Abstract
Significance
Successful treatment of wounds relies on precise control and continuous monitoring of the wound-healing process. Wet or moist treatment of wounds has been shown to promote re-epithelialization and result in reduced scar formation, as compared to treatment in a dry environment.
Recent Advances
By treating wounds in a controlled wet environment, delivery of antimicrobials, analgesics, other bioactive molecules such as growth factors, as well as cells and micrografts, is allowed. The addition of growth factors or transplantation of cells yields the possibility of creating a regenerative wound microenvironment that favors healing, as opposed to excessive scar formation.
Critical Issues
Although several manufacturers have conceived products implementing the concept of moist wound healing, there remains a lack of commercial translation of wet wound-healing principles into clinically available products. This can only be mitigated by further research on the topic.
Future Directions
The strong evidence pointing to the favorable healing of wounds in a wet or moist environment compared to dry treatment will extend the clinical indications for this treatment. Further advances are required to elucidate by which means this microenvironment can be optimized to improve the healing outcome.

Johan P.E. Junker, PhD
Scope and Significance
This review highlights clinical findings regarding treatment of wounds in a moist or wet environment. Moreover, the possible modulation of the microenvironment of the healing wound is discussed, with some examples of current research related to the topic.
Translational Relevance
Successful treatment of wounds relies on precise control and continuous monitoring of the wound-healing process. This review summarizes the impact and implications of wet or moist treatment of skin wounds. The wet microenvironment, in which the wound surface acts as a highly permeable membrane has been suggested to be advantageous for accelerated wound healing in regard to the epithelialization rate and healing outcome. Various treatment solutions can be introduced in the liquid, at known concentrations. This renders the possibility of exact determination of uptake and the rate of elimination, as well as analysis of any byproducts from the wound-healing process.
Clinical Relevance
Wet or moist treatment of wounds has been shown to promote re-epithelialization and result in reduced scar formation, as compared to treatment in a dry environment. The inflammatory reaction is reduced in the wet environment, thereby limiting injury progression. Several studies have compared wet, moist, and dry healing. A wet or moist incubator-like microenvironment provides the fastest healing with fewest aberrations and least scar formation. These clinically relevant observations allow one to resolve translational experiments and relevance to further enhance and define the parameters important to accelerating wound healing.
Discussion of Findings and Relevant Literature
Definition of the wound microenvironment
The wound microenvironment is defined as the environment exterior to the wound and in direct contact with its surface. It is defined as dry when there is no barrier to contain the extracellular fluid and extracellular matrix in the wound. It is described as moist when a moisture-containing controlled hydration dressing is used to cover the surface of the wound. Finally, it is described as wet when covered with an impermeable membrane that is sealed to the periphery of the wound with adhesives.
Treatment of wounds in a moist environment
There are numerous examples throughout history with exacting efforts to utilize moist wound healing in the earliest of medical writings. The modern concept of employing a moist environment for the treatment of wounds was introduced in the early 1960s by Winter et al.1 Here it was shown in a pig model that the rate of epithelialization after wounding was twice as fast when treated with a moist dressing as compared to dry conditions. This, at the time of a new concept, was in opposition with the generally accepted idea that a dry environment was best for wounds to fight infection. One year later, Hinman and Maibach applied the moist dressing concept in the study of experimental human skin wounds.2 Since then, moist dressings have become the standard method for care for chronic wounds.3 A moist environment has been proven to facilitate the healing process of the wound by preventing dehydration and enhancing angiogenesis and collagen synthesis together with increased breakdown of dead tissue and fibrin. This improves the aesthetics of the wound, while decreasing pain. The moist environment has not been shown to increase the risk of infection, as compared to traditional dry therapies.4,5
Improved wound healing in a moist environment under controlled hydration could be attributed to a variety of mechanisms. These include easier migration of epidermal cells on a moist surface,6 faster epithelialization,7 but also the prolonged presence of proteinases and growth factors.8 Clinical studies suggest that wound fluid from wounds healing under moist conditions stimulates keratinocyte proliferation7 and fibroblast growth9 with subsequent preservation of growth factors for wound repair10–13 (Table 1).
Table 1.
Comparison between different parameters of wound healing under dry versus moist healing conditions
| Dry Environment | Moist Environment | References | |
|---|---|---|---|
| Microscopic aspects | |||
| Cellular migration | − | + | 6 |
| Keratinocyte proliferation | − | + | 7 |
| Fibroblast proliferation | − | + | 9 |
| Growth factors activity | − | + | 8,10 |
| Angiogenesis | − | + | 12 |
| Collagen synthesis | − | + | 4 |
| Dead tissue and fibrin | + | − | 4,13 |
| Duration of inflammatory and proliferative phases | + | − | 11 |
| Clinical aspects | |||
| Incidence of infection | − | − | 4,5 |
| Pain | + | − | 4,13 |
| Wound aesthetics and quality | − | + | 4,13 |
The pluses and minuses in the table do not reflect quantitative descriptions or absolute values, but rather serve to illustrate healing conditions under dry and moist environments in comparison to each other.
