Abstract
Significance
Proteases and their inhibitors contribute to the balance between extracellular matrix (ECM) degradation and deposition, creating an equilibrium that is essential for the timely and coordinated healing of cutaneous wounds. However, when this balance is disrupted, wounds are led into a state of chronicity characterized by abundant levels of proteases and decreased levels of protease inhibitors.
Recent Advances
Researchers have sought to investigate the roles of proteases within both acute and chronic wounds and how the manipulation of protease activity may aid healing. Indeed, numerous wound dressings have been developed that target such proteases in an attempt to promote wound healing.
Critical Issues
The normal tissue response to injury involves a complex interaction between cells and cellular mediators. In particular, the inflammatory response is augmented in chronic wounds which are characterized by elevated levels of proinflammatory cytokines and proteases. While controlling levels of inflammation and protease expression is a critical part of normal wound healing, elevated and prolonged expression of proteases produced during the inflammatory phase of healing can lead to excessive ECM degradation associated with impaired healing.
Future Directions
It seems plausible that future research should aim to investigate the ways in which proteases may be targeted as an alternative therapeutic approach to wound management and to assess the benefits and draw-backs of utilizing wound fluids to assess wound progression in terms of proteolytic activity.

Steven L. Percival, PhD
Scope and Significance
This review highlights the key roles of proteases in wound healing. It aims to provide readers with an overview of the control mechanisms of protease expression within the wound environment and the ways in which excessive protease expression and activation can lead to a delay in the healing process of cutaneous wounds. The control of proteases within the wound milieu via the administration of novel wound dressings is one particular recent focal point of wound management.
Translational Relevance
Acute wounds normally heal in a sequenced and timely manner, characterized by four major phases (namely, hemostasis, inflammation, proliferation, and remodeling). Comparatively, chronic wounds remain in one particular stage of healing (usually the inflammatory phase), which disrupts the normal balance between deposition and degradation of extracellular matrix (ECM) components. The degradation and remodeling of the ECM by proteases, particularly the matrix metalloproteinases (MMPs), is a key element of tissue repair and plays a role in various wound-healing mechanisms.
Clinical Relevance
While controlled expression of MMPs is a critical part of normal wound healing, elevated and prolonged expression can lead to excessive ECM degradation associated with impaired healing. Variations in the proteolytic activity between acute and chronic wounds have been highlighted by a number of investigators; the levels and activity of proteases within the wound milieu are important in establishing the status of a wound and its progression toward healing. Wound dressings aimed at sequestering host proteases within the wound milieu is one particular area of interest as an alternative wound management strategy.
Discussion of Findings and Relevant Literature
Background
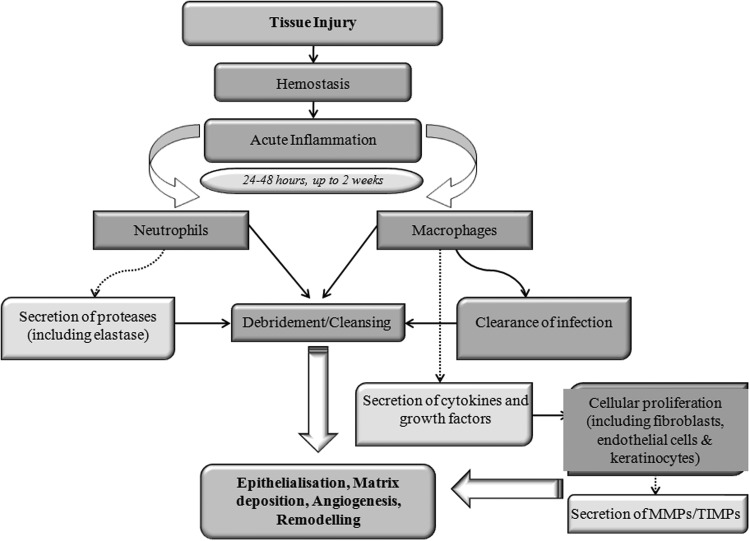
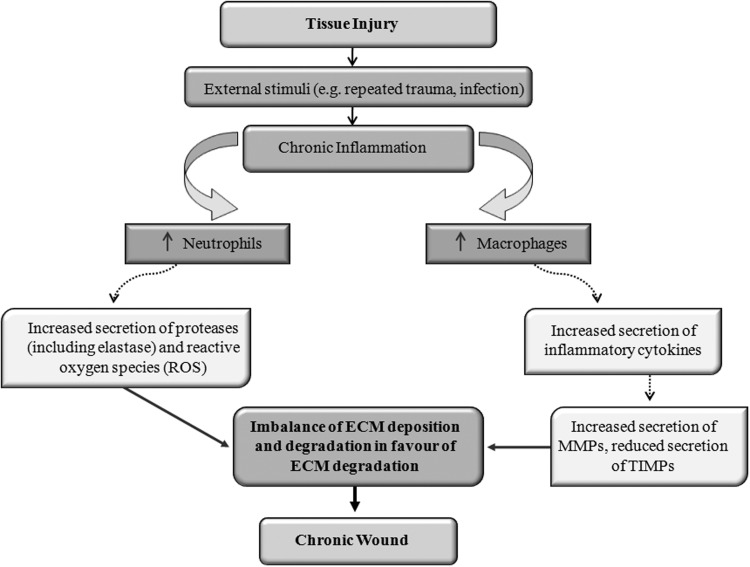
The normal tissue response to injury and the resulting wound-healing process involves an orderly sequence of important pathways that are orchestrated via the movement of specialized cells and secretion of signaling molecules.1 The end point of normal wound healing is the reformation of skin integrity through epithelialization and remodeling, resulting in the production of functional scar tissue (Fig. 1). While acute wounds normally heal in a sequenced and timely manner, characterized by four major phases (namely, hemostasis, inflammation, proliferation, and remodeling), chronic wounds remain in one particular stage of healing (usually the inflammatory phase), disrupting the normal balance between deposition and degradation of ECM components (Fig. 2).1
Figure 1.
