Abstract
Objectives
Anthracyclines and taxanes are often used as first-line chemotherapy treatments in patients with breast cancer. There are, however, significant toxicity and side effects associated with these therapies. Previous studies have demonstrated that active hexose-correlated compound (AHCC) reduces such side effects. The present study explored the beneficial effects of AHCC on adverse events in patients receiving adjuvant chemotherapy for breast cancer.
Subjects
Forty-one women who were treated with anthracyclines and taxanes at Nagumo Clinic in Tokyo from October 2004 to March 2011 were selected for this study.
Outcome measures
We compared the occurrence of adverse events in patients who received AHCC with those who did not receive AHCC. Using Fisher's exact tests, we also compared the worst-grade adverse events in each treatment cycle. Generalized estimating equations were employed to compare longitudinal changes, and the use of granulocyte colony-stimulating factor, in the two groups was analyzed using Student's t-test.
Results
We found that, compared to the control group, the AHCC group had significantly fewer neutrophil-related events (odds ratio, 0.30; p=0.016), significantly lower use of granulocyte colony-stimulating factor, and a higher (although not significant) rate of adverse events associated with γ-glutamyl transpeptidase.
Conclusions
AHCC has the potential to reduce the severity of neutropenia induced by breast cancer chemotherapy and the use of G-CSF during chemotherapy.
Introduction
Among women in Japan, breast cancer is the most common malignant tumor. While anthracyclines and taxanes are used as first-line chemotherapy in the treatment of these patients,1, 2 both are associated with significant toxicity and side effects, including nausea, vomiting, hair loss, bone marrow suppression, hepatotoxicity, cardiac toxicity, peripheral neuropathy, and lipid disorders. Although there are some treatments for nausea and vomiting, 3–5 there are only a few methods of preventing such side effects. Unfortunately, severe side effects may warrant discontinuation or delay of chemotherapy. Moreover, these side effects may have adverse effects on health-related quality of life (HRQOL).6, 7
Active hexose-correlated compound (AHCC), which has been used as a dietary supplement in various countries, is an extract of Lentinula edodes, one of the basidiomycete family of fungi. The main components of AHCC are α-glucans, which are derived from the processed mushrooms. These glucans are thought to provide a carbohydrate that stimulates immune response.8,9
Previous experiments using rodents have demonstrated that AHCC reduces such chemotherapy side effects as bone marrow suppression, hepatotoxicity, and nephrotoxicity.10–12 There have also been several studies with healthy adults that have confirmed the clinical safety and tolerability of AHCC. In these studies, only low incidences of mild and transient side effects have been found.8,13 Matsui et al. have found AHCC reduces the recurrence of hepatocellular carcinoma and adverse events associated with chemotherapy in a human prospective cohort study.14 However, the mechanisms of action of AHCC are still poorly understood, and conflicting evidence suggests the need for further investigation.15
This study aimed to explore the beneficial effects of AHCC on adverse events in female patients receiving adjuvant chemotherapy for breast cancer.
Materials and Methods
Study design
We reviewed the medical records of women treated for breast cancer at Nagumo Clinic in Tokyo from September 2004 to March 2011. From this review, we selected female patients who fulfilled the following criteria: (1) were between the ages of 20 and 64, (2) had pathologically proven breast cancer, and (3) were receiving doxorubicin and cyclophosphamide (AC therapy), followed by taxane-based regimens. From medical records and the clinic's computer database system, we collected the following baseline characteristics: administration of AHCCs, age, body mass index (BMI), tumor size, clinical stage of TNM-UICC,16 estrogen status, and progesterone receptor status.
AHCC treatment
Patients who had taken AHCC during chemotherapy were analyzed as the AHCC group. Each patient in this group had taken a total of 1.0 g of AHCC orally after each meal. Patients who had not taken AHCC were analyzed as the control group.
