Abstract
Purpose
An increasing number of cancer patients are choosing Complementary and Alternative Medicine (CAM) as an active way to manage the physical, psychological, and spiritual consequences of cancer. This trend parallels a movement to understand how a difficult experience, such as a cancer diagnosis, may help facilitate positive growth, also referred to as benefit finding. Little is known about the associations between the use of CAM and the ability to find benefit in the cancer experience.
Methods
We conducted a cross-sectional survey of medical oncology outpatients in an urban academic cancer center. Patients completed measures of CAM use and benefit finding following a diagnosis of cancer. A hierarchical regression, adjusting for covariates, was performed to evaluate the unique contribution of CAM use on benefit finding. The relationship between specific CAM modalities and benefit finding was explored.
Results
Among 316 participants, 193 (61.3%) reported CAM use following diagnosis. Factors associated with CAM use were female gender (p=0.005); college, or higher, education (p=0.09); breast cancer diagnosis (p=0.016); and being 12 to 36 months post-diagnosis (p=0.017). In the hierarchical regression, race contributed the greatest unique variance to benefit finding (23%), followed by time from diagnosis (18%), and age (14%). Adjusting for covariates, CAM use uniquely accounted for 13% of the variance in benefit finding. Individuals using energy healing and healing arts reported significantly more benefit than nonusers. Special diet, herbal remedies, vitamin use, and massage saw a smaller increase in benefit finding, while acupuncture, chiropractic, homeopathy, relaxation, yoga, and tai chi were not significantly associated with benefit finding.
Conclusions
Patients who used CAM following a cancer diagnosis reported higher levels of benefit finding than those who did not. More research is required to evaluate the causal relationship between CAM use, benefit finding, and better psychosocial well-being.
Introduction
Approximately two-thirds of cancer patients report using complementary and alternative medicine (CAM) at some point before, during, or after their cancer treatment.1 CAM, as defined by the National Center for Complementary and Alternative Medicine (NCCAM), is a diverse group of medical and health-care systems, practices, and products that may not yet be incorporated into conventional medicine.2 The types of treatments or therapies that are considered part of CAM can be organized into the following categories: natural products (herbs, vitamins, minerals); mind/body medicine (meditation, yoga); body-based approaches (massage, chiropractic); whole medical systems (acupuncture, Ayurveda, traditional Chinese medicine, homeopathy), and energy healing (reiki). As the evidence base of certain CAM treatments has grown, patients are increasingly interested in comprehensive, integrative cancer care that considers not only physical but also psychological and spiritual well-being.3
CAM use as a form of active coping
Although it was once thought to be used only by individuals dissatisfied with conventional cancer treatments, research has demonstrated that CAM is used more frequently alongside and in tandem with mainstream medicine.4 The typical reasons for utilizing CAM interventions are the promotion of wellness, disease prevention, and symptom management (hot flashes, pain, insomnia, etc.).1 Recently researchers have turned their attention to psychological and spiritual reasons for choosing CAM. The need to lessen feelings of helplessness and to “do something” to better manage persistent symptoms or to potentially influence future cancer risk appears to be particularly important in the period following chemotherapy or radiotherapy.5 Active coping strategies include psychological or behavioral efforts to modify the thoughts or feelings associated with the stressful event. In the case of cancer, active coping frequently includes seeking information, making changes to diet and lifestyle, increasing available social support, and learning techniques to reduce perceived stress. Individuals with cancer who engage in active coping strategies report improved emotional and physical well-being.6,7 A desire for increased control and a preference for a more active and collaborative role in treatment decisions has been consistently linked to CAM use.8,9 In this way, then, the use of CAM can be thought of as a form of active coping.
