j.breast.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai-zhao Z. Flaws B. Boulder, Colorado: Blue Poppy Press; 1989. The Treatment of Cancer by Integrated Chinese-Western Medicine. [Google Scholar]
- 29.Watanabe T. Nakaya N. Kurashima K, et al. Constipation, laxative use and risk of colorectal cancer: the Miyagi Cohort Study. Eur J Cancer. 2004;40:2109–2115. doi: 10.1016/j.ejca.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Shemerovskii KA. Constipation—a risk factor for colorectal cancer. Klin Med (Mosk) 2005;83:60–64. [PubMed] [Google Scholar]
- 31.Meng W. Cai SR. Zhou L, et al. Performance value of high risk factors in colorectal cancer screening in China. World J Gastroenterol. 2009;15:6111–6116. doi: 10.3748/wjg.15.6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin D. Physical activity benefits and risks on the gastrointestinal system. South Med J. 2011;104:831–837. doi: 10.1097/SMJ.0b013e318236c263. [DOI] [PubMed] [Google Scholar]
- 33.Simons CC. Schouten LJ. Weijenberg MP, et al. Bowel movement and constipation frequencies and the risk of colorectal cancer among men in the Netherlands Cohort Study on Diet and Cancer. Am J Epidemiol. 2010;172:1404–1414. doi: 10.1093/aje/kwq307. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y. Taos, New Mexico: Redwing Book Company; 2009. Practical Therapeutics of Traditional Chinese medicine; pp. 434–437. [Google Scholar]
- 35.Walker RA. Immunohistochemical markers as predictive tools for breast cancer. J Clin Pathol. 2008;61:689–696. doi: 10.1136/jcp.2006.041830. [DOI] [PubMed] [Google Scholar]
- 36.Lerwill MF. Current practical applications of diagnostic immunohistochemistry in breast pathology. Am J Surg Pathol. 2004;28:1076–1091. doi: 10.1097/01.pas.0000126780.10029.f0. [DOI] [PubMed] [Google Scholar]