Abstract
Significance
The number of patients with nonhealing wounds has rapidly accelerated over the past 10 years in both the United States and worldwide. Some causative factors at the macro level include an aging population, epidemic numbers of obese and diabetic patients, and an increasing number of surgical procedures. At the micro level, chronic inflammation is a consistent finding.
Recent Advances
A number of treatment modalities are currently used to accelerate wound healing, including energy-based modalities, scaffoldings, the use of mechano-transduction, cytokines/growth factors, and cell-based therapies. The use of stem cell therapy has been hypothesized as a potentially useful adjunct for nonhealing wounds. Specifically, mesenchymal stem cells (MSCs) have been shown to improve wound healing in several studies. Immune modulating properties of MSCs have made them attractive treatment options.
Critical Issues
Current limitations of stem cell therapy include the potentially large number of cells required for an effect, complex preparation and delivery methods, and poor cell retention in targeted tissues. Comparisons of published in-vitro and clinical trials are difficult due to cell preparation techniques, passage number, and the impact of the micro-environment on cell behavior.
Future Directions
MSCs may be more useful if they are preactivated with inflammatory cytokines such as tumor necrosis factor alpha or interferon gamma. This article will review the current literature with regard to the use of stem cells for wound healing. In addition the anti-inflammatory effects of MSCs will be discussed along with the potential benefits of stem cell preactivation.

William J. Ennis, DO, MBA
Scope and Significance
The purpose of this review is to provide the reader with a brief understanding of the causative factors for nonhealing wounds and to understand how stem cells may play a therapeutic role. The review covers some basic definitions of stem cells but focuses primarily on the use of mesenchymal stem cells (MSCs). The authors reviewed relevant papers over the past 10 years that were published in English; however, this is not an exhaustive review. A review of important animal and clinical trials are described in which MSCs are used to heal wounds and/or to improve the quality of healing. The immune modulating features of MSCs are described, in particular the anti-inflammatory effects that MSCs have on the wound healing process.
Translational Relevance
As in any other area of scientific advancement, therapeutic approaches for wound healing involving MSCs are based on a number of critical prior scientific streams from related fields. Biomedical engineering research has led to the development of scaffoldings that can mimic a natural endogenous dermal structure. These advances allow for a more efficient delivery of stem cells into the wound environment. The advancements in stem cell research highlighted potential therapeutic options, including the ability of stem cells to directly differentiate into specific cells as well as their ability to provide necessary cues for the recruitment of different cell types needed in the regenerative process. Basic science research in the area of fibrosis and scar formation highlighted the importance of chronic inflammation and the negative impact of an over active repair process. Investigators studying various organ systems report similar unifying biochemical processes involving tissue repair and regeneration. Fetal healing research led to the understanding that a limited inflammatory response could lead to scarless healing. Using a systems biology approach, the MSC has been targeted as an ideal candidate to assist in the healing process. The basic science research in each of the previously mentioned scientific areas has an immediate potential bench to bedside application.
Clinical Relevance
Currently, there are numerous therapeutic options for the treatment of nonhealing wounds but very little supporting evidence. Despite the growing number of treatments, there is only a 50–60% healing rate in most clinical applications with equally high rates of recidivism. The process of tissue repair and scar formation has negative clinical implications in heart, lung, liver, and brain tissue, in addition to dermal wound healing. As observed in fetal healing, the optimal regenerative healing environment is possible when inflammation is minimized but not absent. Systemic treatments aimed at global immune suppression can improve healing, but the quality of healing may be compromised through weakened scar formation. MSCs can “sense” the degree of inflammation in the micro-environment and respond by release of growth factors and cytokines to reduce the inflammatory process using real-time biochemical cues. If effective, reducing inflammation to an appropriate level to allow healing to proceed should also result in improved tensile strength and scar quality, thereby reducing recidivism.
Discussion of Findings and Relevant Literature
Wound healing: normal process and factors impeding healing
Wound healing requires the successful completion of an orchestrated series of tightly controlled biochemical and cellular events to achieve healing. Overlapping phases of hemostasis, inflammation, proliferation, and remodeling are common to wounds of all etiologies. A chronic wound develops when a wound fails to heal within an expected time frame and fails to achieve functional closure. There are many factors that impede healing, including co-morbid clinical conditions, aging, poor tissue perfusion, malnutrition, unrelieved pressure to the surface of the wound, immune suppression, malignancy, infection, obesity, and a number of medications. The usual patient with a nonhealing wound has a combination of several of the factors mentioned earlier, making any one therapeutic option unlikely to succeed. One common thread with almost all nonhealing wounds is a persistent inflammatory state. Macrophages, known to mediate inflammation, influence healing in a positive way through increasing angiogenesis, decreasing bacterial loads, phagocytosing debris, and providing matrix deposition. If, however, a persistent inflammatory state develops in which the macrophages are dysregulated and become skewed toward a type I inflammatory phenotype, there is an increase in inflammatory cytokine release, increased protease secretion, and a reduction in endogenous local growth factors, impeding progress toward wound repair and regeneration.1 Current therapeutic approaches for patients with nonhealing wounds start with medical stabilization of the patient, including the correction of any and all possible underlying comorbid conditions listed earlier. Standard of care also includes debridement of any nonviable tissue and the use of dressings that provide a moist healing environment. Both synthetic and biological scaffoldings have been implanted into the wound bed in an effort to recreate a favorable environment for native cells to infiltrate and accelerate the healing process. Applying external strain to the wound, as occurs in nature, has also been used over the past decade with increasing regularity. While each of these components are required to achieve healing, in nature, they proceed simultaneously instead of in the sequential method in which clinicians currently apply them. This concept was summarized nicely in a paper by Gurtner et al.2 (Fig. 1). Another potential explanation for the nonhealing wound is the presence of intrinsically dysfunctional or senescent cells that are incapable of responding to normal biochemical signals. These findings have led investigators to consider the use of cell-based therapy as a solution for the chronic nonhealing wound. Allogeneic cells have been used for the treatment of venous ulcers and diabetic foot ulcers with good success.3 Initially thought to be replacements for autologous skin grafts, biological skin substitutes are now known to act in a paracrine fashion, stimulating the wound to progress toward healing. World-wide economic pressures lead to an increasing focus on not only clinical outcomes but also achieving these outcomes in a cost-effective patient-centered manner. A more regenerative healing process should also theoretically lead to decreased wound recidivism compared with the usual process of tissue repair, which is traditionally marked by scar formation.