Active coping and benefit finding
Despite the negative physical and psychological consequences of the diagnosis and subsequent treatment of cancer, some individuals have reported positive changes, interpersonal benefits, and spiritual growth regardless of prognosis.10 The identification of such positive outcomes has been referred to as “benefit finding” or, alternately, “meaning making” or “post-traumatic growth.” There is ongoing debate over how to define these terms and how to best distinguish between them.11 For the purposes of this paper, we will refer to the overall process of evaluating and assigning benefit or meaning to one's experience as benefit finding. The benefits reported by individuals diagnosed with cancer include better psychological functioning, improved relationships with others, increased appreciation for life, and greater personal strength.12–14 Because active coping encourages the individual to engage in efforts to manage distress in constructive ways, it is one method of facilitating benefit finding.15,16 As active coping and benefit finding are related to improved psychosocial well-being, it is important to better understand the association between specific coping behaviors, such as the use of CAM, and the ability to find benefit in the cancer experience.
Benefit finding and CAM use
Emerging research suggests that particular CAM therapies may encourage a positive reframing of the cancer experience and lead to benefit finding. In a randomized, controlled clinical trial of 61 breast cancer patients, for example, Chandwani et al. found that a 12-session yoga program during and after radiotherapy enhanced benefit finding three months after treatment.17 A cross-sectional study of 614 heterogeneous cancer survivors found that those who used CAM were more likely to report enhancements in hopefulness, positive changes, and purpose in life.18 To better understand the associations between benefit finding and CAM use, we conducted a cross-sectional study in which we characterized the factors related to CAM use, examined the demographic and clinical predictors of benefit finding following a diagnosis of cancer, and explored which CAM therapies were associated with greater self-reported benefits.
Methods
Study design and patient population
Our study, which was conducted at three outpatient oncology clinics (Breast, Lung, Gastrointestinal) at the Abramson Cancer Center of the University of Pennsylvania Health System in Philadelphia, was a cross-sectional survey of a large, heterogeneous sample of cancer patients. Participants were at least 18 years of age, had a primary diagnosis of cancer and a Karnofsky score of 60 or greater (i.e., ambulatory). The approval of their oncologists and the ability to understand and provide informed consent in English was also required. Trained research assistants screened medical records and approached potential participants in the waiting area of the oncology clinics. After the informed consent process, each participant completed the questionnaire battery. The study was approved by the Institutional Review Board of the University of Pennsylvania.
Benefit finding
The benefit finding scale assesses the perception of life benefits after a cancer diagnosis.19 The scale is unidimensional and consists of 14 items, making it an abbreviated version of the original 20-item scale designed by Tomich and Helgeson.20 Patients were asked to rate on a 4-point Likert scale how much their attitudes and behaviors had changed due to having cancer. Each question began with the statement, “Having had cancer..,” and continued with a potential positive change that might logically arise from dealing with cancer in the following categories: personal priorities, daily activities, and family. The scale has acceptable internal, convergent, and discriminant validity.19–21
CAM use
To measure CAM use, we modified questions from the 2002 National Health Interview Survey (NHIS). Individuals were asked, “Have you used the following CAM therapies since your cancer diagnosis?” The categories included 12 common CAM therapies (e.g., herbs, relaxation techniques, massage, chiropractic, acupuncture, yoga, tai chi, etc.), and an “Other” category for participants to record the use of a CAM therapy not listed.
Statistical analysis
Analysis was conducted using STATA 11.0 (StataCorp, College Station, TX) and IBM SPSS Statistics for Windows 20.0 (IBM Corp, Armonk, NY). CAM data was dichotomized into use or no use. Univariate analyses were used to compare patients who did and did not use CAM on demographic and clinical variables. Variables with p<0.10 through univariate analysis were entered into the multivariate model to adjust for the influence of potential covariates. Hierarchical regression was performed to evaluate the association between CAM use and benefit finding, adjusting for significant predictors in the univariate analyses. Independent samples t-tests examined levels of benefit finding in individuals who endorsed the use of specific CAM therapies compared with those who did not. All analyses were two-sided with p<0.05 indicating statistical significance.
Results
Of the 382 consecutive patients screened for eligibility based on the initial criteria, 339 (88.7%) agreed to participate. Of the 43 (11%) patients who declined, 6 (1.6%) did so due to lack of time to complete the survey. The remaining 37 (9.7%) were not interested in the research. In addition, 9 patients withdrew consent, and 14 did not return the survey, resulting in a final sample of 316 with a response rate of 83% among eligible subjects.