Stages involved in normal cutaneous wound repair. Following initial tissue injury, hemostasis commences, the end product of which is fibrin clot formation. Inflammation then proceeds, leading to the debridement and cleansing of the wound area before cellular proliferation and migration. Cytokines and growth factors play a pivotal role in the wound-healing process, mediating cellular signaling, and healing pathways. Macrophages and neutrophils act to debride and cleanse the wound area via the clearance of contaminating microorganisms and foreign material. Proteases, including matrix metalloproteinases (MMPs) are secreted, which aid in the remodeling of the extracellular matrix (ECM). A balance between ECM degradation and deposition is maintained via the secretion of both MMPs and tissue inhibitors of metalloproteinases (TIMPs).
Figure 2.
Stages involved in the development of a chronic cutaneous wound. Following the initial tissue injury, external stimuli, including bacterial contamination and infection cause a prolonged and augmented inflammatory response. This in turn leads to an elevated level of inflammatory cytokines and proteases. An imbalance in the deposition and degradation of the ECM is created, leading the wound into a state of chronicity.
The degradation and remodeling of the ECM by proteases, particularly the MMPs, is a key element of tissue repair and plays a role in the influx of leukocytes, angiogenesis and re-epithelialization (Fig. 3).2 MMPs, which are expressed by a number of cell types during different phases of healing (including inflammatory cells, fibroblasts, endothelial cells, and keratinocytes) are a family of zinc endopeptidases (part of the metzincin superfamily) capable of degrading the components of the ECM and facilitate many of the pathways leading to the regeneration of injured tissues, including the clearance of damaged protein and destruction of the provisional matrix, facilitation of cellular migration to the wound area and granulation tissue formation.3–7 MMPs not only act in the direct remodeling of ECM components, but also degrade growth factors and their receptors as well as angiogenic factors and ultimately influence cellular behavior.2 While controlled expression of MMPs is a critical part of normal wound healing, elevated and prolonged expression disrupts the balance between tissue breakdown and repair, leading to excessive ECM degradation associated with impaired healing.2,7 Indeed, chronic wounds display ECM deficits and growth factor abnormalities.2 A number of stimuli prolong elevated protease levels, including the presence of bacteria and biofilms, damaged tissue and foreign material.8
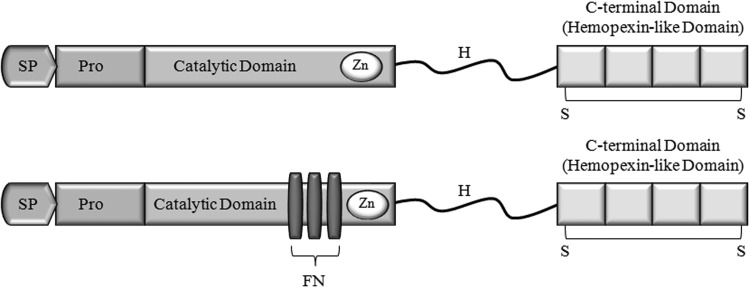
Figure 3.
Simple domain structure of the MMPs important in cutaneous wound healing. The upper image represents the simple hemopexin domain-containing MMPs (including collagenases and stromeolysins). The lower image represents the gelatinases, MMP-2 and MMP-9. MMPs consist of a signal peptide (SP), a propeptide (light gray), which prevent enzyme activity, a catalytic domain, which contains the zinc binding site (dark gray), and a hemopexin-like c-terminal domain, which is made up of 4 repeat units joined by a disulfide bridge. The hemopexin-like c-terminal domain and the catalytic domain are connected by a hinge region (H). The gelatinases (MMP-2 and MMP-9) have an insert in the catalytic domain consisting of three fibronectin type II repeats (FN).
Other proteases involved in wound healing include the serine proteases expressed by neutrophils, including elastase, cathepsin G, and urokinase-type plasminogen activator (uPA).9–11 Plasminogen also has a key role in the healing of wounds, particularly in the re-epithelialization process.12 As highlighted by Wlaschek et al.,13 the serine proteases can degrade growth factors, thus reducing their bioactivity; platelet-derived growth factor-AB degradation was abolished and its bioactivity was maintained following incubation with chronic wound fluid plus a serine protease inhibitor. Similarly, degradation of growth factors following incubation with chronic wound fluids was noted by Yager et al., 14 yet this was inhibited by the addition of broad-spectrum serine proteinase inhibitors and specific inhibitors of neutrophil elastase.
