Abstract
Significance
The skin interfollicular epidermis (IFE) is an organism's first line of defense against a harmful environment and physical damage. During homeostasis and wound repair, the IFE is rejuvenated constantly by IFE stem cells (SCs) that are capable of both proliferation and differentiation. However, the identity and behavior of IFE SCs remain controversial.
Recent Advances
Two opposing theories exist regarding homeostasis of the IFE. On the basis of morphological and proliferative characteristics, one posits that the IFE is composed of a discrete epidermal proliferative unit comprised of ∼10 transit-amplifying (TA) cells and a centrally located SC in the basal layer. The other suggests that homeostasis of the IFE is maintained by a single progenitor population in the basal layer. A recent study has challenged these two apparently distinct models and demonstrated that the basal layer of the IFE contains both SCs and TA cells, which make distinct contributions to tissue homeostasis and repair. Moreover, phosphorylation levels of the transcription factor p63, the master regulator of the proliferative potential of epidermal SCs, can be used to distinguish self-renewing SCs from TA cells with more limited proliferative potential.
Critical Issues
As technologies advance, IFE SCs can be identified at a single-cell level. Refinements of their identification and characterization are critical, not only for SC biology but also for the development of novel clinical applications.
Future Directions
Understanding the signaling pathways that control self-renewal and differentiation of IFE SCs will aid in developing novel cell-based therapeutics targeting degenerative epidermal diseases and wound repair.

Makoto Senoo, PhD
Scope and Significance
In this review, I discuss some of the major findings that have advanced our understanding of the behavior of epidermal stem cells (SCs) and their immediate progeny, transit-amplifying (TA) cells. Particular emphasis is paid to those in the interfollicular epidermis (IFE), in light of their importance in homeostasis and tissue regeneration after injury. I also discuss the recent key findings on the regulation of p63, a transcription factor essential for the maintenance of the proliferative potential of epithelial SCs in both homeostatic and disease conditions of the epidermis, such as chronic wound healing.
Translational Relevance
The role of epidermal SCs in contributing to homeostatic maintenance of the skin and wound repair has been well acknowledged for many years. Over the past decade, characterization of SCs and their differentiating progeny has been successfully refined, owing to the development of nucleotide-labeling and lineage-tracing methodologies. Elucidating the molecular mechanisms controlling the behavior of these cell types will provide novel strategies for the treatment of traumatic and degenerative skin diseases. As p63 is highly expressed in SCs of other epithelial tissues as well as many types of tumors of epithelial origin, these studies will also contribute to our understanding of a wide array of SC-related epithelial diseases.
Clinical Relevance
The cutaneous epidermis provides the first line of protection against environmental assaults. However, chronic wounds affect over 6 million patients in the United States alone (2% of the population), and this number is expected to increase with the rapid expansion of elderly, diabetic, and obese populations. A wide array of management options is being developed, including tissue-engineered skin products, topical application of growth factors, negative pressure, electrical stimulation, and ultrasonography therapies. However, the ultimate results of these exogenous treatments are still far from satisfactory. Therapeutic modulation of autologous SCs and/or their differentiating progeny may help to accelerate tissue repair with a more desirable outcome.
Introduction
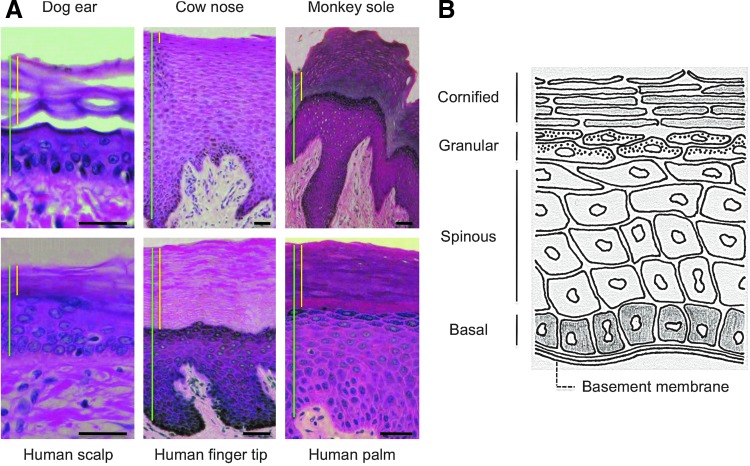
Skin is composed of an underlying dermis of mesodermal origin and overlaying epidermis of ectodermal origin. Epidermis is a stratified epithelium comprised of a basal layer of proliferative cells and suprabasal layers of more differentiated cells that undergo terminal differentiation after a few rounds of cell division.1 The overall structure of the skin epidermis is well conserved across different mammalian species and body sites (Fig. 1A).
Figure 1.