Participant characteristics related to CAM use
Table 1 describes the demographic, medical, and CAM use characteristics of the sample (N=316). The overall mean age of the sample was 58 years, and women comprised two-thirds (64.6%) of the participants. Caucasians made up 76.9% of the sample, followed by African Americans, 17.7%, Asians, 2.5%, Hispanic individuals, 1.9%, and “Other,”1.0%. Within the sample, 27.7% of the participants reported an education status of high school or less and 72.3% had college or graduate education. Overall, 31.1% of the participants were diagnosed with lung cancer, 28.6% with breast cancer, 27.3% with gastrointestinal cancer, and 13.0% with other types of cancer.
Table 1.
Demographic Information and Complementary and Alternative Medicine Use
| |
|
CAM use |
No CAM use |
|
||
|---|---|---|---|---|---|---|
| N (%) | N | % | N | % | p-Value | |
| Total | 316 | 193 | 61.3 | 122 | 38.7 | |
| Age, years | 0.118 | |||||
| ≤65 | 220 (69.6) | 141 | 64.1 | 79 | 35.9 | |
| >65 | 96 (30.4) | 52 | 54.7 | 43 | 45.3 | |
| Gender | 0.005 | |||||
| Male | 112 (35.4) | 57 | 50.9 | 55 | 49.1 | |
| Female | 204 (64.6) | 136 | 67.0 | 67 | 33.0 | |
| Race/ethnicity | 0.636 | |||||
| White | 243 (76.9) | 150 | 62.0 | 92 | 38.0 | |
| Nonwhite* | 73 (23.1) | 43 | 58.9 | 30 | 41.1 | |
| Educational Level | 0.093 | |||||
| High school or less | 87 (27.7) | 46 | 53.5 | 40 | 46.5 | |
| College or above | 227 (72.3) | 145 | 63.9 | 82 | 36.1 | |
| Employment | 0.776 | |||||
| Not employed | 175 (56.3) | 106 | 60.9 | 68 | 39.1 | |
| Employed | 136 (43.7) | 85 | 62.5 | 51 | 37.5 | |
| Cancer Type | 0.016 | |||||
| Breast | 90 (28.6) | 65 | 72.2 | 25 | 27.8 | |
| GI | 86 (27.3) | 48 | 55.8 | 38 | 44.2 | |
| Lung | 98 (31.1) | 51 | 52.6 | 46 | 47.4 | |
| Other | 41 (13.0) | 29 | 61.5 | 12 | 29.3 | |
| Cancer Stage | 0.957 | |||||
| Localized disease | 146 (46.6) | 89 | 61.4 | 56 | 38.6 | |
| Metastatic disease | 167 (53.4) | 103 | 61.7 | 64 | 38.3 | |
| Surgery | 0.043 | |||||
| No | 142 (45.1) | 78 | 55.3 | 63 | 44.7 | |
| Yes | 173 (54.9) | 115 | 66.5 | 58 | 33.5 | |
| Radiation | 0.221 | |||||
| No | 168 (53.3) | 98 | 58.3 | 70 | 41.7 | |
| Yes | 147 (46.7) | 95 | 65.1 | 51 | 34.9 | |
| Chemotherapy | 0.738 | |||||
| No | 34 (10.8) | 20 | 58.8 | 14 | 41.2 | |
| Yes | 281 (89.2) | 173 | 61.8 | 107 | 38.2 | |
| Time since diagnosis | 0.018 | |||||
| ≤12 months | 141 (45.5) | 76 | 53.9 | 65 | 46.1 | |
| >12 and ≤36 months | 80 (25.8) | 58 | 72.5 | 22 | 27.5 | |
| >36 months | 89 (28.7) | 58 | 65.2 | 31 | 34.8 | |
P-values in boldface type are statistically significant.
Mostly African American.
CAM, complementary and alternative medicine.
