Abstract
Objectives
To explore perceptions of health and illness, and the use of complementary and alternative medicine (CAM), among people with type 2 diabetes and/or cardiovascular disease (CVD), and relate these to quality of life.
Design
A self-administered survey was delivered by mail and internet. The questionnaire was designed from data generated from qualitative research and other sources, to collect information on health-status, CAM use, and health and illness perceptions. Quality of life was compared among participants using Assessment of Quality of Life (AQoL-4D).
Subjects
Adults with type 2 diabetes and/or CVD residing in Victoria, Australia, whether or not they used CAM therapies as well as conventional medical treatment.
Outcome measures
Comparisons were made of AQoL-4D utility scores, demographic, health and wellbeing status, care-seeking and health management behaviors, and behavioral and illness perception variables. A range of descriptive statistical and predictive modeling techniques were used to assess significant associations (p<0.01) between CAM-user and non-user populations.
Results
From a sample of 2766 people, 45.1% had used CAM in the past 12 months and the remainder had never used CAM; ages ranged from 20-96 years. CAM-users tended to report lower (worse) quality of life measures than non-users, and a greater number of chronic conditions in addition to diabetes and CVD. Despite this, CAM-users reported their illness perception and behavioral change more positively than non-users. There was little difference between CAM and non-users in use of prescription medications despite CAM-users greater disease burden.
Conclusion
Higher CAM use and low quality of life appear to reflect comorbidity and poor general health. Greater evidence is needed of how CAM use might support chronic illness prevention and complement chronic disease management, with important policy implications concerning the integration of CAM therapies with mainstream health services.
Introduction
Complementary and alternative medicine (CAM) is increasingly used as a component of self-care. Studies conducted in Canada, the United States, Australia, and elsewhere document the common use of CAM among oncology patients, people with arthritis, patients with HIV/AIDS, middle aged women, and people with chronic conditions that were responding poorly to conventional treatment.1–6 A survey conducted in Australia in the early 2000s with patients in complementary healthcare settings also showed that psychological, gynecological, and endocrine disorders were common reasons for seeking treatment and that 78% used CAM for a chronic or recurrent condition.7,8
Little is known about how people with chronic conditions manage their continuing need for medical care or how long-term adherence to conventional prescriptions influences or is affected by CAM therapy. This article focuses on patients with type 2 diabetes mellitus (DM) and cardiovascular disease (CVD), for whom medication, dietary change, and health monitoring can be lifelong.
Before data collection, the hypothesis was that CAM users who, by their use of CAM, actively self-managed their condition, might rate better on an objective Quality of Life (QoL) measure than CAM nonusers. The findings instead revealed that QoL scores tended to be worse for CAM users but also that, in contrast, they rated their perceptions of health more positively than did nonusers. With the purpose of providing comprehensive descriptive results profiling the differences and similarities between CAM users and nonusers within a population of people with DM or CVD, this study compared demographic characteristics, health and well-being, health management, and behavioral and illness perception attributes and used regression modeling to identify predictors of CAM use. Although significant demographic differences were seen between CAM users and nonusers, the differences in behavior and illness perceptions suggests the need for further investigation into why CAM users self-rate their perceptions more positively than do nonusers, despite similar or worse QoL ratings.
Materials and Methods
Design
The Complementary and Alternative Medicine, Economics, Lifestyle and Other Therapeutic (CAMELOT) approaches for chronic conditions project used a mixed-methods design to investigate care-seeking, self-management, and use of CAM by people with DM or CVD.9–12 In 2009, in-depth interviews were conducted with 69 people with DM or CVD who used CAM. Findings from the interviews informed the development of the survey instrument. Between April and July 2010, self-report survey data were obtained from people residing in Victoria, Australia, who had DM or any CVD (including angina, atherosclerosis, heart bypass surgery [or similar procedure], stroke) or the CVD risk factors hypertension or hypercholesterolemia. CAM use was not a prerequisite for survey participation.
Questionnaire
The questionnaire comprised 71 questions across five sections: use of health services, use of CAM, health insurance, health and lifestyle preferences, and respondent demographic characteristics (Supplementary materials are available online at http://www.liebertpub.com/acm). It incorporated the four-dimension Assessment of Quality of Life (AQoL-4D) questionnaire, which uses 12 questions to measure independent living, relationships, senses, and mental health.13,14 AQoL-4D provided an objective, quantifiable measure (utility score) of respondents' QoL. The study questionnaire also incorporated 10 items from Moss-Morris and colleagues' revised illness perception questionnaire.15
Sampling
The questionnaire was distributed widely, in hard copy and via the Internet, to capture a broad sample of responders. The breakdown of survey returns is published elsewhere.10 In total, 2625 valid postal and 290 valid online returns were received (n=2915). Of the valid postal surveys, 2203 were from a random sample of 10,000 Victorian National Diabetes Services Scheme (NDSS) registrants with type 2 DM, 166 were from Heart Support Australia members, and 256 were from responders who learned of the survey from such sources as media (newspaper advertisements, public notice boards), community organizations, and CAM and medical practitioners.