Basic treatment
All patients received AC therapy followed by one of four taxane-based regimens: weekly paclitaxel (PTX) therapy, weekly docetaxel (DTX) therapy, conventional PTX therapy, and conventional DTX therapy. The AC therapy consisted of doxorubicin (60 mg/m2 of body surface area, administered intravenously) and cyclophosphamide (600 mg/m2 of body surface area, administered by intravenous [IV] drip infusion), every 3 weeks for 4 cycles. For PTX therapy, paclitaxel (80 mg/m2 administered by IV drip infusion for 1 h or more) was given weekly for 12 weeks. For DTX therapy, 30 mg/m2 of docetaxel was administered in the same manner as the weekly PTX therapy. Patients in the conventional PTX or DTX groups received, every 3 weeks, for 4 doses, either 125 mg/m2 of PTX by IV drip infusion for 3 h or 100 mg/m2 of DTX by IV drip infusion for 1 h. Dexamethasone and 5-HT3–receptor antagonists were administered to all patients to prevent nausea and vomiting. Granulocyte colony-stimulating factor (G-CSF) was administered if patients had absolute neutrophil counts of <1000/mm3 and fever, or if they had an absolute neutrophil count of <500/mm3.
Measurements and other observations
We measured aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GTP), total cholesterol (T-Chol), triglycerides (TG), white blood cell (WBC) count, and neutrophil count during every course of chemotherapy. AST, ALT and γ-GTP were employed as diagnostic markers for hepatotoxicity, T-Chol and TG for lipid disorder, and WBC count and neutrophil count for bone marrow suppression.
Using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE),17 we then evaluated adverse events according to AST increase, ALT increase, GGT increase, TG increase, T-Chol increase, WBC count decrease, and neutrophil count decrease. Under the NCI-CTCAE standard, AST increase and ALT increase are defined as: grade 1 (>upper limit of normal [ULN]–3.0×ULN); grade 2 (>3.0–5.0×ULN); grade 3 (>5.0–20.0×ULN); grade 4 (>20.0×ULN). GGT increase is defined as grade 1 (>ULN–2.5×ULN); grade 2 (>2.5–5.0×ULN); grade 3 (>5.0–20.0×ULN); grade 4 (>20.0×ULN). TG increase is defined as: grade 1 (150–300 mg/dl); grade 2 (>300– 500 mg/dl); grade 3 (>500–1000 mg/dl); grade 4 (>1000 mg/dl); grade 5 (death). T-Chol increase is defined as: grade 1 (>ULN–300 mg/dl); grade 2 (>300– 400 mg/dl); grade 3 (>400–500 mg/dl); grade 4 (>500 mg/dl). WBC count decrease is defined as: grade 1 (<lower limits of normal [LLN]–3000/mm3); grade 2 (<3000–2000/mm3); grade 3 (<2000–1000 /mm3); grade 4 (<1000/mm3). Neutrophil count decrease is defined as: grade 1 (<LLN–1500/mm3); grade 2 (<1500–000 /mm3); grade 3 (<1000–500/mm3); grade 4 (<500 /mm3). We defined grade 2 or higher toxicities as clinically significant adverse events.
Generally, before the administration of chemotherapy drugs, an adequate WBC count is required. To achieve this count, G-CSF is widely used. Therefore, the CTCAE grade on the day of chemotherapy administration may not reflect the presence of leukocytopenia or neutropenia. To solve this problem, we proposed that G-CSF usage act as a surrogate marker of leukocytopenia and neutropenia. The use of G-CSF during the course of weekly paclitaxel therapy was examined in both the AHCC group and the control group and then compared.
Statistical analysis
The power-based sample size calculation was performed using the results of the Spierings et al. clinical trial.8 Approximately 40 patients were necessary in order to detect a 40% difference in the recurrence of adverse events between the AHCC group and the control group—that is, 15% in the AHCC group and 55% in the control group—with a one-sided significance level of 0.05 and 80% power.