Differentiation of the interfollicular epidermal (IFE) cells. (A) Histology of the epidermis at different body sites in various mammalian species. Shown are images of hematoxylin–eosin-stained sections. Vertical bars in green and yellow indicate total epidermis and the cornified layers, respectively. Bars=50 μm. (B) Schematic representation of the stratified layers of the IFE. The proliferative basal layer is adjacent to the basement membrane at the base. The three differentiation stages include the spinous layer, granular layer, and the cornified layer at the surface of the epidermis. Color images available online at www.liebertpub.com/wound
Stratification of the epidermis initiates during embryonic development and continues throughout life. Some basal cells divide asymmetrically, withdraw from the cell cycle, and detach from the basement membrane to initiate terminal differentiation. The process involves the outward movement of basal cells toward the surface of the skin (Fig. 1B). The basement membrane serves not only as a physical boundary between epithelial cells and dermal cells but also as a proliferation-promoting platform, as it is rich in both extracellular matrix proteins and growth factors.1 The basal cells differentiate first to spinous cells, then to granular cells, and ultimately to enucleated cornified cells that are shed from the surface of the skin. The minimum transit time of basal cells to the cornified layer is estimated to be 28 days in humans and 8–9.5 days in mice.2,3 During homeostasis and wound repair, lost cells are eventually replaced in a process initiated by proliferative basal cells consisting of both SCs and TA cells, early progeny of SCs.
Despite their importance in SC biology and clinical applications, it has been challenging to identify and characterize epidermal SCs and TA cells to determine whether the skin epidermis is maintained by single type of progenitor or by a stem/TA cell hierarchy. Recent advancements in label-retaining and lineage-tracing methodologies have contributed to our understanding of the intrinsic properties of SCs and their differentiated progeny in their native environment.
Discussion of Findings and Relevant Literature
Stem Cells in the Epidermis
Recent studies have shown that there are a number of SC repositories within the adult epidermis, including cells from the bulge region of the hair follicles (HFs) and keratinocytes of the IFE and sebaceous gland.1 Several lines of evidence suggest that multipotent SCs in the epidermis reside within the bulge region of the HFs,1 although clonal analysis of individual bulge cells will be necessary to determine if all bulge SCs are multipotent, or if the bulge harbors a mixture of multiple types of unipotent SCs.
Nucleotide pulse labeling has been used to demonstrate the slow-cycling nature of bulge SCs.4,5 This pulse-chase concept has been adapted to lineage-tracing models utilizing transgenic mice that express an inducible Cre recombinase under the control of either the keratin-15 (K15) or the Leu-rich repeat-containing G-protein-coupled receptor-5 (Lgr5) promoter, crossed onto the Rosa26 locus (R26)-floxed-reporter strain, in which a conditional allele of β-galactosidase or enhanced green fluorescent protein (EGFP) is targeted to the Rosa26 locus.6,7
While K15 was the first molecular marker of the bulge cells,6 Lgr5 is a recently identified marker for SCs in various epithelial tissues, including the HFs.8
These studies have shown that genetically marked slow-cycling bulge SCs contribute to the HF in the long term. Importantly, such genetic tracing experiments also indicate that upon wounding, ∼25%–50% of cells in the repaired IFE are of HF origin.9–11 In sharp contrast, marked cells contribute only minimally, if at all, to homeostatic maintenance of the IFE, underscoring the existence of a distinct SC population in the IFE. This notion has been exemplified by the analysis of Edaradd mutant mice, whose tail skin completely lacks HFs, due to an impaired ectodysplasin receptor (Edar)-signaling pathway.12 The clonogenic potential of Edaradd mutant tail skin is significantly lower than that of normal skin, but is nevertheless able to slowly repair its wounds. Notably, the absence of the HF input leads to recruitment of epidermal cells from a wider area surrounding the wound, suggesting that IFE SCs sense the need to heal wounds without an input from bulge SCs. We also know that injuries on hairless body sites such as the palm of the hand, the sole of the foot, and the lips can heal quite efficiently. Together, these observations indicate that surface epidermis harbors its own SC pool that maintains homeostasis and responds to wounding.
Monitoring SCs in the IFE
The epidermal proliferative unit
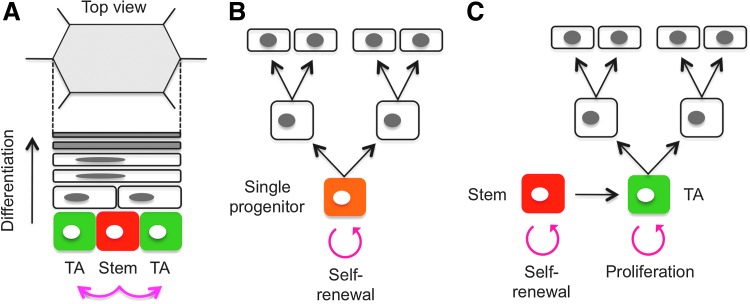
Although researchers have known for years that SCs exist within the basal layer of the IFE, it has not been clear whether all basal cells are SCs or whether the basal layer is composed of heterogeneous cell populations of which only a small number of cells are SCs. Based on architectural and proliferation studies, it was proposed in the 1970s that the IFE is organized into discrete epidermal proliferative units (EPUs).13,14 Each EPU is comprised of ∼10 tightly packed basal cells, giving rise to increasingly larger and flatter cells that are stacked into a hexagonal column at the skin surface, leading to the hypothesis that EPUs consist of one centrally placed slow-cycling SC surrounded by short-lived TA cells (Fig. 2A). In recent studies, clonal marking of IFE cells by retroviruses or transgenesis has confirmed long-lived columns of labeled IFE cells that reach from the basal to the cornified layer of the epidermis.15 Thus, the EPU model has gained wide acceptance to address homeostatic maintenance of the IFE. However, as described below, this EPU model has been the subject of debate in more recent studies.16
Figure 2.