Figure 1.
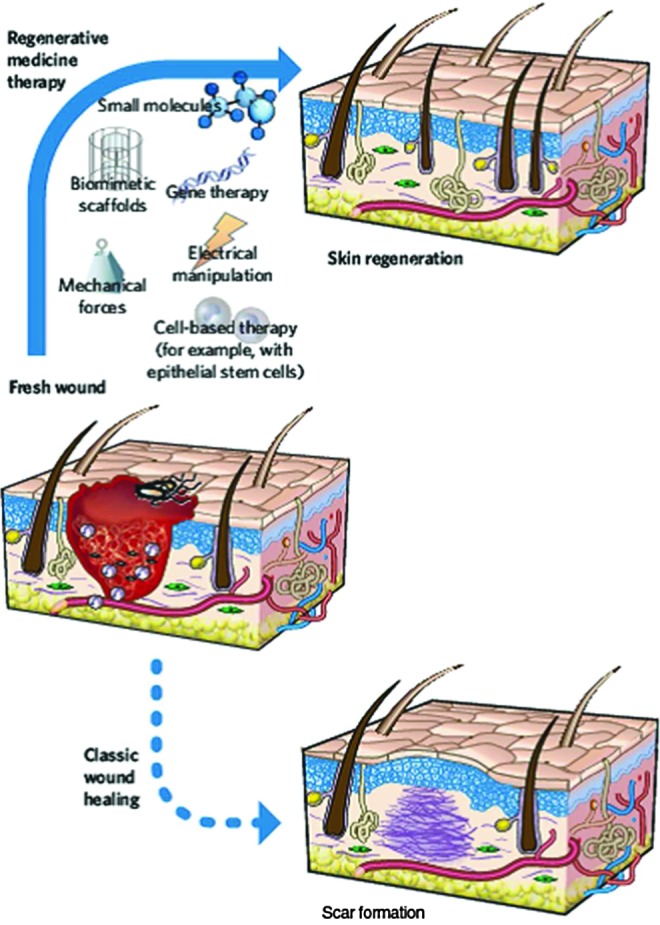
Potential therapies for reducing scar formation during wound repair. To manipulate wound repair to become more regenerative than scar forming, strategies include the use of biomimetic scaffolds, the manipulation of the mechanical environment (e.g., negative pressure wound therapy to increase healing) or the electrical environment, the administration of small molecules, the use of gene-therapy approaches, and the use of cell-based strategies (including administration of epithelial stem cells.) All of these elements have been demonstrated to have an effect on in vitro and in vivo models of wound healing as single-agent therapies. In theory, many of these elements could be combined to recreate a receptive environment (or soil) to promote regeneration. Combining these with the appropriate stem cells (or “seed”) will undoubtedly alter the result of wound healing in humans. (Reprinted with permission from Gurtner et al.2) To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Epidermal cellular therapies versus stem cells: strengths and weaknesses
Coverage for wounds with autologous split thickness skin grafting has been available since the late 1800s. The procedure is limited, however, by the size of healthy available skin, the time it takes for engraftment, and the healing of the donor site. Cultured epidermal cell sheets, developed in the 1970s, were an initial attempt to overcome some of these limitations.4 These sheets were costly and time consuming to create, limiting their clinical utility. Cadaveric skin, a true allograft, can provide immediate coverage but is ultimately rejected. Cadaveric dermal tissues and synthetic dermal substitutes have provided clinicians with more options in recent years but still require skin grafting for ultimate closure. Cultured bilayer and single-cell biological dressings have emerged, each with randomized controlled trial data to support their efficacy in nonhealing wounds.5,6 Despite these advances, overall healing rates continue to remain at 50–70%, and wound recurrence is high regardless of wound etiology. Since stem cells have great proliferative capacity and the ability for self-renewal as well as the ability to differentiate into a number of different tissue types, stem cells became a desirable therapeutic option for nonhealing wounds.
While fetal or embryonic stem cells demonstrate extensive proliferative capacity, self-renewal, and totipotency, or the ability to differentiate into all cell types, due to ethical and logistical issues, most research and development is now predominantly focused on adult stem cells. Adult stem cells are multipotent, indicating that they can differentiate into a number of cell types but not all cell types, and have proliferative capacity limited to the lifespan of the organism.7 Studies in bone marrow-derived stem cells have provided the greatest clinical safety and efficacy experiences among all adult stem cell sources. In the bone marrow, two stem cell types have been clinically developed. The first, hematopoietic stem cells (HSCs) give rise to all blood components. These cells have been clinically developed for bone marrow transplantation, but the CD34+ sub-population, which can give rise to endothelial cells, has recently been investigated in clinical trials to speed wound healing, predominantly due to its potential for enhanced angiogenic activity. The remaining part of this review will focus on adult MSC, as in addition to their pro-angiogenic capabilities, these cells can modify host inflammatory responses, thereby having the potential to reduce time spent in unproductive inflammatory response by switching to pro-regenerative activities.
