Abstract
Background
Blood vessel formation is fundamental to development, while its dysregulation can contribute to serious disease. Expectations are that hundreds of millions of individuals will benefit from therapeutic developments in vascular biology. MSCs are central to the three main vascular repair mechanisms.
Sources of data
Key recent published literature and ClinicalTrials.gov.
Areas of agreement
MSCs are heterogeneous, containing multi-lineage stem and partly differentiated progenitor cells, and are easily expandable ex vivo. There is no single marker defining native MSCs in vivo. Their phenotype is strongly determined by their specific microenvironment. Bone marrow MSCs have skeletal stem cell properties. Having a perivascular/vascular location, they contribute to vascular formation and function and might be harnessed to regenerate a blood supply to injured tissues.
Areas of controversy
These include MSC origin, phenotype and location in vivo and their ability to differentiate into functional cardiomyocytes and endothelial cells or act as vascular stem cells. In addition their efficacy, safety and potency in clinical trials in relation to cell source, dose, delivery route, passage and timing of administration, but probably even more on the local preconditioning and the mechanisms by which they exert their effects.
Growing points
Understanding the origin and the regenerative environment of MSCs, and manipulating their homing properties, proliferative ability and functionality through drug discovery and reprogramming strategies are important for their efficacy in vascular repair for regenerative medicine therapies and tissue engineering approaches.
Areas timely for developing research
Characterization of MSCs' in vivo origins and biological properties in relation to their localization within tissue niches, reprogramming strategies and newer imaging/bioengineering approaches.
Keywords: mesenchymal stem/stromal cells, pericytes, adventitial cells, regenerative medicine, cancer, vasculogenesis, angiogenesis, arteriogenesis, tissue engineering, transplantation
Background
Cells now categorized as mesenchymal stem/stromal cells (MSCs) were identified over 4 decades ago in murine and guinea pig bone marrow by Friedenstein et al.1–3 and subsequently in human bone marrow4 as a non-haemopoietic, tissue culture plastic adherent sub-fraction of bone marrow cells. These cells formed clonogenic fibroblastoid-like colonies or CFU-F (colony-forming units-fibroblastoid) in vitro. Early studies revealed that these bone marrow MSCs possessed osteogenic potential in vivo.1 Bone marrow fragments containing MSCs or MSCs themselves were subsequently demonstrated to support haemopoiesis in vivo5 or in vitro,6 with post-natal bone marrow fragments and cells also giving rise to bone, fat, cartilage and fibrous tissue after transplantation into an in vivo ectopic site. In 1991, Caplan7 coined the phrase ‘mesenchymal stem cells’ to describe the ability of these cells to generate cartilage and bone, while, in 1999, Pittenger et al.8 demonstrated their multi-potentiality for forming adipogenic, chrondrogenic and osteogenic lineages after clonogenic expansion in vitro. These cells were also subsequently referred to as skeletal stem cells since they contain skeletal tissue progenitor cells.9 More recently, Lin and Lue10 have suggested the terminology, vascular stem cell, to describe MSCs resident in vessel walls, although substantial proof for their stem cell properties and their ability to also generate all vascular lineages is required to confirm this definition.
As a key component of the bone marrow haemopoietic stem/progenitor cell (HSPC) niche,11–18 bone marrow MSCs have also been shown to be organizers or regulators of haemopoietic stem cell (HSC) function as well as blood vessel formation and function.12,17–20 In agreement with this latter aspect is the identification of MSCs in the vascular niche of the bone marrow and of MSC-like cells as perivascular or adventitial cells in a variety of foetal and post-natal tissues.10,21–24 While this localization of MSCs or MSC-like cells adjacent to or within the vasculature might suggest a role in blood vessel formation by direct differentiation into endothelial cells10 and/or as supporting niche cells20 for vascular (re-)generation, it is also compatible with them being potential modulators of hostile injury microenvironments through their immunomodulatory and anti-inflammatory properties and their ability to limit inflammatory damage.25–28 From the above, it is understandable that these MSCs or MSC-like cells have generated substantial interest in the medical areas of transplant, regenerative medicine and cancer treatment because of their multi-potency and multi-functionality.
Although MSCs are best known from their isolation and culture from human bone marrow, similar cells have been isolated from such tissues as peripheral blood,29 cord blood,30 umbilical cord derived Wharton's jelly,31–34 adipose tissue,10,35,36 amniotic fluid,37,38 placenta,39,40 foetal tissues,41–44 dental pulp,45 periosteum,46,47 synovial fluid and membrane,47–49, articular cartilage,50 skeletal muscle and dermis,51,52 lungs53 and from a variety of other foetal and post-natal tissues.10,13–16,18,21–24,29 Additionally, MSC-like cells have been described in murine compact bone54–56 and the heart.57,58 Notwithstanding that these human MSC and MSC-like cells also have distinct source-dependent features, they all express certain surface markers (CD90, CD105 and CD73 positive, and lack of HLA-DR, CD45, CD19, CD14 or CD11b and CD19 or CD79), have similar transcriptomes, all adhere to plastic, all differentiate into all or some of the classical mesenchymal lineages, chondrocytes, adipocytes and osteoblasts, and, in most cases modulate the immune response.17,26–28,59–64 Sourcing of MSC from living donors can be invasive as illustrated for bone marrow and amniotic fluid. The non-invasive sourcing from umbilical cord, cord blood and placenta procured after birth has the advantage of not posing any risk to the donor. Moreover, these sources have a minimal risk of viral infections and few ethical concerns associated with their procurement.65 Once sourced from various tissues, the ease of culture of MSCs or MSC-like cells, their in vitro proliferative potential and their ability to home to sites of injury in vivo are even more interesting in terms of their use as cell therapeutics. As indicated and apart from their defining characteristics, MSCs and MSC-like cells are heterogeneous populations of cells, and their function, efficacy and differentiation status change in relation to the microenvironment in which they find themselves. In vivo, this microenvironment can represent different stages of ontogeny or can change from normal to injured or regenerative tissues. Several recent reviews have emphasized the need for more robust definitions of MSCs derived from various tissues,17,59 such as defining their source, species derivation, whether they are primary or cultured cells, their in vitro clonogenic and differentiation capacities and their transcriptome, proteome and secretome profiles under defined conditions. These detailed characteristics might be related to differences in in vivo efficacy and will hopefully predict the latter.
In this review, unless otherwise specified, the terminology ‘MSCs’ will refer to the heterogeneous population of mesenchymal stem/stromal cells. Discussions will concentrate primarily on human MSCs or MSC-like cells with reference to murine studies and will address the function of MSCs in regulating blood vessel formation as one of their central effects. In the studies described below, we will use haemopoietic, cardiovascular and skin repair as exemplars where MSCs or MSC-like cells regulate blood vessel formation. As such, they play a key role in the revascularization of regenerating tissues and are being studied for their therapeutic potential. In this context, their relationship to perivascular adventitial cells and pericytes is crucial to acknowledge and will also be reviewed.
The blood vessel supportive properties of MSCs
Blood vessel (re-)generation occurs by different mechanisms including vasculogenesis (de novo blood vessel formation from endothelial precursors or angioblasts), angiogenesis (the sprouting of existing vessels or intussusceptive angiogenesis) and arteriogenesis (the growth of collateral vessels).66–69 These are illustrated in Fig. 1. MSCs and myeloid cells have been demonstrated to enhance the de novo formation of stable vasculature by endothelial colony-forming cells in surrogate models of vasculogenesis in vitro and in vivo.19,70–80 Arteriogenesis is one of the most powerful revascularization mechanisms in adults leading to an increase in the lumen of existing small collaterals and eventually reperfusion of tissues downstream of a vascular obstruction.69 Arteriogenesis is thought to be initiated by shear stress-induced activation of endothelial cells in the vascular wall, with subsequent macrophage and lymphocyte recruitment and adhesion, remodelling of the vascular wall by released proteases and cell proliferation, prior to neointimal formation. MSCs, by releasing angiogenic factors and proteases, have been reported to stimulate this process, as illustrated for example in the ischaemic hind limb model studies.81–83 Finally, sprouting angiogenesis, which occurs in response to ishaemia and hypoxia, is characterized by extracellular matrix degradation and detachment of mural cells (pericytes or MSC-like cells) from capillaries and microvessels (<100 μm in diameter). This allows the endothelial tip cells to become invasive and to form filopodia and lammellipodia in response to guidance cues, while stalk cells which lie behind the tip cells proliferate, extend the vessels and form extracellular matrix, junctions and lumens.66,67,84,85 Once the tip cells anastomose or inosculate with other tip cells,86 vessel maturation takes place and this involves the commencement of blood flow, mural cell recruitment and extracellular matrix deposition.67,68,87 Here, the mural cells come into direct contact with capillaries and microvascular cells and stabilize the tubular network. These mural cells, which are also denoted by some as pericytes, have been demonstrated in the human to give rise to multi-potent MSCs in vitro.22,23 These pericytes or MSC-like cells are reported to express CD146 in humans.12,13 In contrast to capillaries, the vascular wall of human arteries and veins is composed of three layers, the lumen facing tunica intima, the tunica media and the outer tunica adventitia. The adventitial layer especially contains cells with properties of and which give rise to multi-lineage MSCs in vitro.24 Unlike pericytes, these adventitial cells are reported to be CD146–.22–24 In the remainder of this review, these pericytes and perivascular adventitial cells, which contain cells reminiscent or equivalent to bone marrow MSCs, will be referred to as MSC-like cells, although their heterogeneity and potentiality will be discussed in subsequent sections.
Fig. 1.