Dyson et al. compared the effects of moist and dry conditions on dermal repair in a porcine model. An adhesive polyurethane dressing was used as a moist dressing, while gauze dressing exposed to air ensured dry healing conditions. They showed that both the inflammatory and proliferative phases of dermal repair were shorter for wounds under moist conditions when compared with those healing under dry conditions.11 Four years later, the same group performed another comparative study between moist and dry environments, investigating the process of angiogenesis during dermal repair. They concluded that wounds that were allowed to heal in a moist environment were revascularized at a greater rate than those maintained under dry conditions. Angiogenesis also occurred in a more orderly fashion in moist wounds.12 A study by Vogt et al. also used a porcine model to compare the effect of moist, wet and dry environments on wound repair. It found that moist wounds exhibited a higher number of subepidermal neutrophils than wet wounds 5 days postwounding, which may have been caused by the hydrocolloid dressing used (Table 2).13 Moist and wet healing environment resulted in less necrosis, faster healing, and better quality of healing than the dry environment.13
Table 2.
Subepithelial infiltrate in moist and wet wounds
| Time After Wounding | Wet (Mean±SEM; n=8) | Moist (Mean±SEM; n=8) | p |
|---|---|---|---|
| Day 5 | |||
| All | 171±8.90 | 183.50±17.40 | n.s. |
| Lymphocytes | 16.40±4.30 | 17.10±2.80 | n.s. |
| Fibroblasts | 124.00±10.60 | 132.60±7.90 | n.s. |
| Macrophages | 12.60±2.70 | 12.26±4.80 | n.s. |
| Neutrophils | 2.40±0.40 | 6.20±1.30 | 0.0156 |
| Others | 15.60±2.40 | 16.90±2.90 | n.s. |
| Day 7 | |||
| All | 127.00±2.90 | 149.00±5.60 | 0.0003 |
| Lymphocytes | 5.70±0.70 | 8.57±2.00 | n.s. |
| Fibroblasts | 107.60±2.60 | 119.30±3.00 | 0.0031 |
| Macrophages | 5.40±37.00 | 7.59±1.00 | n.s. |
| Neutrophils | 1.87±0.29 | 4.00±1.70 | n.s. |
| Others | 7.59±0.50 | 11.60±1.60 | 0.0343 |
Values represent number of cells/10−6 mm2. (Adapted from Vogt et al.13)
n.s., not significant; SEM, standard error of the mean.
Manufacturers have taken the cue from researchers following Winter's postulate and worked to provide today's market with a wide range of moist dressings such as hydrocolloids that absorb the wound fluid under a semiocclusive dressing,5,14 foams,15,16 alginates,17 and hydrogels.18,19 Dumville et al. performed four systematic reviews of the Cochrane database on each of the four dressing families to evaluate its role in the healing of diabetic ulcers.20–23 A systematic review by Wiechula suggests that moist wound-healing products have distinct clinical advantages over nonmoist products in the management of split-thickness skin donor sites.24
Treatment of wounds in a wet environment
The success of wet wound healing was espoused in 1861, where a report published by Hebra described the treatment of burns using “continuous baths.”25 Patients with major open burn wounds were treated by submersion in bathtubs. The treatment would continue for extended periods of time, and was found to reduce pain and weight loss. The patients survived until treatment in water was discontinued. During the Second World War, Bunyon, a Medical Officer and Lieutenant in the British Navy, treated wounded soldiers using the “envelope” method.26 This procedure also relied on the employment of fluid surrounding the wounded area. These historical examples represent two of the first described strategies to treat wounds in a wet environment.
When Bunyan added bleach to the fluid surrounding the wounds, he noticed less pain, reduced amount of necrotic tissue, and fewer infections in the patients who received the envelope treatment. Since then, several irrigation systems have been developed, for instance by Svedman,27 Kinetic Concepts Incorporated,28 and Zamirowski.29 Owens and Wenke suggested that earlier irrigation in a contaminated wound resulted in superior bacterial removal in their goat model.30 The authors, however, found in another study that irrigants other than saline solutions or high-pressure devices may not have the best clinical outcome regarding bacterial removal.31 Another article by the same group concludes that pulsed lavage is a more effective and efficient method of irrigation to remove bacteria in a complex musculoskeletal wound.32
To expand the utility of the treatments, antimicrobial solutions have been used as irrigation fluid. Collectively, these efforts illustrate the difficulties of delivering precise amounts of antimicrobials and antibiotics via an irrigation system. In practice, the concentrations of antimicrobials are kept quite low to avoid the possibility of toxicity. The above examples also illustrate the difficulties in producing a cost-effective device serving as a vehicle for the liquid.
In his study published in 1983, Svedman compared wound healing under saline irrigation with conventional saline dressings and concluded that wound contraction was similar under both conditions, but the wound blood flow, measured by a laser Doppler flowmeter, increased earlier with irrigation.33
In 1992, Breuing et al. published a study comparing the healing of partial-thickness burns and excisional porcine skin wounds in a wet environment to dry control wounds.34 The wet environment was achieved by encapsulating the wounds in a transparent chamber attached to the skin peripheral to the wound. Liquid containing antibiotics were delivered into the chamber through a port and left in place between 1 and 5 days. Outcome parameters studied included the amount of necrosis and exudates produced from the wound, as well as the amount of protein content and electrolytes in the wound fluid. The authors concluded that the depth of necrosis of the dermal wound bed was significantly reduced in wet wounds when using the wound chamber compared to a dry environment. Seven days after wounding, the necrosis in the partial-thickness wounds treated in a dry environment was 866 μm compared to zero in the wet environment. The study continued to attempt to identify markers of healing in the wounds. The wound fluid was collected daily, and the amounts of potassium, calcium, and total protein were assayed. The protein amount in the wound exudate reached zero at the time of complete re-epithelialization of the wounds. This was observed in the healing of burns as well as excisional wounds. In summary, the wound chamber protected the wound and stopped the injury progression in terms of necrosis. No adverse effects were noted on a normal skin or the wound itself.34
Modulating the wound microenvironment
By treating wounds in a controlled wet environment, delivery of antimicrobials, analgesics, other bioactive molecules such as growth factors, as well as cells and micrografts, is allowed. The objective when applying topical antimicrobial agents is to eradicate contamination, as well as prevent wound colonization. Due to the favorable concentration gradient that is achieved with topical application, the active concentration of antimicrobials at the wound site is increased by orders of magnitude compared to traditionally used IV delivery.35 According to the same principles, local analgesics will make a wound pain free. Before treatment methodologies based on topical administration of high-dose antibiotics are adapted in a clinical setting, thorough studies regarding toxicity and dosing regimens are required. In studies performed by us and others, no negative effects on the healing wound or the healthy surrounding skin have been observed. Clinical studies defining the specific indications and contraindications are the next step in assessing the potential of high-dose topical antibiotics in the treatment of wounds.