MSCs give rise to tissues of mesenchymal origin. Sources of adult stem cells include sites rich with high vascular flow and proliferative and pro-regenerative cellular activity such as the bone marrow, umbilical cord, and amniotic fluid.7 In the marrow, the endosteal and vascular niches serve as the microenvironment for maintaining HSCs; growing evidence indicates that MSCs and HSCs co-regulate activities of each other, coordinating the function of the marrow and, as a consequence, both the inflammatory and pro-regenerative cells released from that repository.8,9 Adipose-derived MSCs are notably different from previously mentioned sources, as they are isolated from tissue, which is considerably less vascularized and is rich in the secretion of pro-inflammatory growth factors.9 Clinical studies demonstrate that cells isolated from bone marrow, cord blood, and adipose tissue differ in their ability for proliferation, differentiation, and gene products. For instance, cord blood MSC are unable to differentiate well into adipose tissue, but are highly proliferative; bone marrow and adipose MSC proliferate at similar rates; however, all have different genomic patterns and products that vary with cell passage and individual. These variabilities in cell source may provide an insight on the variable efficacy observed in patient response to these cellular therapies. There is also still controversy in the field over which surface markers define stem cell status.
Studies in small animal models suggest that stem cells not only improve wound healing when applied alone but also synergize with scaffolds. When adipose-derived MSC were loaded on biological scaffoldings, there was enhanced viability when compared with direct topical application, suggesting a simultaneously multi-modal product, that is, scaffold-loaded stem cells, can augment stem cell efficacy.10 In addition to the options of retrieving MSCs directly from the patient or from a young donor, it is also possible to indirectly develop terminally differentiated cells, such as fibroblasts, from either patient or donor to obtain mesenchymal-like stem cells. Terminally differentiated cells can be reprogrammed to dedifferentiate into a pluripotent cell, via viral transfection, plasmids, synthesized RNAs, or small molecules.11 Using these induced human pluripotent stem cells (iPSCs), investigators have recently coaxed human iPSCs to take on the phenotype of MSCs, helping to attenuate critical limb ischemia in a mouse model.12 There is a low transfection rate and still many unanswered questions as to the immunogenicity of these recently developed cell lines.13
The safety of MSCs has been evaluated in a recent meta-analysis and at this time appear to be safe for clinical use.14 Badavias and Falanga used subcutaneous injections of bone marrow along with topical application of MSCs in an early human study and demonstrated improved healing.15 Falanga et al. subsequently demonstrated improved healing in both human and diabetic mouse models with MSCs using a fibrin spray to apply the cells topically.16 Javazon demonstrated improvement in wound healing as well as decreased pain in a diabetic mouse model using a topical application of stem cells.17 The proposed mechanism of action includes differentiation into epidermal and dermal cells, improved vasculogenesis, immune modulation, and paracrine signaling pathways (Fig. 2).18,19 Limitations to successful therapy, however, include cell delivery methods (IV, topical/spray, scaffold-loaded, or subcutaneous injection), cell viability, heterogenicity in MSC preparations, and inconsistent micro-environmental cues (Fig. 3).18 In one of the only two reported randomized clinical trial using MSCs, Dash et al. treated ischemic and diabetic ulcers and noted both wound healing and decreased pain.20 This study utilized intramuscular injections of MSCs and demonstrated an increase in immature cells, vascularity, and reticulin fibers that were not seen in control cases. Jain et al., however, found no significant improvement in healing using topically applied and subcutaneously injected MSCs in patients with lower extremity ulcers.21 Wu et al. noted both MSC differentiation into epidermal cell lines, in addition to increases in angiogenesis via paracrine signaling in a diabetic mouse model.22 There appears to be bi-directional cross-talk between MSCs and macrophages. This relationship can potentially be manipulated to enhance a more regenerative wound micro-environment.23,24 One of the proposed mechanisms of action for this includes a paracrine-like reprogramming of inflammatory type 1 macrophages to a type 2 anti-inflammatory, pro-regenerative phenotype.25 Alternatively activated macrophages have been shown to increase levels of the anti-inflammatory cytokine Il-10 while simultaneously lowering pro-inflammatory cytokines such as Il-Iβ, Il-17.26
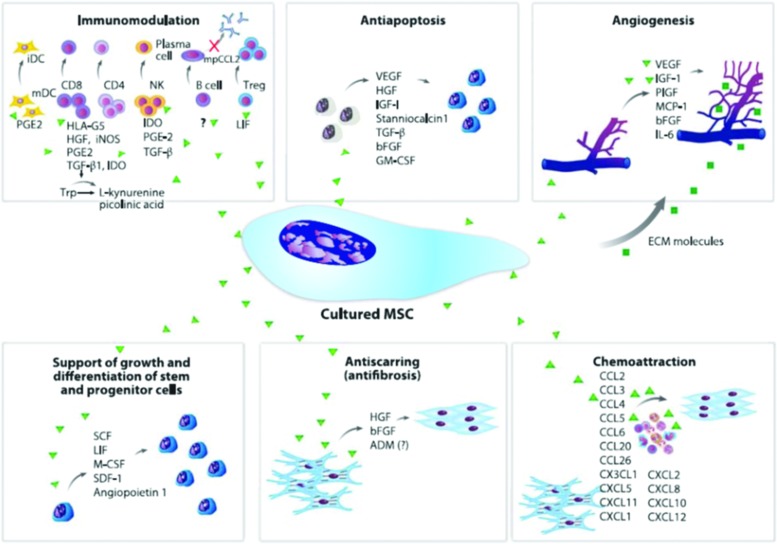
Figure 2.