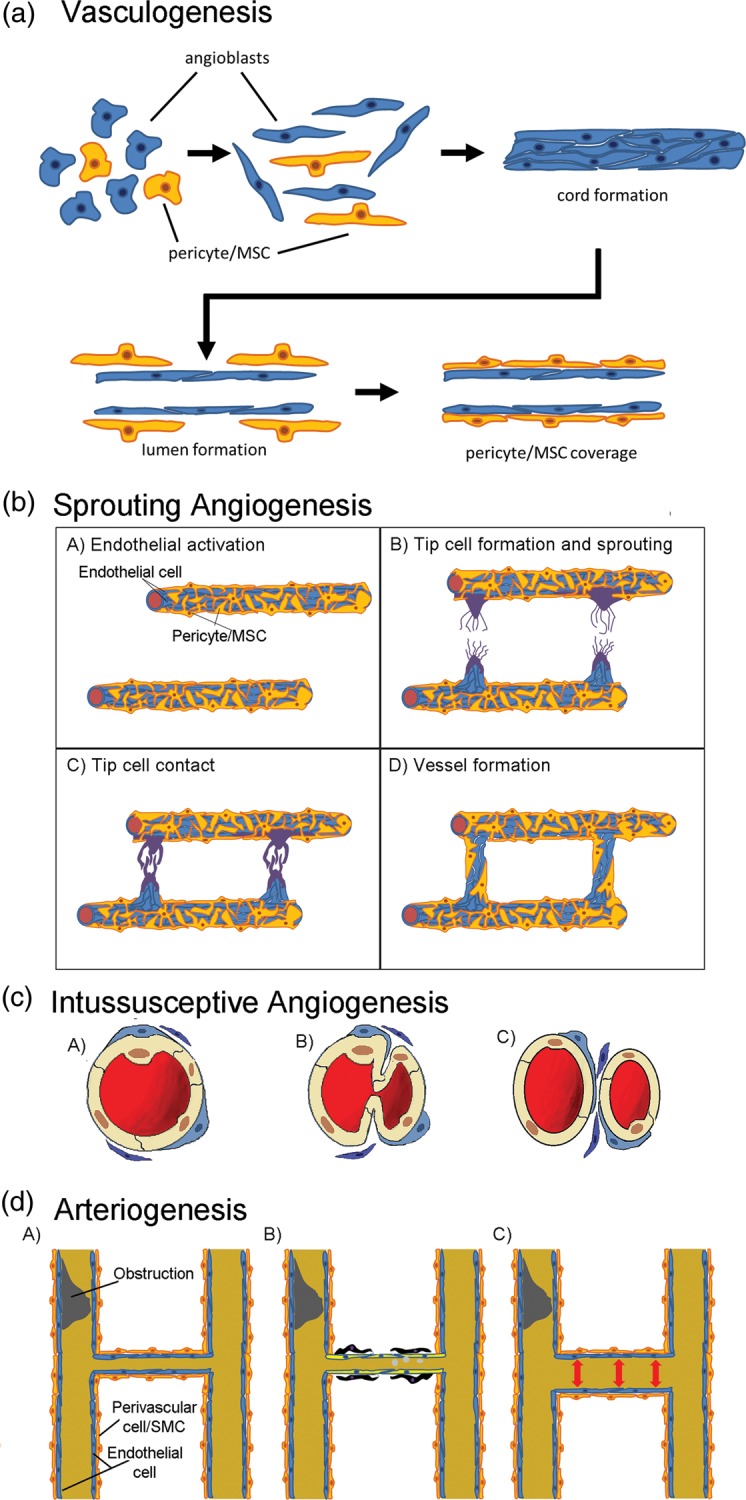
New blood vessel formation. Schematic representation of (a) vasculogenesis or the formation of new vessels de novo from stem/progenitor cells; (b) Sprouting angiogenesis, where endothelial cells respond to ischaemia or hypoxia first by movement of MSCs or pericytes away from the endothelia with the endothelial tip cells extending filopodia or lamellipodia in response to guidance cues (A and B). Endothelial stalk cells then proliferate extending the tip cells and forming a lumen (C) as they inosculate with other extending vessels (C). These vessels are then stabilized by pericyte/MSC recruitment (D); (c) Intussusceptive angiogenesis occurs without endothelial proliferation. The endothelia protrude into the vessel to form a transendothelial bridge with the aid of pericytes/MSC and fibroblastoid cells (B) before separating into two vessels (C); (d) Arteriogenesis can occur in the absence of hypoxia with an increase in luminal diameter and length of pre-existing arterioles following a larger vessel blockage (A) to form larger collateral vessels. It is thought that endothelia in these smaller vessels respond to sheer stress and recruit macrophages and lymphocytes (B). The macrophages degrade the ECM allowing paracrine signalling and regulating interactions between endothelial and perivascular cells [pericytes/MSC/smooth muscle cells (SMC)], and resulting in vascular proliferation (B) and vessel enlargement and stabilization (C).
MSCs derived from murine or human bone marrow cells have the ability to regulate new blood vessel formation, stability and function,19,70–78 and similar effects have been demonstrated with MSC-like cells from murine adipose tissue, skeletal muscle and the heart,79 and from human adipose tissue,75,76,88 the limbal niche,89 the foetal circulation,90 amniotic fluid,74 the vascular wall22–24 and umbilical cord blood.91 Interestingly, second trimester human amniotic fluid MSC-like cells appear to provide better vasculogenic support in an in vivo surrogate model than bone marrow MSCs.74 This might suggest that MSCs at earlier stages of ontogeny are more supportive when compared with adult bone marrow MSCs. This may be due to superior proliferative or homing and retention potential or through their unique secretome profiles. Indeed, amniotic fluid MSC-like cells secrete more than twice as many angiogenic factors as bone marrow MSCs.74 Nevertheless, together with this vascular-supporting function, recent data have demonstrated that human MSCs from umbilical cord blood also show angiogenic potential since they directly self-organize forming new functional vasculature connected with the host circulatory system once implanted in mice.91
MSCs, the bone marrow vascular niche and haemopoietic regeneration
A specialized intact bone marrow sinusoidal vascular niche is now well recognized as being essential for post-natal haemopoiesis and for haemopoietic recovery after bone marrow damage, as exemplified by the response to preconditioning regimes during the treatment of malignancies and prior to transplants.18,92,93 The concept of the HSPC inductive microenvironment or niche was developed over four decades ago94,95 to explain the specific ability of the bone marrow to generate blood cells. In healthy human adults, this allows the production of over 1011–1012 new blood cells on a daily basis. Three anatomical regions, the sinusoids (the vascular niche), the endosteum (the osteoblastic niche) and the haemopoietic tissue proper, have been identified in murine bone marrow.9 Cellular components of HSPC niches (Fig. 2) include MSCs and their osteoblastic and adipocytic progeny, in addition to osteoclasts, macrophages, other haemopoietic cells and sinusoidal endothelial cells.96,97 The vascular wall of the sinusoids in the bone marrow is, however, highly specialized and consists maximally of two cell layers. Below the continuous layer of endothelial cells, a discontinuous layer of other perivascular cells (variously termed MSCs, pericytes or adventitial reticular cells) extends into the bone marrow compartment as an essential part of the vascular niche.14,18 Some studies in mice have supported the view that the osteoblastic niche and the vascular niche are distinct entities with different roles in supporting haemopoiesis.14 It has been proposed that HSCs lying adjacent to the endosteal bone surface which is populated with osteoblasts possess higher proliferative and transplantation capacities, while the vascular niche supports the proliferation and maturation of HSPC subsets.9 Other studies have argued for less distinction between these niches, with both osteoblasts and endothelial cells contributing to a trabecular HSPC niche.9 The rationale for this second model has been based on observations that most murine HSPCs are located close to bone marrow sinusoidal endothelial cells, that sinusoidal endothelial cell modulation affects HSC numbers and that the endosteal region is highly vascularized.9 Although it is thought similar niches exist, the distinction between endosteal and vascular niches in adult human bone marrow is not as clearly defined as in the mouse.
Fig. 2.
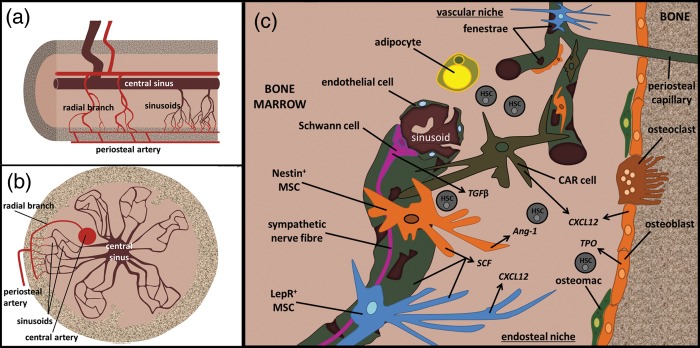
The bone marrow niche. Schematic representation of the haemopoietic niches in long bones. (a) Longitudinal section demonstrating blood flow into the bone and bone marrow and the formation of sinusoidal vessels; (b) Transverse section of bone containing sinusoidal vessels; (c) haemopoietic niches are located near the endosteum of the bone, near sinusoids and in the bone marrow proper. There is controversy as to whether these are distinct niches or a continuum of niches that allow HSPC maintenance, proliferation and differentiation. Of importance to the niche in terms of MSCs or their progeny are nestin+ MSCs, CXCL12 adventitial reticular (CAR) cells, LepR+ MSCs, osteoblasts and adipocytes.
It is now widely accepted that, in the murine bone marrow, MSCs expressing the chemokine CXCL12 play a key role in maintaining undifferentiated HSCs, in HSC homing and survival, and in controlling HSPC proliferation or differentiation.14–16 More recently, conditional knockout studies of CXCL12 and stem cell factor in murine bone marrow niche cells have indicated that different HSPCs reside in specialized niches. More specifically, CXCL12 from murine perivascular MSCs was shown to be required for HSC support, whereas CXCL12 expressing osteoblasts supported early lymphoid progenitors.14,15 The equivalent human bone marrow cells appear to be the subendothelial CD146+CD271+ skeletal progenitor or MSC, which contributes to the vascular niche, and the CD146−CD271+ osteoblast, which contributes to the endosteal niche.12,13 Since perivascular MSCs play a central role in regulating haemopoiesis in post-natal human bone marrow, the question arises as to whether these same cells also regulate bone marrow vasculogenesis and angiogenesis. Sacchetti et al.12 reported that the CD146+ MSC perivascular subset in human bone marrow expressed angiopoietin-1, a secreted factor that plays a pivotal role both in angiogenesis and haemopoiesis. After subcutaneous transplantation into immune compromised mice, the CD146+ MSCs displayed self-renewal capacity and the ability to differentiate into osteoblasts, forming bone and contributing to the structure of the haemopoietic niche via a defined developmental sequence. Bone formation preceded the appearance of a sinusoidal system, and ultimately of haemopoiesis. The CD146+ MSCs were an integral part of this sinusoidal system, residing in the sinusoidal wall. Of note, gene knockdown of angiopoietin-1 in these CD146+ cells limited their ability to regulate microvessel assembly, suggesting a key role in the vascular remodelling of the bone marrow.12 Chan et al. reported the existence of a murine CD45−Tie2−αV+CD105+Thy1− foetal bone skeletal progenitor subset, which following transplantation under the adult mouse kidney capsule, recruited blood vessels, generated ectopic bone and ultimately gave rise to a marrow cavity populated by HSC, suggesting that niche formation is dependent on endochondral ossification.98 VEGFR-2 played an essential role in bone marrow sinusoidal formation. In both cases, the generation of bone alone was not sufficient to initiate niche formation and HSC engraftment, while recruitment of host-derived vasculature was critical to this process. Building on this, we analysed the clonal murine CXCL12 and angiopoietin-1-expressing MS-5 bone marrow-derived MSC cell line and demonstrated that this clonally derived MSC could not only regulate human haemopoiesis, but also promote vasculogenesis and angiogenesis from primary human endothelial precursors in vitro.19 This provides a simplified clonal surrogate model with which to analyse factors that promote both human blood vessel formation and haemopoiesis and where CXCL12 has been shown to play a key role in both processes. Our studies also demonstrate that both human bone marrow MSCs and second trimester amniotic fluid MSCs, which secrete CXCL12, can also promote human vasculogenesis and angiogenesis in vitro and in vivo.74 Such studies may facilitate the use of MSC-derived cellular and molecular therapies for vascular repair of damaged bone marrow and for improving haemopoietic recovery following myeloablation and transplantation.