The addition of growth factors or transplantation of cells yields the possibility of creating a regenerative wound microenvironment that favors healing, as opposed to excessive scar formation.
Principally, the liquid in the chamber becomes a reservoir and acts as a sustained release system. The tissue absorption is easily deducted from the remaining concentration of the agent in the chamber after a certain time.
Antibiotics
In a clinical study by Vranckx et al., wet wound treatment was used in patients who had either not responded to conventional treatment or had wounds that were very difficult to treat with conventional methods (Fig. 1).35 The wounds were covered by wound chambers and vancomicin and gentamicin were diluted in saline and injected into the wound chamber. Antibiotic concentrations at the wound surface of up to 2,500 times the minimum inhibitory concentration for common bacteria could be achieved, while still limiting the total dose in the wound chamber to one single IV dose of the antibiotic for the particular patient. When the concentration of antibiotics was measured 2 days later, 20% or more of the original concentration remained in the chamber fluid. Since vancomicin and gentamicin are both excreted through glomerular filtration, systemic absorption of the drugs from the chamber fluid is the probable route of elimination. To prevent any systemic toxicity, the infusion of antibiotics in the chamber was limited to one daily i.v. dose per day for a particular patient. In 71% of these 28 wounds, complete healing was achieved during the planned treatment period.35 The wound chamber treatment was not found to cause any injury to the wound itself or to the surrounding intact skin.35
Figure 1.

Chronic venous stasis ulcer treated in a wet environment. (A) Wound after two failed debridements and split-thickness skin graft. (B) The wound chamber with antibiotics covering the wound. (C) The healed wound 3 weeks after débridement and split-thickness skin graft. (Reprinted with permission from Vranckx et al.35) To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Antifungal agents, such as amphotericin B, can also be introduced in the chamber. Further studies are required to evaluate their utility and possible toxicity.
Analgesics
Pain is a considerable problem in chronic wounds.36,37 An ibuprofen foam has been developed to provide both a moist environment for wound healing together with the reduction of temporary and persistent wound pain.38 A study by Cigna et al. suggests that the integration of ibuprofen with a polyurethane bio-occlusive dressing in the management of donor sites of split thickness skin grafts (STSG) resulted in faster wound healing compared to gauze dressings and almost eliminated pain and discomfort in all patients treated.39 This has also been investigated in venous leg ulcers in a randomized control double-blind clinical study.40 In the wet wound chamber environment analgesics, such as lidocaine, can be introduced to mitigate pain.
Growth factors
A number of growth factors have been showed to promote wound healing in animal models, most noteworthy are the epidermal (EGF), fibroblast (FGF), keratinocyte (KGF), platelet-derived (PDGF), and nerve (NGF) growth factors, as well as transforming growth factors (TGF) alpha and beta (Table 3).41–57 The only growth factor reported to have a substantial effect when applied topically to wounds in humans is the PDGF-BB, in promoting healing of refractory nondiabetic ulcers.58 Studies have shown that transplantation of keratinocytes along with ex vivo gene delivery of the EGF can increase healing of porcine full-thickness wounds (Fig. 2).59 Transplantation of insulin-like growth factor 1 (IGF-1)-expressing keratinocytes improves the healing of porcine full-thickness wounds. In summary, topical administration of growth factors seems to be somewhat effective, although the half-life and biological activity of introduced factors are sometimes difficult to predict.
Table 3.
Limited list of selected growth factors for modulation of wound microenvironment
| Growth Factor | In Vitro Effects | Products for Clinical Use |
|---|---|---|
| PDGF | Stimulates the production of other growth factors. Assumes a role in remodeling. |
Chemicon (Millipore) Becaplermin gel 0.01% (rhPDGF) |
| TGF-β | Regulates cellular migration and proliferation. Proteinase expression. Fibronectin binding interactions. Terminates cell proliferation. Stimulates collagen production. |
None |
| IGF-1 | Assumes a role in fibroblast proliferation and migration. Stimulates matrix production. |
None |
| VEGF | Stimulates angiogenesis. | None |
| Basic FGF (aka FGF-2) | Stimulates angiogenesis. Regulates cell migration and proliferation. |
None |
| KGF-1 (aka FGF-7)KGF-2 (aka FGF-10) | Enhances proliferation and migration of keratinocytes. Are downregulated in fetal wound repair. |
None |
| NGF | Regulates angiogenesis. Enhances functional properties of various inflammatory cells, including neutrophils, macrophages, and mast cells. Accelerates cutaneous wound-healing rate. |
None |
| EGF | Stimulates keratinocytes to produce hyaluronic acid. Stimulates fibroblast function. Implicated in cellular motility and migration during wound repair. Implicated in formation of granulation tissue. |
None |
All of the effects mentioned in the table above were studied in vitro or in animal models, and have yet to be proven clinically in humans, except for PDGF.