Paracrine effects of cultured mesenchymal stem cells (MSCs). The secretion of a broad range of bioactive molecules is now believed to be the main mechanism by which MSCs achieve their therapeutic effect. This mechanism can be divided into six main actions: immunomodulation, antiapoptosis, angiogenesis, support of the growth and differentiation of local stem and progenitor cells, antiscarring, and chemoattraction. Although the number of molecules known to mediate the paracrine action of MSCs increases every day, only a few factors that are secreted by cultured MSCs are shown. The immunomodulatory effects of MSCs consist of inhibition of the proliferation of CD8+ and CD4+ T lymphocytes and natural killer (NK) cells, suppression of immunoglobulin production by plasma cells, inhibition of maturation of dendritic cells (DCs), and stimulation of the proliferation of regulatory T cells. The secretion of prostaglandin E2 (PGE2), human leukocyte antigen G5(HLA-G5), hepatocyte growth factor (HGF), inducible nitric oxide synthase (iNOS), indoleamine 2,3-dioxygenase (IDO), transforming growth factor Beta (GFFB), leukemia-inhibitory factor (LIF), and interleukin (IL)-10 contributes to this effect. MSCs can also limit apoptosis, and the principal bioactive molecules responsible for this process are HGF, TGF-B, vascular endothelial growth factor (VEGF), insulin like growth factor (IGF)-1, stanniocalcin 1, and granulocyte macrophage colony-stimulating factor (GM-CSF). MSCs stimulate local angiogenesis by secretion of extracellular matrix (ECM) molecules, VEGF, IGF-1, phosphatidylinositol-glycan biosynthesis class F protein (PIGF), monocyte chemoattractant protein 1 (MCP-1), basic fibroblast growth factor (bFGF), and IL-6; they also stimulate mitosis of tissue-intrinsic progenitor or stem cells by secretion of stem cell factor (SCF), macrophage colony-stimulating factor (MCSF), stromal cell-derived factor (SDF-1), LIF, and angiopoietin 1. Moreover, HGF and bFGF (and possibly adrenomedullin) produced by MSCs contribute to the inhibition of scarring caused by ischemia. Finally, a group of at least 15 chemokines produced by MSCs can elicit leukocyte migration to the injured area, which is important for normal tissue maintenance. (Reprinted with permission from Singer and Caplan.19) To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
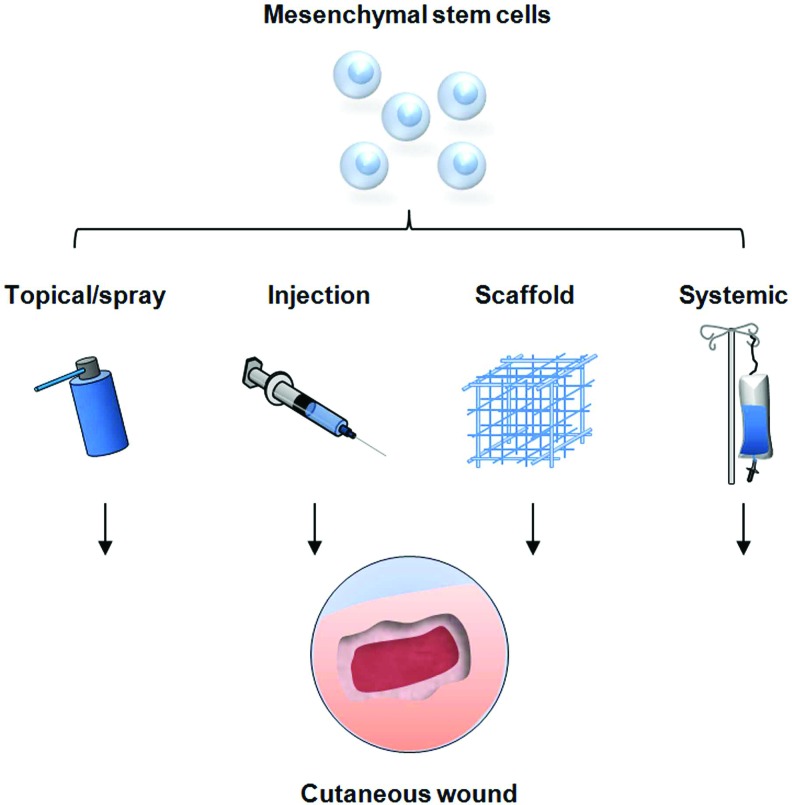
Figure 3.
Strategies for mesenchymal cell delivery to cutaneous wounds. Traditional techniques include local injection of cells into the soft tissue, direct topical application, and systemic delivery via injection into the peripheral circulation. These methods have resulted in improved wound healing but are limited by sub-optimal cell survival and engraftment. Novel delivery methods are being developed, utilizing tissue scaffolds to optimize stem cell function and maximize the therapeutic potential for cellular therapy. (Reprinted with permission from Chen et al.18) To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
It is known that aging reduces both the rate and quality of healing in adults.26 The number of MSCs also decline with advancing age in humans. Using a mouse model, Lee et al. demonstrated that the tensile strength of a healed wound in an aged animal could be improved to the level of a young animal with the use of MSCs.27 In this study, a lower number of MSCs were used than in previous reports, and the authors theorize that preactivation with interferon gamma (IFG) might have induced increased potency of the cells. In addition, Lee et al. blocked the positive effect of MSCs on improved tensile strength by chemically depleting the macrophages in both the young and aged animals, further supporting the importance of MSC/macrophage interactions (Fig. 4). Investigators have found improved scar formation, increased tensile strength and vascularity in a number of animal models.28–31 The same group, using an incisional wound mouse model, further evaluated cytokine levels from treatments using fibroblasts, nonactivated MSCs, and activated MSCs.* The authors noted that the presence of transforming growth factor β3 (TGFβ3), a growth factor typically found in scarless healing, is enhanced and prolonged with treatment of activated MSCs when compared with naïve MSCs or fibroblasts.