To date, preclinical surrogate models (immunodeficient mice, foetal sheep, non-human primates) have demonstrated that co-transplanting human HSPCs with human bone marrow MSCs or foetal lung-derived MSC-like cells, or non-human primate CD34+ HSPCs and bone marrow MSCs can improve HSPC engraftment and haemopoietic reconstitution.99–101 In clinical trials, co-transplantation of human cultured bone marrow MSCs with peripheral blood or umbilical cord blood HSPCs have given variable results with some studies demonstrating enhanced HSPC engraftment102–106 and haemopoietic recovery, while others do not.107 Notwithstanding the normal and specific localization of MSCs in the marrow vascular niche, the variability of the observed co-transplantation effects is not fully understood but may be due to differences in study design, patient population, and the efficacy and source of the MSCs used. However, where positive effects in terms of efficacy have been demonstrated, it is unknown if the MSC co-transplants directly or indirectly enhance bone marrow revascularization and the recovery of the vascular niche. Although chemoradiotherapy conditioning before haemopoietic stem cell transplantation in general induces grave destruction of the marrow including its vascular architecture, infused MSCs are usually not substantially found back in the bone marrow. Therefore, modulation of endogenous repair instead of actual differentiation of MSC into vascular structures might be more likely. On the other hand, while MSC co-infusion seems most effective with limited HSC grafts, they might also facilitate homing of HSC to the bone marrow. Alternatively, the observations described may merely reflect an improved efficacy and potency of the cell products used. This latter conclusion might be supported by particularly noteworthy recent evidence that it is possible, using peptidomimetic ligands, to direct human MSCs to the bone where their engraftment is enhanced in xenotransplant models.108
MSCs for cardiovascular and skin repair as exemplars
Cardiovascular disease is a leading cause of morbidity and mortality worldwide,23,109,110 but less known is the fact that in the UK and USA alone chronic wounds currently affect over 6.7 million patients.111,112 This burden is growing rapidly as a result of an ageing population and a sharp rise in the incidence of diabetes and obesity worldwide. Additionally, the treatment of full-thickness skin loss (e.g. in burn injuries) is a major clinical challenge, with morbidity, scarring and contracture being significant problems.112
Medical interventions for cardiovascular disease include thrombolytic therapy, percutaneous or surgical revascularization, and regenerative therapies using cells and/or biologics.23,113–116 For MSC-based therapies, bone marrow MSCs and MSC-like cell containing populations from adipose tissue and the vascular wall (e.g. umbilical cord and saphenous vein at the time of coronary artery bypass grafting) have been used either in surrogate models of myocardial infarction, cardiac myopathies, stroke or limb ischaemia, or in clinical trials. The use of MSCs in clinical trials treating cardiovascular diseases is listed on ClinicalTrials.gov and summarized in Table 1. Those listed on ClinicalTrials.gov include the use of human allogeneic cardiac or cardiosphere-derived cells (ALLSTAR; RECONSTRUCT; CADUCEUS) or autologous cardiac stem or progenitor cells (SCIPIO; ALCADIA; TICAP). A number of these trials use or plan to use human cardiac stem cells selected for c-kit (CD117) positivity or cardiac or cardiosphere-derived cells that have been reported to contain c-kit+ cells and other progenitor cells (e.g. MSC-like cells). Many of the clinical trials listed are conducted as safety or feasibility studies and will require further studies on the optimization of their efficacy.117–119 The outcome of the POSEIDON-Pilot trial (autologous vs. allogeneic MSCs) demonstrated improved New York Heart Association classification and quality of life indicators, but not left ventricular function, in patients receiving autologous bone marrow MSCs,120,121 and the effects were most significant with the lowest dosage of cells (2 × 106 per patient). Thus, the benefit of transplantation of MSCs in this clinical setting is currently unclear. Short-term follow-up (6–12 months) demonstrated significant increases in such parameters as viable myocardium and regional contractility for the CADUCEUS trial117 and improved left ventricular systolic function and reduced scar size for the SCIPIO trial.118 The use of MSCs in treating limb ischaemia has also been recently reviewed,115,116 and there are currently five clinical trials aimed at using bone marrow or adipose tissue MSCs for treating ischaemic cerebral stroke and peripheral vascular disease listed on Clinical Trials.gov.
Table 1.
Clinical trials using MSCs to treat cardiovascular diseases
| Clinical Trial | Disease treated | Therapeutic agent | Current status |
|---|---|---|---|
|
NCT01392105 SEED-MSC |
Acute myocardial infarction | Human autologous bone marrow MSCs | Randomized Completed |
| NTC00587990 Prometheus |
Chronic ischaemic left ventricular dysfunction secondary to myocardial infarction and undergoing CABG | Human autologous adult MSCs | Randomized Completed |
|
NCT01392625 Poseidon DCM |
Non-ischaemic dilated cardiomyopathy | Human autologous vs. allogeneic bone marrow MSCs | Randomized Recruiting |
|
NCT01087996 Poseidon-Pilot study |
Chronic ischaemic left ventricular dysfunction secondary to myocardial infarction | Human autologous vs. allogeneic bone marrow MSCs | Randomized Completed |
|
NCT00768066 TAC-HFT |
Chronic ischaemic left ventricular dysfunction and heart failure secondary to myocardial infarction | Human autologous bone marrow MSCs vs. MNCs | Randomized Completed |
|
NCT01291329 WJ-MSC-AMI |
ST-segment elevation acute myocardial infarction | Human allogeneic umbilical cord Wharton's jelly-derived MSCs | Randomized Completed |
|
NCT01076920 MESAMI |
Chronic myocardial ischaemia and left ventricular dysfunction | Human autologous bone marrow MSCs | Active, not recruiting |
| NCT01710888 | Ischaemic dilated cardiomyopathy | Human autologous bone marrow MSCs | Randomized Recruiting |
| NCT00883727 | ST-segment elevation acute myocardial infarction | Human adult allogeneic MSCs | Randomized Completed |
|
NCT01449032 MyStromalCell Trial |
Chronic myocardial ischaemia | Human cultured adipose-derived MSCs | Randomized Recruiting |
|
NCT00442806 APOLLO-01 |
ST-segment elevation acute myocardial infarction | Human autologous adipose-derived stem cells | Randomized Not recruiting |
|
NCT01502514 ADI-ME-CHF-002 |
Ischaemic congestive heart failure | Human autologous adipose-derived stem cells | Non-randomized Recruiting |
|
NCT00426868 PRECISE-01 |
Ischaemic heart disease; coronary arteriosclerosis; cardiovascular disease; coronary disease; coronary artery disease | Human autologous adipose-derived stem cells | Randomized Not recruiting |
| Heart disease; blocked arteries; coronary ischemia; coronary disease; coronary artery disease; coronary atherosclerosis | Human autologous bone marrow MNCs and MSCs (MESENDO) | Randomized
|
*Listed on ClinicalTrials.gov; CABG, coronary artery bypass graft; do not include bone marrow MNCs or HSPCs alone.
Clinical trials have progressed without necessarily understanding the mechanism nor fully demonstrating the efficacy of the cell product in preclinical models.17 Moreover, the surrogate models used may not be fully representative of the human disease. Furthermore, while engraftment and therapeutic benefit may both be seen, there is not a great deal of evidence that MSC engraftment itself is always beneficial. At least one study has demonstrated that autologous cardiac-derived cells from neonatal rats and which contained MSC-like cells, when administered into rats intramyocardially and subsequently systemically following myocardial infarction were retained in the heart and improved capillary density and cardiac function.122 A further study combining human foetal with human bone marrow MSCs in an immunosuppressed swine model of myocardial infarction showed cell retention in the heart, reduction in scar size, engraftment of cells into vessels, vascular proliferation and improved left ventricular ejection fraction.123 In other surrogate models of myocardial infarction or peripheral vascular disease which have been recently reviewed or described elsewhere,22,23,123 human foetal aortic, CD34+CD31− adult saphenous vein, CD146+CD45−CD56−CD34− foetal or adult muscle-derived pericytes or MSC-like cells improved function and promoted blood vessel formation in injured tissue.