Relevant basic science
Research has shown that molecular differences exist between acute and chronic wound microenvironments, including differences in the levels of proinflammatory cytokines and growth factors11 and matrix components. Hyaluronic acid (HA), for example, is a major component of early granulation tissue and its degradation products seem to be proactive in wound healing.15,16 There is also variation in the levels of HA between fetal and adult wound healing, with persistently higher levels of HA present in fetal wounds, which ultimately heal without scarring.15,17
The formation of a scar is a natural phenomenon that occurs following normal wound healing. However, for a wound to progress to a fibrous scar, a complex interaction between proinflammatory cytokines, growth factors, proteases and their inhibitors and ECM components must occur, which regulates the cellular activity within the wound and its ultimate closure.18
In terms of the proteolytic environment of chronic wounds, the prolonged exposure of the wound tissues to proinflammatory cytokines may act to stimulate the production of MMPs, while inhibiting the synthesis of tissue inhibitors of metalloproteinases (TIMPs).11 While interleukin (IL)-1 and tumor necrosis factor (TNF)-α enhance collagenase secretion, chronic exposure of skin cells to these cytokines is a contributing factor in connective tissue disease.19
A number of researchers have sought to investigate the roles of proinflammatory and anti-inflammatory cytokines in the activation or suppression of proteases within the wound environment. The deposition of connective tissue during the wound-healing process can be affected by TNF-α, since this cytokine influences the synthesis of collagen, MMPs, and TIMPs.18 The differences in levels of the proinflammatory cytokines in wound fluids between acute and chronic human wounds were highlighted by Tarnuzzer and Schultz20; levels of IL-1β and TNF-α were approximately 100-fold higher in chronic wound fluids when compared to levels in mastectomy fluids, indicating an imbalance of proinflammatory cytokines within the chronic wound. Additionally, Ito et al.21 found that at a low concentration, TNF increased TIMP production by human fibroblasts, whereas at higher concentrations this increased synthesis was hampered in a dose-dependent manner. Collagenase and stromeolysin production was also stimulated by TNF. The results therefore suggested that TNF acted to modulate ECM degradation via the modulation of MMP and TIMP production. IL-1α, another inflammatory cytokine, on the other hand, induced both MMP and TIMP production.21 These results, however, varied depending on the source of the fibroblasts used and the concentration of cytokines, indicating a more complex effect on protease modulation and regulation. Barone et al.22 compared the levels of MMP-1 and IL-1α in both chronic and acute wound fluids. They found that clinical healing of the chronic wounds correlated with a decrease in IL-1α and collagenase activities.22 Similarly, Vincenti et al.23 used rabbit synovial fibroblasts to examine the molecular mechanisms of IL-1β-mediated collagenase gene expression and found that stimulation of these cells with recombinant human IL-1β resulted in an increase in collagenase mRNA expression in an in vitro culture model, while increasing collagenase transcript stability. It has also been shown that the NF-kB pathway (which is activated by IL-1 and TNF-α) is required for the activation of collagenase-1 (MMP-1) transcription in rabbit synovial fibroblasts.19 The monocyte chemoattractant protein-1 has also been shown to upregulate MMP-1 and MMP-2 mRNA expression in human skin fibroblasts via the endogenous production of transforming growth factor-β; a parallel induction of IL-1 α mRNA expression in dermal fibroblasts was also noted.24 However, TIMP-1 mRNA expression also increased alongside the elevated MMP expression. In contrast, Silvestre et al.25 found that IL-10, an anti-inflammatory cytokine, affects MMP activity; IL-10−/− mice demonstrated increased MMP activity, which in turn mediates an increase in ischemia-induced angiogenesis via the vascular endothelial growth factor. Collectively, these data sets highlight the complex nature of MMP regulation and modulation within the wound environment and emphasize the need for continued investigation into the therapeutic inhibition of MMPs and the indirect pathways that lead to their activation.
MMPs play a pivotal role in normal wound healing via the degradation of various ECM components, which facilitates the migration of cells and remodeling of the wound.3,26 Protease activity is essential for cutaneous wound healing, as demonstrated by the retarded wound healing seen in mice deficient in plasminogen, the serine protease precursor, and mice treated with a metalloprotease inhibitor.9 Neutrophil elastase has also demonstrated antimicrobial properties against a range of wound-associated bacteria.27 It is when the balance between ECM degradation and deposition is disrupted, however, in part, due to a disruption in the equilibrium of the production or activation of proteases and their respective inhibitors that wounds become chronic. Chronic wounds are maintained in a state of persistent inflammation, characterized by abundant levels of proteases and neutrophils, which themselves secrete proteases (including collagenase and elastase), which further augment connective tissue breakdown.28 It is important, therefore, to assess and control the level of inflammation in chronic wounds to prevent further tissue destruction.
A number of cell types contribute to the proteolytic environment of chronic wounds. Keratinocytes, which reside at the wound edge, along with fibroblasts and endothelial cells all coordinate the progressive breakdown of ECM components via the production of different protease classes within the chronic wound, yet the invading neutrophils and macrophages are considered the major source of such enzymatic activity.28 In addition to the MMPs present within the chronic wound environment, a number of serine proteinases are present at the wound site, including cathepsin G, uPA, and neutrophil elastase; the proteolytic wound environment has demonstrated significance in the degradation of important matrix components and growth factors.14,28–32 The influx of cells involved in wound healing combined with the increase in proinflammatory cytokines, which in turn induce MMP expression and downregulate TIMP expression, collectively cause an environment consistent with an excess of MMP activity.
