Abstract
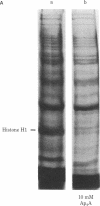
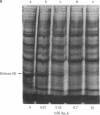
We have identified a proteolytic system that selectively degrades histone H1 in normal human lymphocytes. Treatment of permeabilized human lymphocytes with a series of nucleotides produced a marked decrease in their histone H1 content compared to untreated cells. The nucleotide-stimulated process was selective for histone H1 because gel electrophoresis showed that almost all other lymphocyte protein bands remained constant while histone H1 disappeared. The elimination of histone H1 appears to be the result of proteolysis by a trypsin-like enzyme because it was inhibited by phenylmethylsulfonyl fluoride, antipain, soybean trypsin inhibitor, and diisopropyl fluorophosphate. Proteolysis was stimulated by P1,P4-di(adenosine-5') tetraphosphate, P1,P3-di(adenosine-5') triphosphate, P1,P5-di(adenosine-5') pentaphosphate, adenosine 5'-tetraphosphate, ATP, adenosine 5'-[alpha, beta-methylene]triphosphate, adenosine 5'-[beta, gamma-methylene]triphosphate, ADP, CTP, GTP, UTP, dATP, or pyrophosphate, whereas AMP, adenosine, adenosine diphosphoribose, NAD+, cAMP, or sodium phosphate did not show this stimulation of proteolysis. ATP, [alpha, beta-methylene]ATP, [beta, gamma-methylene]ATP, and pyrophosphate all stimulated proteolysis, suggesting that a pyrophosphate linkage was necessary for this process. Thus, resting human lymphocytes contain a trypsin-like protease that is stimulated by nucleotides or pyrophosphate to selectively degrade histone H1.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen M. W., Ballal N. R., Goldknopf I. L., Busch H. Protein A24 lyase activity in nucleoli of thioacetamide-treated rat liver releases histone 2A and ubiquitin from conjugated protein A24. Biochemistry. 1981 Mar 3;20(5):1100–1104. doi: 10.1021/bi00508a009. [DOI] [PubMed] [Google Scholar]
- Aoyagi T., Takeuchi T., Matsuzaki A., Kawamura K., Kondo S. Leupeptins, new protease inhibitors from Actinomycetes. J Antibiot (Tokyo) 1969 Jun;22(6):283–286. doi: 10.7164/antibiotics.22.283. [DOI] [PubMed] [Google Scholar]
- Bartley J., Chalkley R. Further studies of a thymus nucleohistone-associated protease. J Biol Chem. 1970 Sep 10;245(17):4286–4292. [PubMed] [Google Scholar]
- Berger N. A., Adams J. W., Sikorski G. W., Petzold S. J., Shearer W. T. Synthesis of DNA and poly(adenosine diphosphate ribose) in normal and chronic lymphocytic leukemia lymphocytes. J Clin Invest. 1978 Jul;62(1):111–118. doi: 10.1172/JCI109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger N. A., Johnson E. S. DNA synthesis in permeabilized mouse L cells. Biochim Biophys Acta. 1976 Feb 18;425(1):1–17. doi: 10.1016/0005-2787(76)90211-2. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Heller H., Elias S., Haas A. L., Hershko A. ATP-dependent conjugation of reticulocyte proteins with the polypeptide required for protein degradation. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1365–1368. doi: 10.1073/pnas.77.3.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciehanover A., Hod Y., Hershko A. A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1100–1105. doi: 10.1016/0006-291x(78)91249-4. [DOI] [PubMed] [Google Scholar]
- DeMartino G. N., Goldberg A. L. Identification and partial purification of an ATP-stimulated alkaline protease in rat liver. J Biol Chem. 1979 May 25;254(10):3712–3715. [PubMed] [Google Scholar]
- Eickbush T. H., Watson D. K., Moudrianakis E. N. A chromatin-bound proteolytic activity with unique specificity for histone H2A. Cell. 1976 Dec;9(4 Pt 2):785–792. doi: 10.1016/0092-8674(76)90141-0. [DOI] [PubMed] [Google Scholar]