For the measured parameters, we defined adverse events of grade 2 or higher as clinically significant. We subsequently compared the proportions of adverse events in the AHCC group and the control group. For statistical analysis, we defined the significance level as 95% and used Fisher's exact test to compare the worst-grade adverse events. Generalized estimating equations (GEEs) were also used to analyze the longitudinal changes in the occurrence of adverse events (grades 2–4) between the AHCC group and the control group. We applied logistic regression with adjustments for age (below and above the age of 50) and BMI (below and above a BMI of 25). Robust variances were used to account for the correlation of outcomes for the same patient. We used JMP, version 9 and R 2.10.1 (SAS Institute, Inc., Cary, NC), for Fisher's exact test and Student's t-test. Sample size calculation and GEE analysis was performed using the SAS software, version 9.3.
Ethical considerations
This study was performed in accordance with the Helsinki Declaration.18 The study protocol was approved by the ethical review board of Nagumo Clinic in Tokyo, which allowed us to analyze the data without obtaining patient consent because the study involved no intervention and direct patient identifiers, such as name and medical record number, were not collected from the computer database.
Results
Patient characteristics
We retrospectively analyzed 41 female patients, ages 31 to 61, who underwent adjuvant chemotherapy, including anthracyclines and taxanes, for breast cancer at the Nagumo Clinic in Tokyo between October 2004 and March 2011. As shown in Table 1, the patients' characteristics in the AHCC group and the control group were well balanced. The mean age was 43.6 years (range, 33–52 years) in the AHCC group and 45.1 years (range, 31–61 years) in the control group. The mean tumor caliper measurement was 2.94 cm (range, 0.5–8.5 cm) in the AHCC group and 2.86 cm (range, 0.3–10.0 cm) in the control group. Most patients had stage I, IIA, or IIB disease. More than 75% of the patients were estrogen-receptor positive. Of the 41 patients, 35 received weekly PTX therapy, 4 received conventional PTX therapy, 1 received weekly DTX therapy, and 1 received conventional DTX therapy after AC therapy.
Table 1.
Patient Characteristics
| Characteristic | AHCCa(n=18) | Control (n=23) |
|---|---|---|
| Age | ||
| Mean | 43.6 | 45.1 |
| Range | 33 – 52 | 31 – 61 |
| Tumor size (cm) | ||
| Mean | 2.94 | 2.86 |
| Range | 0.5 – 8.5 | 0.3 – 10 |
| Clinical stage | ||
| I | 3 | 6 |
| II A | 9 | 8 |
| II B | 4 | 7 |
| III A | 1 | 2 |
| Unknown | 1 | 0 |
| Estrogen status | ||
| Positive | 14 | 18 |
| Negative | 3 | 5 |
| Unknown | 1 | 0 |
| Progesterone receptor status | ||
| Positive | 13 | 17 |
| Negative | 4 | 6 |
| Unknown | 1 | 0 |
AHCC, active hexose-correlated compound.
NCI-CTCAE grade
An analysis of comparisons of worst-grade adverse events between the two groups was performed using Fisher's exact test (Table 2). Although not statistically significant, patients in the AHCC group had fewer adverse events associated with TG than patients in the control group (5.5% in the AHCC group and 34.6% in the control group, p=0.054). Patients in the AHCC group also had fewer adverse events with T-Chol than patients in the control group (5.5% in the AHCC group and 17.3% in the control group, p=0.363), although this, too, was not statistically significant. The AHCC group did have more adverse events associated with γ-GTP than the control group (28% in the AHCC group and 13% in the control group, p=0.267). Again, however, this was not statistically significant.
Table 2.