PDGF, platelet-derived growth factor; TGF-β, transforming growth factor beta; IGF-1, insulin-like growth factor 1; VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor; KGF, keratinocyte growth factor; NGF, nerve growth factor; EGF, epidermal growth factor.
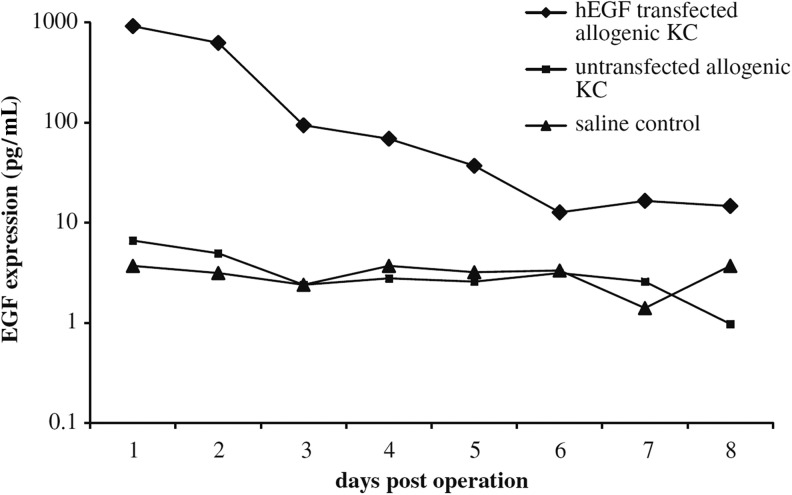
Figure 2.
In vivo expression of human epidermal growth factor (hEGF) in wound fluid retrieved daily from wound chambers and determined by ELISA. EGF expression: 920 pg/mL hEGF on day 1 and 624.5 pg/mL on day 2. In control wounds, EGF expression reached 6.6 pg/mL on day 1. (Reprinted with permission from Vranckx et al.59) KC, keratinocytes.
Transplantation of cells and micrografts in a wet/moist environment
Vogt et al. studied the transplantation of cells in a wet wound environment.60 It was found that single-cell suspensions of keratinocytes survive, proliferate, migrate to the surface of the wound, forming a new mature epidermis (Fig. 3). The neoepidermis has a basement membrane, basal layer, and undergoes the normal process of differentiation. The formation of these structures by the transplanted cells was confirmed by using transfected keratinocytes. In this study setup, low levels of antibiotics were used to reduce the risks of growth of resident opportunistic flora.
Figure 3.
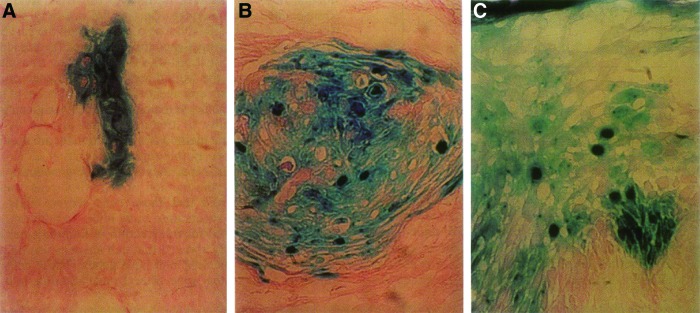
Reconstitution of new epithelium of porcine full-thickness wounds. X-Gal staining to demonstrate lacZ expression. (A) LacZ keratinocytes are first seen in clusters on the bottom of the wounds on day 4, (B) having migrated upward on day 6, and (C) are present in all layers of the epithelium by day 8. (Reprinted with permission from Vogt et al.60) To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Similar results can be achieved when subjecting autologous donor skin to mechanical mincing instead of enzymatic digestion and culture expansion. Skin minced into submillimeter particles and transplanted in a wet environment, will survive and contribute to re-epithelialization, regardless of orientation.61 Hackl et al. demonstrated the possible utility of a 100-fold expansion of a skin graft with complete wound coverage within 14 days in porcine wounds (Fig. 4).61 Kiwanuka et al. compared healing times and outcomes of full-thickness porcine excisional wounds treated with cultured autologous keratinocytes, STSGs, or minced skin micrografts.62 Transplantation of micrografts yielded results similar to STSGs in regard to re-epithelialization, scar formation, epidermal maturity, and the Vancouver Scar scale scores.
Figure 4.