Among the participants, 61.3% reported CAM use. Women were more likely to use CAM than men (67.0% vs. 50.9% for men, p=0.005). Patients with an educational level of college or higher were also more likely to use CAM than those with an educational level of high school or less (63.9% vs. 53.5%, p=0.093). Across cancer types, patients with breast cancer were the most likely to use CAM (72.2%), while those with lung cancer were the least likely (52.6%, p=0.016). Patients who were 12 to 36 months postdiagnosis showed greater levels of CAM use than patients who were less than 12 months postdiagnosis or more than 36 months postdiagnosis (72.5% vs. 53.9% and 65.2%, p=0.018). No significant difference was seen in CAM use in relation to employment, cancer stage, chemotherapy use, or survivor status.
Factors related to benefit finding
Table 2 summarizes the demographic and clinical factors associated with benefit finding. Significantly higher levels of benefit finding were reported in patients younger than 65 years of age (p=0.001), and in women (p=.019). Benefit finding was also higher among nonwhite patients than white patients (p<0.001). Patients who were more than 36 months postdiagnosis described more benefits than those who were 12 to 36 months postdiagnosis (p=0.001). No significant difference in benefit finding was seen across educational level, employment status, cancer type, cancer stage, surgery history, radiation history or chemotherapy. Patients who used CAM from the time of their cancer diagnosis had a 9.1% increase in benefit finding compared with nonusers (p=0.003).
Table 2.
Demographic and Clinical Predictors of Benefit Finding
| |
Benefit finding score |
|
|
|---|---|---|---|
| Mean | SD | p-Value | |
| Age, years | 0.001 | ||
| ≤65 | 2.76 | 0.68 | |
| >65 | 2.49 | 0.60 | |
| Gender | 0.019 | ||
| Male | 2.56 | 0.62 | |
| Female | 2.75 | 0.68 | |
| Race/ethnicity | <0.001 | ||
| White | 2.60 | 0.66 | |
| Nonwhite* | 2.95 | 0.63 | |
| Educational Level | 0.179 | ||
| High school or less | 2.77 | 0.73 | |
| College or above | 2.65 | 0.64 | |
| Employment | 0.833 | ||
| Not employed | 2.67 | 0.69 | |
| Employed | 2.69 | 0.64 | |
| Cancer Type | 0.637 | ||
| Breast | 2.72 | 0.71 | - |
| GI | 2.73 | 0.66 | 0.966 |
| Lung | 2.61 | 0.64 | 0.274 |
| Other | 2.67 | 0.67 | 0.698 |
| Cancer Stage | 0.937 | ||
| Localized disease | 2.69 | 0.68 | |
| Metastatic disease | 2.68 | 0.66 | |
| Surgery | 0.114 | ||
| No | 2.61 | 0.65 | |
| Yes | 2.74 | 0.68 | |
| Radiation | 0.508 | ||
| No | 2.66 | 0.70 | |
| Yes | 2.71 | 0.63 | |
| Chemotherapy | 0.175 | ||
| No | 2.53 | 0.62 | |
| Yes | 2.70 | 0.67 | |
| Time since cancer diagnosis | 0.005 | ||
| ≤12 months | 2.56 | 0.70 | - |
| >12 and ≤36 months | 2.69 | 0.61 | 0.174 |
| >36 months | 2.86 | 0.61 | 0.001 |
| CAM use | 0.003 | ||
| No | 2.54 | 0.68 | |
| Yes | 2.77 | 0.64 | |
P-values in boldface type are statistically significant.
Mostly African American.
In order to determine whether CAM use accounted for unique variance in benefit finding, the covariates of race, age, gender, and time from diagnosis were entered into the first step of a hierarchical regression model (Table 3). In total, these variables explained 12% of the variance in benefit finding (F (4, 300)=10.072, p<.001). CAM use was then entered in step 2 and accounted for a significant increase in the amount of explained variance (R2 change=0.016; F change (1, 299)=5.635, p=.018). In the full model, which included the four covariates and one predictor, gender was no longer a significant predictor of benefit finding. Race contributed the most unique variance to the total R2 (23%), followed by time from diagnosis (18%), age≤65 years (14%). CAM use contributed 13% to the total variance accounted for in the model.
Table 3.