Comparisons of Worst-Grade Adverse Events During Chemotherapy
| Control n(%) | AHCCan(%) | P-valueb | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | 0 | 1 | 2 | 3 | 4 | 5 | 0 | 1 | 2 | 3 | 4 | 5 | ||||||||||
| ASTc increase | 10 | (43.4) | 12 | (52.1) | 1 | (4.3) | — | — | — | 9 | (50) | 9 | (50.0) | — | — | — | — | 1 | ||||
| ALTd increase | 6 | (26.0) | 15 | (65.2) | 1 | (4.3) | 1 | (4.3) | — | — | 8 | (44.4) | 8 | (44.4) | 2 | (11.1) | — | — | — | 1 | ||
| GGTe increase | 15 | (65.2) | 5 | (21.7) | 1 | (4.3) | 2 | (8.6) | — | — | 8 | (44.4) | 5 | (27.7) | 2 | (11.1) | 3 | (16.6) | — | — | 0.267 | |
| TGf increase | 7 | (30.4) | 8 | (34.7) | 6 | (26.0) | 2 | (8.6) | — | — | 5 | (27.7) | 12 | (66.6) | 1 | (5.5) | — | — | — | 0.054 | ||
| T-Cholg increase | 7 | (30.4) | 12 | (52.1) | 4 | (17.3) | — | — | — | 6 | (33.3) | 11 | (61.1) | 1 | (5.5) | — | — | — | 0.363 | |||
| WBCh decrease | 5 | (21.7) | 5 | (21.7) | 10 | (43.4) | 3 | (13.0) | — | — | 2 | (11.1) | 5 | (27.7) | 10 | (55.5) | 1 | (5.5) | — | — | 1 | |
| Neutrophil decrease | 2 | (25.0) | 0 | (0.0) | 4 | (50.0) | 2 | (25.0) | — | — | 2 | (11.1) | 3 | (16.7) | 8 | (44.4) | 4 | (22.2) | 1 | (5.6) | — | 1 |
AHCC, active hexose-correlated compound.
P-values based on Fisher's exact test.
AST, aspartate aminotransferase.
ALT, alanine aminotransferase.
GGT, γ-glutamyl transpeptidase.
TG, triglycerides.
T-Chol, total cholesterol.
WBC, white blood cells.
Using the GEE method, we found that the AHCC group experienced significantly fewer adverse neutrophil events than the control group (odds ratio, 0.30; 95% confidence interval [CI], 0.1 –0.80; p=0.016) (see Table 3). An analysis of worst-grade adverse events showed that the AHCC group had fewer events associated with TG (odds ratio, 0.23; 95% CI, 0.03–1.86; p=0.169) and T-Chol (odds ratio, 0.15; 95% CI, 0.02–1.25; p=0.079), but more events associated with γ-GTP (odds ratio, 2.07; 95% CI, 0.31–14.06; p=0.456), than the control group, although these differences were not statistically significant.
Table 3.
Comparisons of Occurrence of Adverse Events During Chemotherapy
| Odds ratioa(95% CI) | P-value | |
|---|---|---|
| ASTb | — | — |
| ALTc | 1.66 (0.19 – 14.24) | 0.642 |
| γ-GTPd | 2.07 (0.31 – 14.06) | 0.456 |
| TGe | 0.23 (0.03 – 1.86) | 0.169 |
| T-Cholf | 0.15 (0.02 – 1.25) | 0.079 |
| WBCg | 1.17 (0.47 – 2.92) | 0.744 |
| Neutrophil | 0.30 (0.11 – 0.80) | 0.016 |
Odds ratio based on generalized estimating equations (GEE).
AST, aspartate aminotransferase.
ALT, alanine aminotransferase.
γ-GTP, γ-glutamyl transpeptidase.
TG, trigylcerides.
T-Chol, total cholesterol.
WBC, white blood cell.