Clinical and scientific evidence
The variations in proteolytic activity between acute and chronic wounds have been addressed by a number of investigators. In 1999, Trengove11 and team explored these variations in acute surgical and chronic wounds of varying aetiology using substrate assays and gelatin zymography. Results indicated a clear increase in MMP activity within chronic wound fluids as compared to acute wound fluids; elevation in the levels of the gelatinases MMP-9 and MMP-2 were observed in chronic wound fluids with a paralleled higher degradation of the epidermal growth factor in chronic wound fluid samples. Furthermore, the elevated levels of MMP activity decreased as healing occurred. This work corroborated previous investigation by Yager et al.;33 both MMP-9 and MMP-2 were found to be elevated in fluids from pressure ulcers as compared to those levels found in fluids from healing acute surgical wounds. Levels of total and active collagenase were greater in the nonhealing wound fluids.33 While the classification and substrate specificity of MMP-2 and MMP-9 is a subject of debate, these two MMPs are considered as particularly important factors in the remodeling and re-epithelialization of wounds.34,35 Wound fluid samples collected from elective surgery patients were used to detect levels of MMPs via gelatin zymography and quenched fluorescent substrate hydrolysis, and MMP and TIMPs protein levels were determined via ELISAs in a study by Baker and Leaper.36 The profiles of proteinases, their inhibitors, and relevant cytokines were analyzed; differences in proteinase and inhibitor expression were found to exist among acute surgical wound fluid samples; however, the total MMP activity did not vary significantly between samples. Inflammatory cytokines and growth factors also varied in expression between acute wound types, highlighting differences not only between acute and chronic wound environments, but also between wound sites.36 An augmentation of MMP-9 within chronic wound fluid proportionate to acute wound fluid has also been linked with a poor clinical status in terms of ulcer healing34; again, gelatin zymography, along with MMP inhibitor studies and ELISAs were used in this particular study to identify an elevated proteolytic activity in chronic wound fluid compared with acute wound fluid and human serum. Compared with minimal levels of MMP-1, -8, and -13, MMP-9 was found to be the most prominent MMP in chronic wound fluid. However, as mentioned in previous studies, the heterogeneity of wound fluid samples obtained from varied sources should be considered in such studies. In comparison, MMP-8 has been shown to be an important collagenase in the nonhealing wound; Nwomeh et al.37 demonstrated an elevated level of active MMP-8 in chronic wound fluids compared with acute wound fluids. However, these levels of enzyme activity varied between wound fluid samples collected from different chronic wounds.37 While wound fluids provide researchers with a useful way of measuring various markers of wound progression, deviations in wound care protocols and sampling techniques all contribute to further variation in results.34,38 The detection of high protease activity, however, is considered to be the best available biochemical marker of wound progression toward healing for both acute and chronic wounds.8
Not only are the levels of proteases important in establishing the status of a wound and its progression toward healing, but also the activity of these enzymes must be considered. Ladwig et al.39 reported on assays of pro- and activated MMP-2 and -9 and TIMP-1 and -2 in fluids and biopsies of 56 pressure ulcer patients and correlated their findings with progression of the ulcers toward healing; overall, a decrease in the MMP/TIMP ratio was associated with healing. The proteolytic activity in wound fluid derived from chronic venous leg ulcers has also been explored. Expression of uPA was detected in the active forms in chronic wound fluid, but was found to be present in an inhibitor complex in wound fluids from healing wounds. Furthermore, MMP-9 overexpression in chronic wound fluid was noted in parallel to the presence of uPA in the active forms, compared with blood-derived sera and acute wound fluid, suggesting a proteolytic cascade.40 Neely et al.41 used an animal model of impaired wound healing to compare levels of latent and active MMP-2 and MMP-9 in wound samples; full-thickness excisional wounds in genetically diabetic healing impaired mice and nondiabetic, nonhealing impaired littermates were analyzed for 25 days postwounding for gelatinase activity. Results indicated a general increase in gelatinase activity postwounding. However, both latent and active MMP-2 and MMP-9 levels were augmented in healing impaired wounds as compared to the nonhealing impaired wounds. Lobmann et al.42 utilized a human diabetic wound model to study the differences in the MMP activity between acute and chronic wound biopsies. Concentrations of MMP-1, -2, -8, -9, and -14 were increased in diabetic foot ulcer biopsies as compared to traumatic wound biopsies. In addition, the TIMP-2 concentration was reduced in diabetic wounds compared with nondiabetic wounds, further enhancing the proteolytic environment of chronic nonhealing wounds.
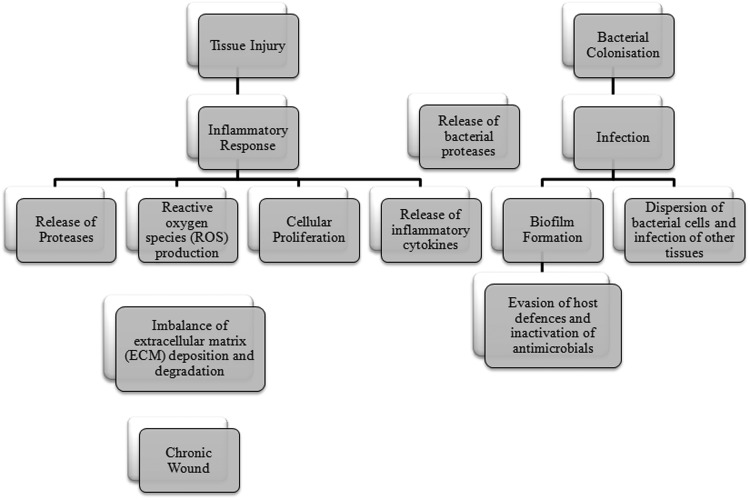
The extent to which bacterial proteases contribute to the overall poteolytic activity in chronic wounds and how these proteases could be targeted as a therapeutic option is a topic of more recent investigation. Chronic wounds are commonly colonized by invading bacteria, causing further perturbation of healing via the enhancement of the prolonged inflammatory response and endotoxin production; as summarized by Enoch and Price,43 chronic wounds are often subjected to invasive infection from multiple bacterial species, which often form biofilms within the wound milieu. The continued presence of bacteria within the wound leads to a further prolonged inflammatory response causing further increases in proinflammatory mediators, which can act to upregulate host protease expression (see Fig. 4). Bacteria may also contribute to the proteolytic nature of chronic wounds via the production of their own proteases, which in turn may enhance the host protease activity.44,45
Figure 4.