Similar mechanisms might also be responsible for healing skin wounds. There is accumulating evidence that MSC-like cells are also essential for the formation and stabilization of new vessels within healing skin wounds.84,85 On the other hand, MSCs might also enhance skin wound healing through anti-inflammatory and anti-apoptotic effects, by enhancing keratinocyte migration, by promoting a vascularized granulation matrix and through ECM deposition increasing the tensile strength of repairing wounds, both traumatic and in chronic non-healing ulcers.112,124,125 In this respect, MSCs have also been reported to enhance wound healing in diabetic tissue.126,127 In a study examining the efficacy of bone marrow MSCs in such chronic non-healing ulcers, autologous implantation of bone marrow MSCs accelerated the healing process and improved clinical parameters (pain-free walking and wound size) significantly, emphasizing the beneficial effect of topical therapy.126 Allied to this, acute pathology, in particular as a result of burns, causes a breach in the integrity of the skin that may also benefit from the vasculogenic properties of MSCs. However, a problem remains as to how to ensure that these cells reach their target area in a timely fashion and in sufficient numbers to maximize their therapeutic benefit. Although the infusion of MSCs may provide a reasonable therapeutic effect by their homing capacity, skin wounds provide the ideal candidate for a topical delivery approach. Ways in which MSCs have been previously been delivered to a wound have included an acrylic acid polymer carrier (ppAAC),73,128 a fibrin-based spray129 and numerous collagen or fibrin-based hydrogels containing a mixture of both MSCs and endothelial cells.111 The ppAAc carrier transferred bone marrow MSCs to decellularized human dermis in vitro and delivered adipose-derived MSC-like cells to full-thickness murine wounds with high efficiency—>80% of cells transferred by 3 days. Moreover, the presence of these cells significantly accelerated wound healing, achieving levels equivalent to the MSCs being delivered intradermally.128 In order to produce the MSC-containing fibrin spray,129 a single bone marrow aspirate of 35–50 ml was obtained, and cultured autologous bone marrow MSC were applied up to four times to chronic wounds using a fibrin polymer spray system. Application resulted in significant decreases in size to chronic human wounds, whilst topical application of autologous MSCs also stimulated closure of full-thickness wounds in diabetic mice. Perhaps more importantly, GFP tracking of the human bone marrow MSCs in mouse wounds showed GFP+ cells associated with blood vessels, suggesting the involvement of these cells in vascularization of the healing wound.128
MSC secretome, microvesicles and tissue engineering as complementary therapeutic approaches
Paracrine effects of MSCs and MSC-like cells have been highlighted as important effector mechanisms in promoting blood vessel formation and endogenous cardiovascular and wound repair. While arteriogenesis and/or angiogenesis and anti-inflammatory effects will be significant targets in adult life, vasculogenesis may also be a likely target in ex vivo newly engineered tissues. Hence, harnessing the secretome of MSCs or MSC-like cells is another approach which may add value to cellular therapeutics. As with cell therapies, the studies reviewed recently by Ranganath et al. and our own studies demonstrate that the MSC secretome, at least in vitro, is dependent on cell source, purity and preconditioning by microenvironmental factors such as growth factors, small molecules and hypoxia (Table 2).19,74,130,131 Approaches to improve revascularization have included the direct use of individual cytokines such as VEGF, FGF4 and EPO in clinical trials, but these have not generally replicated the efficacy observed in preclinical models.129 Further improvements may be achieved by better secretome analysis and characterizing the optimal MSC or MSC-like population, by controlling the release of factors, and identifying MSCs or MSC-like cells in the context of three-dimensional (3D) scaffolds or spheroids that can alter MSC function. The latter approach may be used in tissue engineering approaches but may also be exemplary of better understanding the signalling and transcriptional mechanisms that regulate the MSC secretome in the vascular niches in health and disease.
Table 2.
Differences and similarities between the secretome of two sources of human mesenchymal stromal cells as assessed using angiogenesis antibody arrays*
| Secreted molecules | Amniotic fluid | Bone marrow | Some reported functions† |
|---|---|---|---|
| Angiogenin | + | + | A member of the pancreatic ribonuclease family; vasculogenesis |
| Angiopoietin-1 | + | + | Ligand for Tie-2; proangiogenic; endothelial cell chemotaxi; survival; sprouting and stabilization; vessel maturation |
| Angiopoietin-2 | + | − | Ligand for Tie-2; alone promotes vascular regression and destabilizes endothelial cell–perivascular cell interactions. With VEGF, Ang-2 promotes neovascularization. |
| Angiostatin | + | − | Plasminogen cleavage fragment; angiogenic inhibitor; inhibitor of endothelial cell proliferation and migration |
| Amphiregulin | + | − | EGF-like ligand which signals through the EGFR; enhanced lymphangiogenesis |
| Artemin | + | − | Member of the GNDF ligand family; promotes angiogenesis |
| Tissue Factor | + | − | Coagulation factor III/CD142; promotes neovascularization and stabilization through CCL2; enhances transcription of VEGF and decreases transcription of the thrombospondins |
| CXCL16 | + | + | Proangiogenic |
| DPPIV (CD26) | + | − | Membrane-bound oligo-peptidase acting on and modulating the proangiogenic chemokine CXCL12 |
| Epidermal growth factor | + | − | Anti-apoptotic; enhances MSC proliferation and survival; enhanced lymphangiogenesis |
| EG-VEGF | + | − | Prokineticin 1; proangiogenic |
| Endostatin | + | − | Collagen XVIII cleavage fragment; inhibitor of angiogenesis and of endothelial cell proliferation and migration; promotes endothelial apoptosis and G1 arrest |
| Endothelin-1 | + | + | Proangiogenic and prolymphoangiogenic |
| Endoglin (CD105) | + | − | Auxillary TGF-β1 receptor that modulates TGF-β1 and β3 responses; role in vascular development, angiogenesis and vascular homeostasis |
| FGF-7 | + | + | Arteriogenesis |
| FGF acidic/FGF-1 | + | − | Proangiogenic |
| FGF basic/FGF-2 | + | − | Vascular regeneration; induces vascular networks and stimulates arteriogenesis |
| FGF-4 | + | − | Proangiogenic |
| GDNF | + | − | Glial-derived neurotrophic factor; proangiogenic |
| GM-CSF | + | − | Proangiogenic |
| Heparin binding-EGF | + | + | Proangiogenic |
| Hepatocyte growth factor | + | − | Proangiogenic |
| IL-1β | + | − | Proangiogenic; lymphangiogenesis |
| IL-8 | + | − | Proangiogenic |
| TGF-β1 | + | − | Angiogenic inhibitor |
| Leptin | + | − | Promotes vascular tubule formation |
| MCP-1 | + | − | CCL2; promotes neovascularization and stabilization |
| MIP-1α | + | − | CCL3; reported to act on macrophages or other cells to stimulate vessel formation |
| MMP-8 | + | − | Collagenase 2 cleaves collagen type I, II and III; angiogenesis |
| MMP-9 | + | − | Gelatinase B cleave both collagens and gelatins; neovascularization and angiogenesis |
| NRG1-β1 | + | − | Angiogenesis; arteriogenesis |
| Pentraxin-3 (PTX3) | + | + | Proangiogenic |
| PD-ECGF | + | − | Thymidine phosphorylase; promotes angiogenesis |
| PDGF-AA | + | + | MSC proliferation; enhances angiogenesis |
| PDGF-AB/PDGF-BB | + | − | Induces vascular networks and stimulates arteriogenesis |
| Persefin | + | − | Member of the GNDF ligand family |
| Platelet factor 4 (PF4) | + | − | Angiogenic inhibitor |
| PlGF | + | + | Proangiogenic |
| Prolactin | + | − | Proangiogenic in intact form |
| Serpin B5 | + | − | Maspin; member of the serine protease inhibitor family; negative regulator of angiogenesis |
| Serpin E1 | + | + | PAI-1; member of the serine protease inhibitor family; inhibitor of uPA; negative regulator of angiogenesis; maintains microvascular integrity |
| Serpin F1 | + | + | member of the serine protease inhibitor family; negative regulator of angiogenesis |
| TIMP-1 | + | + | Negative regulator of angiogenesis |
| TIMP-4 | + | + | Negative regulator of angiogenesis |
| Thrombospondin-1 | + | + | Anti-angiogenic; inhibits endothelial cell proliferation |
| Thrombospondin-2 | + | − | Anti-angiogenic; inhibits endothelial cell migration and tubule formation |
| uPA | + | + | Endothelial cell proliferation, migration and tubule formation |
| Vasohibin | + | − | Negative feedback regulator of angiogenesis |
| VEGF | + | + | Proangiogenic |
| VEGF-C | + | − | Regulates lymphangiogenesis |
Studies were conducted using angiogenic antibody arrays and 24-h conditioned media obtained from MSCs from human second trimester amniotic fluid or from bone marrow cultured in EMB2 medium with low serum (0.5%) as described by Roubelakis et al.74 and which had been shown to support vasculogenesis/angiogenesis in vitro and in vivo (+: presence, − no detectable expression).
*In separate experiments, CXCL12 was also detected although this was not present in the antibody array.
†Functions may vary depending on tissue or environmental context.
Intercellular communication between MSC or MSC-like cells and their interacting cells can also be modulated via the production of micro- or nano- cellular membrane vesicles, which can carry mediators as well as genetic information (mRNA, miRNA) between cells.132 The ability of such microvesicles to stimulate angiogenesis has been described both in vitro and in vivo and emerging evidence indicates that microRNAs (miRNAs) play a significant role in vascular biology, as well as regulating other facets of tissue repair.133,134 Microvesicles sort and contain multiple effector molecules, while miRNAs regulate multiple targets or pathways and can be enhanced or suppressed with small molecule mimics or antagomirs. Such small molecules may thus also be used to potentially control MSC behaviour (e.g. endothelial tubule formation, migration and proliferation) and to improve neovascularization, and cardiovascular and skin formation and function. miRNAs involved in MSC-mediated neovascularization include miR-210, miR-126, miR-221/222, miR-296, miR-320, miR-18a, miR-17-5, miR-132, miR-92a and miR-let-7b, with Table 3 listing examples of known targets and as recently reviewed or described.23,133,134 As an example of effector mechanisms, miR-126 plays an important in the development of blood vessels and vascular integrity by regulating Spred1 and PI3R2, negative regulators of the MAP kinase and PI3K signalling pathway.135,136 An additional example relates to intramyocardial injected saphenous vein MSC-like cells which produce miR-132, resulting in improved cardiac function in an acute myocardial infarcted murine model, a response attributed in part to increased neovascularization.137 miRNA studies are being rapidly translated into clinical use, principally in biomarker analyses or miRNA signatures as diagnostics, with 124 clinical trials involving miRNAs listed on ClinicalTrials.com.133 Further miRNA network analysis in functional models, however, might lead to alternative approaches to actually enhance and regulate blood vessel formation as therapy.
Table 3.
miRNAs affecting angiogenesis
| miRNA | Target |
|---|---|
| Positive effects | |
| miR-201 | NPTX1, Ephrin A3 |
| miR-126 | SPRED-1, PIK3R2, VCAM-1 |
| miR-18a* | TSP-1 |
| miR017-5* | TIMP-1 |
| miR-132 | P120RasGAP, PTEN |
| Let-7b | TIMP-1 |
| miR-424 | Cullin 2 |
| Negative effects | |
| miR-221/222 | c-kit |
| miR-296 | HGF-regulated tyrosine kinase substrate |
| miR-320 | IGF-1 |
| miR-92a | Integrin alpha5 |
*In Dicer depleted cells.