CAM Use and Benefit Finding
| Step | Predictor | B | SE | β | t | p | R2 | R2 change | sr2 inc* | |
|---|---|---|---|---|---|---|---|---|---|---|
| Benefit Finding | 1 | Age≤65 | −.221 | .080 | −.152 | −2.767 | .006 | .118 | −.141 | |
| Female gender | .099 | .078 | .071 | 1.280 | .202 | .052 | ||||
| Nonwhite race | .348 | .085 | .222 | 4.077 | .000 | .227 | ||||
| >36 months post diagnosis | .151 | .043 | .193 | 3.487 | .001 | .178 | ||||
| 2 | CAM use | .178 | .075 | .130 | 2.374 | .018 | .135 | .016 | .128 |
Full model.
Specific CAM interventions and benefit finding
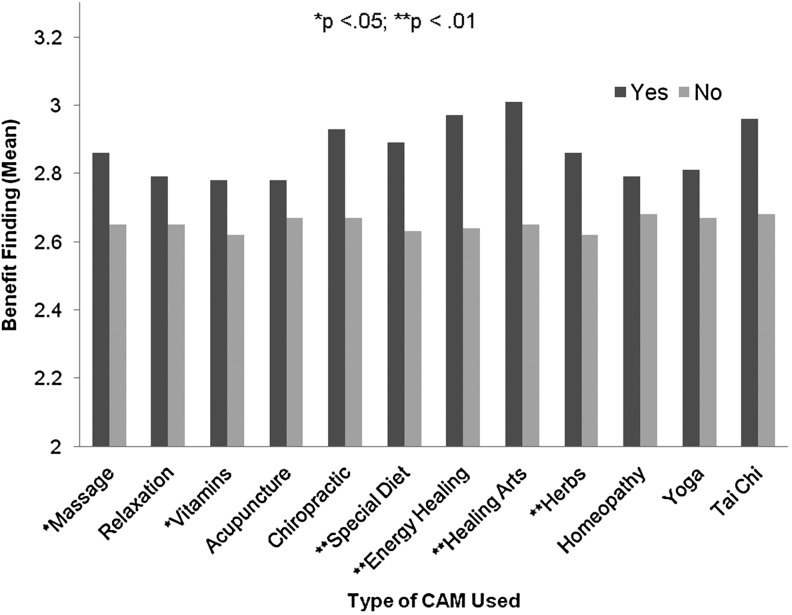
Among the CAM modalities measured (see Figure 1), individuals using special diets (t (1, 304)=−2.828, p=.005), energy healing (t (1, 308)=−2.925, p=.004), healing arts (t (1, 307)=−2.902, p=.004), and herbs (t (1, 307)=−2.715, p=.007), reported higher levels of benefit finding than individuals not using these therapies. The use of massage (t (1, 308)=−2.201; p=.03) and vitamins (t (1, 308)=−2.141, p=.03) were also associated with higher levels of benefit finding, although to a lesser degree. Individuals using relaxation strategies, acupuncture, chiropractic, homeopathy, yoga, and Tai Chi did not report higher levels of benefit finding than nonusers. Considering the exploratory nature of these analyses, a familywise error rate correction was not applied; however, even with a Bonferroni correction (p=.004), reported benefits would remain statistically higher in those individuals using energy healing and healing arts than in those who did not.
FIG. 1.
Specific complementary and alternative medicine modalities and benefit finding.
Discussion
In this cross-sectional study, the use of CAM therapies was associated with increased benefit finding in a heterogeneous sample of cancer patients. Benefit finding increased as the time from diagnosis increased and was more prevalent among individuals who were less than age 65 and those whose race was nonwhite. Individuals using CAM therapies tended to be female, have a college education or more, and a diagnosis of breast cancer. Other factors significantly associated with CAM use included having had surgery and being at least 12 months postdiagnosis. The observed demographic patterns in CAM use were consistent with other published studies 22,23.
We hypothesize that CAM use represents an active coping strategy for the management of cancer-related symptoms and distress. As active coping strategies have been linked to benefit finding,8,9 it follows that encouraging evidence-based CAM use may represent a unique way to facilitate the growth of benefit finding. Bann, Sirois, and Walsh suggest there are three components of the CAM provider-patient relationship that may contribute to greater patient perception of benefit.24 First, patient-centered care has been recognized for its role in positive therapeutic outcomes and is considered an integral part of CAM. Second, there is some evidence that patients perceive their relationship with their CAM provider as more emotionally supportive than their relationship with their oncologist. They are apt to report feeling more accepted, trusted, and cared for by their CAM provider.25 Last, patient empowerment is a key component that distinguishes CAM from conventional biomedicine.