- Etlinger J. D., Goldberg A. L. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977 Jan;74(1):54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornells M., Subirana J. A. Specific degradation of histones H1 and H3. Biochem Biophys Res Commun. 1977 Sep 9;78(1):217–221. doi: 10.1016/0006-291x(77)91242-6. [DOI] [PubMed] [Google Scholar]
- Haas A. L., Warms J. V., Hershko A., Rose I. A. Ubiquitin-activating enzyme. Mechanism and role in protein-ubiquitin conjugation. J Biol Chem. 1982 Mar 10;257(5):2543–2548. [PubMed] [Google Scholar]
- Hagiwara H., Miyazaki K., Matuo Y., Yamashita J., Horio T. Novel protease bound with chromatins in normal and tumorous tissues of rats. Biochem Biophys Res Commun. 1980 Jun 16;94(3):988–995. doi: 10.1016/0006-291x(80)91332-7. [DOI] [PubMed] [Google Scholar]
- Hatcher V. B., Norin A. J. Expression of protease and protease-inhibitory activity in human mononuclear leukocyte cultures. Effect of conA stimulation. Exp Cell Res. 1982 Dec;142(2):471–476. doi: 10.1016/0014-4827(82)90392-5. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A., Rose I. A. Resolution of the ATP-dependent proteolytic system from reticulocytes: a component that interacts with ATP. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3107–3110. doi: 10.1073/pnas.76.7.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhn M., Albert W., Grummt F. Diadenosine tetraphosphate hydrolase from mouse liver. Purification to homogeneity and partial characterization. J Biol Chem. 1982 Mar 25;257(6):3003–3006. [PubMed] [Google Scholar]
- Ito S., Shizuta Y., Hayaishi O. Purification and characterization of poly(ADP-ribose) synthetase from calf thymus. J Biol Chem. 1979 May 10;254(9):3647–3651. [PubMed] [Google Scholar]
- Krueger R. C. The inhibition of a chromatin-bound proteolytic activity. Arch Biochem Biophys. 1982 Oct 15;218(2):619–622. doi: 10.1016/0003-9861(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Lennox R. W., Cohen L. H. The histone H1 complements of dividing and nondividing cells of the mouse. J Biol Chem. 1983 Jan 10;258(1):262–268. [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport E., Zamecnik P. C. Presence of diadenosine 5',5''' -P1, P4-tetraphosphate (Ap4A) in mamalian cells in levels varying widely with proliferative activity of the tissue: a possible positive "pleiotypic activator". Proc Natl Acad Sci U S A. 1976 Nov;73(11):3984–3988. doi: 10.1073/pnas.73.11.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Suda H., Aoyagi T., Hamada M., Takeuchi T., Umezawa H. Antipain, a new protease inhibitor isolated from actinomycetes. J Antibiot (Tokyo) 1972 Apr;25(4):263–266. doi: 10.7164/antibiotics.25.263. [DOI] [PubMed] [Google Scholar]
- Suda H., Aoyagi T., Takeuchi T., Umezawa H. Letter: A thermolysin inhibitor produced by Actinomycetes: phospholamidon. J Antibiot (Tokyo) 1973 Oct;26(10):621–623. doi: 10.7164/antibiotics.26.621. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Aoyagi T., Morishima H., Matsuzaki M., Hamada M. Pepstatin, a new pepsin inhibitor produced by Actinomycetes. J Antibiot (Tokyo) 1970 May;23(5):259–262. doi: 10.7164/antibiotics.23.259. [DOI] [PubMed] [Google Scholar]
- Watson D. K., Moudrianakis E. N. Histone-dependent reconstitution and nucleosomal localization of a nonhistone chromosomal protein: the H2A-specific protease. Biochemistry. 1982 Jan 19;21(2):248–256. doi: 10.1021/bi00531a008. [DOI] [PubMed] [Google Scholar]
- Wilkinson K. D., Urban M. K., Haas A. L. Ubiquitin is the ATP-dependent proteolysis factor I of rabbit reticulocytes. J Biol Chem. 1980 Aug 25;255(16):7529–7532. [PubMed] [Google Scholar]