The most complicated and most recent approaches to cardiovascular and skin therapies are the ex vivo generation of larger tissue or organ segments, possibly even into complete transplantable organs. The cardiac-related engineering approaches, which have been reviewed recently, include (i) cultured cell sheets that are generated without scaffolds and can be stacked, (ii) the reconstruction of decellularized tissues or organs reseeded with autologous cells from the recipient and (iii) the use of smart biocompatible and, where relevant, biodegradable scaffolds or biogels derived artificially and which may possess porous, pre-patterned, perfused channels, flexible and/or elastic 3D designs or niches, with ordered geometry and containing key biologics.138–150 The latter may allow the delivery of cells to the damaged heart in injectable hydrogels, or following mechanical or electrical stimulation of cells in the hydrogels or porous scaffolds and each approach cited above is amenable to recent advances in bioreactor and in imaging technologies which assess revascularization.151–153 Of note from these studies, fabricated multilayered cell sheets-derived in vitro are more easily vascularized than thick scaffolds. Indeed, Sekine et al.145 demonstrated that these sheets inosculate with host vasculature after in vivo transplantation. Other approaches are to specifically engineer myoblast sheets with factors which promote blood vessel formation and improve cardiac function upon transplantation into the host,143,144 or to create a preformed 3D vascular niche that promotes revascularization and hence survival of the cardiac patch.146,147 In addition, optimizing the inner walls of the synthetic tubules, for example with proteoglycans and anchor peptides to better capture MSCs or other vascular cells and their products, aims to allow functional vessels to extend more deeply within biocompatible scaffolds and to permit more rapid inosculation with host vessels. Finally where organ transplant remains the only option for treatment, and given the dearth of organs available, a whole organ bioengineering approach using decellularized organ scaffolds, where the tissue microarchitecture is maintained and which can be reseeded with autologous cells, is another important option being examined, although this research area will take considerable time to reach translation.147,150 Indeed, Taylor and colleagues147,150 have pioneered whole organ tissue engineering using naturally occurring decellularized 3D biological heart scaffolds seeded with relevant cell subsets. Similarly engineered skin substitutes (whether artificial or from de-cellularized dermis) for treating extensive full-thickness skin loss following burn injury, which allow the regeneration of the dermis and epidermis while minimizing scarring, would meet the challenge of maintaining a blood supply to the graft. Combinations of MSC or MSC-like cells with biologics and reprogramming, bioreactor and imaging technologies are likely to eventually synergize with revascularization in endogenous repair.138,139,151,152,154–156
Safety issues and related other uses of vascular regeneration stimulating MSC cell therapies
Using MSC or other progenitors, particularly if derived from embryonic or reprogrammed stem cells, with proliferative and differentiation capacity as a cell therapy should acknowledge ectopic tumour formation as a possible side effect.154,157 The safety challenges that need to be overcome before induced pluripotent cell-derived MSCs are used clinically have recently been reviewed and the safety of human products has been highlighted as ‘the most important criterion for human application’.154 Moreover, a general concern of vascular regenerative therapies involves promotion of angiogenesi-dependent malignancies. Cancers affect more than one in three individuals in their lifetime (www.cancer researchuk.org) and one of the hallmarks of some cancers is the induction of aberrant angiogenesis, which can occur in solid and benign tumours and also in haematological malignancies.158–160 The homeostatic balance between pro- and anti-angiogenic mechanisms in normal tissues is shifted in favour of proangiogenic signals emanating from cancer cells, or following their induction in tumour niche cells by cancer cells, thereby leading to an ‘angiogenic switch’. This angiogenic switch results in abnormal angiogenesis, is regulated by hypoxia and is associated with increased perivascular cell recruitment.159–161 MSC-like cells have been linked to this angiogenic switch process by the secretion of proangiogenic factors, such as fibroblast growth factor 2, VEGF, angiogenin, TGFβ, IL-6, IL-8, hepatocyte growth factor and platelet-derived growth factor BB (PDGF-BB), as well as by contributing to the recruitment of circulating vascular progenitor cells.162–165 Other mechanisms that can contribute to tumour vascularization include vascular co-option and mimicry.166,167 In the former case, tumour growth co-opts the pre-existing vasculature, a process followed by reactive stimulation of angiogenesis. In the latter case, tumour cells themselves form a capillary-like vessel network. Stromal/MSC-like cells may have a differing and pivotal role in both processes and moreover may affect the outcomes of anti-angiogenic therapies.167 Where specific tumour-orientated homing and incorporation of MSCs have been demonstrated in various preclinical cancer models, there is evidence that this can even be enhanced by irradiation and/or by cytokines or chemokine gradients generated at the tumour site.163–165 On the basis of these observations and with the exception of their use to treat severe graft versus host disease following allografts for haematological malignancies where the benefits and safety of MSCs have been reported, all vascular (re-)generating therapies should be used with caution in patients with active or recent malignancies.
On the other hand, this specific homing of MSCs towards tumour vasculature has also led to the proposed use of MSCs as carriers for anti-cancer gene delivery. This might be particularly handy, where the gene expression is driven by tissue-specific promoters or enhancers, and where their effects are targeted to the site of tumour angiogenesis. The rationale is to achieve more efficient and directed targeting of chemo- and gene therapies or to use these as an image-guided tool for detecting advanced solid tumours with multiple metastases. In any case, electrophoration and other non-viral MSC modifications that are needed for such therapies have not yet been explored and may indeed be a more likely clinical route. Gene transduction efficiency into MSCs using conventional adenoviral vectors has been improved 10-fold using modified adenoviral vectors containing the RGD (Arg–Gly–Asp) motif.168 Using this approach, Kanehira et al.168 demonstrated that intravenous injection of MSCs expressing NK4, an antagonist of hepatocyte growth factor, inhibited multiple lung tumors and prolonged the survival of tumor-bearing mice without inducing adverse effects. Other examples of transgenes transduced into bone marrow MSCs and inhibiting angiogenesis in surrogate in vivo models of solid tumours following gancyclovir administration include the HSV-tk suicide gene under the control of Tie-2 or CCL5 transcriptional regulatory elements. These and other approaches aimed at targeting the tumour cells have recently been reviewed by Keung et al.,165 with evidence in some but not all cases of reducing angiogenesis and/or tumour load. However, the use of genetically engineered MSCs in cancer treatment has been met by a general concern related to biosafety and feasibility. Insertional mutagenesis of introduced transgenes and thus potential tumorigenicity in this respect need to be acknowledged. On the other hand, as with all vascular modulatory therapies, such MSCs may also enhance tumour growth by increasing the vasculature. The cellular heterogeneity of MSCs, variability in their chemokine or adhesion receptor expression and hence homing properties, and their diverse functions within different host-tumour microenvironments make their effect largely unpredictable and may affect the safety and efficacy profiles of these approaches.165
The heterogeneity of MSCs: does this affect their therapeutic efficacy?
MSCs or MSC-like cells can be sourced from a variety of tissues and are often used as heterogeneous populations of cells in autologous or allogeneic transplant settings. This heterogeneity can affect their potency, safety, tissue specific efficacy, regulation and mechanism of action. Additionally and as in all cell therapies, in vitro expansion of small cell subsets to clinical doses is difficult and potentially might result in a loss of efficacy with population doublings. Cultured human MSCs have been shown to express a different gene signature during culture and when compared with enriched mesenchymal stem cells.169–173 Additionally, MSCs from different locations, at different stages of differentiation and at various stages of ontogeny demonstrate both similarities and differences in their surface marker profiles, multi-potentiality, transcriptome, proteome and secretome fingerprints, their growth abilities and potency in their functional efficacy. A first attempt to create some uniformity in the field was the International Society of Cellular Therapy proposal in 2005 on three minimal criteria defining human bone marrow MSCs: (i). tissue culture plastic adherence, (ii) phenotype (specifically expression of surface markers CD90, CD105 and CD73, together with lack of HLA-DR as well as haemopoietic surface markers such as CD45, CD19, CD14 or CD11b and CD19 or CD79), and (iii) capacity to differentiate into adipogenic, osteogenic and chrondrogenic lineages.62,63 Although these criteria can be applied to all MSC-like cells, they do not include markers for many other in vitro measurable phenotypic characteristics of MSC and MSC-like cells which indeed differ between cells sourced from different tissues and which vary in relation to in vitro (and in vivo) microenvironmental cues notwithstanding the fact that the minimal criteria are still fulfilled. Neither the 2005 criteria nor the newly discovered markers allow for the prospective identification and purification of such cells prior to in vitro expansion culture as these markers separately also occur on other cell types and as there is currently no single cell surface marker available to allow pure mesenchymal stem cell identification or isolation. Mesenchymal stromal cell subsets, however, can be enriched when using a combination of positive or negative selection markers. For example, CFU-Fs have been enriched from both lin−/CD271+/CD45−/CD146− and lin−/CD271+/CD45−/CD146+ bone marrow cell fractions and CD271 identifies a small population of cells with an MSC-like phenotype that are found as either perivascular (CD146+) or bone-lining cells in the bone marrow (CD146−).12,13,169
The vascular or perivascular localization of MSCs or MSC-like cells has led to the proposal that such cells might be categorized as vascular stem cells.10,174,175 However, as has been proposed, such cells, at least in vitro, should then have the ability to generate endothelial cells and pericytes in capillaries and endothelial and vascular smooth muscle cells in larger vessels, as well as generating mesenchymal cells with tri-lineage potential (bone, cartilage, fat). Interestingly, recent research has demonstrated that autologous cells can be reprogrammed to vascular stem or at least progenitor cells, which can stimulate coronary collateral vessel growth or be used to engineer durable vessels in surrogate in vivo models.176–178 The importance of MSCs is further supported by an earlier demonstration that epidermal growth factor (EGF) pre-conditioned MSCs stimulate collateral growth of coronary vessels in a rodent model.179 The main issue that still remains is that more detailed investigations on the phenotype and functionality of MSCs or MSC-like cells will further illustrate donor, acquisition site, culture methods, passage and microenvironment modulated heterogeneity of these cells, which can even persist within one culture. However, so far there is no good evidence as to which in vitro markers and functionality are of importance for final in vivo therapeutic efficacy. In this respect, we also need conclusive evidence as to whether the vessel-associated MSC subsets or vascular stem/stromal cells are more efficacious as a vascular regenerative therapy, for example by generating all vascular lineages in vivo or by providing stromal/microenvironmental support for vascular regeneration in vivo. Clonal repopulation assays in vivo that are reminiscent of those studies carried out in defining haemopoietic stem cells could give us clues in this respect.