While currently the role of the CAM provider in the patient's support network and its effect on health outcomes is vague, it may well prove to be a valuable concept in the effort to better understand CAM treatment effects. A Swiss study of 318 primary-care practices found that patients said their CAM providers had more time, were more interested in their concerns, had better communication and listening skills, were more available, and were more thorough in their examination than conventional medical practioners.26 As a result, patients reported significantly greater satisfaction with their treatment and found it a more positive experience. In their sample of 131 cancer patients, Scrignaro, Barni, and Magrin demonstrated that perceived support was significantly associated with benefit finding.27 They suggest that the experience of support that encourages autonomy, competence, and relatedness is an important facilitator of positive growth and benefit finding. It would seem evidence is beginning to support the impact of CAM use on coping and positive treatment outcomes, such as benefit finding.
Although, as we have reported, race was not a significant predictor of CAM use, it did contribute the greatest variance (23%) to the level of perceived benefit. This is a novel finding, given that many of the studies examining benefit finding to date have use samples largely comprised of white women.14,28,29 The data available, although limited, supports differential benefit finding in racial populations.20 In an evaluation of a cognitive-behavioral stress management program for men with prostate cancer, Penedo et al. report that Black and Hispanic individuals endorsed higher preintervention levels of benefit finding, regardless of income or education, than white individuals.30 Future qualitative and quantitative research is required to understand the reasons for this difference.
Notable in our results is the apparent difference among CAM modalities and their perceived benefits. In particular, energy healing and healing arts are correlated with the greatest increase in benefit finding. Special diet, herbal remedies, vitamin use, and massage saw a lesser increase in benefit finding, while acupuncture, chiropractic, homeopathy, relaxation, yoga, and tai chi were not significantly associated with enhanced benefit finding. It is possible that different reasons for utilizing CAM play a role in the tendency to experience benefits. Grzywacz et al. show that most CAM users who employ mind-body techniques, such as energy healing and healing arts, do so to promote health and prevent illness, while most of those who use acupuncture and chiropractic care do so to treat an existing condition.31 Following this line of reasoning, however, we would expect to find a positive association between benefit finding and other mind-body therapies, such as yoga. Clearly additional research is needed to understand the mechanisms by which certain CAM modalities promote positive coping strategies and increased perceived benefit.
The cross-sectional design of this study precludes any causal conclusions concerning the relationship between benefit finding and CAM use. It is possible that the benefits described by CAM users are not a result of CAM use but rather a part of its use. In other words, the relationship may be bidirectional rather than unidirectional. For example, while vitamin use can be considered a CAM modality in itself, patients may consider daily vitamin intake a positive change arising from their cancer experience. Statistical mediation models may represent the next step in understanding the role of CAM in promoting positive health outcomes.
By having a large, ethnically diverse sample and the use of a validated instrument, this study, despite its limitations, contributes to the very limited literature on benefit finding and CAM in significant ways. Our results suggest different CAM modalities affect benefit finding to different extents, and greater understanding of these variances might enhance biopsychosocial approaches to cancer treatment and improve future cancer treatment regimens.
Acknowledgments
Dr. Mao's work is supported by a grant from the National Institutes of Health (1K23 AT004112-05). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Garland's work is supported by a postdoctoral fellowship and the Bisby award from the Canadian Institutes of Health Research.
Disclosure Statement
No competing financial interests exist.
References
- 1.Mao JJ. Palmer CS. Healy KE. Desai K. Amsterdam J. Complementary and alternative medicine use among cancer survivors: a population-based study. J Cancer Surviv. 2011;5:8–17. doi: 10.1007/s11764-010-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.What is complementary and alternative medicine? [homepage on the Internet] National Center for Complementary and Alternative Medicine. http://nccam.nih.gov./health/whatiscam. [Oct 17;2012 ]. http://nccam.nih.gov./health/whatiscam Updated 2011.