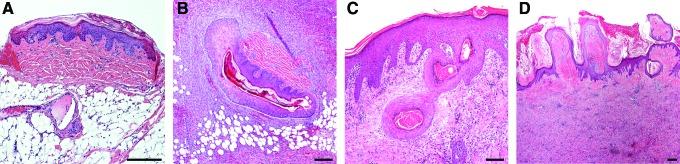
Hematoxylin and eosin–stained section showing porcine wound transplanted with micrografts. (A) A micrograft on the wound bed 1 h after transplantation. (B) A micrograft in wound 6 days after transplantation showing the micrograft with proliferating keratinocytes. (C) Micrografts in wound 10 days after transplantation. The stratum corneum of four different micrografts is surrounded by keratinocytes in different stages of migration. (D) Wound 14 days after transplantation showing the dermis and stratum corneum of transplanted micrografts in various stages of transepidermal elimination. Bar equals 200 μm in all panels. (Reprinted with permission from Hackl et al.61) To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Moist/wet treatment reduces scarring
Healing of adult human skin wounds is associated with varying degrees of scar formation.63 Scarring can be correlated with the intensity and duration of inflammation during healing.64 In 2009, Reish et al. published an experimental study performed in porcine wounds in which inflammation, in terms of numbers of inflammatory cells/high-power field 3 days after wounding, was compared in wounds treated either in a wound chamber or with gauze.65 Inflammation was greatly reduced in the wounds treated in the wound chamber, and there was a very strong correlation between the number of inflammatory cells on day 3 and the amount of scarring that had developed by day 28 (Fig. 5).65 Wet wounds created under sterile conditions, here treated with low concentrations of antibiotics, exhibited a significantly smaller macroscopic scar surface area in all experimental wound groups compared with dry wounds. Wounds with a greater inflammatory cell infiltrate could be predicted to develop more residual scarring.
Figure 5.
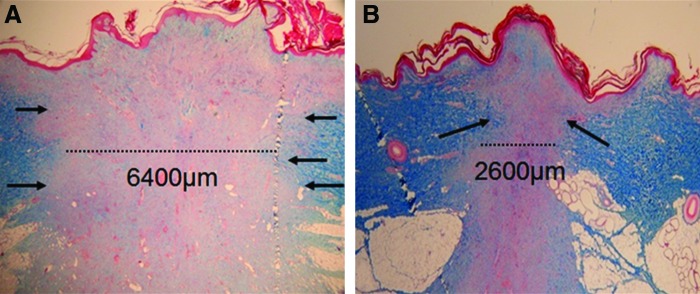
Wet wound healing reduces inflammation and scar formation. Masson's trichrome staining of (A) dry and (B) wet porcine wounds 28 days postwounding. The dry wound has a significantly greater width of scar tissue compared with the wet wound. Arrows indicate scar tissue borders. (Reprinted with permission from Reish et al.65) To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Take Home Messages.
Wet or moist wound treatment significantly reduces the time required for re-epithelialization, and leads to reduced inflammation, necrosis, and subsequent scar formation, as has been demonstrated in several large animal studies, as well as limited clinical studies
Wet or moist treatments have no adverse effects on the wound itself, or the surrounding tissue
A wet environment can allow for precise delivery of antimicrobial agents and analgesics to the healing wound
Wet environment has been shown to support transplanted cells and micrografts in large animal models and one clinical case, thereby accelerating healing
Soluble agents, for instance, growth factors or bioactive molecules, can be introduced in a highly controlled manner in a wet wound-healing environment
Abbreviations and Acronyms
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- HPF
high-power field
- IGF-1
insulin-like growth factor 1
- KGF
keratinocyte growth factor
- NGF
nerve growth factor
- PDGF
platelet-derived growth factor
- STSG
split thickness skin graft
- TGF
transforming growth factor
Author Disclosure and Ghostwriting
E.E. has filed a number of patents in the fields of negative pressure treatment and transplantation, and is also a member of an LLC that deals with wound healing. J.P.E.J., R.A.K., and E.J.C. have no competing financial interests. No ghostwriters were used to write this article.
About the Authors
Dr. Johan Junker is the Director of Wound Healing and Tissue Engineering Research at Brigham and Women's Hospital, and Instructor of Surgery at Harvard Medical School. His background is in tissue engineering, regenerative medicine, and wound healing. Dr. Rami Kamel is a research fellow at Brigham and Women's Hospital and Harvard Medical School. Dr. E.J. Caterson is a plastic surgeon at Brigham and Women's Hospital and Instructor of Surgery at Harvard Medical School. He is involved in clinical and experimental efforts to modulate wound healing and tissue engineer skin. Dr. Elof Eriksson is Chief of the Division of Plastic Surgery at Brigham and Women's Hospital, and Joseph E. Murray professor of Plastic and Reconstructive Surgery at Harvard Medical School. Dr. Eriksson has conducted wound-healing research for more than three decades.