Maintenance of the epidermal homeostasis. (A) Diagram of the epidermal proliferative unit (EPU). A centrally located putative slow-cycling stem cell (SC) (red) occasionally divides, giving rise to an SC daughter and a transit-amplifying (TA) cell (green) within the basal layer. The TA cells undergo several rounds of cell division before terminally differentiating. Each EPU is comprised of ∼10 basal cells, giving rise to increasingly larger and flatter suprabasal cells that are stacked into a hexagonal column at the surface. (B) Diagram of the single-progenitor model. Clayton et al. utilized an inducible AhCreER/R26-floxed-EYFP reporter system to label epidermal cells.17 In this model, homeostasis of the IFE is maintained by a single-progenitor population (orange) without involving a TA cell pool, and the average number of labeled basal cells increases linearly with time. (C) Diagram of the stem/TA cell hierarchy model. Mascré et al. utilized two functionally different promoters (K14 and Inv) that target distinct epidermal progenitor populations.21 The average size of persisting Inv-CreER-targeted clones with basement membrane attachment increases linearly with time, whereas the number of surviving clones progressively falls, suggesting that the Inv promoter marks actively proliferating TA cells (green). In contrast, although K14-CreER-targeted clones abruptly expand in size immediately after induction in the basal layer, their growth rapidly decelerates, and survival is long term, suggesting that the K14 promoter marks both TA cells and slow-cycling SCs (red). This new model argues against the single-progenitor theory and supports a hierarchical organization of stem/TA cells in the IFE. Color images available online at www.liebertpub.com/wound
The single-progenitor model
According to the stem/TA cell hypothesis, the IFE contains slowly cycling basal SCs that produce TA cells with a more limited proliferative potential. However, an alternative model of epidermal homeostasis has recently been proposed based on quantitative lineage tracing in the tail epidermis of mice, where the EPU was originally described. In this study, Clayton et al. utilized inducible in vivo genetic marking of epidermal cells and proposed a new model in which a single population of progenitor cells maintains homeostasis of the epidermis without involving a TA cell pool (Fig. 2B).17
To label epidermal cells, animals transgenic for the tamoxifen-regulated mutant of Cre recombinase, expressed from the control of the β-naphthoflavone-inducible ubiquitous CYP1A1 promoter (AhCreER), were crossed onto the R26-floxed-EYFP reporter strain. Cells expressing enhanced yellow fluorescent protein (EYFP) and their labeled progenitors were detected in confocal whole-mount microscopy after a single injection of a combination of the inducing drugs.
Using this system, the authors investigated the behavior of labeled clones that persisted long term. At 2 weeks postinduction, expression of EYFP was detected in ∼1 in 600 basal cells, but not in the bulge region of the HFs. If individual SCs retained their self-renewal capacity, the stem/TA cell model predicts that the clone-size distribution in the basal layer would remain constant, a presumptive characteristic of a single EPU. However, analysis of cohorts of mice demonstrated that the average number of basal cells within persisting clones increased linearly with time, leading to a new model in which the IFE is maintained by a single type of epidermal progenitor cell that undergoes an unlimited number of cell divisions.17 Similar conclusions were drawn from a separate study of mouse ear epidermis,18 further discounting the existence of a discrete population of slow-cycling SCs as proposed in the EPU hypothesis.13,14
More recently, the single progenitor theory has been tested in a study of mouse esophageal epithelium that consists of layers of keratinocytes.19 Similar to the skin, proliferation of esophageal epithelium is confined to the basal layer. As proliferating cells differentiate, they exit the cell cycle and migrate to the tissue surface from which they are sloughed. The lack of secondary structures, such as glands or crypts that form an SC niche in other epithelia,20 makes the esophagus an ideal organ in which to assess the contribution of basal cells to epidermal homeostasis.
To investigate whether slow-cycling or quiescent epithelial SCs exist in the esophageal epithelium, the authors used a transgenic label-retaining cell assay in Rosa26rtTA/TetO-HGFP mice, in which expression of a Histone–2B/EGFP fusion protein (HGFP) is seen throughout the tissue upon induction with doxycycline (Dox). When Dox is withdrawn, HGFP is diluted two fold in each cell division. Quantification of the HGFP signals in the esophageal epithelium revealed strikingly homogeneous cell division in the basal layer at a rate of approximately twice per week. After a 4-week chase, only 0.4% of the basal cells retained label, of which essentially all cells were positive for CD45, a marker for Langerhan's cells and lymphocytes. Based on these findings, the authors conclude that there are no slow-cycling or quiescent SCs in the esophageal epithelium.19
To further address this issue, the authors used inducible Cre-lox-based genetic marking as described above.17,18 The fate of single-cell-derived clones was followed in cohorts of induced mice at multiple time points over a year postinduction. These studies revealed that the average size of persisting clones with basal cell labeling increased linearly with time, while persisting clone numbers decreased through differentiation. These data are consistent with those in the IFE, and support the conclusion that homeostasis of the esophageal epithelium is maintained by a single type of progenitor cells and does not involve TA cells.