Effects of bacterial colonization and infection on cutaneous wound healing. Invading bacteria release proteases, which augment the host's inflammatory response, in turn contributing to the proteolytic nature of a chronic wound.
Many pathogenic bacteria secrete a range of proteases of the serine, cysteine, and metallo type.46,47 These proteases act as virulence factors and facilitate colonization, evasion of host immune defenses and dissemination of bacteria to other parts of the infected tissues and also attainment of nutrients via tissue degradation.46 As reviewed by Miyoshi and Shinoda,48 exogenous proteases particularly derived from opportunistic pathogens can disrupt the host's proteinase−proteinase inhibitor systems thus disturbing the balance of generation and degradation of various important host components. The generation of necrotic or hemorrhagic tissue damage via the digestion of host structural components at the site of infection, enhanced vascular permeability, generation of inflammatory mediators, and resulting dissemination of bacteria systemically are all possible outcomes of bacterial infection at the wound site as a result of bacterial protease production.48 The development of bacterial protease inhibitors as a therapeutic alternative to traditional antibiotics has been suggested as a future target of wound infection.46,47
Clinical perspective and innovation
In terms of the clinical impact of chronic infections and elevated protease levels within the wound milieu, the diabetic foot ulcer is a prime example of the consequences of such imbalances in the chronic wound environment. Indeed, the diabetic foot syndrome represents a major problem in the health care of diabetic patients, often leading to amputation.5 Local therapy with protease inhibitors has been shown to aid healing of chronic wounds. The treatment of diabetic foot ulcers with doxycycline, an antibiotic that is also a competitive inhibitor of MMPs and can also act to reduce inflammation,5 has been shown to aid healing via the sequestration of proteases, including elastase and MMPs.49
In recent years, the development of wound dressings aimed at sequestering host proteases within the wound milieu has emerged. In particular, the regulation of MMPs in the chronic wound is a concept central to a number of modern wound dressings. Such dressings range from products containing a protease substrate used to hamper excess protease levels, to products containing components that act to directly inhibit proteases within the wound environment.
For example, the use of wound dressings that incorporate a collagen and oxidized regenerated cellulose (ORC) matrix, which act to bind and inactivate MMPs has been shown to stimulate wound repair. The collagen in these dressings acts as a sacrificial substrate for the proteases within the wound area, while the ORC with multiple negative charges counteracts the positive charges associated with the metal ions of MMPs, destroying the three-dimensional structure of these proteases.50 Indeed, Cullen et al.51 demonstrated a reduction in neutrophil-derived elastase, plasmin, and MMPs when this type of dressing was added to human chronic wound fluid derived from diabetic foot ulcers in an ex vivo model, compared to wet gauze. Similarly, Veves et al.,52 noted the potential of this dressing type for use in chronic wounds; the authors highlighted the benefits of a combination of collagen and ORC in terms of protease inhibition without affecting the growth factor activity. The result of their study indicated the benefits of the test dressing when used in the early stages (diabetic foot ulcers less than 6 months old) of ulcer development. Smeets et al.53 found that venous leg ulcers treated with an ORC/collagen matrix demonstrated a reduction in the levels of elastase, plasmin, and gelatinase activity compared with venous ulcers treated with a hydrocolloid dressing, as measured by the protease activities within the wound fluid. Comparatively, human neutrophil elastase has been targeted with oleic acid/albumin formulations.54,55 Another example of a dressing type that acts to control the microenvironment of the wound incorporates a healing accelerator, nano-oligosaccharide factor, which has antiprotease properties and is released from the dressing upon contact with chronic wound fluid; this type of dressing aims to sequester the protease activity within the nonhealing wound.56 Alternatively, Adhirajan et al.57 demonstrated the potential of modified gelatin microspheres with a catechol MMP inhibitor combined with antimicrobials incorporated into a collagen scaffold to combine the benefits of infection control and attenuation of proteases in diabetic wound tissue lysates.
Silver dressings have also been used as an alternative method of sequestering proteases within the wound, while also maintaining infection control; while the exact mechanism of action of silver in terms of protease inactivation is not yet fully understood, it is postulated that silver may act to displace zinc from MMPs.58 In vitro studies have shown that silver is effective in reducing MMP-2 and MMP-9 concentrations.58 Wright et al.59 explored the effects of nanocrystalline silver-coated wound dressings on contaminated full-thickness wounds using a porcine model in terms of infection control and protease sequestration. Results of this study revealed that this dressing effectively reduced levels of MMPs and decreased the time to develop a full granulation bed compared with control wounds.
Ratios of proteases and their inhibitors have also been used as predictive markers of a wound's progression toward healing. Indeed, Muller et al.7 described how the MMP-1/TIMP-1 ratio could be used as an indicator of wound healing in diabetic foot ulcers. Schultz and Gibson60 also noted that wound fluids from different wound types may display different patterns of proteases, inhibitors, and cytokines that may reflect differences in healing progression, and so MMP/TIMP expression and activation may prove useful biomarkers of wound status.
Take-Home Messages.