References
- 1.Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293. doi: 10.1038/193293a0. [DOI] [PubMed] [Google Scholar]
- 2.Hinman CD. Maibach H. Effect of air exposure and occlusion on experimental human skin wounds. Nature. 1963;200:377. doi: 10.1038/200377a0. [DOI] [PubMed] [Google Scholar]
- 3.Korting HC. Schollmann C. White RJ. Management of minor acute cutaneous wounds: importance of wound healing in a moist environment. J Eur Acad Dermatol Venereol. 2011;25:130. doi: 10.1111/j.1468-3083.2010.03775.x. [DOI] [PubMed] [Google Scholar]
- 4.Field FK. Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S. doi: 10.1016/0002-9610(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 5.Singh A. Halder S. Menon GR. Chumber S. Misra MC. Sharma LK. Srivastava A. Meta-analysis of randomized controlled trials on hydrocolloid occlusive dressing versus conventional gauze dressing in the healing of chronic wounds. Asian J Surg. 2004;27:326. doi: 10.1016/S1015-9584(09)60061-0. [DOI] [PubMed] [Google Scholar]
- 6.Eaglstein WH. Moist wound healing with occlusive dressings: a clinical focus. Dermatol Surg. 2001;27:175. doi: 10.1046/j.1524-4725.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- 7.Madden MR. Nolan E. Finkelstein JL. Yurt RW. Smeland J. Goodwin CW. Hefton J. Staiano-Coico L. Comparison of an occlusive and a semi-occlusive dressing and the effect of the wound exudate upon keratinocyte proliferation. J Trauma. 1989;29:924. doi: 10.1097/00005373-198907000-00004. discussion 930. [DOI] [PubMed] [Google Scholar]
- 8.Svensjo T. Pomahac B. Yao F. Slama J. Eriksson E. Accelerated healing of full-thickness skin wounds in a wet environment. Plast Reconstr Surg. 2000;106:602. discussion 613. [PubMed] [Google Scholar]
- 9.Katz MH. Alvarez AF. Kirsner RS. Eaglstein WH. Falanga V. Human wound fluid from acute wounds stimulates fibroblast and endothelial cell growth. J Am Acad Dermatol. 1991;25:1054. doi: 10.1016/0190-9622(91)70306-m. [DOI] [PubMed] [Google Scholar]
- 10.Kerstein MD. Moist wound healing: the clinical perspective. Ostomy Wound Manage. 1995;41:37S. discussion 45S. [PubMed] [Google Scholar]
- 11.Dyson M. Young S. Pendle CL. Webster DF. Lang SM. Comparison of the effects of moist and dry conditions on dermal repair. J Invest Dermatol. 1988;91:434. doi: 10.1111/1523-1747.ep12476467. [DOI] [PubMed] [Google Scholar]
- 12.Dyson M. Young SR. Hart J. Lynch JA. Lang S. Comparison of the effects of moist and dry conditions on the process of angiogenesis during dermal repair. J Invest Dermatol. 1992;99:729. doi: 10.1111/1523-1747.ep12614460. [DOI] [PubMed] [Google Scholar]
- 13.Vogt PM. Andree C. Breuing K. Liu PY. Slama J. Helo G. Eriksson E. Dry, moist, and wet skin wound repair. Ann Plast Surg. 1995;34:493. doi: 10.1097/00000637-199505000-00007. discussion 499. [DOI] [PubMed] [Google Scholar]
- 14.Barnett A. Berkowitz RL. Mills R. Vistnes LM. Comparison of synthetic adhesive moisture vapor permeable and fine mesh gauze dressings for split-thickness skin graft donor sites. Am J Surg. 1983;145:379. doi: 10.1016/0002-9610(83)90206-4. [DOI] [PubMed] [Google Scholar]
- 15.Carter K. Hydropolymer dressings in the management of wound exudate. Br J Community Nurs. 2003;8(suppl 10) doi: 10.12968/bjcn.2003.8.Sup3.11579. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher J. The application of foam dressings. Nurs Times. 2003;99:59. [PubMed] [Google Scholar]
- 17.Piacquadio D. Nelson DB. Alginates. A “new” dressing alternative. J Dermatol Surg Oncol. 1992;18:992. doi: 10.1111/j.1524-4725.1992.tb02773.x. [DOI] [PubMed] [Google Scholar]
- 18.Eisenbud D. Hunter H. Kessler L. Zulkowski K. Hydrogel wound dressings: where do we stand in 2003? Ostomy Wound Manage. 2003;49:52. [PubMed] [Google Scholar]
- 19.Kickhofen B. Wokalek H. Scheel D. Ruh H. Chemical and physical properties of a hydrogel wound dressing. Biomaterials. 1986;7:67. doi: 10.1016/0142-9612(86)90092-x. [DOI] [PubMed] [Google Scholar]
- 20.Dumville JC. Deshpande S. O'Meara S. Speak K. Hydrocolloid dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2012;(2):CD009099. doi: 10.1002/14651858.CD009099.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Dumville JC. Deshpande S. O'Meara S. Speak K. Foam dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2011;(1):CD009111. doi: 10.1002/14651858.CD009111.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Dumville JC. O'Meara S. Deshpande S. Speak K. Alginate dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2012;(2):CD009110. doi: 10.1002/14651858.CD009110.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Dumville JC. O'Meara S. Deshpande S. Speak K. Hydrogel dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2011;(1):CD009101. doi: 10.1002/14651858.CD009101.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Wiechula R. The use of moist wound-healing dressings in the management of split-thickness skin graft donor sites: a systematic review. Int J Nurs Pract. 2003;9:S9. doi: 10.1046/j.1322-7114.2003.00417.x. [DOI] [PubMed] [Google Scholar]