CAM
In the questionnaire, CAM use was defined as the “use of CAM products or visits to CAM practitioners, including: naturopathy, homoeopathy, herbal medicine, nutritional medicine (including vitamin or mineral supplements), aromatherapy, reflexology, massage therapies, Ayurveda, acupuncture, Chinese medicine, spiritual healing, meditation, Tai chi, yoga, and many others.” Other CAM therapies specified included chiropractic, osteopathy, prayer, aromatherapy, and color and music therapy. Questions referred to CAM use “ever” or “in the last 12 months”; type of use as “CAM practitioner” or “CAM product”; and reason for use as “any condition” or specifically for “treatment of type 2 diabetes or heart/cardiovascular conditions.”
Analysis
IBM SPSS Statistics, version 20 (SPSS Inc., College Station, Texas), was used to explore assumptions of normality and perform statistical analyses. The association between CAM use, age, sex, and AQoL-4D was assessed by using univariate analysis of variance. Comparisons of demographic characteristics, health status and management, and behavioral and illness perception factors between CAM users and nonusers were performed by using contingency tables with chi-square tests for independence and z-tests to compare column proportions (α<0.01), along with independent sample t-tests. Binary logistic regression was used to calculate odds ratios and 95% confidence intervals (CIs) of CAM use for demographic, health status, and management variables. The variables that showed an association between CAM users and nonusers with a p-value<.05 were then entered, one by one, into a binary logistic regression procedure to assess independent predictors of CAM use.
To interpret the relationship between variables and CAM use, a p-value≥.05 indicated a non–statistically significant association (and, therefore, similarity between groups), and a p-value<.01 indicated a significant association (i.e., a difference; this provided for more conservative reporting than p<.05). Contingency tables report “valid” percentages (missing cases ignored); for consistency, percentage reporting outside of contingency tables uses “valid” percentages, and the number of missing cases is noted if it represents more than 6.0% of the sample. Unless specified, “CAM user” refers to respondents who used CAM in the last 12 months, and “CAM nonuser” refers to participants who did not use CAM in the previous 12 months. Income is reported in 2010 Australian dollars. A total of 149 respondents who used CAM in their lifetime, but not in the prior 12 months, were excluded, for a total sample of 2766 people.
Results
Sample profile and CAM use
The majority of respondents had DM (91.1%); were male (54.8%); were Australian born (68.8%); lived in a major city (59.6%); lived with a spouse, partner, or family (77.1%); were married (69.2%); were not employed (68.0%); had a low annual household income (≤$50,000) (70.4%; data missing for 312 respondents); attended secondary school as highest education (58.4%); had a mean age of 65.3 years (range, 20–96 years); and had never used CAM (54.9%; n=1519). Of the 1247 respondents who had used CAM in the last 12 months, 346 (29.5%) used it to treat their DM or CVD. Most CAM users used products (94.6%; n=1180), most often vitamins, minerals, and other nutritional supplements; 96.9% used them in addition to prescription pharmaceuticals. CAM practitioners were consulted by 54.5% (n=671) of CAM users.11 Multiple reasons were given for first using CAM, including suggestion from a doctor, pharmacist, or other health professional (42.7%); belief that CAM would improve their condition (39.4%); belief that CAM was not harmful (33.3%); desire to take more control of their own health (31.3%); preference for using “natural things” (27.8%); suggestion from family or friend (23.8%); and dissatisfaction with the healthcare system or pharmaceutical treatments (18.2%).
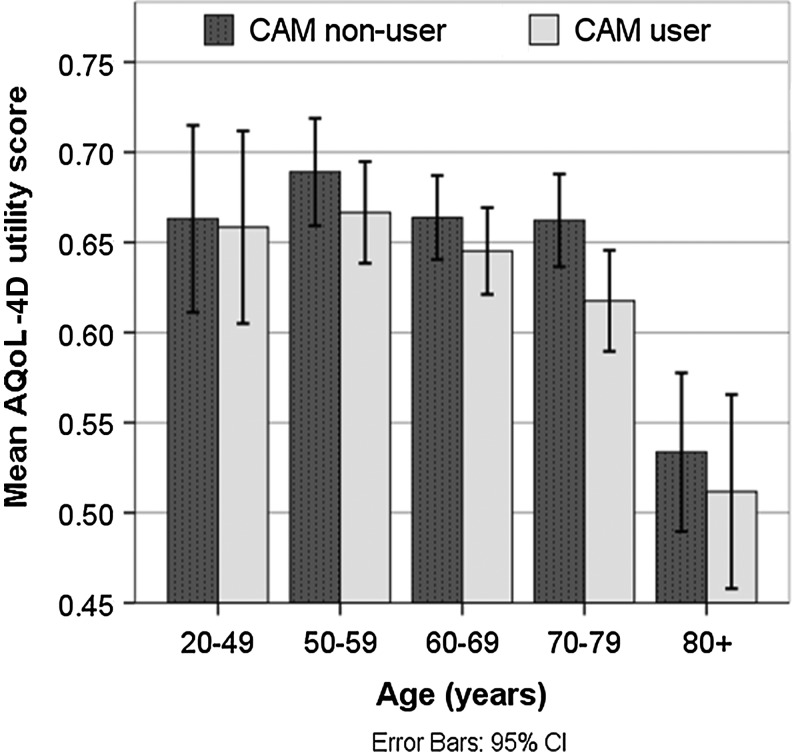
Quality of life
The closer an AQoL-4D score is to 1, the greater (better) the QoL measure. Across all ages, our survey respondents' mean AQoL-4D scores were lower than those of the general population (mean for all CAM users, 0.63; mean for nonusers, 0.65; mean for general population, 0.83).14 This was expected because our respondents had at least one chronic disease. Although the trend of CAM users having lower AQoL-4D utility scores persisted across age groups (Fig. 1), the association was not statistically significant (p>0.05). However, the “mental health” dimension (which assessed quality of sleep; anxiety, worry, depression; and pain), which contributed to the overall AQoL-4D utility score, did have a statistically significant association with CAM use (p=.001). When AQoL-4D utility score was included in logistic regression modeling, it did emerge as a statistically significant predictor of CAM use (see later section on predictors of CAM use).