Frequency of G-CSF usage
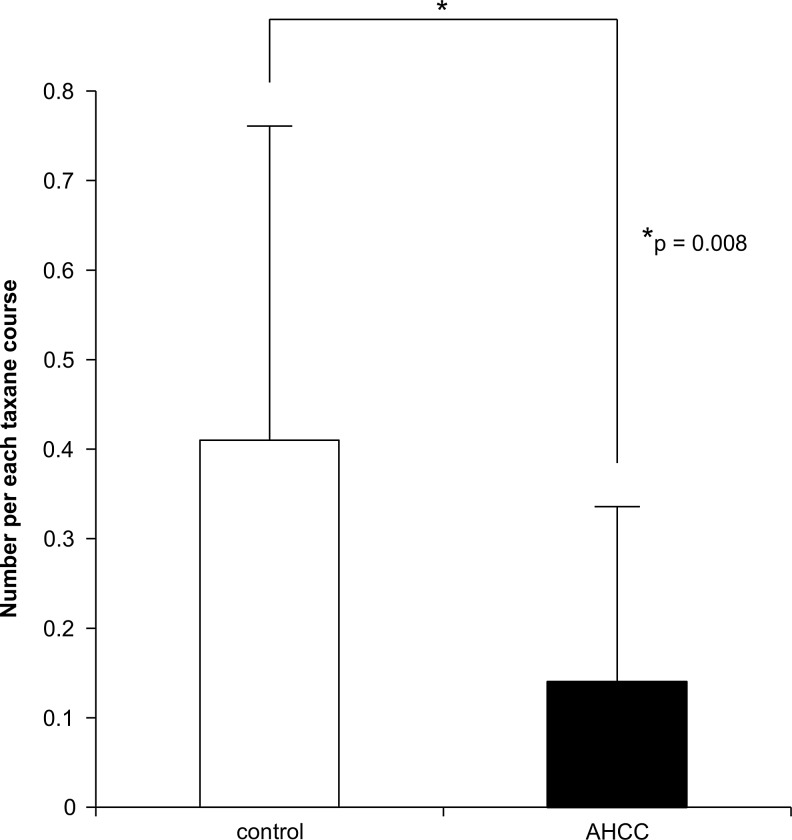
We calculated the number of G-CSF usages per taxane infusion in the patients receiving weekly paclitaxel therapy. According to Student's t-test, the AHCC group had significantly lower usage of G-CSF per administration of taxane therapy than did the control group (0.14 in the AHCC group and 0.41 in the control group, p=0.008) (see Fig. 1).
FIG. 1.
The average number of G-CSF usages per administration of taxane infusion in patients receiving weekly paclitaxel therapy. AHCC, active hexose-correlated compound; G-CSF, granulocyte colony-stimulating factor.
Discussion
The present study explored the effects of AHCC on breast cancer patients who were treated with adjuvant chemotherapy. Using GEE to analyze CTCAE-grade adverse events, we found the AHCC group experienced significantly fewer adverse neutrophil events than the control group. Furthermore, during weekly taxane therapy, a significantly lower usage of G-CSF was observed in the AHCC group than in the control group. These results are in agreement with those of Shigama et al., who showed that AHCC improved bone marrow suppression in mice treated with several anticancer drugs, including adriamycin and cyclophosphamide.10 In an analysis of comparisons of worse-grade adverse neutrophil events, however, we found no significant difference between the AHCC group and the control group. One possible explanation is that GEE analysis uses data from individual, repeated measures, and considers the longitudinal nature of the data.
The mechanism by which AHCC improves bone marrow suppression remains unclear. One previous study showed that maitake β-glucans (MBG) promoted bone marrow cell viability and protected the bone marrow stem cell colony formation unit from adriamycin-induced hematopoietic toxicity.19 It is speculated that the beneficial effects of AHCC on bone marrow suppression may be mediated by a mechanism similar to that of MBG, although the predominant component of AHCC is partially acetylated α-glucans, and not β-glucans. Whatever the mechanism, these results suggest that AHCC has the potential to increase the neutrophil count during bone marrow suppression. Through this beneficial effect, it may be possible to increase the intensity of chemotherapy and achieve a better clinical outcome.