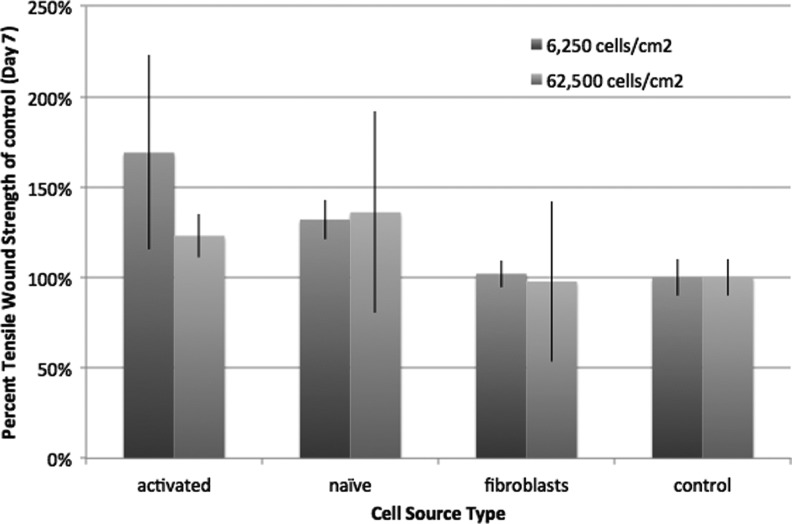
Figure 4.
MSCs activated with interferon gamma provided increased tensile strength at lower cell doses (6,250 cells/cm2) when compared with naive MSC or fibroblasts. At higher cell doses (62,500 cells/cm2), there were no statistical differences between interferon gamma or naive MSCs, suggesting that the feasibility of lower cell doses could be achieved if the cells were actuated before administration.
Inflammation and the impact of preactivation
As stated earlier, chronic inflammation is one of the hallmarks of a chronic nonhealing wound. Wound healing usually progresses through hemostasis, inflammation, proliferation, and, finally, a remodeling phase. The initial platelet plug that forms results in a temporary provisional matrix for cells to invade and transform into a mature granulating bed. Neutrophils, mast cells, and monocytes arrive in the matrix through a process that is enhanced by vasodilation and a marked increase in permeability secondary to mast cell histamine release.32 Monocytes differentiate into macrophages and due to their plasticity can be influenced by the microenvironment to polarize toward either a classical or an alternately activated phenotype.1 While neutrophils and macrophages are crucial for defense, in the absence of infection, the reactive oxygen species (ROS) and other inflammatory mediators released by these cells can retard healing. The pro-inflammatory conditions surrounding the nonhealing wound have been compared with the micro-environmental conditions noted in cancer. In fact, tumors have been referred to as wounds that do not heal.33 While inflammation is necessary to initiate wound healing, it is equally important that micro-environmental signals are generated for the termination of this phase. Therefore, researchers are now able to apply lessons learned from modulating inflammation in cancer to nonhealing wounds.34 In addition, many chronic conditions share the pathophysiology of inflammation, tissue repair, and, ultimately, fibrosis.35 The effect of MSCs on inflammation has potential therapeutic implications for a myriad of clinical conditions.
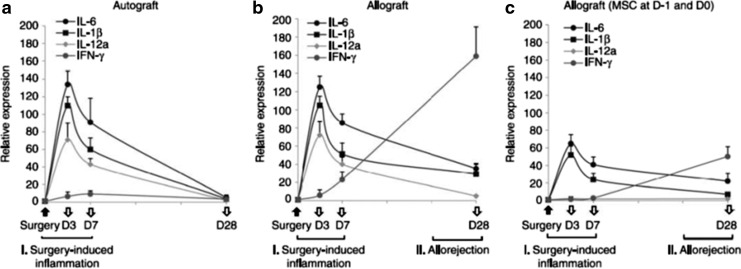
After prolonged myocardial ischemia, cells undergo necrosis, apoptosis, or can remain in a hibernating or stunned state. These concepts have been applied to the processes noted in chronic wound healing.36 After myocardial infarction, cells express damage-associated proteins (DAMPs) that attract and activate various components of the immune system.37 Attempts at pharmacological therapy to lower inflammation have resulted in poor healing, scar formation, and, ultimately, heart failure.38 These findings led investigators to research alternative methods for reducing inflammation without compromising healing39 (Fig. 5). There are several molecules and pathways that have been implied in MSC-dependent immunomodulation, including indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), nitric oxide (NO), TGFβ1, and interleukin 10 (IL-10).39 IV MSCs were found to decrease myocardial infarction size in a mouse model, despite the fact that the majority of cells were filtered by the lung.40 After trapping in the lungs, MSCs up-regulated the expression of the anti-inflammatory protein tumor necrosis factor inducible gene siRNA6 (TSG-6). Confirmation of this mechanism of action was proved by achieving similar outcomes when IV infusions of purified TSG-6 were utilized. In another organ system, the allergic response to inflammatory airway disease was reduced by the use of human iPSCs in a mouse model.41 A reduction in nasal eosinophilia, levels of Th-2 cytokines, and serum IgE levels were appreciated using human iPSCs in this trial. In ophthalmology, both early surgical inflammatory and delayed immune responses were blunted through IV MSCs in a mouse model of both auto and allograft corneal transplantation.42 Allografted animals demonstrated significantly lower levels of inflammatory cytokines during the surgical-induced early inflammatory period and the delayed allorejection time frame when MSCs were used (Fig. 6). Interestingly, as in the myocardial infarction studies, the MSCs were found to be trapped in the lung. The trapped MSCs were also found in this study to secrete TSG-6. IV-MSCs had no effect on small interfering RNA (siRNA) TSG-6 knockdown mice while IV injection of recombinant TSG-6 was equally as effective in preventing both early and late rejection of corneal allografts, further confirming the paracrine nature of the MSC response. In another trial, MSCs given to mice just before inducing sepsis using a cecal perforation model reduced mortality and preserved critical organ function.43
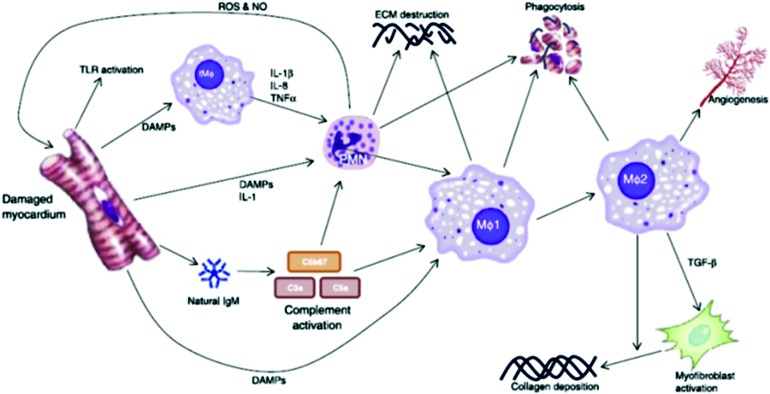
Figure 5.