Fig. 1.
Mean Assessment of Quality of Life (AQoL-4D) utility scores by age and complementary and alternative medicine (CAM) use, with 95% confidence intervals.
Demographic factors
Mean age differed little between groups, with CAM users slightly younger than nonusers (mean age±standard deviation, 64.5±10.9 years versus 66.0±11.3 years). CAM users were similar to nonusers (p≥0.05) in many aspects, including employment status; income, whether born in Australia, relationship status (e.g., married/defacto, widowed, single), living arrangements (e.g., living alone, living with partner/family), and having a medical benefits card (issued to people with low income or war veterans). CAM users and nonusers showed distinct differences (p<.001) in sex, highest level of education, occupation, and holding of private health insurance (Table 1). Just 36.6% of male respondents used CAM, and women were more likely to use CAM then men. CAM users were more likely to work (or have previously worked) in professional or clerical/administrative roles, and nonusers were more likely to have worked in technical or manual occupations. Australian Standard Geographical Classification remoteness categories, based on postal codes, indicated no significant association between rurality and CAM use. However, just 36.8% of people living in outer regional areas used CAM compared with 45.7% of people who lived in metropolitan or inner regional areas.
Table 1.
Population Profile
| Variable | CAM nonuser, n (%) | CAM user, n (%) | Odds ratio (95% confidence interval) | p-Value |
|---|---|---|---|---|
| Sexa | ||||
| Male | 953 (63.0)* | 550 (44.7)** | 1 | |
| Female | 559 (37.0)* | 681 (55.3)** | 2.11 (1.81–2.46) | <.001 |
| Age groupa | ||||
| 20–49 y | 117 (7.7)* | 99 (8.0)* | 1 | .002 |
| 50–69 y | 806 (53.3)* | 732 (59.5)** | 1.07 (0.81–1.43) | .627 |
| ≥70 y | 589 (39.0)* | 399 (32.4)** | 0.80 (0.60–1.08) | .141 |
| Highest educationa | ||||
| Secondary school | 954 (64.4)* | 624 (51.1)** | 1 | <.001 |
| Certificate, diploma or undergraduate | 333 (30.3)* | 471 (38.6)** | 1.60 (1.36–1.89) | <.001 |
| Postgraduate | 79 (5.3)* | 125 (10.2)** | 2.42 (1.79–3.26) | <.001 |
| Household incomea,b | ||||
| ≤$50,000 | 948 (71.3)* | 779 (69.2)* | 1 | .255 |
| $50,001–$100,000 | 285 (21.4)* | 245 (21.8)* | 1.05 (0.86–1.27) | .651 |
| ≥$100,001 | 96 (7.2)* | 101 (9.0)* | 1.28 (0.95–1.72) | .101 |
| Employed | ||||
| No | 1038 (69.2)* | 812 (66.3)* | 1 | |
| Yes | 461 (30.8)* | 413 (33.7)* | 1.14 (0.97–1.34) | .109 |
| Occupation (current or previous) | ||||
| Other or never worked | 96 (6.6)* | 64 (5.3)* | 1 | <.001 |
| Manager | 274 (18.9)* | 189 (15.7)* | 1.04 (0.72–1.49) | .855 |
| Professional | 288 (19.8)* | 352 (29.2)** | 1.83 (1.29–2.61) | .001 |
| Technician or trade | 190 (13.1)* | 115 (9.5)** | 0.91 (0.61–1.34) | .629 |
| Community/personal service | 85 (5.8)* | 83 (6.9)* | 1.47 (0.945–2.27) | .087 |
| Clerical or administrative | 201 (13.8)* | 231 (19.1)** | 1.72 (1.19–2.49) | .004 |
| Sales worker | 90 (6.2)* | 59 (4.9)* | 0.98 (0.62–1.55) | .942 |
| Machine operator or laborer | 229 (15.8)* | 114 (9.4)** | 0.75 (0.51–1.10) | .140 |
| Area of residencea | ||||
| Major city | 895 (59.0)* | 753 (60.4)* | 1 | .045 |
| Inner regional | 491 (32.3)* | 417 (33.4)* | 1.01 (0.86–1.19) | .910 |
| Outer regional | 132 (8.7)* | 77 (6.2)* | 0.69 (0.52–0.93) | .016 |
| Born in Australia | ||||
| No | 484 (32.5)* | 361 (29.6)* | 1 | |
| Yes | 1003 (67.5)* | 860 (70.4)* | 1.15 (0.98–1.35) | .104 |
| Health concession carda | ||||
| No | 480 (33.1)* | 443 (36.8)* | 1 | |
| Yes | 972 (66.9)* | 762 (63.2)* | 0.85 (0.72–1.00) | .046 |
| Private health insurancea | ||||
| No | 757 (50.5)* | 523 (42.6)** | 1 | |
| Yes | 743 (49.5)* | 704 (57.4)** | 1.37 (1.18–1.60) | <.001 |
Column proportions were compared by using z-tests. Where the asterisks (*,**) in the rows are different, the proportions differ significantly from each other at p<.01.