- 3.Arthur K. Belliard JC. Hardin SB. Knecht K. Chen CS. Montgomery S. Practices, attitudes, and beliefs associated with complementary and alternative medicine (CAM) use among cancer patients. Integr Cancer Ther. 2012;11:232–242. doi: 10.1177/1534735411433832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sollner W. Maislinger S. DeVries A. Steixner E. Rumpold G. Lukas P. Use of complementary and alternative medicine by cancer patients is not associated with perceived distress or poor compliance with standard treatment but with active coping behavior: a survey. Cancer. 2000;89:873–880. doi: 10.1002/1097-0142(20000815)89:4<873::aid-cncr21>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Vapiwala N. Mick R. Hampshire MK. Metz JM. DeNittis AS. Patient initiation of complementary and alternative medical therapies (CAM) following cancer diagnosis. Cancer J. 2006;12:467–474. doi: 10.1097/00130404-200611000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Cheng C. Chan NY. Chio JH. Chan P. Chan AO. Hui WM. Being active or flexible? Role of control coping on quality of life among patients with gastrointestinal cancer. Psychooncology. 2012;21:211–218. doi: 10.1002/pon.1892. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro JP. McCue K. Heyman EN. Dey T. Haller HS. Coping-related variables associated with individual differences in adjustment to cancer. J Psychosoc Oncol. 2010;28:1–22. doi: 10.1080/07347330903438883. [DOI] [PubMed] [Google Scholar]
- 8.Lengacher CA. Bennett MP. Kip KE. Gonzalez L. Jacobsen P. Cox CE. Relief of symptoms, side effects, and psychological distress through use of complementary and alternative medicine in women with breast cancer. Oncol Nurs Forum. 2006;33:97–104. doi: 10.1188/06.ONF.97-104. [DOI] [PubMed] [Google Scholar]
- 9.Henderson JW. Donatelle RJ. The relationship between cancer locus of control and complementary and alternative medicine use by women diagnosed with breast cancer. Psychooncology. 2003;12:59–67. doi: 10.1002/pon.636. [DOI] [PubMed] [Google Scholar]
- 10.Barskova T. Oesterreich R. Post-traumatic growth in people living with a serious medical condition and its relations to physical and mental health: A systematic review. Disabil Rehabil. 2009;31:1709–1733. doi: 10.1080/09638280902738441. [DOI] [PubMed] [Google Scholar]
- 11.Sumalla EC. Ochoa C. Blanco I. Posttraumatic growth in cancer: Reality or illusion? Clin Psychol Rev. 2009;29:24–33. doi: 10.1016/j.cpr.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Costa RV. Pakenham KI. Associations between benefit finding and adjustment outcomes in thyroid cancer. Psychooncology. 2012;21:737–744. doi: 10.1002/pon.1960. [DOI] [PubMed] [Google Scholar]
- 13.Thambyrajah C. Herold J. Altman K. Llewellyn C. “Cancer doesn't mean curtains”: Benefit finding in patients with head and neck cancer in remission. J Psychosoc Oncol. 2010;28:666–682. doi: 10.1080/07347332.2010.516812. [DOI] [PubMed] [Google Scholar]
- 14.Mols F. Vingerhoets AJ. Coebergh JW. van de Poll-Franse LV. Well-being, posttraumatic growth and benefit finding in long-term breast cancer survivors. Psychol Health. 2009;24:583–595. doi: 10.1080/08870440701671362. [DOI] [PubMed] [Google Scholar]
- 15.Thornton AA. Owen JE. Kernstine K, et al. Predictors of finding benefit after lung cancer diagnosis. Psychooncology. 2012;21:365–373. doi: 10.1002/pon.1904. [DOI] [PubMed] [Google Scholar]
- 16.Llewellyn CD. Horney DJ. McGurk M, et al. Assessing the psychological predictors of benefit finding in patients with head and neck cancer. Psychooncology Epub. 2011. Sept. http://www.onlinelibrary.wiley.com/doi/:10.1002/pon.2065/pdf. http://www.onlinelibrary.wiley.com/doi/:10.1002/pon.2065/pdf [DOI] [PubMed]