Chronic wounds remain in one particular stage of healing (usually the inflammatory phase), which disrupts the normal balance between deposition and degradation of ECM components
The degradation and remodeling of the ECM by proteases, particularly MMPs, is a key element of tissue repair
Molecular differences exist between acute and chronic wound microenvironments, including differences in the levels of proteases, proinflammatory cytokines, growth factors, and matrix components
Prolonged exposure of the wound tissues to proinflammatory cytokines may act to stimulate the production of MMPs, while inhibiting the synthesis of TIMPs
Many pathogenic bacteria secrete a range of proteases, of the serine, cysteine, and metallo type that acts as virulence factors
Wound fluids provide researchers with a useful way of measuring various markers of wound progression; however, deviations in wound care protocols and sampling techniques contribute to variation in results
Abbreviations and Acronyms
- ECM
extracellular matrix
- ELISA
enzyme-linked immunosorbent assay
- HA
hyaluronic acid
- IL
interleukin
- MMP
matrix metalloproteinase
- ORC
oxidized regenerated cellulose
- TIMP
tissue inhibitor of metalloproteinase
- TNF
tumor necrosis factor
- uPA
urokinase-type plasminogen activator
Acknowledgments and Funding Sources
No funding sources were obtained for this review article.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Sara McCarty gained her BSc in Biomedical Sciences in 2008 from the University of Chester, United Kingdom. Since then, Sara has undertaken positions as a research technician and enjoyed gaining experience within the field of dermatology. She is currently completing an MPhil in the role of proteases in wound healing at the University of Liverpool, United Kingdom. Prof. Steven Percival holds a PhD in medical microbiology and biofilms, a BSc in Applied Biological Sciences, Postgraduate Certificate in Education, diploma in Business Administration, an MSc in Public Health and an MSc in Medical and Molecular Microbiology. He is also a fellow of the Institute of Biomedical Science. Early in his career, Steven held R&D positions at The British Textile Technology Group PLC, followed then by 6 years as a senior university lecturer in medical microbiology and later the positions of Director of Research and Development and Chief Scientific Officer at Aseptica, Inc. and senior clinical fellowships at the Centers for Disease Control, Atlanta, and Leeds Teaching Hospitals Trust, Leeds, United Kingdom. More recently, Steven held senior R&D positions at Bristol Myers Squibb, Advanced Medical Solutions PLC and held an honorary Professorship at West Virginia University. In 2011, Steven joined Scapa Healthcare PLC as Vice President of Global Healthcare R&D. He has written over 250 scientific publications and conference abstracts on biofilms, antimicrobials, and infection control and has authored and edited six and biofilms textbooks on microbiology.
References
- 1.Diegelmann RF. Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 2.Schultz GS. Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DG. Jude EB. The role of matrix metalloproteinases in wound healing. J Am Podiatr Med Assoc. 2002;92:12. doi: 10.7547/87507315-92-1-12. [DOI] [PubMed] [Google Scholar]
- 4.Heissig B. Hattori K. Friedrich M. Rafii S. Werb Z. Angiogenesis: vascular remodelling of the extracellular matrix involves metalloproteinases. Curr Opin Hematol. 2003;10:136. doi: 10.1097/00062752-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Lobmann R. Schultz G. Lehnert H. Proteases and the diabetic foot syndrome: mechanisms and therapeutic implications. Diabetes Care. 2005;28:461. doi: 10.2337/diacare.28.2.461. [DOI] [PubMed] [Google Scholar]
- 6.Mott JD. Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller M. Trocme C. Lardy B. Morel F. Halimi S. Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet Med. 2008;25:419. doi: 10.1111/j.1464-5491.2008.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harding K. Armstrong DG. Barrett S. Kaufman H. Lázaro-Martinez JL. Mayer D. Moore Z. Queen D. Romanelli M. Schultz G. Serena T. Sibbald G. Snyder R. Strohal R. Vowden K. Vowden P. Zamboni P. London: Wounds International; 2011. International consensus. The role of proteases in wound diagnostics. An expert working group review. [Google Scholar]
- 9.Lund LR. Romer J. Bugge TH. Nielsen BS. Frandsen TL. Degen JL. Stephens RW. Danø K. Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO J. 1999;18:4645. doi: 10.1093/emboj/18.17.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yager DR. Nwomeh BC. The proteolytic environment of chronic wounds. Wound Repair Regen. 1999;7:433. doi: 10.1046/j.1524-475x.1999.00433.x. [DOI] [PubMed] [Google Scholar]
- 11.Trengove NJ. Stacey MC. Macauley S. Bennett N. Gibson J. Burslem F. Murphy G. Schultz G. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7:442. doi: 10.1046/j.1524-475x.1999.00442.x. [DOI] [PubMed] [Google Scholar]
- 12.Romer J. Bugge TH. Pyke C. Lund LR. Flick MJ. Degen JL. Dano K. Impaired wound healing in mice with a disrupted plasminogen gene. Nat Med. 1996;2:287. doi: 10.1038/nm0396-287. [DOI] [PubMed] [Google Scholar]
- 13.Wlaschek M. Peus D. Achterberg V. Meyer-Ingold W. Scharffetter-Kochanek K. Protease inhibitors protect growth factor activity in chronic wounds. Br J Dermatol. 1997;137:646. doi: 10.1111/j.1365-2133.1997.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 14.Yager DR. Chen SM. Ward SI. Olutoye OO. Diegelmann RF. Cohen K. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen. 1997;5:23. doi: 10.1046/j.1524-475X.1997.50108.x. [DOI] [PubMed] [Google Scholar]
- 15.Gailit J. Clark RAF. Wound repair in the context of extracellular matrix. Curr Opin Cell Biol. 1994;6:717. doi: 10.1016/0955-0674(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 16.Price RD. Myers S. Leigh IM. Navsaria HA. The role of hyaluronic acid in wound healing. Am J Clin Dermatol. 2005;6:393. doi: 10.2165/00128071-200506060-00006. [DOI] [PubMed] [Google Scholar]