Revival of the stem/TA cell theory
Although the single-progenitor model is attractive in its simplicity, it is important to note that, if present, a small quiescent population of SCs would be undetectable in the analyses described above. Indeed, a new study by Mascré et al. utilizing two different promoters that function in distinct epidermal progenitor populations clearly demonstrated that a stem/TA cell hierarchy differentially contributes to IFE homoeostasis (Fig. 2C).21 In these studies, a very low dose of tamoxifen given to R26-floxed-EYFP mice that were crossed to mice expressing CreER under the control of the keratin-14 promoter (K14-CreER) promoted EYFP expression at clonal density in the basal layer of tail IFE. In contrast, when CreER was expressed under the control of the involucrin promoter (Inv-CreER), EYFP-expressing cells were predominantly in the suprabasal layers, although EYFP-expressing basal cells were also observed at a low frequency. The basal cells marked in each case were both IFE progenitors, as both populations were able to proliferate and differentiate to form columns of labeled cells that span the basal to cornified layer. From a detailed study of the transition of the labeled cells, coupled with mathematical modeling, the authors deduced that both K14-CreER- and Inv-CreER-targeted progenitors follow identical cell division patterns: ∼80% divide asymmetrically, yielding one daughter SC/progenitor and one more differentiated cell, whereas the remaining divisions are equally balanced between symmetric duplication and symmetric differentiation, consistent with other reports in the IFE cells and esophageal epithelium.17,19
Despite their similar division patterns, many of the clones marked by K14-CreER survived up to 1 year postinduction, whereas the vast majority of the Inv-CreER-marked clones were progressively lost in a relatively short term, suggesting that K14-CreER-targeted clones contain cells with a higher proliferative potential than Inv-CreER-marked progenitors. DNA microarray analysis comparing these two populations supports this notion. To exclude differentiated cells from each subpopulation, the authors used fluorescence-activated cell sorter–purified EYFP-positive immature α6+CD34− cells. Gene ontology classification revealed that genes preferentially expressed in K14-CreER-marked α6+CD34− cells were enriched in functional groups involved in cell cycle regulation, chromosome segregation, cell proliferation, and DNA repair. By contrast, Inv-CreER-targeted α6+CD34− cells preferentially expressed genes known to control epidermal cell differentiation and keratinization.
The functional difference between these two distinct pools of progenitors became more apparent in long-term chase analyses of their behavior within the labeled columns. The average size of surviving clones marked by the Inv-CreER (as measured by their total size and labeled basal cells) grew linearly over the 48-week time course, whereas the number of surviving clones progressively fell. Such behavior mirrors that reported previously using an ubiquitous promoter,17 and indicates that Inv-CreER marks the actively cycling TA cell population, in which stochastic cell loss through differentiation is compensated for by duplication. In contrast, although K14-CreER-targeted clones abruptly expanded in size beyond that of Inv-CreER-marked clones immediately after induction, their growth in the basal layer rapidly decelerated over the first few weeks, showing only a modest expansion at later times. These observations suggest that the K14 promoter targets both actively cycling TA cells and slowly dividing SCs.
Together, this new study argues against the single-progenitor theory and proposes the existence of a hierarchical organization of stem/TA cells that differentially contribute to homeostasis of the IFE.
Maintenance of Epidermal Stem Cells
Epidermal SC culture
The key features of SCs are their immense capacity for self-renewal and differentiation. The presence of SCs in adult tissues was first demonstrated in the hematopoietic system in the 1960s,22 where the so-called colony-forming units were produced by short-term culture of isolated murine bone marrow cells that were then individually transplanted into immune-deficient recipients to reconstitute the hematopoietic system. In the 1970s, long-term passage of SCs in vitro began with pioneering work in clonogenic cultures of human epidermal SCs.23 Successful utilization of cultured human keratinocytes for burn therapy and the absence of skin cancers in patients engrafted decades ago suggest that long-term in vitro culture of epidermal SCs maintains their proliferative potential, yet avoids malignant transformation.
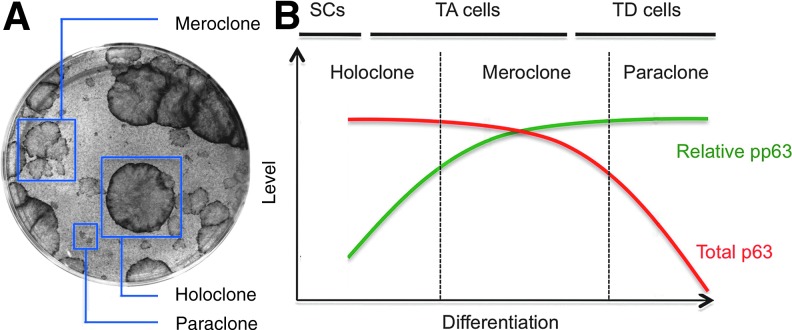
During in vitro culture, epidermal cells form three types of clones: holoclones, meroclones, and paraclones, in decreasing order of proliferative potential and increasing order of differentiation (Fig. 3A).24 Holoclones consist primarily of SCs, as they are morphologically immature, express high levels of SC markers such as β1 integrin and K14, and exhibit the greatest proliferative potential.24,25 A long-term culture of holoclones generates meroclone-producing cells, committed epidermal progenitors with more limited proliferative potential. Paraclones are composed of terminally differentiated cells that are not capable of proliferation and express high levels of markers for terminal differentiation such as Inv.24 As such, these cultures are ideal not only for expanding epidermal SCs for therapeutic options but also for studying SC self-renewal and differentiation.
Figure 3.