Attraction and activation of the immune system after myocardial infarction. The first cells activated after myocardial infarction are the local macrophages. Soon after, damage associated proteins (DAMPs) complement system and IL-1 attract neutrophils, which enter the damage tissue to clear the debris. Within days, large numbers of macrophages infiltrate the tissue, clearing both the debris and activating the reparative pathways. The M1 macrophages are the first to arrive and have a pro-inflammatory character. They are followed by the anti-inflammatory M2 macrophage. Lymphocytes arrive relatively late on the scene, due to the lengthy process of activation. (Reprinted with permission from van den Akker et al.39) To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 6.
Time course of gene expression levels in the cornea after transplantation surgery. Real-time reverse transcription (RT-PCR) showed that the levels of proinflammatory cytokines (interleukin IL-6,IL-1B, and IL-2) were up-regulated at similar levels in (a) autografts and (b) allografts at days 3 and 7 after transplantation, which defines the early phase of surgery-induced inflammation. The levels of T-cell derived cytokines (IFN-gamma) were elevated through day 28 in allografts, but not in autografts, which indicates the late phase of the allogeneic immune rejection. In allografts that received intravenous human MSCs (c), levels of IL-6, IL-1b, and IL-12a were significantly lower at days 3 and 7 and levels of IFN-gamma were markedly decreased at day 28 compared with autografts or allografts that did not receive hMSCs. n=5 at each time-point in all experimental groups. (Reprinted with permission from Oh et al.42)
MSC can respond to differential levels of inflammation by correspondingly altering both function and phenotype. At low exposures of IFG, a highly inflammatory cytokine found in wounds and other sites of tissue injury, MSC can up-regulate surface expression of major histocompatibility complex Class II and adopt the ability to present antigen and promote an inflammatory response.44,45 At higher doses of IFG, MSC become more immunosuppressive, releasing greater amounts of indoleamine 2,3-dioxygenase, Cox-2, and PGE-2.46,47 We observed increased immunosuppressive potential of IFG activated MSC when compared with naïve MSC in reducing the mortality of ongoing graft versus host disease and in preventing mortality when given early in the course of the disease.48 Reduction of early inflammatory responses in wounds combined with regenerative signals may be beneficial in triggering a more rapid course of early repair and regeneration in adult wound healing, as this process more closely resembles fetal healing, in which wounds heal by regeneration without contracture or scar. MSC effects are largely paracrine in nature.23,49 IFG activation appears to augment this paracrine function by increased MSC production of cytokines, chemokines, and growth factors. We propose that such activation may provide a new and more potent method of accelerating tissue regeneration.
Take-Home Messages.
Inflammation is a necessary component of the normal wound healing process but if unchecked and persistent, can lead to a nonhealing wound.
There are many macro and micro-level confounding variables that contribute to a nonhealing wound. This helps explain why therapies that target a single process or factor often fail to improve healing in clinical trials.
MSCs can provide the right biochemical cues depending on how they sense the wound bed micro-environmental signals. This individualized approach could help reduce inflammation and, therefore, tissue repair with its expected high scar tissue levels.
Preactivation of MSCs might offer a method of improving the potency of these cells without the need for additional cell numbers.
There is an increasing awareness that the clinical benefit from MSCs is through paracrine functioning and not terminal differentiation of the cells.
The use of scaffoldings and other methods may improve cell viability and durability, which is currently a major limitation for stem cell use.