- 25.Hebra F. Ueber kontinuierliche allgemeine Bäder und deren Anwendung bei Behandlung von Verbrennungen. Allg Wien med Ztg. 1861;6:351. [Google Scholar]
- 26.Bunyan J. Treatment of burns and wounds by the envelope method. Br Med J. 1941;2:1. doi: 10.1136/bmj.2.4200.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zelen CM. Stover B. Nielson D. Cunningham M. A prospective study of negative pressure wound therapy with integrated irrigation for the treatment of diabetic foot ulcers. Eplasty. 2011;11:e5. [PMC free article] [PubMed] [Google Scholar]
- 28.D'Hondt M. D'Haeninck A. Dedrye L. Penninckx F. Aerts R. Can vacuum-assisted closure and instillation therapy (VAC-Instill therapy) play a role in the treatment of the infected open abdomen? Tech Coloproctol. 2011;15:75. doi: 10.1007/s10151-010-0662-4. [DOI] [PubMed] [Google Scholar]
- 29.Ahearn C. Intermittent NPWT and lower negative pressures—exploring the disparity between science and current practice: a review. Ostomy Wound Manage. 2009;55:22. [PubMed] [Google Scholar]
- 30.Owens BD. Wenke JC. Early wound irrigation improves the ability to remove bacteria. J Bone Joint Surg Am. 2007;89:1723. doi: 10.2106/JBJS.F.01210. [DOI] [PubMed] [Google Scholar]
- 31.Owens BD. White DW. Wenke JC. Comparison of irrigation solutions and devices in a contaminated musculoskeletal wound survival model. J Bone Joint Surg Am. 2009;91:92. doi: 10.2106/JBJS.G.01566. [DOI] [PubMed] [Google Scholar]
- 32.Svoboda SJ. Bice TG. Gooden HA. Brooks DE. Thomas DB. Wenke JC. Comparison of bulb syringe and pulsed lavage irrigation with use of a bioluminescent musculoskeletal wound model. J Bone Joint Surg Am. 2006;88:2167. doi: 10.2106/JBJS.E.00248. [DOI] [PubMed] [Google Scholar]
- 33.Svedman P. Irrigation treatment of leg ulcers. Lancet. 1983;2:532. doi: 10.1016/s0140-6736(83)90567-6. [DOI] [PubMed] [Google Scholar]
- 34.Breuing K. Eriksson E. Liu P. Miller DR. Healing of partial thickness porcine skin wounds in a liquid environment. J Surg Res. 1992;52:50. doi: 10.1016/0022-4804(92)90278-8. [DOI] [PubMed] [Google Scholar]
- 35.Vranckx JJ. Slama J. Preuss S. Perez N. Svensjo T. Visovatti S. Breuing K. Bartlett R. Pribaz J. Weiss D. Eriksson E. Wet wound healing. Plast Reconstr Surg. 2002;110:1680. doi: 10.1097/01.PRS.0000033181.56887.61. [DOI] [PubMed] [Google Scholar]
- 36.Cooper SM. Hofman D. Burge SM. Leg ulcers and pain: a review. Int J Low Extrem Wounds. 2003;2:189. doi: 10.1177/1534734603260556. [DOI] [PubMed] [Google Scholar]
- 37.McMullen M. The relationship between pain and leg ulcers: a critical review. Br J Nurs. 2004;13:S30. doi: 10.12968/bjon.2004.13.Sup4.16357. [DOI] [PubMed] [Google Scholar]
- 38.Jorgensen B. Friis GJ. Gottrup F. Pain and quality of life for patients with venous leg ulcers: proof of concept of the efficacy of Biatain-Ibu, a new pain reducing wound dressing. Wound Repair Regen. 2006;14:233. doi: 10.1111/j.1743-6109.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- 39.Cigna E. Tarallo M. Bistoni G. Anniboletti T. Trignano E. Tortorelli G. Scuderi N. Evaluation of polyurethane dressing with ibuprofen in the management of split-thickness skin graft donor sites. In Vivo. 2009;23:983. [PubMed] [Google Scholar]
- 40.Gottrup F. Jorgensen B. Karlsmark T. Sibbald RG. Rimdeika R. Harding K. Price P. Venning V. Vowden P. Junger M. Wortmann S. Sulcaite R. Vilkevicius G. Ahokas TL. Ettler K. Arenbergerova M. Reducing wound pain in venous leg ulcers with Biatain Ibu: a randomized, controlled double-blind clinical investigation on the performance and safety. Wound Repair Regen. 2008;16:615. doi: 10.1111/j.1524-475X.2008.00412.x. [DOI] [PubMed] [Google Scholar]
- 41.Andree C. Swain WF. Page CP. Macklin MD. Slama J. Hatzis D. Eriksson E. In vivo transfer and expression of a human epidermal growth factor gene accelerates wound repair. Proc Natl Acad Sci USA. 1994;91:12188. doi: 10.1073/pnas.91.25.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beck LS. DeGuzman L. Lee WP. Xu Y. Siegel MW. Amento EP. One systemic administration of transforming growth factor-beta 1 reverses age- or glucocorticoid-impaired wound healing. J Clin Invest. 1993;92:2841. doi: 10.1172/JCI116904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benn SI. Whitsitt JS. Broadley KN. Nanney LB. Perkins D. He L. Patel M. Morgan JR. Swain WF. Davidson JM. Particle-mediated gene transfer with transforming growth factor-beta1 cDNAs enhances wound repair in rat skin. J Clin Invest. 1996;98:2894. doi: 10.1172/JCI119118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breuing K. Andree C. Helo G. Slama J. Liu PY. Eriksson E. Growth factors in the repair of partial thickness porcine skin wounds. Plast Reconstr Surg. 1997;100:657. doi: 10.1097/00006534-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 45.Brown GL. Curtsinger L. Jurkiewicz MJ. Nahai F. Schultz G. Stimulation of healing of chronic wounds by epidermal growth factor. Plast Reconstr Surg. 1991;88:189. [PubMed] [Google Scholar]