Phosphorylation of p63 during epidermal SC differentiation. (A) Clonogenic culture of epidermal SCs. In these cultures, epidermal cells form three types of clones: holoclones, meroclones, and paraclones in decreasing order of the proliferative potential and increasing order of differentiation. Holoclones consist primarily of SCs, as they are morphologically immature and exhibit the greatest proliferative potential, while meroclone-producing cells represent committed progenies with more limited proliferative potential. Paraclones are composed of terminally differentiated cells that are not capable of proliferation. (B) Increase in p63 phosphorylation during epidermal SC differentiation. Expression of p63 is uniformly high in holoclones, while meroclones express reduced levels, and paraclones show only modest levels of p63. Before the decrease in p63 expression, p63 phosphorylation increases in centrally located cells within holoclones, which in turn leads to the generation of meroclone-forming cells in the subsequent passage. Thus, p63 phosphorylation levels distinguish self-renewing epidermal SCs from TA cells with more limited proliferative potential.25 pp63, phosphorylated p63; TD, terminally differentiated. Color images available online at www.liebertpub.com/wound
p63: The master regulator of epidermal SCs
Upon searching for a homolog of the tumor suppressor p53 and its first homolog p73, we and other groups discovered the second p53 homolog, p63 (called variably Ket, p40, p51, p63, and p73L in the original literature), in the late 1990s.26 Unlike p53, p63 mutations in human cancers are very rare. Instead, p63 mutations are responsible for a variety of inheritable ectodermal dysplasias that exhibit abnormalities in many ectodermal tissues, including the skin.27 Mutant mice lacking p63 are severely compromised in skin development.28,29 The p63 gene encodes both a transactivating version (TAp63) and a dominant-negative isoform (ΔNp63), due to the usage of two different promoters. Amino terminal-specific functions of p63 were discerned by generating isoform-specific knockout mice, which demonstrate that ΔNp63, but not TAp63, is responsible for the phenotypes seen in p63-null mice.30,31 In contrast, analysis of TAp63-specific knockout mice has revealed that this isoform has diverse functions, including the protection of female germ cells from apoptosis,32 prevention of metastasis of epithelial tumors,33 and suppression of aging and obesity.30,34 However, as both TAp63 and ΔNp63 can be alternatively spliced at the carboxyterminus to produce α-, β-, γ-, δ-, and ɛ-isoforms,35,36 further investigation is needed to elucidate specific functions of each specific isoform. Notably, however, ΔNp63α is the dominant isoform expressed in the basal layer of epithelial tissues35 and has been the focus in epithelial SC biology.
Using clonogenic cultures of epidermal SCs, we have shown previously that p63 plays an essential role in the maintenance of SC proliferative potential.37 While expression of p63 is uniformly high in holoclones, meroclones express reduced levels, and paraclones show only modest levels of p63 (Fig. 3B). Consistent with this observation, knockdown of p63 by shRNA-mediated silencing in holoclone-forming cells leads to more profound generation of meroclones and paraclones with increased expression of keratinocyte differentiation markers.37 Using the same clonogenic cultures, it was demonstrated earlier that human epidermal cells that exhibit higher levels of β1 integrin had greater proliferative potential than other β1 integrin-positive basal cells.38 These two findings provide a potential link between cell intrinsic regulation by p63 and signaling through transmembrane core components of focal adhesions, which are required for basement membrane assembly, cell adhesion, and cell proliferation. Notably, p63 appears to directly regulate expression of extracellular matrix adhesion molecules, including β1 integrin.39
Although the mechanisms underlying the regulation of p63 expression are not fully understood, recent studies have shown that p63 expression is reduced at both protein and mRNA levels during the basal-to-suprabasal transition. For example, suprabasal signaling through Notch receptors leads to the suprabasal repression of p63 and inhibition of proliferation.40 Similarly, the ubiquitin (Ub) protein ligase (E3) Itch, preferentially expressed in the suprabasal layers, ubiquitilates, and degrades p63.41 The involvement of microRNAs (miRNAs) provides an additional layer of complexity to the regulation of p63 levels. miR-203, which targets and degrades the p63 mRNAs, is evolutionarily conserved and is expressed suprabasally.42 Moreover, ectopic expression of miR-203 in basal cells induces premature differentiation and diminishes proliferative potential, whereas in its absence, cell proliferation is no longer restricted to the basal layer.42 The collective view emerging from these studies is that the transition of epidermal cells from the basal to suprabasal layers activates a switch in fate determination.
However, as exemplified in the stem/TA cell model, epidermal basal cells are heterogeneous, and the SC differentiation program initiates within the basal layer. This is particularly interesting in light of our own finding that cells expressing high levels of p63 (p63hi) in the IFE basal layer exhibit a wide range of p63 phosphorylation levels.25 As phosphorylation of p63 ultimately leads to its degradation by proteasome-mediated pathways,43 these findings led us to hypothesize that increased p63 phosphorylation marks the initiation of SC differentiation. Indeed, close inspection of holoclones revealed that while p63 levels were uniformly high throughout the clones, phosphorylation of p63 was variable and higher toward the clone center. Propagation in serial passages of isolated peripheral and central cells showed that p63hi cells with low phosphorylation had significantly higher proliferative potential than p63hi cells with high phosphorylation.25 These data clearly indicate that phosphorylation of p63 inversely correlates with the proliferative potential and that phosphorylation levels of p63 distinguish self-renewing SCs from TA cells with more limited proliferative potential (Fig. 3B).