- 17.Mast BA. Flood LC. Haynes JH. Depalma RL. Cohen IK. Diegelmann RF. Krummel TM. Hyaluronic acid is a major component of the matrix of fetal rabbit skin and wounds: implications for healing by regeneration. Matrix. 1991;11:63. doi: 10.1016/s0934-8832(11)80228-3. [DOI] [PubMed] [Google Scholar]
- 18.Mast BA. Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen. 1996;4:411. doi: 10.1046/j.1524-475X.1996.40404.x. [DOI] [PubMed] [Google Scholar]
- 19.Barchowsky A. Frleta D. Vincenti MP. Integration of the NF-kappaB and mitogen-activated protein kinase/AP-1 pathways at the collagenase-1 promoter: divergence of IL-1 and TNF-dependent signal transduction in rabbit primary synovial fibroblasts. Cytokine. 2000;12:1469. doi: 10.1006/cyto.2000.0743. [DOI] [PubMed] [Google Scholar]
- 20.Tarnuzzer RW. Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996;4:321. doi: 10.1046/j.1524-475X.1996.40307.x. [DOI] [PubMed] [Google Scholar]
- 21.Ito A. Sato T. Iga T. Mori Y. Tumor necrosis factor bifunctionally regulates matrix metalloproteinases and tissue inhibitor of metalloproteinases (TIMP) production by human fibroblasts. FEBS Lett. 1990;269:93. doi: 10.1016/0014-5793(90)81127-a. [DOI] [PubMed] [Google Scholar]
- 22.Barone EJ. Yager DR. Pozez AL. Olutoye OO. Crossland MC. Diegelmann RF. Cohen IK. Interleukin-1alpha and collagenase activity are elevated in chronic wounds. Plast Reconstr Surg. 1998;102:1023. [PubMed] [Google Scholar]
- 23.Vincenti MP. Coon CI. Lee O. Brinckerhoff CE. Regulation of collagenase gene expression by IL-1β requires thranscriptional and post-transcriptional mechanisms. Nucleic Acids Res. 1994;22:4818. doi: 10.1093/nar/22.22.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto T. Eckes B. Mauch C. Hartmann K. Krieg T. Monocyte chemoattractant protein-1 enhances gene expression and synthesis of matrix metalloproteinase-1 in human fibroblasts by an autocrine IL-1α loop. J Immunol. 2000;164:6174. doi: 10.4049/jimmunol.164.12.6174. [DOI] [PubMed] [Google Scholar]
- 25.Silvestre JS. Mallat Z. Tamarat R. Duriez M. Tedgui A. Levy BI. Regulation of matrix metalloproteinase activity in ischemic tissue by interleukin-10. Circ Res. 2001;89:259. doi: 10.1161/hh1501.094269. [DOI] [PubMed] [Google Scholar]
- 26.McCarty SM. Cochrane CA. Clegg PD. Percival SL. The role of endogenous and exogenous enzymes in chronic wounds: a focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair Regen. 2012;20:125. doi: 10.1111/j.1524-475X.2012.00763.x. [DOI] [PubMed] [Google Scholar]
- 27.Cole AM. Shi J. Ceccarelli A. Kim YH. Park A. Ganz T. Inhibition of neutrophil elastase prevents cathelicidin activation and impairs clearance of bacteria from wounds. Blood. 2001;97:297. doi: 10.1182/blood.v97.1.297. [DOI] [PubMed] [Google Scholar]
- 28.Eming SA. Krieg T. Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 29.Grinnell F. Ho CH. Wysocki A. Degradation of fibronectin and vitronectin in chronic wound fluid: analysis by cell blotting, immunoblotting, and cell adhesion assays. J Invest Dermatol. 1992;98:410. doi: 10.1111/1523-1747.ep12499839. [DOI] [PubMed] [Google Scholar]
- 30.Grinnell F. Zhu M. Fibronectin degradation in chronic wounds depends on the relative levels of elastase, α1-proteinase inhibitor, and α2-macroglobulin. J Invest Dermatol. 1996;106:335. doi: 10.1111/1523-1747.ep12342990. [DOI] [PubMed] [Google Scholar]
- 31.Lauer G. Sollberg S. Cole M. Flamme I. Stürzebecher J. Mann K. Krieg T. Eming SA. Expression and proteolysis of vascular endothelial growth factor is increased inn chronic wounds. J Invest Dermatol. 2000;115:12. doi: 10.1046/j.1523-1747.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- 32.Roth D. Piekarek M. Paulsson M. Christ H. Bloch W. Krieg T. Davidson JM. Eming SA. Plasmin modulates vascular endothelial growth factor-A-mediated angiogenesis during wound repair. Am J Pathol. 2006;168:670. doi: 10.2353/ajpath.2006.050372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yager DR. Zhang LY. Liang HX. Diegelmann RF. Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol. 1996;107:743. doi: 10.1111/1523-1747.ep12365637. [DOI] [PubMed] [Google Scholar]
- 34.Rayment EA. Upton Z. Shooter GK. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol. 2008;158:951. doi: 10.1111/j.1365-2133.2008.08462.x. [DOI] [PubMed] [Google Scholar]
- 35.Rowe RG. Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Baker EA. Leaper DJ. Proteinases, their inhibitors, and cytokine profiles in acute wound fluid. Wound Repair Regen. 2000;8:392. doi: 10.1111/j.1524-475x.2000.00392.x. [DOI] [PubMed] [Google Scholar]
- 37.Nwomeh BC. Liang HX. Cohen IK. Yager DR. MMP-8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res. 1999;81:189. doi: 10.1006/jsre.1998.5495. [DOI] [PubMed] [Google Scholar]
- 38.Staiano-Coico L. Higgins PJ. Schwartz SB. Zimm AJ. Goncalves J. Wound fluids: a reflection of the state of healing. Ostomy Wound Manage. 2000;46(Supp 1A):85S. [PubMed] [Google Scholar]
- 39.Ladwig GP. Robson MC. Liu R. Kuhn MA. Muir DF. Schultz GS. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen. 2002;10:26. doi: 10.1046/j.1524-475x.2002.10903.x. [DOI] [PubMed] [Google Scholar]
- 40.Wysocki A. Kusakabe AO. Chang S. Tuan TL. Temporal expression of urokinase plasminogen activator, plasminogen activator inhibitor and gelatinase-B in chronic wound fluid switches from a chronic to acute wound profile with progression to healing. Wound Repair Regen. 1999;7:154. doi: 10.1046/j.1524-475x.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- 41.Neely AN. Clendening CE. Gardner J. Greenhalgh DG. Gelatinase activities in wounds of healing-impaired mice versus wounds of non-healing-impaired mice. J Burn Care Rehabil. 2000;21:395. doi: 10.1097/00004630-200021050-00001. [DOI] [PubMed] [Google Scholar]
- 42.Lobmann R. Ambrosch A. Schultz G. Waldmann K. Schiweck S. Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002;45:1011. doi: 10.1007/s00125-002-0868-8. [DOI] [PubMed] [Google Scholar]
- 43.Enoch S. Price P. Cellular, molecular, biochemical differences in the pathophysiology of healing between acute wounds, chronic wounds, wounds in the aged. World Wide Wounds. 2004. Aug 13, www.worldwidewounds.com/2004/august/Enoch/Pathophysiology-Of-Healing.html. [Aug 11;2013 ]. www.worldwidewounds.com/2004/august/Enoch/Pathophysiology-Of-Healing.html [online]; available at.