Conclusions
MSCs are localized in the vascular niche in bone marrow, but also found as MSC-like cells around adult vessels (also termed pericytes and adventitial cells) and there is substantial evidence that they play a pivotal role in regulating blood vessel formation and function through multiple mechanisms such as vasculogenesis, arteriogenesis and angiogenesis. Although MSCs or MSC-like cells have been safely used and do not pose the ethical concern of embryonic stem cells, their effects in clinical studies cannot be delineated to specific mechanisms. These might include different simultaneously acting MSC-induced mechanisms. Immunomodulation towards a more repair-friendly microenvironment, actual differentiation into vascular tissue and paracrine or systemic release of vasculogenic, angiogenic and/or arteriogenic-stimulating factors should in this respect be acknowledged. Additionally, the results of preclinical studies have been shown to not only depend on the model chosen and the endogenous repair capacity of the cardiovascular tissue in vivo, but also on cell source, administration route, timing of cell delivery and cell dosage and with these specific homing and retention mechanisms. Clinical studies on necessarily heterogeneous patients adds many variables (e.g. inflammatory and disease status, co-morbidities, concomitant medication etc.) and may explain the differences in the results observed so far. Future studies will undoubtedly lead to a more defined MSC product and more personalized and tissue specific approaches to the use of these cells in regenerative medicine.
Funding
The authors wish to acknowledge the support of NHS Blood and Transplant, the National Institute of Health Research under its Programme Grants Scheme RP-PG-0310-10001 and RP-PG-0310-10003 and Restore Burns and Wound Healing Trust. This article summarises independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (Grant Reference Number RP-PG-0310-1001 and -1003). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Acknowledgement
We would like to thank Mrs Wendy Slack for expert assistance with formatting the references.
References
- 1.Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and haemopoietic tissues. Transplantation. 1968;6:230–47. [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse haemopoietic organs. Exp Hematol. 1976;4:267–74. [PubMed] [Google Scholar]
- 4.Castro-Malaspina H, Gay RE, Resnick G, et al. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56:289–301. [PubMed] [Google Scholar]
- 5.Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–40. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Dexter TM, Allen TD, Lajtha LG. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91:335–44. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 7.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 8.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–47. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 9.Bianco P. Minireview: the stem cell next door: skeletal and haemopoietic stem cell ‘niches’ in bone. Endocrinol. 2011;152:2957–62. doi: 10.1210/en.2011-0217. [DOI] [PubMed] [Google Scholar]
- 10.Lin CS, Lue TF. Defining vascular stem cells. Stem Cells Dev. 2013;22:1018–26. doi: 10.1089/scd.2012.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a haemopoietic microenvironment. Cell. 2007;131:324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Tormin A, Li O, Brune JC, et al. CD146 expression on primary nonhaemopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117:5067–77. doi: 10.1182/blood-2010-08-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–35. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–30. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugiyama T, Kohara H, Noda M, et al. Maintenance of the haemopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanoun M, Frenette PS. This niche is a maze; an amazing niche. Cell Stem Cell. 2013;12:391–2. doi: 10.1016/j.stem.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou B, Tsaknakis G, Coldwell KE, et al. A novel function for the haemopoietic supportive murine bone marrow MS-5 mesenchymal stromal cell line in promoting human vasculogenesis and angiogenesis. Br J Haematol. 2012;3:299–311. doi: 10.1111/j.1365-2141.2012.09050.x. [DOI] [PubMed] [Google Scholar]
- 20.Watt SM, Athanassopoulos A, Harris AL, et al. Human endothelial stem/progenitor cells, angiogenic factors and vascular repair. J R Soc Interface. 2010;7(Suppl 6):S731–51. doi: 10.1098/rsif.2010.0377.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–9. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crisan M, Corselli M, Chen WC, et al. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16:2851–60. doi: 10.1111/j.1582-4934.2012.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vono R, Spinetti G, Gubernator M, et al. What's new in regenerative medicine: split up of the mesenchymal stem cell family promises new hope for cardiovascular repair. J Cardiovasc Transl Res. 2012;5:689–99. doi: 10.1007/s12265-012-9395-2. [DOI] [PubMed] [Google Scholar]
- 24.Corselli M, Chen CW, Sun B, et al. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21:1299–308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–5. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell. 2010;7:431–42. doi: 10.1016/j.stem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Dazzi F, Lopes L, Weng L. Mesenchymal stromal cells: a key player in ‘innate tolerance’? Immunol. 2012;137:206–13. doi: 10.1111/j.1365-2567.2012.03621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nauta A, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 29.He Q, Wan C, Li G. Concise review: multipotent mesenchymal stromal cells in blood. Stem Cells. 2007;25:69–77. doi: 10.1634/stemcells.2006-0335. [DOI] [PubMed] [Google Scholar]
- 30.Erices A, Conget P, Minguell J. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–42. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 31.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–92. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 32.Sarugaser R, Lickorish D, Baksh D, et al. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220–9. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- 33.Troyer DL, Weiss ML. Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591–9. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Bruyn C, Najar M, Raicevic G, et al. A rapid, simple, and reproducible method for the isolation of mesenchymal stromal cells from Wharton's jelly without enzymatic treatment. Stem Cells Dev. 2011;20:547–57. doi: 10.1089/scd.2010.0260. [DOI] [PubMed] [Google Scholar]
- 35.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2011;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 36.Strioga M, Viswanathan S, Darinskas A, et al. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–52. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 37.In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–49. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 38.Trohatou O, Anagnou NP, Roubelakis MG. Human amniotic fluid stem cells as an attractive tool for clinical applications. Curr Stem Cell Res Ther. 2013;8:125–32. doi: 10.2174/1574888x11308020003. [DOI] [PubMed] [Google Scholar]
- 39.Ilic N, Brooke G, Murray P, et al. Manufacture of clinical grade human placenta-derived multipotent mesenchymal stromal cells. Methods Mol Biol. 2011;698:89–106. doi: 10.1007/978-1-60761-999-4_8. [DOI] [PubMed] [Google Scholar]
- 40.Pilz GA, Ulrich C, Ruh M, et al. Human term placenta-derived mesenchymal stromal cells are less prone to osteogenic differentiation than bone marrow-derived mesenchymal stromal cells. Stem Cells Dev. 2011;20:635–46. doi: 10.1089/scd.2010.0308. [DOI] [PubMed] [Google Scholar]
- 41.Zhang ZY, Teoh SH, Hui JH, et al. The potential of human fetal mesenchymal stem cells for off-the-shelf bone tissue engineering application. Biomaterials. 2012;33:2656–72. doi: 10.1016/j.biomaterials.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 42.Campagnoli C, Roberts IA, Kumar S, et al. Identification of mesenchymal stem/progenitor cells in human first- trimester fetal blood, liver, and bone marrow. Blood. 2011;98:2396–402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 43.Jones GN, Moschidou D, Puga-Iglesias TI, et al. Ontological differences in first compared to third trimester human fetal placental chorionic stem cells. PLoS One. 2012;7:e43395. doi: 10.1371/journal.pone.0043395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joshi M, B Patil P, He Z, et al. Fetal liver-derived mesenchymal stromal cells augment engraftment of transplanted hepatocytes. Cytotherapy. 2012;14:657–69. doi: 10.3109/14653249.2012.663526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–35. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 46.Nakahara H, Dennis JE, Bruder SP, et al. In vitro differentiation of bone and hypertrophic cartilage from periosteal-derived cells. Exp Cell Res. 1991;195:492–503. doi: 10.1016/0014-4827(91)90401-f. [DOI] [PubMed] [Google Scholar]
- 47.De Bari C, Dell'Accio F, Karystinou A, et al. A biomarker-based mathematical model to predict bone-forming potency of human synovial and periosteal mesenchymal stem cells. Arthritis Rheum. 2008;58:240–50. doi: 10.1002/art.23143. [DOI] [PubMed] [Google Scholar]
- 48.Jones EA, English A, Henshaw K, et al. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum. 2004;50:817–27. doi: 10.1002/art.20203. [DOI] [PubMed] [Google Scholar]
- 49.Karystinou A, Dell'Accio F, Kurth TB, et al. Distinct mesenchymal progenitor cell subsets in the adult human synovium. Rheumatology. 2009;48:1057–64. doi: 10.1093/rheumatology/kep192. [DOI] [PubMed] [Google Scholar]
- 50.Dowthwaite GP, Bishop JC, Redman SN, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–97. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 51.Bosch P, Musgrave DS, Lee JY, et al. Osteoprogenitor cells within skeletal muscle. J Orthop Res. 2000;18:933–44. doi: 10.1002/jor.1100180613. [DOI] [PubMed] [Google Scholar]
- 52.Young HE, Steele TA, Bray RA, et al. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001;264:51–62. doi: 10.1002/ar.1128. [DOI] [PubMed] [Google Scholar]
- 53.Chow K, Fessel JP, Kaoriihida-Stansbury, et al. Dysfunctional resident lung mesenchymal stem cells contribute to pulmonary microvascular remodeling. Pulm Circ. 2013;3:31–49. doi: 10.4103/2045-8932.109912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo Z, Li H, Li X, et al. In vitro characteristics and in vivo immunosuppressive activity of compact bone-derived murine mesenchymal progenitor cells. Stem Cells. 2006;24:992–1000. doi: 10.1634/stemcells.2005-0224. [DOI] [PubMed] [Google Scholar]
- 55.Zaidi M, Sun L, Blair HC. Special stem cells for bone. Cell Stem Cell. 2012;10:233–4. doi: 10.1016/j.stem.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 56.Park D, Spencer JA, Koh BI, et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10:259–72. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chong JJ, Chandrakanthan V, Xaymardan M, et al. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell. 2011;9:527–40. doi: 10.1016/j.stem.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pelekanos RA, Li J, Gongora M, et al. Comprehensive transcriptome and immunophenotype analysis of renal and cardiac MSC-like populations supports strong congruence with bone marrow MSC despite maintenance of distinct identities. Stem Cell Res. 2012;8:58–73. doi: 10.1016/j.scr.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–16. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 60.Covas DT, Panepucci RA, Aparecida M, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exper Hem. 2008;36:642–54. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 61.Bosch J, Houben AP, Radke TF, et al. Distinct differentiation potential of ‘MSC’ derived from cord blood and umbilical cord: are cord-derived cells true mesenchymal stromal cells? Stem Cells Dev. 2012;21:1977–88. doi: 10.1089/scd.2011.0414. [DOI] [PubMed] [Google Scholar]