In vivo, expression of p63 is generally high in the basal layer and is reduced in the suprabasal layers; however, some suprabasal cells express p63 at levels that are equivalent to p63hi basal cells. Interestingly, virtually all p63hi suprabasal cells exhibit high p63 phosphorylation.25 Together with clonogenic analysis of the proliferative potential, these findings suggest that p63hi basal cells with low phosphorylation represent SCs, while p63hi cells with high phosphorylation represent more differentiated progenitor cells that are found in both the basal and suprabasal layers. Investigation of the signals responsible for p63 phosphorylation should provide essential insight into self-renewal and differentiation of epidermal SCs in future studies.
Epidermal Stem Cells and Wound Healing
As discussed above, the IFE is capable of tissue regeneration upon injury. However, the relative contribution of IFE SCs and TA cells to wound healing is currently unknown. To investigate their respective contributions during tissue repair, Mascré et al. used the system described above to mark basal progenitors by titrating the dose of tamoxifen so as to label Inv-CreER and K14-CreER epidermis at roughly the same density.21 Animals were then subjected to full-thickness wounding of the tail epidermis, and the contribution of marked cells during wound repair was analyzed by whole-mount confocal microscopy. K14-CreER-labeled cells formed large clones with a broad basal attachment and a large number of differentiated cells that persisted long term within the wound. In contrast, Inv-CreER-targeted cells formed a small cluster of cells in the wound, and the majority of clones quickly lost basal attachment. These data demonstrate that K14-CreER-marked SCs are capable of extensive tissue regeneration, whereas Inv-CreER-labeled TA cells have only a limited contribution to wound healing.
Although these studies indicate that SCs contribute substantially to the repair of the tissue, TA cells make only a limited contribution. However, it was not ascertained if the K14-CreER-labeled cells that expand in response to injury represent SCs or TA cells. Indeed, the majority of epidermal cells in healing wounds in human express high levels of p63.44 Our recent analysis of the changes in p63 phosphorylation during wound repair suggests that these expanding cells in wounds are TA cells, as they exhibit high levels of p63 phosphorylation (Suzuki and Senoo, unpublished work). Notably, in contrast to the low levels of p63 expression in the suprabasal layers of unperturbed epidermis, the majority of IFE cells in repairing wounds express high levels of p63. These findings suggest that during wound healing, phosphorylated p63 is protected from degradation, ensuring continued proliferation of TA cells during wound repair. In support, a short pulse with 5-bromo-2′-deoxyuridine revealed that newly formed TA cells within wounds exhibit the highest proliferative response among all p63-positive IFE cells. These observations support a model in which wounding stimulates SCs to produce more TA cells, which then contribute to tissue repair by proliferation and differentiation.
Together, these studies suggest that the roles of IFE SCs and TA cells during wound repair are likely distinct: SCs produce highly proliferative juvenile TA cells through their self-renewal activity, whereas TA cells play an immediate role in reconstituting damaged tissue. As such, the hyperproliferation and incomplete differentiation of suprabasal cells observed in nonhealing wounds45 may result from deregulation of TA cell functions. Indeed, activation of c-myc promotes pathogenesis of chronic wounds caused by enhanced SC differentiation to TA cells.46,47 As p63 and c-myc share common SC regulatory pathways such as the Wnt-signaling pathway,48,49 elucidation of their cooperative roles should promote our understanding of SC differentiation in homeostasis and wound repair. Notably, the Wnt-signaling pathway is activated during wound healing,49 and it would be interesting to investigate whether modulation of the Wnt-signaling pathway can control SC differentiation and/or TA cell activity by altering p63 phosphorylation during tissue repair of the epidermis.
Concluding Remarks
Identification and characterization of SCs and TA cells, which together are responsible for tissue homeostasis and wound repair of the IFE, have been challenging. Technical advancement in recent years has contributed to our understanding of the intrinsic properties of SCs and their differentiating progeny in their native environment. Although many questions remain to be answered, each approach has brought refined insights into these fascinating and clinically important tissue residents. As our understanding of SC biology progresses through basic science research, novel cell-based therapies will continue to emerge to improve the clinical outcome in regenerative medicine of the epidermis. Targeting the specific function of SCs and TA cells and their regulatory machineries may lead to better therapeutic strategies for wound treatment in the future.
Take-Home Messages.
SCs have an immense capacity for self-renewal and differentiation, properties that contribute to homeostasis and wound repair of the IFE. Despite their importance in SC biology and clinical applications, it has been challenging to identify SCs and their differentiating progenies in the IFE.
Whether the IFE is maintained by single type of progenitor or by a stem/TA cell hierarchy is controversial. The most recent lineage-tracing analysis has revealed the existence of both SCs and TA cells that differentially contribute to homeostasis and wound repair of the IFE.
The transcription factor p63 plays an essential role in maintenance of the proliferative potential of epidermal SCs, and its expression is tightly controlled during SC differentiation into TA cells. In particular, post-translational modification of p63 (i.e., phosphorylation) serves as a marker for the early transition of IFE SCs into differentiating progenies with a more limited proliferative potential.
Detailed characterization of IFE SCs and TA cells will lead to novel therapeutic approaches in which specific functions of SCs and TA cells can be targeted to improve clinical outcome in the treatment of epidermal degenerative diseases such as chronic wounding.