Summary
Wound healing is a complex process that requires an orchestrated biochemical series of events to occur to achieve tissue closure. The process of healing in most adult tissues results in significant scar formation as a part of the normal repair process. Fetal healing, in which tissue is replaced without scar formation, is known to proceed with minimal inflammation. Current devices and dressings, therefore, have targeted inflammation as a method to emulate the fetal healing environment. Clinicians currently start with moist dressings and then move through a series of treatment options of increasing complexity if there is either an absent or less than adequate healing response. This sequential treatment method fails to capture the natural complex processes that are simultaneously proceeding in the in-vivo setting. The wound care industry has generated an enormous amount of products, dressings, devices, and, more recently, scaffoldings and cell-based therapies but to date, only 50–70% of wounds heal within randomized controlled trials. Stem cells offer a novel therapeutic option that differs dramatically from current treatment options. MSCs can be deployed within scaffoldings, topically or by injection, to sense the microenvironment and influence the healing process in a more regenerative manner through paracrine functioning. With preactivation of MSCs, it is thought that fewer cells are required and that their paracrine effects are heightened. There are those that raise questions about safety, but many patients have received stem cell therapy and meta-analysis reports confirm that assumption. The next big thing for wound care will be to create off the shelf, allogeneic, activated MSCs that can be delivered in a biological scaffolding to promote a more regenerative healing process. It should follow, that with less scar and increased tensile strength that recidivism will decrease. As payment for healthcare begins to focus on outcomes, clinicians will need to achieve quality healing, low recurrence, and patient-centered outcomes in a cost-effective manner. It may prove that the initial expense of stem cell therapy has long-term cost-effective outcomes.
Abbreviations and Acronyms
- DAMPs
damage-associated proteins
- HSCs
hematopoietic stem cells
- IDO
indoleamine 2,3-dioxygenase
- IFG
interferon gamma
- IL-10
interleukin 10
- iPSC
induced human pluripotent stem cell
- MSC
mesenchymal stem cell
- NO
nitric oxide
- PGE2
prostaglandin E2
- siRNA
small interfering RNA
- TSG-6
tumor necrosis factor inducible gene siRNA6
Footnotes
Szilagyi E, Neri F, Kim W, Makhlouf T, Douglas GW, Sears M, Acosta A, Lee S, Rahman A, Rowan D, Turbelidze A, Wietecha M, DiPietro L, Balla A, Ennis W, and Bartholomew A: Effects of interferon gamma–activated mesenchymal stem cells. University of Illinois Hospital and Health Sciences System, Chicago, IL, 2012; submitted to Cytokine, May 2013.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
William J. Ennis, DO, MBA, is a professor of clinical surgery and the chief of the section of wound healing and tissue repair at the University of Illinois Hospital and Health Sciences System (UIHHS) in Chicago, IL. He has been practicing wound care for 22 years and has lectured and published extensively. He cofounded the American College of Wound Healing and Tissue Repair, a nonprofit organization focused on creating a formal specialty of wound healing. Audrey Sui, BA, is a Notre Dame graduate, and currently serves as the research assistant in the Section of Wound Healing and Tissue Repair at UIHHS. Audrey is helping conduct numerous clinical trials and coordinate collaborative basic science research within the department. Amelia Bartholomew, MD, MPH, is a transplant surgeon and currently director of research in the Department of Surgery at UIHHS. Her research is focused on stem cell therapeutics, immune-modulation, and translational science. Dr. Bartholomew's research on the anti-inflammatory properties of MSCs as well as the impact of preactivation with IFG have been widely cited.
References
- 1.Mirza R. Koh TJ. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine. 2011;56:256. doi: 10.1016/j.cyto.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Gurtner GC. Werner S. Barrandon Y. Longaker MT. Wound repair and regeneration. Nature. 2008;453:314. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 3.Limova M. Active wound coverings: bioengineered skin and dermal substitutes. Surg Clin North Am. 2010;90:1237. doi: 10.1016/j.suc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Rheinwald JG. Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 5.Falanga V. Sabolinski M. A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 1999;7:201. doi: 10.1046/j.1524-475x.1999.00201.x. [DOI] [PubMed] [Google Scholar]
- 6.Marston WA. Hanft J. Norwood P. Pollak R Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701. doi: 10.2337/diacare.26.6.1701. [DOI] [PubMed] [Google Scholar]
- 7.Weissman IL. Anderson DJ. Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001;17:387. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- 8.Shiozawa Y. Havens AM. Pienta KJ. Taichman RS. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia. 2008;22:941. doi: 10.1038/leu.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caplan AI. Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 10.Lam MT. Nauta A. Meyer NP. Wu JC. Longaker MT. Effective delivery of stem cells using an extracellular matrix patch results in increased cell survival and proliferation and reduced scarring in skin wound healing. Tissue Eng Part A. 2013;19:738. doi: 10.1089/ten.tea.2012.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10:678. doi: 10.1016/j.stem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Lian Q. Zhang Y. Zhang J. Zhang HK. Wu X. Zhang Y. Lam FF. Kang S. Xia JC. Lai WH. Au KW. Chow YY. Siu CW. Lee CN. Tse HF. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 13.Zhao T. Zhang ZN. Rong Z. Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 14.Lalu MM. McIntyre L. Pugliese C. Fergusson D. Winston BW. Marshall JC. Granton J. Stewart DJ Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badiavas EV. Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510. doi: 10.1001/archderm.139.4.510. [DOI] [PubMed] [Google Scholar]