- 62.Horwitz EM, Le Blanc K, Dominici M, et al. International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–5. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 63.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 64.Melief SM, Zwaginga JJ, Fibbe WE, et al. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2:455–63. doi: 10.5966/sctm.2012-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watt SM. Comprehensive Biotechnology. 2nd edn. Vol. 5. Kidlington, UK: Elsevier B.V.; 2011. Umbilical cord blood stem cell banking; pp. 397–406. Moo-Young M (editor-in-chief) [Google Scholar]
- 66.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–87. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 67.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Makanya AN, Hlushchuk R, Djonov VG. Intussusceptive angiogenesis and its role in vascular morphogenesis, patterning, and remodeling. Angiogenesis. 2009;12:113–23. doi: 10.1007/s10456-009-9129-5. [DOI] [PubMed] [Google Scholar]
- 69.van Royen N, Piek JJ, Schaper W, et al. A critical review of clinical arteriogenesis research. J Am Coll Cardiol. 2009;55:17–25. doi: 10.1016/j.jacc.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 70.Melero-Martin JM, De Obaldia ME, Kang SY, et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kachgal S, Putnam AJ. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogen. 2011;14:47–59. doi: 10.1007/s10456-010-9194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Athanassopoulos A, Tsaknakis G, Newey SE, et al. Microvessel networks in pre-formed in artificial clinical grade dermal substitutes in vitro using cells from haematopoietic tissues. Burns. 2012;38:691–701. doi: 10.1016/j.burns.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 73.Walker NG, Mistry AR, Smith LE, et al. A chemically defined carrier for the delivery of human mesenchymal stem/stromal cells to skin wounds. Tissue Eng Part C Methods. 2012;18:143–55. doi: 10.1089/ten.tec.2011.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roubelakis MG, Tsaknakis G, Pappa KI, et al. Spindle shaped human mesenchymal stem/stromal cells from amniotic fluid promote neovascularization. PLoS One. 2013;8:e54747. doi: 10.1371/journal.pone.0054747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Traktuev DO, Prater DN, Merfeld-Clauss S, et al. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res. 2009;104:1410–20. doi: 10.1161/CIRCRESAHA.108.190926. [DOI] [PubMed] [Google Scholar]
- 76.Merfeld-Clauss S, Gollahalli N, March KL, et al. Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng Part A. 2010;16:2953–66. doi: 10.1089/ten.tea.2009.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Au P, Tam J, Fukumura D, et al. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–8. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Melero-Martin JM, De Obaldia ME, Allen P, et al. Host myeloid cells are necessary for creating bioengineered human vascular networks in vivo. Tissue Eng Part A. 2010;16:2457–66. doi: 10.1089/ten.tea.2010.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin RZ, Moreno-Luna R, Zhou B, et al. Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis. 2012;15:443–55. doi: 10.1007/s10456-012-9272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Traktuev DO, Merfeld-Clauss S, Li J, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 81.Kim SW, Han H, Chae GT, et al. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger's disease and ischemic limb disease animal model. Stem Cells. 2006;24:1620–6. doi: 10.1634/stemcells.2005-0365. [DOI] [PubMed] [Google Scholar]
- 82.Lian Q, Zhang Y, Zhang J, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–23. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 83.Smadja DM, d'Audigier C, Guerin CL, et al. Angiogenic potential of BM MSCs derived from patients with critical leg ischemia. Bone Marrow Transplant. 2012;47:997–1000. doi: 10.1038/bmt.2011.196. [DOI] [PubMed] [Google Scholar]
- 84.Siekmann AF, Affolter M, Belting HG. The tip cell concept 10 years after: New players tune in for a common theme. Exp Cell Res. 2013;319:1255–63. doi: 10.1016/j.yexcr.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 85.Ribatti D, Crivellato E. Sprouting angiogenesis’, a reappraisal. Dev Biol. 2012;372:157–65. doi: 10.1016/j.ydbio.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 86.Cheng G, Liao S, Kit Wong H, et al. Engineered blood vessel networks connect to host vasculature via wrapping and tapping anastomosis. Blood. 2011;118:4740–9. doi: 10.1182/blood-2011-02-338426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davis GE, Stratman AN, Sacharidou A, et al. Molecular basis for endothelial lumen formation and tubulogenesis during vasculogenesis and angiogenic sprouting. Int Rev Cell Mol Biol. 2011;288:101–65. doi: 10.1016/B978-0-12-386041-5.00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cai X, Lin Y, Hauschka PV, et al. Adipose stem cells originate from perivascular cells. Biol Cell. 2011;103:435–47. doi: 10.1042/BC20110033. [DOI] [PubMed] [Google Scholar]
- 89.Li GG, Zhu YT, Xie HT, et al. Mesenchymal stem cells derived from human limbal niche cells. Invest Ophthalmol Vis Sci. 2012;53:5686–97. doi: 10.1167/iovs.12-10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang ZY, Teoh SH, Chong MS, et al. Neo-vascularization and bone formation mediated by fetal mesenchymal stem cell tissue-engineered bone grafts in critical-size femoral defects. Biomaterials. 2010;31:608–20. doi: 10.1016/j.biomaterials.2009.09.078. [DOI] [PubMed] [Google Scholar]
- 91.Roura S, Bagó JR, Soler-Botija C, et al. Human umbilical cord blood-derived mesenchymal stem cells promote vascular growth in vivo. PLoS One. 2012;7:e49447. doi: 10.1371/journal.pone.0049447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hooper AT, Butler JM, Nolan DJ, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–74. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Slayton WB, Li XM, Butler J, et al. The role of the donor in the repair of the marrow vascular niche following hematopoietic stem cell transplant. Stem Cells. 2007;25:2945–55. doi: 10.1634/stemcells.2007-0158. [DOI] [PubMed] [Google Scholar]
- 94.Wolf NS, Trentin JJ. Hemopoietic colony studies. V. Effect of hemopoietic organ stroma on differentiation of pluripotent stem cells. J Exp Med. 1968;127:205–14. doi: 10.1084/jem.127.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 96.Bianco P. Back to the future: moving beyond ‘mesenchymal stem cells. J Cell Biochem. 2011;112:1713–21. doi: 10.1002/jcb.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lander AD, Kimble J, Clevers H, et al. What does the concept of the stem cell niche really mean today? BMC Biol. 2012;10:19. doi: 10.1186/1741-7007-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chan C, Chen C, Luppen C, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–4. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Almeida-Porada G, Porada CD, Tran N, et al. Cotransplantation of human stromal cell progenitors into preimmune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in bone marrow at later time points after transplantation. Blood. 2000;95:3620–7. [PubMed] [Google Scholar]
- 100.Noort WA, Kruisselbrink AB, In't Anker PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002;30:870–8. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- 101.Masuda S, Ageyama N, Shibata H, et al. Cotransplantation with MSCs improves engraftment of HSCs after autologous intra-bone marrow transplantation in nonhuman primates. Exp Hematol. 2009;37:1250–7. doi: 10.1016/j.exphem.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 102.Ball LM, Bernardo ME, Roelofs H, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical haemopoietic stem-cell transplantation. Blood. 2007;110:2764–7. doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- 103.Bernardo ME, Cometa AM, Locatelli F. Mesenchymal stromal cells: a novel and effective strategy for facilitating engraftment and accelerating hematopoietic recovery after transplantation? Bone Marrow Transplant. 2012;47:323–9. doi: 10.1038/bmt.2011.102. [DOI] [PubMed] [Google Scholar]
- 104.Lee SH, Lee MW, Yoo KH, et al. Co-transplantation of third-party umbilical cord blood-derived MSCs promotes engraftment in children undergoing unrelated umbilical cord blood transplantation. Bone Marrow Transplant. 2013;48:1040–5. doi: 10.1038/bmt.2013.7. [DOI] [PubMed] [Google Scholar]
- 105.Koc ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–16. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 106.Macmillan ML, Blazar BR, DeFor TE, et al. Transplantation of ex-vivo culture-expanded parental haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: results of a phase I-II clinical trial. Bone Marrow Transplant. 2009;43:447–54. doi: 10.1038/bmt.2008.348. [DOI] [PubMed] [Google Scholar]
- 107.Gonzalo-Daganzo R, Regidor C, Martin-Donaire T, et al. Results of a pilot study on the use of third-party donor mesenchymal stromal cells in cord blood transplantation in adults. Cytotherapy. 2009;11:278–88. doi: 10.1080/14653240902807018. [DOI] [PubMed] [Google Scholar]
- 108.Guan M, Yao W, Liu R, et al. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med. 2012;18:456–62. doi: 10.1038/nm.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clifford DM, Fisher SA, Brunskill SJ, et al. Long-term effects of autologous bone marrow stem cell treatment in acute myocardial infarction: factors that may influence outcomes. PLoS One. 2012;7:e37373. doi: 10.1371/journal.pone.0037373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Korhonen M, Jolkkonen J. Intravascular cell therapy in stroke patients:where the cells go and what they do. Regen Med. 2013;8:93–5. doi: 10.2217/rme.13.7. [DOI] [PubMed] [Google Scholar]
- 111.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair. 2009;17:763–71. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Markeson D, Pleat JM, Sefalian AM, et al. Scarring, stem cells, scaffolds and skin repair. J Tiss Eng Regen Med. 2013 doi: 10.1002/term.1841. (in press) [DOI] [PubMed] [Google Scholar]
- 113.King A. Stem cells. Could it be TIME to abandon BMCs? Nat Rev Cardiol. 2013;10:8. doi: 10.1038/nrcardio.2012.170. [DOI] [PubMed] [Google Scholar]
- 114.Penn MS. Are stem cells the teacher or the student? Curr Opin Organ Transplant. 2012;17:663–9. doi: 10.1097/MOT.0b013e32835a5aad. [DOI] [PubMed] [Google Scholar]
- 115.Ohno T, Kaneda H, Nagai Y, et al. Regenerative medicine in critical limb ischemia – current and future directions. J Ather Thromb. 2012;19:883–9. doi: 10.5551/jat.12906. [DOI] [PubMed] [Google Scholar]
- 116.Lasala GP, Minguell JJ. Vascular disease and stem cell therapies. Br Med Bull. 2011;98:187–97. doi: 10.1093/bmb/ldr017. [DOI] [PubMed] [Google Scholar]