- 17.Chandwani KD. Thornton B. Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8:43–55. [PubMed] [Google Scholar]
- 18.Mao JJ. Cronholm PF. Stein E. Straton JB. Palmer SC. Barg FK. Positive changes, increased spiritual importance, and complementary and alternative medicine (CAM) use among cancer survivors. Integr Cancer Ther. 2010;9:339–347. doi: 10.1177/1534735410387419. [DOI] [PubMed] [Google Scholar]
- 19.Tomich PL. Helgeson VS. Nowak Vache EJ. Perceived growth and decline following breast cancer: a comparison to age-matched controls 5-years later. Psychooncology. 2005;14:1018–1029. doi: 10.1002/pon.914. [DOI] [PubMed] [Google Scholar]
- 20.Tomich PL. Helgeson VS. Is finding something good in the bad always good? Benefit finding among women with breast cancer. Health Psychol. 2004;23:16–23. doi: 10.1037/0278-6133.23.1.16. [DOI] [PubMed] [Google Scholar]
- 21.Antoni MH. Lehman JM. Kilbourn KM, et al. Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychol. 2001;20(1):20–32. doi: 10.1037//0278-6133.20.1.20. [DOI] [PubMed] [Google Scholar]
- 22.Anderson JG. Taylor AG. Use of complementary therapies for cancer symptom management: Results of the 2007 national health interview survey. J Altern Complement Med. 2012;18:235–241. doi: 10.1089/acm.2011.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walshe R. James EL. MacDonald-Wicks L, et al. Socio-demographic and medical correlates of the use of biologically based complementary and alternative medicines amongst recent Australian cancer survivors. Prev Med. 2012;54:23–26. doi: 10.1016/j.ypmed.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Bann CM. Sirois FM. Walsh EG. Provider support in complementary and alternative medicine: exploring the role of patient empowerment. J Altern Complement Med. 2010;16:745–752. doi: 10.1089/acm.2009.0381. [DOI] [PubMed] [Google Scholar]
- 25.Cartwright T. Torr R. Making sense of illness: The experiences of users of complementary medicine. J Health Psychol. 2005;10:559–572. doi: 10.1177/1359105305053425. [DOI] [PubMed] [Google Scholar]
- 26.Busato A. Kunzi B. Differences in the quality of interpersonal care in complementary and conventional medicine. BMC Complement Altern Med. 2010;10:63. doi: 10.1186/1472-6882-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scrignaro M. Barni S. Magrin ME. The combined contribution of social support and coping strategies in predicting post-traumatic growth: a longitudinal study on cancer patients. Psychooncology. 2011;20:823–831. doi: 10.1002/pon.1782. [DOI] [PubMed] [Google Scholar]
- 28.Lechner SC. Carver CS. Antoni MH. Weaver KE. Phillips KM. Curvilinear associations between benefit finding and psychosocial adjustment to breast cancer. J Consult Clin Psychol. 2006;74:828–840. doi: 10.1037/0022-006X.74.5.828. [DOI] [PubMed] [Google Scholar]
- 29.Tartaro J. Roberts J. Nosarti C. Crayford T. Luecken L. David A. Who benefits? Distress, adjustment and benefit-finding among breast cancer survivors. J Psychosoc Oncol. 2005;23:45–64. doi: 10.1300/j077v23n02_04. [DOI] [PubMed] [Google Scholar]
- 30.Penedo FJ. Molton I. Dahn JR, et al. A randomized clinical trial of group-based cognitive-behavioral stress management in localized prostate cancer: development of stress management skills improves quality of life and benefit finding. Ann Behav Med. 2006;31:261–270. doi: 10.1207/s15324796abm3103_8. [DOI] [PubMed] [Google Scholar]
- 31.Grzywacz JG. Lang W. Suerken C. Quandt SA. Bell RA. Arcury TA. Age, race, and ethnicity in the use of complementary and alternative medicine for health self-management: evidence from the 2002 national health interview survey. J Aging Health. 2005;17:547–572. doi: 10.1177/0898264305279821. [DOI] [PubMed] [Google Scholar]