Abbreviations and Acronyms
- CreER
tamoxifen-dependent Cre recombinase
- Dox
doxycycline
- Edar
ectodysplasin receptor
- EGFP
enhanced green fluorescent protein
- EPU
epidermal proliferative unit
- EYFP
enhanced yellow fluorescent protein
- HF
hair follicle
- IFE
interfollicular epidermis
- Inv
involucrin
- K14
keratin-14
- K15
keratin-15
- Lgr5
Leu-rich repeat-containing G-protein-coupled receptor 5
- pp63
phosphorylated p63
- R26
Rosa26 locus
- SC
stem cell
- TA
transit-amplifying
- TD
terminally differentiated
Acknowledgments
I thank my colleagues at the University of Pennsylvania School of Veterinary Medicine: Drs. Susan Volk and Leslie King, for reading of the manuscript and helpful comments; and Dr. Nancy Gartland, for slides of various epidermal tissues. I also wish to apologize to our colleagues whose contributions were not cited due to formatting constraints.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the author listed. No ghostwriters were used to write this article.
About the Author
Makoto Senoo is an Assistant Professor of Developmental Biology in the Department of Animal Biology at the University of Pennsylvania School of Veterinary Medicine, Philadelphia, Pennsylvania. He is also an investigator at the Institute for Regenerative Medicine of the University of Pennsylvania. His laboratory is investigating the role of p63 in epithelial stem cells during development, homeostasis, and tissue regeneration.
References
- 1.Blanpain C. Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nature Rev Mol Cell Biol. 2009;10:207. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoath SB. Leahy DG. The organization of human epidermis: functional epidermal units and phi proportionality. J Invest Dermatol. 2003;121:1440. doi: 10.1046/j.1523-1747.2003.12606.x. [DOI] [PubMed] [Google Scholar]
- 3.Potten CS. Saffhill R. Maibach HI. Measurement of the transit time for cells through the epidermis and stratum corneum of the mouse and guinea-pig. Cell Tissue Kinet. 1987;20:461. doi: 10.1111/j.1365-2184.1987.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 4.Cotsarelis G. Sun TT. Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 5.Braun KM. Niemann C. Jensen UB. Sundberg JP. Silva-Vargas V. Watt FM. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130:5241. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 6.Morris RJ. Liu Y. Marles L. Yang Z. Trempus C. Li S. Lin JS. Sawicki JA. Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 7.Jaks V. Barker N. Kasper M. van Es JH. Snippert HJ. Clevers H. Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 8.Schuijers J. Clevers H. Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J. 2012;31:2685. doi: 10.1038/emboj.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito M. Liu Y. Yang Z. Nguyen J. Liang F. Morris RJ. Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 10.Levy V. Lindon C. Harfe BD. Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005;9:855. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Levy V. Lindon C. Zheng Y. Harfe BD. Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 12.Langton AK. Herrick SE. Headon DJ. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol. 2006;128:1311. doi: 10.1038/sj.jid.5701178. [DOI] [PubMed] [Google Scholar]
- 13.Mackenzie IC. Relationship between mitosis and the ordered structure of the stratum corneum in mouse epidermis. Nature. 1970;226:653. doi: 10.1038/226653a0. [DOI] [PubMed] [Google Scholar]
- 14.Potten CS. The epidermal proliferative unit: the possible role of the central basal cell. Cell Tissue Kinet. 1974;7:77. doi: 10.1111/j.1365-2184.1974.tb00401.x. [DOI] [PubMed] [Google Scholar]
- 15.Strachan LR. Ghadially R. Tiers of clonal organization in the epidermis: the epidermal proliferation unit revisited. Stem Cell Rev. 2008;4:149. doi: 10.1007/s12015-008-9020-6. [DOI] [PubMed] [Google Scholar]
- 16.Doupé DP. Jones PH. Interfollicular epidermal homeostasis: dicing with differentiation. Exp Dermatol. 2012;21:249. doi: 10.1111/j.1600-0625.2012.01447.x. [DOI] [PubMed] [Google Scholar]
- 17.Clayton E. Doupé DP. Klein AM. Winton DJ. Simons BD. Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- 18.Doupé DP. Klein AM. Simons BD. Jones PH. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev Cell. 2010;18:317. doi: 10.1016/j.devcel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Doupé DP. Alcolea MP. Roshan A. Zhang G. Klein AM. Simons BD. Jones PH. A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science. 2012;337:1091. doi: 10.1126/science.1218835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs E. Horsley V. Ferreting out stem cells from their niches. Nat Cell Biol. 2011;13:513. doi: 10.1038/ncb0511-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mascré G. Dekoninck S. Drogat B. Youssef KK. Brohée S. Sotiropoulou PA. Simons BD. Blanpain C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- 22.McCulloch EA. Till JE. Perspectives on the properties of stem cells. Nat Med. 2005;11:1026. doi: 10.1038/nm1005-1026. [DOI] [PubMed] [Google Scholar]
- 23.Green H. The birth of therapy with cultured cells. Bioessays. 2008;30:897. doi: 10.1002/bies.20797. [DOI] [PubMed] [Google Scholar]