- 117.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bolli R, Chugh AR, D'Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–57. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 119.Williams AR, Hatzistergos KE, Addicott B, et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–23. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–79. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carr CA, Stuckey DJ, Tan JJ, et al. Cardiosphere-derived cells improve function in the infarcted rat heart for at least 16 weeks—an MRI study. PLoS One. 2011;6:e25669. doi: 10.1371/journal.pone.0025669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen CW, Okada M, Proto JD, et al. Human pericytes for ischemic heart repair. Stem Cells. 2013;31:305–16. doi: 10.1002/stem.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jackson WM, Nesti LJ, Tuan RS. Concise review: clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl Med. 2012;1:44–50. doi: 10.5966/sctm.2011-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maxson S, Lopez EA, Yoo D, et al. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1:142–9. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dash NR, Dash SN, Routray P, et al. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuven Res. 2009;12:359–66. doi: 10.1089/rej.2009.0872. [DOI] [PubMed] [Google Scholar]
- 127.Blum A, Balkan W, Hare JM. Advances in cell-based therapy for peripheral vascular disease. Atherosclerosis. 2012;223:269–77. doi: 10.1016/j.atherosclerosis.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 128.Jiang D, Qi Y, Walker NG, et al. The effect of adipose tissue derived MSCs delivered by a chemically defined carrier on full-thickness cutaneous wound healing. Biomaterials. 2013;34:2501–15. doi: 10.1016/j.biomaterials.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 129.Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng Part A. 2007;13:1299–312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 130.Ranganath SH, Levy O, Inamdar MS, et al. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–58. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martin-Rendon E, Hale SJ, Ryan D, et al. Transcriptional profiling of human cord blood CD133+ and cultured bone marrow mesenchymal stem cells in response to hypoxia. Stem Cells. 2007;25:1003–12. doi: 10.1634/stemcells.2006-0398. [DOI] [PubMed] [Google Scholar]
- 132.Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:1–11. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anand S. A brief primer on microRNAs and their roles in angiogenesis. Vasc Cell. 2013;5:2. doi: 10.1186/2045-824X-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wen Z, Zheng S, Zhou C, et al. Bone marrow mesenchymal stem cells for post-myocardial infarction cardiac repair: microRNAs as novel regulators. J Cell Mol Med. 2012;16:657–71. doi: 10.1111/j.1582-4934.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fish JE, Santoro MM, Morton SU, et al. miR-126 regulates angiogenic signaling and vascular integrity. Develop Cell. 2008;15:272–84. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang S, Aurora AB, Johnson BA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Develop Cell. 2008;15:261–71. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Katare R, Riu F, Mitchell K, et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132/novelty and significance. Circ Res. 2011;109:894–906. doi: 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vunjak-Novakovic G, Lui KO, Tandon N, et al. Bioengineering heart muscle: a paradigm for regenerative medicine. Annu Rev Biomed Eng. 2011;13:245–67. doi: 10.1146/annurev-bioeng-071910-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Udelsman BV, Maxfield MW, Breuer CK. Tissue engineering of blood vessels in cardiovascular disease: moving towards clinical translation. Heart. 2013;99:454–60. doi: 10.1136/heartjnl-2012-302984. [DOI] [PubMed] [Google Scholar]
- 141.Arenas-Herrera JE, Ko IK, Atala A, et al. Decellularization for whole organ bioengineering. Biomed Mater. 2013;8:014106. doi: 10.1088/1748-6041/8/1/014106. [DOI] [PubMed] [Google Scholar]
- 142.Iyer RK, Chiu LL, Vunjak-Novakovic G, et al. Biofabrication enables efficient interrogation and optimization of sequential culture of endothelial cells, fibroblasts and cardiomyocytes for formation of vascular cords in cardiac tissue engineering. Biofabrication. 2012;4:035002. doi: 10.1088/1758-5082/4/3/035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Uchinaka A, Kawaguchi N, Hamada Y, et al. Transplantation of elastin-secreting myoblast sheets improves cardiac function in infarcted rat heart. Mol Cell Biochem. 2012;368:203–14. doi: 10.1007/s11010-012-1361-4. [DOI] [PubMed] [Google Scholar]
- 144.Uchinaka A, Kawaguchi N, Hamada Y, et al. Transplantation of myoblast sheets that secrete the novel peptide SVVYGLR improves cardiac function in failing hearts. Cardiovasc Res. 2013;99:102–10. doi: 10.1093/cvr/cvt088. [DOI] [PubMed] [Google Scholar]
- 145.Sekine H, Shimizu T, Sakaguchi K, et al. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013;4:1399–409. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chachques JC. Bio-hybrid tissue engineering for cellular cardiomyoplasty: future directions. Methods Mol Biol. 2013;1036:151–62. doi: 10.1007/978-1-62703-511-8_13. [DOI] [PubMed] [Google Scholar]
- 147.Haneef K, Lila N, Benadda S, et al. Development of bioartificial myocardium by electrostimulation of 3D collagen scaffolds seeded with stem cells. Heart Int. 2012;7:e14. doi: 10.4081/hi.2012.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tulloch NL, Muskheli V, Razumova MV, et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Eschenhagen T, Eder A, Vollert I, et al. Physiological aspects of cardiac tissue engineering. Am J Physiol Heart Circ Physiol. 2012;303:H133–43. doi: 10.1152/ajpheart.00007.2012. [DOI] [PubMed] [Google Scholar]
- 150.Ott HC, Matthiesen TS, Goh SK, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 151.Roura S, Gálvez-Montón C, Bayes-Genis A. Bioluminescence imaging: a shining future for cardiac regeneration. J Cell Mol Med. 2013;17:693–703. doi: 10.1111/jcmm.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vila OF, Bagó JR, Navarro M, et al. Calcium phosphate glass improves angiogenesis capacity of poly(lactic acid) scaffolds and stimulates differentiation of adipose tissue-derived mesenchymal stromal cells to the endothelial lineage. Biomed Mater Res A. 2013;101:932–41. doi: 10.1002/jbm.a.34391. [DOI] [PubMed] [Google Scholar]
- 153.Bagó JR, Aguilar E, Alieva M, et al. In vivo bioluminescence imaging of cell differentiation in biomaterials: a platform for scaffold development. Tissue Eng Part A. 2013;19:593–603. doi: 10.1089/ten.tea.2012.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jung Y, Bauer G, Nolta JA. Concise review: Induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. Stem Cells. 2012;30:42–7. doi: 10.1002/stem.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tuan RS. Regenerative medicine in 2012: the coming of age of musculoskeletal tissue engineering. Nat Rev Rheumatol. 2013;9:74–6. doi: 10.1038/nrrheum.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Didié M, Christalla P, Rubart M, et al. Parthenogenetic stem cells for tissue-engineered heart repair. J Clin Invest. 2013;123:1285–98. doi: 10.1172/JCI66854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Carpenter L, Carr C, Yang CT, et al. Efficient differentiation of human induced pluripotent stem cells generates cardiac cells that provide protection following myocardial infarction in the rat. Stem Cells Dev. 2012;21:977–86. doi: 10.1089/scd.2011.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bernardo ME, Fibbe WE. Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders. Ann N Y Acad Sci. 2012;1266:107–17. doi: 10.1111/j.1749-6632.2012.06667.x. [DOI] [PubMed] [Google Scholar]
- 159.Giuliani N, Storti P, Bolzoni M, et al. Angiogenesis and multiple myeloma. Cancer Microenviron. 2011;4:325–37. doi: 10.1007/s12307-011-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Goel S, Wong AH, Jain RK. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb Perspect Med. 2012;2:a006486. doi: 10.1101/cshperspect.a006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Goel S, Fukumura D, Jain RK. Normalization of the tumor vasculature through oncogenic inhibition: An emerging paradigm in tumor biology. Proc Natl Acad Sci USA. 2012;109:E1214. doi: 10.1073/pnas.1203794109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Razmkhah M, Jaberipour M, Erfani N, et al. Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-beta1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol. 2011;266:116–22. doi: 10.1016/j.cellimm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 163.Corcoran KE, Trzaska KA, Fernandes H, et al. Mesenchymal stem cells in early entry of breast cancer into bone marrow. PLoS One. 2008;3:e2563. doi: 10.1371/journal.pone.0002563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Quante M, Tu SP, Tomita H, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–72. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Keung EZ, Nelson PJ, Conrad C. Concise review: genetically engineered stem cell therapy targeting angiogenesis and tumor stroma in gastrointestinal malignancy. Stem Cells. 2013;31:227–35. doi: 10.1002/stem.1269. [DOI] [PubMed] [Google Scholar]
- 166.De Spiegelaere W, Casteleyn C, Van den Broeck W, et al. Intussusceptive angiogenesis: a biologically relevant form of angiogenesis. J Vasc Res. 2012;49:390–404. doi: 10.1159/000338278. [DOI] [PubMed] [Google Scholar]
- 167.Raza A, Franklin MJ, Dudek AZ. Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am J Hematol. 2010;85:593–8. doi: 10.1002/ajh.21745. [DOI] [PubMed] [Google Scholar]
- 168.Kanehira M, Xin H, Hoshino K, et al. Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Ther. 2007;14:894–903. doi: 10.1038/sj.cgt.7701079. [DOI] [PubMed] [Google Scholar]
- 169.Corselli M, Chin CJ, Parekh C, et al. Perivascular support of human haemopoietic cells. Blood. 2013;121:2891–901. doi: 10.1182/blood-2012-08-451864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jones EA, Kinsey SE, English A, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349–60. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 171.Izadpanah R, Kaushal D, Kriedt C, et al. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68:4229–38. doi: 10.1158/0008-5472.CAN-07-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Charbord P, Livne E, Gross G, et al. Human bone marrow mesenchymal stem cells: a systematic reappraisal via the genostem experience. Stem Cell Res. 2011;7:32–42. doi: 10.1007/s12015-010-9125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Churchman SM, Ponchel F, Boxall SA, et al. Transcriptional profile of native CD271+ multipotential stromal cells: evidence for multiple fates, with prominent osteogenic and Wnt pathway signaling activity. Arthritis Rheum. 2012;64:2632–43. doi: 10.1002/art.34434. [DOI] [PubMed] [Google Scholar]
- 174.Tang Z, Wang A, Yuan F, et al. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012;3:875. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bautsch VL. Stem cells and the vasculature. Nat Med. 2011;17:1437–43. doi: 10.1038/nm.2539. [DOI] [PubMed] [Google Scholar]
- 176.Samuel R, Daheron L, Liao S, et al. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc Natl Acad Sci USA. 2013;110:12774–9. doi: 10.1073/pnas.1310675110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Faber JE. Reprogrammed endothelial cells: cell therapy for coronary collateral growth? Circ Res. 2012;110:192–4. doi: 10.1161/CIRCRESAHA.111.261495. [DOI] [PubMed] [Google Scholar]
- 178.Yin L, Ohanyan V, Pung YF, et al. Induction of vascular progenitor cells from endothelial cells stimulates coronary collateral growth. Circ Res. 2012;110:241–52. doi: 10.1161/CIRCRESAHA.111.250126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Belmadani S, Matrougui K, Kolz C, et al. Amplification of coronary arteriogenic capacity of multipotent stromal cells by epidermal growth factor. Arterioscler Thromb Vasc Biol. 2009;29:802–8. doi: 10.1161/ATVBAHA.109.186189. [DOI] [PMC free article] [PubMed] [Google Scholar]