- 46.Buckley A. Davidson JM. Kamerath CD. Woodward SC. Epidermal growth factor increases granulation tissue formation dose dependently. J Surg Res. 1987;43:322. doi: 10.1016/0022-4804(87)90088-6. [DOI] [PubMed] [Google Scholar]
- 47.Dubay DA. Wang X. Kuhn MA. Robson MC. Franz MG. The prevention of incisional hernia formation using a delayed-release polymer of basic fibroblast growth factor. Ann Surg. 2004;240:179. doi: 10.1097/01.sla.0000131576.12153.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eming SA. Whitsitt JS. He L. Krieg T. Morgan JR. Davidson JM. Particle-mediated gene transfer of PDGF isoforms promotes wound repair. J Invest Dermatol. 1999;112:297. doi: 10.1046/j.1523-1747.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- 49.Galeano M. Deodato B. Altavilla D. Cucinotta D. Arsic N. Marini H. Torre V. Giacca M. Squadrito F. Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia. 2003;46:546. doi: 10.1007/s00125-003-1064-1. [DOI] [PubMed] [Google Scholar]
- 50.Greenhalgh DG. Sprugel KH. Murray MJ. Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990;136:1235. [PMC free article] [PubMed] [Google Scholar]
- 51.Hebda PA. Klingbeil CK. Abraham JA. Fiddes JC. Basic fibroblast growth factor stimulation of epidermal wound healing in pigs. J Invest Dermatol. 1990;95:626. doi: 10.1111/1523-1747.ep12513528. [DOI] [PubMed] [Google Scholar]
- 52.Lynch SE. Williams RC. Polson AM. Howell TH. Reddy MS. Zappa UE. Antoniades HN. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J Clin Periodontol. 1989;16:545. doi: 10.1111/j.1600-051x.1989.tb02334.x. [DOI] [PubMed] [Google Scholar]
- 53.Mellin TN. Mennie RJ. Cashen DE. Ronan JJ. Capparella J. James ML. Disalvo J. Frank J. Linemeyer D. Gimenez-Gallego G. Thomas KA. Acidic fibroblast growth factor accelerates dermal wound healing. Growth Factors. 1992;7:1. doi: 10.3109/08977199209023933. [DOI] [PubMed] [Google Scholar]
- 54.Muangman P. Muffley LA. Anthony JP. Spenny ML. Underwood RA. Olerud JE. Gibran NS. Nerve growth factor accelerates wound healing in diabetic mice. Wound Repair Regen. 2004;12:44. doi: 10.1111/j.1067-1927.2004.012110.x. [DOI] [PubMed] [Google Scholar]
- 55.Nanney LB. Epidermal and dermal effects of epidermal growth factor during wound repair. J Invest Dermatol. 1990;94:624. doi: 10.1111/1523-1747.ep12876204. [DOI] [PubMed] [Google Scholar]
- 56.Okwueze MI. Cardwell NL. Pollins AC. Nanney LB. Modulation of porcine wound repair with a transfected ErbB3 gene and relevant EGF-like ligands. J Invest Dermatol. 2007;127:1030. doi: 10.1038/sj.jid.5700637. [DOI] [PubMed] [Google Scholar]
- 57.Robson MC. Phillips LG. Thomason A. Robson LE. Pierce GF. Platelet-derived growth factor BB for the treatment of chronic pressure ulcers. Lancet. 1992;339:23. doi: 10.1016/0140-6736(92)90143-q. [DOI] [PubMed] [Google Scholar]
- 58.Harrison-Balestra C. Eaglstein WH. Falabela AF. Kirsner RS. Recombinant human platelet-derived growth factor for refractory nondiabetic ulcers: a retrospective series. Dermatol Surg. 2002;28:755. doi: 10.1046/j.1524-4725.2002.02004.x. discussion 759. [DOI] [PubMed] [Google Scholar]
- 59.Vranckx JJ. Hoeller D. Velander PE. Theopold CF. Petrie N. Takedo A. Eriksson E. Yao F. Cell suspension cultures of allogenic keratinocytes are efficient carriers for ex vivo gene transfer and accelerate the healing of full-thickness skin wounds by overexpression of human epidermal growth factor. Wound Repair Regen. 2007;15:657. doi: 10.1111/j.1524-475X.2007.00272.x. [DOI] [PubMed] [Google Scholar]
- 60.Vogt PM. Thompson S. Andree C. Liu P. Breuing K. Hatzis D. Brown H. Mulligan RC. Eriksson E. Genetically modified keratinocytes transplanted to wounds reconstitute the epidermis. Proc Natl Acad Sci USA. 1994;91:9307. doi: 10.1073/pnas.91.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hackl F. Bergmann J. Granter SR. Koyama T. Kiwanuka E. Zuhaili B. Pomahac B. Caterson EJ. Junker JP. Eriksson E. Epidermal regeneration by micrograft transplantation with immediate 100-fold expansion. Plast Reconstr Surg. 2012;129:443e. doi: 10.1097/PRS.0b013e318241289c. [DOI] [PubMed] [Google Scholar]
- 62.Kiwanuka E. Hackl F. Philip J. Caterson EJ. Junker JP. Eriksson E. Comparison of healing parameters in porcine full-thickness wounds transplanted with skin micrografts, split-thickness skin grafts, and cultured keratinocytes. J Am Coll Surg. 2011;213:728. doi: 10.1016/j.jamcollsurg.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 63.Atiyeh BS. Amm CA. El Musa KA. Improved scar quality following primary and secondary healing of cutaneous wounds. Aesthetic Plast Surg. 2003;27:411. doi: 10.1007/s00266-003-3049-3. [DOI] [PubMed] [Google Scholar]
- 64.Robson MC. Barbul A. Guidelines for the best care of chronic wounds. Wound Repair Regen. 2006;14:647. doi: 10.1111/j.1524-475X.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 65.Reish RG. Zuhaili B. Bergmann J. Aflaki P. Koyama T. Hackl F. Waisbren E. Canseco JA. Verma KD. Eriksson E. Yao F. Modulation of scarring in a liquid environment in the Yorkshire pig. Wound Repair Regen. 2009;17:806. doi: 10.1111/j.1524-475X.2009.00546.x. [DOI] [PubMed] [Google Scholar]