Variable included in the logistic regression model.
11.3% missing.
CAM, complementary and alternative medicine.
Health and well-being status
Table 2 compares variables related to respondents' health and well-being status. Within the past 5 years, 66.4% of CAM users had one or more comorbid chronic conditions in addition to DM or CVD, compared with 48.9% of the nonuser group (8.1% missing), including anxiety or depression, food allergy or intolerance, or “other” (a category that included arthritis and musculoskeletal problems). CAM users were also more likely to report pain and poor sleep. However, the groups did not significantly differ in self-reported health status (excellent to poor), length of time since diagnosis of DM or CVD, body–mass index, or need for assistance with personal care tasks.
Table 2.
Health and Well-Being Status
| Variable | CAM nonuser, n (%) | CAM user, n (%) | Odds ratio (95% confidence interval) | p-Value |
|---|---|---|---|---|
| Diagnosisa | ||||
| DM and risk factors | 975 (64.2)* | 768 (61.6)* | 1 | <.001 |
| Risk factors only | 19 (1.3)* | 66 (5.3)** | 4.41 (2.63–7.41) | <.001 |
| CVD only | 66 (4.3)* | 96 (7.7)** | 1.85 (1.33–2.56) | <.001 |
| DM and CVD | 459 (30.2)* | 317 (25.4)** | 0.88 (0.74–1.04) | .133 |
| Years with DM/CVD | ||||
| <5 | 325 (21.4)* | 291 (23.3)* | 1 | |
| ≥5 | 1194 (78.6)* | 956 (76.7)* | 0.89 (0.75–1.07) | .222 |
| Comorbid conditionsb | ||||
| Anxiety or depressiona | 311 (22.5)* | 379 (32.7)** | 1.68 (1.41–2.00) | <.001 |
| Cancer | 129 (9.3)* | 102 (8.8)* | 0.94 (0.72–1.23) | .650 |
| Food allergy or intolerancea | 82 (5.9)* | 130 (11.2)** | 2.01 (1.50–2.68) | <.001 |
| Respiratory conditions | 161 (11.6)* | 163 (14.1)* | 1.24 (0.99–1.57) | .067 |
| Othera | 207 (15.0)* | 387 (33.4)** | 2.85 (2.35–3.45) | <.001 |
| Number of comorbid conditions (in addition to DM/CVD) | ||||
| None (0) | 707 (51.1)* | 388 (33.5)** | 1 | <.001 |
| 1–2 | 622 (45.0)* | 627 (54.1)** | 1.84 (1.56–2.17) | <.001 |
| ≥3 | 54 (3.9)* | 143 (12.3)** | 4.83 (3.45–6.76) | <.001 |
| Feels anxious, worried, or depressedc | ||||
| Generally does not feel this | 819 (54.7)* | 605 (49.3)** | 1 | .003 |
| Slightly | 502 (33.5)* | 429 (35.0)* | 1.16 (0.98–1.37) | .086 |
| Moderately or extremely | 177 (11.8)* | 192 (15.7)** | 1.47 (1.17–1.85) | .001 |
| Paina,c | ||||
| None at all | 676 (45.1)* | 396 (32.3)** | 1 | <.001 |
| Moderate | 678 (46.0)* | 693 (56.6)** | 1.75 (1.48–2.05) | <.001 |
| Severe or unbearable | 144 (9.6)* | 136 (11.1)* | 1.61 (1.24–2.10) | <.001 |
| Sleepa,c | ||||
| No difficulty most of the time | 498 (33.2)* | 300 (24.4)** | 1 | <.001 |
| Interrupted some of the time | 404 (27.0)* | 359 (29.2)* | 1.48 (1.21–1.81) | <.001 |
| Interrupted most nights | 433 (28.9)* | 429 (34.9)** | 1.65 (1.35–2.00) | <.001 |
| In short bursts, awake most of the night | 163 (10.9)* | 140 (11.4)* | 1.43 (1.09–1.86) | .009 |
| Help with personal care tasksc | ||||
| None | 1144 (76.6)* | 940 (77.2)* | 1 | .770 |
| Occasionally | 235 (15.7)* | 180 (14.8)* | 0.93 (0.75–1.15) | .517 |
| Only with more difficult tasks | 70 (4.7)* | 65 (5.3)* | 1.13 (0.80–1.60) | .492 |
| Daily with most or all | 44 (2.9)* | 33 (2.7)* | 0.91 (0.58–1.45) | .697 |
| Body–mass indexb | ||||
| Normal (<25 kg/m2)d | 235 (16.5)* | 228 (19.5)* | 1 | .105 |
| Overweight (25<30 kg/m2) | 535 (37.6)* | 408 (34.8)* | 0.79 (0.63–0.98) | .034 |
| Obese (30 <40 kg/m2) | 547 (38.4)* | 433 (36.9)* | 0.82 (0.65–1.01) | .072 |
| Morbidly obese (≥40 kg/m2) | 107 (7.5)* | 103 (8.8)* | 0.99 (0.72–1.38) | .962 |
| Self-reported health | ||||
| Fair/poor | 551 (36.8)* | 440 (36.0)* | 1 | .903 |
| Good | 642 (42.9)* | 533 (43.6)* | 1.04 (0.88–1.23) | .654 |
| Very good/excellent | 305 (20.4)* | 250 (20.4)* | 1.03 (0.83–1.27) | .807 |
Column proportions were compared by using z-tests. Where the asterisks (*,**) in the rows are different, the proportions differ significantly from each other at p<.01.