We also found that the AHCC group had fewer adverse events associated with TG and T-Chol, although this difference was not statistically significant. She-Fang Ye et al. previously revealed that AHCC suppressed the increased serum corticosterone level induced by oxidative stress.20 It has been speculated that AHCC improves lipid abnormalities through modulation of the serum corticosterone level, which is also affected by anticancer drugs.15 Although the true mechanism is still unclear, our data suggest that AHCC might improve the lipid abnormalities seen in breast cancer patients who are undergoing chemotherapy. Lipid abnormalities during chemotherapy are thought to be induced by anticancer drugs and steroids, which are used as antiemetic drugs. Steroids are widely used to treat chemotherapy-induced nausea and vomiting. Therefore, AHCC may be effective for patients undergoing chemotherapy for various cancers.
Previous studies have shown that AHCC improves ALT abnormalities due to PTX, and AST and ALT abnormalities due to 6-mercaptopurine+methotrexate, respectively, in animals.10,12 Moreover, AHCC has been shown to improve AST, γ-GTP, and cholinesterase abnormalities in patients with hepatocellular carcinoma after surgery.14 Our study did not find any significant AHCC beneficial effect on liver damage during chemotherapy. However, the AHCC group in our study had fewer grade1 or higher ALT abnormalities than the control group had (55.5% vs. 73.8%, p=0.322). It is possible that our small sample size prevented detection of the beneficial effect of AHCC on liver function.
Although the difference was not statistically significant, the AHCC group in our study did experience more adverse events associated with γ-GTP. In contrast, Matsui et al. found that AHCC improved the γ-GTP level in postsurgical patients with hepatocellular carcinoma.14 In our study, the AHCC group had more patients with grade 2 or higher γ-GTP events at the beginning of chemotherapy than the control group (10% vs. 4%). Further studies are needed to fully examine whether AHCC has beneficial effects on γ-GTP.14
Our study results are valuable in clarifying the benefits of AHCC and determining an appropriate sample size for prospective studies. Our sample size was small, and the study was retrospective. Moreover, there was no data on AHCC compliance. Therefore, it is possible that the effect of AHCC was underestimated. Nevertheless, our results show that AHCC has the potential to reduce the adverse events associated with chemotherapy. At present, there is no established prophylactic treatment for hepatotoxicity, lipid disorder, and bone marrow suppression. Our study raises the possibility that AHCC may be a good candidate for such a purpose. Further studies are needed to elucidate other beneficial effects. Among other things, AHCC may improve HRQOL in breast cancer patients through the reduction of adverse events. Future studies will clarify the impact of AHCC on HRQOL for these patients.
Conclusion
AHCC has the potential to improve neutropenia induced by the use of chemotherapy in the treatment of breast cancer and to reduce the use of G-CSF during such treatment. Although the AHCC group in our study experienced more adverse events associated with γ-GTP than the control group, the overall data suggest that AHCC may reduce the toxicity of chemotherapy and may then allow for intensification of the chemotherapy dosage. A prospective trial is needed to study the effects of AHCC further, particularly our finding of a higher, although not significant, number of adverse events associated with γ-GTP in the AHCC group.
Acknowledgments
The authors thank Nagumo Clinic in Tokyo for providing the clinical data and conducting an ethical review of this study.
Disclosure Statement
No competing financial interests exist.