- 24.Barrandon Y. Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki D. Senoo M. Increased p63 phosphorylation marks early transition of epidermal stem cells to progenitors. J Invest Dermatol. 2012;132:2461. doi: 10.1038/jid.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaelin WG. The emerging p53 family gene family. J Natl Cancer Inst. 1999;91:594. doi: 10.1093/jnci/91.7.594. [DOI] [PubMed] [Google Scholar]
- 27.Van Bokhoven H. Melino G. Candi E. Declercq W. p63, a story of mice and men. J Invest Dermatol. 2011;131:1196. doi: 10.1038/jid.2011.84. [DOI] [PubMed] [Google Scholar]
- 28.Mills AA. Zheng B. Wang XJ. Vogel H. Roop DR. Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 29.Yang A. Schweitzer R. Sun D. Kaghad M. Walker N. Bronson RT. Tabin C. Sharpe A. Caput D. Crum C. McKeon F. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 30.Su X. Paris M. Gi YJ. Tsai KY. Cho MS. Lin YL. Biernaskie JA. Sinha S. Prives C. Pevny LH. Miller FD. Flores ER. TAp63 prevents premature aging by promoting adult stem cell maintenance. Cell Stem Cell. 2009;5:64. doi: 10.1016/j.stem.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romano RA. Smalley K. Magraw C. Serna VA. Kurita T. Raghavan S. Sinha S. ΔNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development. 2012;139:772. doi: 10.1242/dev.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suh EK. Yang A. Kettenbach A. Bamberger C. Michaelis AH. Zhu Z. Elvin JA. Bronson RT. Crum CP. McKeon F. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624. doi: 10.1038/nature05337. [DOI] [PubMed] [Google Scholar]
- 33.Su X. Chakravarti D. Cho MS. Liu L. Gi YJ. Lin YL. Leung ML. El-Naggar A. Creighton CJ. Suraokar MB. Wistuba I. Flores ER. TAp63 suppresses metastasis through coordinate regulation of Dicer and miRNAs. Nature. 2010;467:986. doi: 10.1038/nature09459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su X. Gi YJ. Chakravarti D. Chan IL. Zhang A. Xia X. Tsai KY. Flores ER. TAp63 is a master transcriptional regulator of lipid and glucose metabolism. Cell Metab. 2012;16:511. doi: 10.1016/j.cmet.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang A. Kaghad M. Wang Y. Gillett E. Fleming MD. Dötsch V. Andrews NC. Caput D. McKeon F. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 36.Mangiulli M. Valletti A. Caratozzolo MF. Tullo A. Sbisà E. Pesole G. D'Erchia AM. Identification and functional characterization of two new transcriptional variants of the human p63. Nucleic Acids Res. 2009;37:6092. doi: 10.1093/nar/gkp674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Senoo M. Pinto F. Crum CP. McKeon F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 38.Jones PH. Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 39.Carroll DK. Carroll JS. Leong CO. Cheng F. Brown M. Mills AA. Brugge JS. Ellisen LW. p63 regulates an adhesion programme and cell survival in epithelial cells. Nat Cell Biol. 2006;8:551. doi: 10.1038/ncb1420. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen BC. Lefort K. Mandinova A. Antonini D. Devgan V. Della Gatta G. Koster MI. Zhang Z. Wang J. Tommasi di Vignano A. Kitajewski J. Chiorino G. Roop DR. Missero C. Dotto GP. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 2006;20:1028. doi: 10.1101/gad.1406006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossi M. Aqeilan RI. Neale M. Candi E. Salomoni P. Knight RA. Croce CM. Melino G. The E3 ubiquitin ligase Itch controls the protein stability of p63. Proc Natl Acad Sci USA. 2006;103:12753. doi: 10.1073/pnas.0603449103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yi R. Poy MN. Stoffel M. Fuchs E. A skin microRNA promotes differentiation by repressing “stemness”. Nature. 2008;452:225. doi: 10.1038/nature06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westfall MD. Joyner AS. Barbieri CE. Livingstone M. Pietenpol JA. Ultraviolet radiation induces phosphorylation and ubiquitin-mediated degradation of ΔNp63α. Cell Cycle. 2005;4:710. doi: 10.4161/cc.4.5.1685. [DOI] [PubMed] [Google Scholar]
- 44.Noszczyk BH. Majewski ST. p63 expression during normal cutaneous wound healing in humans. Plast Reconstr Surg. 2001;108:1242. doi: 10.1097/00006534-200110000-00022. [DOI] [PubMed] [Google Scholar]
- 45.Morasso MI. Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biol Cell. 2009;97:173. doi: 10.1042/BC20040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stojadinovic O. Brem H. Vouthounis C. Lee B. Fallon J. Stallcup M. Merchant A. Galiano RD. Tomic-Canic M. Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol. 2005;167:59. doi: 10.1016/s0002-9440(10)62953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schäfer M. Werner S. Transcriptional control of wound repair. Annu Re Cell Dev Biol. 2007;23:69. doi: 10.1146/annurev.cellbio.23.090506.123609. [DOI] [PubMed] [Google Scholar]
- 48.Wu N. Rollin J. Masse I. Lamartine J. Gidrol X. p63 regulates human keratinocyte proliferation via MYC-regulated gene network and differentiation commitment through cell adhesion-related gene network. J Biol Chem. 2012;287:5627. doi: 10.1074/jbc.M111.328120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arwert EN. Hoste E. Watt FM. Epithelial stem cells, wound healing and cancer. Nature Rev Cancer. 2012;12:170. doi: 10.1038/nrc3217. [DOI] [PubMed] [Google Scholar]