- 16.Falanga V. Iwamoto S. Chartier M. Yufit T. Butmarc J. Kouttab N. Shrayer D. Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 17.Javazon EH. Keswani SG. Badillo AT. Crombleholme TM. Zoltick PW. Radu AP. Kozin ED. Beggs K. Malik AA. Flake AW. Enhanced epithelial gap closure and increased angiogenesis in wounds of diabetic mice treated with adult murine bone marrow stromal progenitor cells. Wound Repair Regen. 2007;15:350. doi: 10.1111/j.1524-475X.2007.00237.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen JS. Wong VW. Gurtner GC. Therapeutic potential of bone marrow-derived mesenchymal stem cells for cutaneous wound healing. Front Immunol. 2012;3:192. doi: 10.3389/fimmu.2012.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer NG. Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011;6:457. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 20.Dash NR. Dash SN. Routray P. Mohapatra S. Mohapatra PC. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009;12:359. doi: 10.1089/rej.2009.0872. [DOI] [PubMed] [Google Scholar]
- 21.Jain P. Perakath B. Jesudason MR. Nayak S. The effect of autologous bone marrow-derived cells on healing chronic lower extremity wounds: results of a randomized controlled study. Ostomy Wound Manage. 2011;57:38. [PubMed] [Google Scholar]
- 22.Wu Y. Chen L. Scott PG. Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 23.Chen L. Tredget EE. Wu PY. Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freytes DO. Kang JW. Marcos-Campos I. Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114:220. doi: 10.1002/jcb.24357. [DOI] [PubMed] [Google Scholar]
- 25.Kim J. Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gosain A. DiPietro LA. Aging and wound healing. World J Surg. 2004;28:321. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]
- 27.Lee S. Szilagyi E. Chen L. Premanand K. Dipietro LA. Ennis W. Bartholomew AM. Activated mesenchymal stem cells increase wound tensile strength in aged mouse model via macrophages. J Surg Res. 2013;181:20. doi: 10.1016/j.jss.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 28.Badillo AT. Redden RA. Zhang L. Doolin EJ. Liechty KW. Treatment of diabetic wounds with fetal murine mesenchymal stromal cells enhances wound closure. Cell Tissue Res. 2007;329:301. doi: 10.1007/s00441-007-0417-3. [DOI] [PubMed] [Google Scholar]
- 29.Stoff A. Rivera AA. Sanjib Banerjee N. Moore ST. Michael Numnum T. Espinosa-de-Los-Monteros A. Richter DF. Siegal GP. Chow LT. Feldman D. Vasconez LO. Michael Mathis J. Stoff-Khalili MA. Curiel DT. Promotion of incisional wound repair by human mesenchymal stem cell transplantation. Exp Dermatol. 2009;18:362. doi: 10.1111/j.1600-0625.2008.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McFarlin K. Gao X. Liu YB. Dulchavsky DS. Kwon D. Arbab AS. Bansal M. Li Y. Chopp M. Dulchavsky SA. Gautam SC. Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen. 2006;14:471. doi: 10.1111/j.1743-6109.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- 31.Kwon DS. Gao X. Liu YB. Dulchavsky DS. Danyluk AL. Bansal M. Chopp M. McIntosh K. Arbab AS. Dulchavsky SA. Gautam SC. Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. Int Wound J. 2008;5:453. doi: 10.1111/j.1742-481X.2007.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weller K. Foitzik K. Paus R. Syska W. Maurer M. Mast cells are required for normal healing of skin wounds in mice. FASEB J. 2006;20:2366. doi: 10.1096/fj.06-5837fje. [DOI] [PubMed] [Google Scholar]
- 33.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 34.Balkwill FR. Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 35.White ES. Mantovani AR. Inflammation, wound repair, and fibrosis: reassessing the spectrum of tissue injury and resolution. J Pathol. 2013;229:141. doi: 10.1002/path.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ennis WJ. Meneses P. Wound healing at the local level: the stunned wound. Ostomy Wound Manage. 2000;46:39S. ; quiz 49S. [PubMed] [Google Scholar]
- 37.Frantz S. Bauersachs J. Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res. 2009;81:474. doi: 10.1093/cvr/cvn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Timmers L. Sluijter JP. Verlaan CW. Steendijk P. Cramer MJ. Emons M. Strijder C. Grundeman PF. Sze SK. Hua L. Piek JJ. Borst C. Pasterkamp G. de Kleijn DP. Cyclooxygenase-2 inhibition increases mortality, enhances left ventricular remodeling, and impairs systolic function after myocardial infarction in the pig. Circulation. 2007;115:326. doi: 10.1161/CIRCULATIONAHA.106.647230. [DOI] [PubMed] [Google Scholar]
- 39.van den Akker F. Deddens JC. Doevendans PA. Sluijter JP. Cardiac stem cell therapy to modulate inflammation upon myocardial infarction. Biochim Biophys Acta. 2013;1830:2449. doi: 10.1016/j.bbagen.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 40.Lee RH. Pulin AA. Seo MJ. Kota DJ. Ylostalo J. Larson BL. Semprun-Prieto L. Delafontaine P. Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun YQ. Deng MX. He J. Zeng QX. Wen W. Wong DS. Tse HF. Xu G. Lian Q. Shi J. Fu QL. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells. 2012;30:2692. doi: 10.1002/stem.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oh JY. Lee RH. Yu JM. Ko JH. Lee HJ. Ko AY. Roddy GW. Prockop DJ. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther. 2012;20:2143. doi: 10.1038/mt.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nemeth K. Leelahavanichkul A. Yuen PS. Mayer B. Parmelee A. Doi K. Robey PG. Leelahavanichkul K. Koller BH. Brown JM. Hu X. Jelinek I. Star RA. Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Blanc K. Tammik C. Rosendahl K. Zetterberg E. Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 45.Aggarwal S. Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 46.Meisel R. Zibert A. Laryea M. Gobel U. Daubener W. Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 47.English K. Barry FP. Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008;115:50. doi: 10.1016/j.imlet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Polchert D. Sobinsky J. Douglas G. Kidd M. Moadsiri A. Reina E. Genrich K. Mehrotra S. Setty S. Smith B. Bartholomew A. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li QM. Fu YM. Shan ZY. Shen JL. Zhang XM. Lei L. Jin LH. MSCs guide neurite directional extension and promote oligodendrogenesis in NSCs. Biochem Biophys Res Commun. 2009;384:372. doi: 10.1016/j.bbrc.2009.04.147. [DOI] [PubMed] [Google Scholar]