References
- 1.Henderson IC. Berry DA. Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 2.Martin M. Pienkowski T. Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 3.Jantunen IT. Kataja VV. Muhonen TT. An overview of randomised studies comparing 5-HT3 receptor antagonists to conventional anti-emetics in the prophylaxis of acute chemotherapy-induced vomiting. Eur J Cancer. 1997;33:66–74. doi: 10.1016/s0959-8049(96)00276-6. [DOI] [PubMed] [Google Scholar]
- 4.Ioannidis JP. Hesketh PJ. Lau J. Contribution of dexamethasone to control of chemotherapy-induced nausea and vomiting: a meta-analysis of randomized evidence. J Clin Oncol. 2000;18:3409–3422. doi: 10.1200/JCO.2000.18.19.3409. [DOI] [PubMed] [Google Scholar]
- 5.Warr DG. Hesketh PJ. Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2005;23:2822–2830. doi: 10.1200/JCO.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 6.Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res. 2008;27:32. doi: 10.1186/1756-9966-27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browall M. Ahlberg K. Karlsson P, et al. Health-related quality of life during adjuvant treatment for breast cancer among postmenopausal women. Eur J Oncol Nurs. 2008;12:180–189. doi: 10.1016/j.ejon.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Spierings EL. Fujii H. Sun B. Walshe T. A Phase I study of the safety of the nutritional supplement, active hexose correlated compound, AHCC, in healthy volunteers. J Nutr Sci Vitaminol (Tokyo) 2007;53:536–539. doi: 10.3177/jnsv.53.536. [DOI] [PubMed] [Google Scholar]
- 9.Terakawa N. Matsui Y. Satoi S, et al. Immunological effect of active hexose correlated compound (AHCC) in healthy volunteers: a double-blind, placebo-controlled trial. Nutr Cancer. 2008;60:643–651. doi: 10.1080/01635580801993280. [DOI] [PubMed] [Google Scholar]
- 10.Shigama K. Nakaya A. Wakame K, et al. Alleviating effect of active hexose correlated compound (AHCC) for anticancer drug-induced side effects in non-tumor-bearing mice. J Exp Ther Oncol. 2009;8:43–51. [PubMed] [Google Scholar]
- 11.Hirose A. Sato E. Fujii H, et al. The influence of active hexose correlated compound (AHCC) on cisplatin-evoked chemotherapeutic and side effects in tumor-bearing mice. Toxicol Appl Pharmacol. 2007;222:152–158. doi: 10.1016/j.taap.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Sun B. Wakame K. Sato E, et al. The effect of active hexose correlated compound in modulating cytosine arabinoside-induced hair loss, and 6-mercaptopurine- and methotrexate-induced liver injury in rodents. Cancer Epidemiol. 2009;33:293–299. doi: 10.1016/j.canep.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Yin Z. Fujii H. Walshe T. Effects of active hexose correlated compound on frequency of CD4+ and CD8+ T cells producing interferon-γ and/or tumor necrosis factor-α in healthy adults. Hum Immunol. 2010;71:1187–1190. doi: 10.1016/j.humimm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Matsui Y. Uhara J. Satoi S, et al. Improved prognosis of postoperative hepatocellular carcinoma patients when treated with functional foods: a prospective cohort study. J Hepatol. 2002;37:78–86. doi: 10.1016/s0168-8278(02)00091-0. [DOI] [PubMed] [Google Scholar]
- 15.Shah SK. Walker PA. Moore-Olufemi SD, et al. An evidence-based review of a Lentinula edodes mushroom extract as complementary therapy in the surgical oncology patient. J Parenter Enteral Nutr. 2011;35:449–458. doi: 10.1177/0148607110380684. [DOI] [PubMed] [Google Scholar]
- 16.Sobin LH. Gospodarowicz MK. Wittekind C. 7th. New Jersey: Wiley Blackwell; 2009. TNM: Classification of Malignant Tumors. [Google Scholar]
- 17.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [Oct 26;2012 ]. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- 18.World Medical Association. World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. http://www.wma.net/en/30publications/10policies/b3/17c.pdf. [Oct 26;2012 ]. http://www.wma.net/en/30publications/10policies/b3/17c.pdf [DOI] [PubMed]
- 19.Lin H. She YH. Cassileth BR, et al. Maitake beta-glucan MD-fraction enhances bone marrow colony formation and reduces doxorubicin toxicity in vitro. Int Immunopharmacol. 2004;4:91–99. doi: 10.1016/j.intimp.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Ye SF. Wakame K. Ichimura K. Matsuzaki S. Amelioration by active hexose correlated compound of endocrine disturbances induced by oxidative stress in the rat. Endocr Regul. 2004;38:7–13. [PubMed] [Google Scholar]