Variables included in the logistic regression model.
Missing responses: other comorbid conditions, 8.1%; body–mass index, 6.1%.
Questions contribute to the Assessment of Quality of Life utility score.
Includes 12 underweight (body–mass index <18.5 kg/m2).
CVD, cardiovascular disease; DM, diabetes mellitus.
Care-seeking and health management behaviors
On feeling unwell, CAM users were almost twice as likely (odds ratio, 1.9) as nonusers to initially self-manage with bed rest, home remedies, or medications bought at the supermarket or pharmacy than to “do nothing,” whereas nonusers were more likely to report seeing a doctor at the first sign of illness (Table 3). Yet CAM users consulted with more doctors or medical specialists than did nonusers. They were also more likely (p<.001) to be involved in social or health-related support groups, to participate in fitness or exercise programs, and to have exercised in the 2 weeks before the survey than nonusers. CAM use was not statistically significantly associated with frequency of visiting a general medical practitioner, number of different pharmaceutical medications taken, participation in chronic disease self-management, prevention, rehabilitation programs, or smoking.
Table 3.
Care-Seeking and Health Management
| Variable | CAM nonuser, n (%) | CAM user, n (%) | Odds ratio (95% confidence interval) | p-Value |
|---|---|---|---|---|
| First thing does when unwell | ||||
| Do nothing | 820 (54.7)* | 588 (48.0)** | 1 | <.001 |
| Self-manage | 332 (22.2)* | 463 (37.8)** | 1.95 (1.63–2.32) | <.001 |
| See a doctor | 346 (23.1)* | 164 (13.4)** | .66 (0.53–0.82) | <.001 |
| See a natural therapist or nonmedical provider | 0 (0.0) | 10 (0.8) | NA | NA |
| Number of doctors or medical specialists visited (last 12 mo)a | ||||
| 1–2 | 872 (61.6)* | 658 (55.2)** | 1 | .004 |
| 3–4 | 389 (27.5)* | 375 (31.4)* | 1.28 (1.07–1.52) | .006 |
| ≥5 | 155 (10.9)* | 160 (13.4)* | 1.37 (1.07–1.74) | .011 |
| Frequency of visiting GP | ||||
| Once yearly or less | 50 (3.3)* | 48 (3.9)* | 1 | .581 |
| Every 6 mo | 357 (23.8)* | 269 (21.8)* | 0.79 (0.51–1.20) | .266 |
| Every 3 mo | 739 (49.3)* | 622 (50.4)* | 0.88 (0.58–1.32) | .530 |
| At least monthly | 354 (23.6)* | 294 (23.8)* | 0.87 (0.57–1.32) | .504 |
| Number of pharmaceutical medications takena | ||||
| 0 (none) | 33 (2.2)* | 43 (3.5)* | 1 | .160 |
| 1–3 | 421 (28.0)* | 360 (29.3)* | 0.66 (0.41–1.06) | .082 |
| 4–6 | 658 (43.7)* | 518 (42.1)* | 0.60 (0.38–0.97) | .035 |
| ≥7 or more | 394 (26.2)* | 309 (25.1)* | 0.60 (0.37–0.97) | .037 |
| Self-management or rehabilitation programb | ||||
| No | 589 (39.2)* | 442 (35.8)* | 1 | .092 |
| Yes | 851 (56.7)* | 750 (60.7)* | 1.17 (1.00–1.38) | .046 |
| Not sure/don't know | 62 (4.1)* | 43 (3.5)* | 0.92 (0.62–1.39) | .705 |
| Social or health-related support groupa | ||||
| No | 1209 (84.0)* | 829 (69.3)** | 1 | |
| Yes | 230 (16.0)* | 367 (30.7)** | 2.33 (1.93–2.80) | <.001 |
| Fitness or exercise programa | ||||
| No | 990 (66.2)* | 675 (55.1)** | 1 | |
| Yes | 506 (33.8)* | 549 (44.9)** | 1.59 (1.36–1.86) | <.001 |
| Exercised (past 2 wk)a | ||||
| No | 472 (31.5)* | 311 (25.3)** | ||
| Yes | 1025 (68.5)* | 920 (74.7)** | 1.36 (1.15–1.61) | <.001 |
| Smoker | ||||
| No | 1360 (90.7)* | 1137 (92.7)* | ||
| Yes | 140 (9.3)* | 89 (7.3)* | 0.76 (0.58–1.00) | .053 |
Column proportions were compared by using z-tests. Where the asterisks (*,**) in the rows are different, the proportions differ significantly from each other at p<.01.
Variables included in the logistic regression model.
Aggregated responses to three questions: Have you ever taken part in (1) a chronic disease or health self-management course, (2) a diabetes prevention or management program, or (3) a cardiovascular rehabilitation program?