- 44.Lantz MS. Are bacterial proteases important virulence factors? J Periodont Res. 1997;32:126. doi: 10.1111/j.1600-0765.1997.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 45.Sorsa T. Ingman T. Suomalainen K. Haapasalo M. Konttinen YT. Lindy O. Saari H. Uitto V. Identification of proteases from periodontopathogenic bacteria as activators of latent human neutrophil and fibroblast-type interstitial collagenases. Infect Immun. 1992;60:4491. doi: 10.1128/iai.60.11.4491-4495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Supuran CT. Scozzafava A. Mastrolorenzo A. Bacterial proteases: current therapeutic use and future prospects for the development of new antibiotics. Exp Opin Ther Patents. 2001;11:221. [Google Scholar]
- 47.Supuran CT. Scozzafava A. Clare BW. Bacterial protease inhibitors. Med Res Rev. 2002;22:329. doi: 10.1002/med.10007. [DOI] [PubMed] [Google Scholar]
- 48.Miyoshi S. Shinoda S. Bacterial metalloprotease as the toxic factor in infection. J Toxicol Toxin Rev. 1997;16:177. [Google Scholar]
- 49.Chin GA. Thigpin TG. Perrin KJ. Moldawer LL. Schultz GS. Treatment of chronic ulcers in diabetic patients with a topical metalloproteinase inhibitor, doxycycline. Wounds. 2003;15:315. [Google Scholar]
- 50.Ovington LG. Advances in wound dressings. Clin Dermatol. 2007;25:33. doi: 10.1016/j.clindermatol.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Cullen B. Smith R. Mcculloch E. Silcock D. Morrison L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen. 2002;10:16. doi: 10.1046/j.1524-475x.2002.10703.x. [DOI] [PubMed] [Google Scholar]
- 52.Veves A. Sheehan P. Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg. 2002;137:822. doi: 10.1001/archsurg.137.7.822. [DOI] [PubMed] [Google Scholar]
- 53.Smeets R. Ulrich D. Unglaub F. Woltje M. Pallua N. Effect of oxidised regenerated cellulose/collagen matrix on proteases in wound exudate of patients with chronic venous ulceration. Int Wound J. 2008;5:195. doi: 10.1111/j.1742-481X.2007.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edwards JV. Howley P. Cohen IK. In vitro inhibition of human neutrophil elastase by oleic acid albumin formulations from derivatized cotton wound dressings. Int J Pharm. 2004;284:1. doi: 10.1016/j.ijpharm.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Edwards JV. Howley P. Davis R. Mashchak A. Goheen SC. Protease inhibition by oleic acid transfer from chronic wound dressings to albumin. Int J Pharm. 2007;340:42. doi: 10.1016/j.ijpharm.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 56.Timmons J. Evaluating a new foam dressing with a healing accelerator. Wounds UK. 2010;6:88. [Google Scholar]
- 57.Adhirajan N. Shanmugasundaram N. Shanmuganathan S. Babu M. Functionally modified gelatin microspheres impregnated collagen scaffold as novel wound dressing to attenuate the proteases and bacterial growth. Eur J Pharm Sci. 2009;36:235. doi: 10.1016/j.ejps.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 58.Walker M. Bowler PG. Cochrane CA. In vitro studies to show sequestration of matrix metalloproteinases by silver-containing wound care products. Ostomy Wound Manage. 2007;53:18. [PubMed] [Google Scholar]
- 59.Wright JB. Lam K. Buret AG. Olson ME. Burrell RE. Early healing events in a porcine model of contaminated wounds: effects of nanocrystalline silver on matrix metalloproteinases, cell apoptosis, and healing. Wound Repair Regen. 2002;10:141. doi: 10.1046/j.1524-475x.2002.10308.x. [DOI] [PubMed] [Google Scholar]
- 60.Schultz GS. Gibson DJ. Measurement of biomarkers for impaired healing in fluids, tissues. In: Mani R, editor; Romanelli M, editor; Shukla V, editor. Measurements in Wound Healing. New York: Springer; 2013. pp. 243–258. [Google Scholar]