Predictors of CAM use
Factors for which p<.05 from Tables 1 (all), 2, and 3 (selected), were used to assess the effect on the likelihood that respondents would use CAM. The model, containing 17 demographic and health status variables, is statistically significant (chi-square=348.35; df=26; p<.001; n=2156) and correctly classifies 67.6% of respondents (p=.500 in the Hosmer–Lemeshow goodness-of-fit test). Table 4 shows the nine variables that contribute to the model with p<.05. For p<.01, the model predicts that CAM users are more likely to be female than male, have a university education, attend exercise classes and social or support groups, experience moderate pain, have depression or anxiety, and have “other” chronic conditions. When numbers of comorbid conditions (grouped as per Table 2, with “none” as the reference category) were included as an aggregated alternative to the named specific conditions, their p-values were<.001 and odds ratios were similar to those in Table 2; this indicated that increasing numbers of chronic conditions are significant predictors of CAM use after adjustment for other variables. The exploratory modeling also indicated a strong interactional effect between number of chronic conditions, AQoL-4D score, and the specifically identified chronic conditions (e.g., anxiety and depression, allergy, and food intolerance). When AQoL-4D score was included instead of comorbid conditions, pain, and sleep problems, it was a significant predictor of CAM use (chi square=241.10; df=19; p<.001; n=2304); it correctly classifies 67.6% of respondents (p=.893 in the Hosmer–Lemeshow goodness-of-fit test).
Table 4.
Independent Predictors of Complementary and Alternative Medicine Use
| Variable | Odds ratio (95% confidence interval) | p-Value |
|---|---|---|
| Female | 1.85 (1.52–2.25) | <.001 |
| Highest education | ||
| Secondary school | 1 | <.001 |
| Undergraduate | 1.68 (1.37–2.06) | <.001 |
| Postgraduate | 2.27 (1.59–3.26) | <.001 |
| Number of pharmaceutical medications taken | ||
| None | 1 | .007 |
| 1–3 | 0.66 (0.35–1.22) | .185 |
| 4–6 | 0.51 (0.27–0.95) | .035 |
| ≥7 | 0.42 (0.22–0.81) | .010 |
| Pain | ||
| None | 1 | <.001 |
| Moderate | 1.79 (1.45–2.22) | <.001 |
| Severe or unbearable | 1.39 (0.96–2.02) | .085 |
| Has anxiety or depression | 1.50 (1.20–1.87) | <.001 |
| Has "other" chronic conditionsa | 2.35 (1.86–2.97) | <.001 |
| Attends fitness or exercise program | 1.37 (1.10–1.69) | .004 |
| Attends social or support group | 1.83 (1.44–2.33) | <.001 |
| Has private health insurance | 1.24 (1.02–1.52) | .035 |
Perceptions of illness and behavioral change
Survey respondents were asked, with respect to their DM or CVD, to rate a series of value statements beginning with: “Since I was diagnosed…” using a 5-point Likert scale (strongly agree to strongly disagree). The first 13 statements (Table 5) were informed by the in-depth interviews from the study's first phase; the final 10 statements elicited information on illness perception: treatment control, personal control, consequences of illness, and illness timeline.15 There were statistically significant differences between CAM users' and nonusers' mean scores for perceptions of illness, its effect on life, outlook, and behavior, with p<.001 for 9 of 23 statements and p<.01 for a further 8. In each of these instances (excepting having experienced depression, sadness, or loss of hope), CAM users were more likely to strongly agree/disagree (depending on which was the more positive response) to the statements than nonusers, who tended to be less emphatic. We used logistic regression to assess the effect of age, sex, and each perception statement (modeled individually) on CAM use (dependent variable), with a resulting pattern of statistical significance similar to that seen in Table 5. A notable difference, however, was that experiencing depression, sadness, or loss of hope was not a predictor of CAM use (p=.207) after adjustment for age and sex (data not shown).
Table 5.
Behavioral Change and Illness Perception
| |
Mean perception ratinga |
|
|
|---|---|---|---|
| “Since I was diagnosed…” | CAM user | CAM nonuser | p-Value |
| I have made significant diet or lifestyle change | 4.01 | 3.84 | <.001 |
| I am more aware of the value of each day | 3.97 | 3.86 | .001 |
| My health has progressively deterioratedb | 2.60 | 2.61 | .818 |
| My fitness has improvedc | 3.25 | 3.14 | .008d |
| My sense of well-being has improvedc | 3.42 | 3.30 | .001 |
| I know more about health and how my body works | 3.90 | 3.70 | <.001d |
| My quality of life has improvedc | 3.31 | 3.19 | .001d |
| I exercise more | 3.45 | 3.32 | .001 |
| I have become more limited in what I can do | 2.96 | 3.08 | .013 |
| I pay more attention to symptoms or what my body is telling me | 3.96 | 3.82 | <.001d |
| I try to relax more or avoid things that stress me | 3.80 | 3.70 | <.001d |
| I live my life as close as possible to how I did before I was diagnosed | 3.25 | 3.42 | <.001d |
| I have experienced depression, sadness, or loss of hope | 2.74 | 2.58 | .003d |
| My illness has major consequences on my life | 3.40 | 3.30 | .021 |
| My illness has serious financial consequences | 3.00 | 2.86 | .001 |
| There is a lot I can do to control my symptoms | 3.89 | 3.80 | .007 |
| The course of my illness depends on me | 3.93 | 3.92 | .667 |
| Nothing I do will affect my illnessc | 2.12 | 2.32 | <.001d |
| I don't understand my illnessc | 2.12 | 2.28 | <.001d |
| My actions will have no effect on the outcomes of my illnessc | 2.02 | 2.31 | <.001d |
| I expect to have this illness for the rest of my life | 3.91 | 3.96 | .194d |
| My treatment can control my illness | 3.95 | 3.89 | .032 |
| I have a clear picture or understanding of my condition | 3.95 | 3.85 | <.001d |
Rated on scale of 1–5 where 1=strongly disagree, 2=disagree, 3=neither agree nor disagree, 4=agree, 5=strongly agree.
Missing 7.8%.
Missing 6.2<7%.
Significance value for Levene test for equality of variance is <.05, indicating the variances for CAM users and nonusers are not the same; corresponding p-value is provided where equal variance is not assumed.
Mean AQoL-4D measures were also calculated for each Likert response category of each perception statement (data not shown). For most statements for which CAM users nominated the most positive response categories (as explained earlier), they had lower (worse) mean AQoL-4D scores than nonusers. This indicated that despite lower AQoL-4D measures, CAM users perceptions' of illness control and its management tended to be more positive than those of nonusers.
Discussion
In the responder group of people with DM or CVD, being female, being university educated, having chronic conditions in addition to DM or CVD (e.g., anxiety or depression), participation in a fitness or exercise program, social or support group attendance, and objectively measured quality of life (AQoL-4D score) were predictors of CAM use. The comparison of the demographic, health and well-being status, and care-seeking and health management characteristics of respondents indicated many similarities between CAM users and nonusers, including length of time since diagnosis, body–mass index, self-reported health status, frequency of visiting a general practitioner, number of prescribed medications taken, employment status, and income. The findings support other Australian research, which also indicated that CAM users were more likely to be female, to be highly educated, and to have worked in a professional capacity.8,16 CAM use among people with chronic illness is also a common finding,3,17 and other research has found that CAM users report worse health than nonusers.18 Although the CAM-using respondents in the current study had more chronic conditions than nonusers, they did not take more prescribed medications.
The AQoL-4D utility measure does not provide insight into how a person feels about or perceives his or her health, illness, and QoL, so this study explored respondents' subjective beliefs and values to consider how these affected experiences of living with chronic illness. Although CAM users and nonusers reported their general health status similarly, CAM users were more likely (p≤.001) to perceive their situation more positively than nonusers in many ways (Table 5), suggesting that CAM users tended to have a greater sense than nonusers of confidence, empowerment, knowledge, and control relating to their health. This was seen even though one in three (32.7%) CAM users experienced depression or anxiety in the last 5 years, compared with 22.5% of nonusers, and their increased tendency to feel more anxiety, worry, or depression than nonusers.
These findings are supported by other research reported in a systematic review on beliefs involved in CAM use. Those authors concluded that CAM users “want to participate in treatment decisions, are likely to have active coping styles and might believe that they can control their health.”19 Whether an empowered population seeks CAM or CAM use empowers its users is not well understood.19,20 However, the fact that almost a third of the respondents in the current study were motivated to first use CAMs through a desire to take more control of their health suggests that some proactivity and empowerment within this population influence their use of CAM. Although it is not possible to determine the efficacy of CAM use from the current findings, it does appear that CAM was used as part of a broader self-management strategy by people dealing with multiple chronic conditions.
Most survey returns (76%) were from a random sample of people with DM from the NDSS register (25% total response rate, 22% valid). The proportion of male and female surveyed NDSS responders, in 10-year age brackets, was broadly representative of the larger NDSS population;10 however, it is unknown whether CAM users were more likely to complete the survey than nonusers. This limits the generalizability of the findings; there are limited, similar, Australian data on CAM use among people with chronic conditions to contrast. A systematic review of international literature found that the prevalence of CAM use among people with diabetes ranged from 17% to 72.8%. The wide range was due to inconsistency in the definition of CAM and variations in reported timeframes.21 A small Australian survey (n=69) reported prevalence results similar to those in the current study, with 46.3% of people with DM using CAM.22 Australian National Health Survey data (2004–2005) indicated that fewer than 10% of people with asthma, diabetes, or heart or circulatory conditions used CAM,2 but those results are not similar to the current ones because of the short timeframe (2 weeks before the survey) and narrow definition of CAM (vitamin/mineral supplements or natural/herbal treatments) used.
Conclusion
These findings indicate potentially positive effect of CAM use on the outlooks of people with chronic conditions. More detailed analysis is warranted to tease out whether particular types of CAM use correlate with greater positivity and proactivity of users, and how CAM use might affect levels of prescription pharmaceutical use. Given the high use of CAM among general and disease-specific populations, greater evidence of the ways CAMs could support chronic illness prevention and complement chronic disease management might help decrease the burden of chronic disease. This could have important policy implications relating to integration of CAM therapies within mainstream health services.
Supplementary Material
Acknowledgments
Maximilian de Courten (University of Copenhagen) and Ngianga-Bakwin Kandala (University of Warwick) provided us with valuable suggestions for the analysis of the material we present in this article. We thank Diabetes Australia–Victoria, Heart Support Australia, CAMELOT Reference Group members, and CAMELOT Project team members Brian Oldenburg, Vivian Lin, Bruce Hollingsworth, Maximilian de Courten, Nalika Unantenne, Jean Spinks, and Jennifer Moral for their contributions to the study. This work was supported by the Australian National Health and Medical Research Council (NHMRC grant number 491171).
Disclosure Statement
No competing financial interests exist.
References
- 1.Wootton JC. Sparber A. Surveys of complementary and alternative medicine usage: a review of general population trends and specific patient populations. Semin Integr Med. 2003;1:10–24. [Google Scholar]
- 2.Armstrong AR. Thiébaut SP. Brown LJ. Nepal B. Australian adults use complementary and alternative medicine in the treatment of chronic illness: a national study. Aust N Z J Public Health. 2011;35:384–390. doi: 10.1111/j.1753-6405.2011.00745.x. [DOI] [PubMed] [Google Scholar]
- 3.Saydah SH. Eberhardt MS. Use of complementary and alternative medicine among adults with chronic diseases: United States 2002. J Altern Complement Med. 2006;12:805–812. doi: 10.1089/acm.2006.12.805. [DOI] [PubMed] [Google Scholar]
- 4.Adams J. Sibbritt DW. Easthope G. Young AF. The profile of women who consult alternative health practitioners in Australia. Med J Aust. 2003;179:297–300. doi: 10.5694/j.1326-5377.2003.tb05551.x. [DOI] [PubMed] [Google Scholar]
- 5.Markovic M. Manderson L. Wray N. Quinn M. Complementary medicine use by Australian women with gynaecological cancer. Psychooncology. 2006;15:209–220. doi: 10.1002/pon.936. [DOI] [PubMed] [Google Scholar]
- 6.Visser Rd. Grierson J. Use of alternative therapies by people living with HIV/AIDS in Australia. AIDS Care. 2002;14:599–606. doi: 10.1080/0954012021000005425. [DOI] [PubMed] [Google Scholar]
- 7.Lin V. McCabe P. Bensoussan A, et al. The practice and regulatory requirements of naturopathy and western herbal medicine in Australia. Risk Manag Healthc Policy. 2009;2:21–33. doi: 10.2147/RMHP.S4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill S. Bensoussan A. Myers SP, et al. Consumers of naturopathy and western herbal medicine. In: Lin V, editor; Bensoussan A, editor; Myers SP, editor; McCabe P, editor; Cohen M, editor; Hill S, editor. The Practice and Regulatory Requirements of Naturopathy and Western Herbal Medicine. Bundoora: School of Public Health, La Trobe University; 2006. pp. 233–253. [Google Scholar]
- 9.Manderson L. Canaway R. Unantenne N, et al. Care seeking, use of complementary therapies, and self-management among people with type 2 diabetes and cardiovascular disease: CAMELOT Phase I, an ethnographic approach. Aust J Herb Med. 2012;24:10–18. [Google Scholar]
- 10.Manderson L. Oldenburg B. Lin V, et al. Care seeking, complementary therapy and herbal medicine use among people with type 2 diabetes and cardiovascular disease: CAMELOT Phase II, surveying for diversity. Aust J Herb Med. 2012;24:46–55. [Google Scholar]
- 11.Canaway R. Manderson L. Complementary therapy use among Australians with type 2 diabetes or cardiovascular disease. Altern Complement Ther. 2013;19:18–27. doi: 10.1089/act.2013.19110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spinks J. Hollingsworth B. Manderson L, et al. Costs and drivers of complementary and alternative medicine (CAM) use in people with type 2 diabetes or cardiovascular disease. Eur J Integr Med. 2012 Epub ahead of print. [Google Scholar]
- 13.Hawthorne G. Richardson J. Day N. Melbourne: Centre for Health Economics, Monash University; 2009. [Jul 17;2012 ]. Using the Assessment of Quality of Life (AQoL-4D): Abridged Technical Report 12. [Google Scholar]
- 14.Centre for Health Economics, Monash University. Melbourne: Centre for Health Economics, Monash University; 2009. [Jul 17;2012 ]. Assessment of Quality of Life. [Google Scholar]
- 15.Moss-Morris R. Weinman J. Petrie KJ, et al. The revised illness perception questionnaire (IPQ-R) Psychol Health. 2002;17:1–16. [Google Scholar]
- 16.Xue CCL. Zhang AL. Lin V, et al. Complementary and alternative medicine use in Australia: a national population-based survey. J Altern Complement Med. 2007;13:643–650. doi: 10.1089/acm.2006.6355. [DOI] [PubMed] [Google Scholar]
- 17.Metcalfe A. Williams J. McChesney J, et al. Use of complementary and alternative medicine by those with a chronic disease and the general population: results of a national population based survey. BMC Complement Altern Med. 2010:10. doi: 10.1186/1472-6882-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shmueli A. Shuval J. Are users of complementary and alternative medicine sicker than non-users? Evid Based Complement Alternat Med. 2007;4:251–255. doi: 10.1093/ecam/nel076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bishop FL. Yardley L. Lewith GT. A systematic review of beliefs involved in the use of complementary and alternative medicine. J Health Psychol. 2007;12:851–867. doi: 10.1177/1359105307082447. [DOI] [PubMed] [Google Scholar]
- 20.Sasagawa M. Martzen MR. Kelleher WJ. Wenner CA. Positive correlation between the use of complementary and alternative medicine and internal health locus of control. Explore. 2008;4:38–41. doi: 10.1016/j.explore.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang H-Y. Wallis M. Tiralongo E. Use of complementary and alternative medicine among people living with diabetes: literature review. J Adv Nurs. 2007;58:307–319. doi: 10.1111/j.1365-2648.2007.04291.x. [DOI] [PubMed] [Google Scholar]
- 22.Manya K. Champion B. Dunning T. The use of complementary and alternative medicine among people living with diabetes in Sydney. BMC Complement Altern Med. 2012:12. doi: 10.1186/1472-6882-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.