Abstract
A 5-week study was conducted to evaluate commercially available Artemia, Ziegler zebrafish diet, and Calamac diet fed in five different feeding regimes on the growth and reproductive development of 7-month-old zebrafish. Zebrafish were fed to satiation three times daily during the normal work week and twice daily during the weekend and holidays. Zebrafish in dietary groups CCC (Calamac three times daily) and CCA (Calamac twice daily, Artemia once daily) had a significantly (p<0.05) greater weight gain and specific growth rate as compared to all other dietary groups. Male zebrafish in dietary group 5 had significantly larger gonadosomatic index (GSI) values than all other groups, while female zebrafish in dietary group CCC had significantly larger GSI values than all other groups. No differences in the fatty acid content of female gonads were detected. Zebrafish fed solely Artemia had the greatest weight loss and lowest GSI values. Preliminary evidence of protein sparing in zebrafish is reported. Collectively, this study sheds more light into the effects of the use of commercially available feeds and feeding regime on the rearing of zebrafish.
Introduction
The zebrafish (Danio rerio) is an important animal model in genetic and developmental biology research,1 with new applications emerging frequently.2–4 As the use of this animal model advances into new areas of research, it becomes increasingly important to increase our understanding of this species biological requirements.5 An area of great importance is that of nutrition and its effects on reproductive development and performance.
Nutrition has been identified as an important factor in the reproductive performance and overall health of fish species.6 Very little published research has focused on elucidating the role of nutrition on zebrafish growth and reproduction, although there are an estimated 1000 publications annually with zebrafish as the animal model.7 Slowly, improvements in our understanding of zebrafish nutrition are materializing, with emphasis on fatty acids,8,9 purified diet formulation,7,10 and micronutrient needs.11,12 Meanwhile, a small number of studies have evaluated the effects of feeds on larval growth,7,13,14 juvenile growth, and early reproductive performance15 and adult reproductive performance.10,16,17 Yet, without a better understanding of the nutritional needs of zebrafish, the industry must utilize whatever feeds (formulated or live) are commercially available.
The feeds and feeding regimes implemented by laboratories for rearing zebrafish are varied.18 In some cases, feeds and feeding regimes are implemented without a formal evaluation.15 At the National Institutes of Child Health and Human Development (NICHD; National Institutes of Health, Bethesda, MD) Artemia is fed twice, while the Ziegler zebrafish pellet is fed once daily to adult zebrafish, although the rationale for this feeding protocol had never been demonstrated. In a 2-week pilot study, the feeding regime used at NICHD was compared to other commercially available diets that appeared to offer a ω-6/ω-3 fatty acid ratio that would be more favorable for fertilization rates and spawning performance in zebrafish adults.8,9 Zebrafish fed Calamac had comparable spawning rates, fertilization rates, and fecundity values as zebrafish fed the normal feeding regime for NICHD. These results suggested that Calamac, a squid-based diet formulated for rearing larval marine fish, warranted further evaluation.
Previous research has demonstrated the importance of dietary fatty acids, in particular, arachidonic acid (ARA) in the reproductive performance of zebrafish.7–9 The purpose of this study was to evaluate the Calamac diet, on its own and in combination with Artemia, as a possible replacement for the Ziegler diet in NICHD's feeding protocol for zebrafish, and its effects on the fatty acid composition of gonads and the resulting gonadosomatic index (GSI) of adult zebrafish.
Materials and Methods
Strain selection
The AB strain was used in this study due to its popularity in laboratories throughout the region, with particular emphasis in the Dawid laboratory. One thousand five hundred AB fish were generated from a large group, cross raised in mixed sex populations at a density of 10 fish per liter until the start of the study. Starting from 3 months old, these fish were spawned at least once every 3 weeks to ensure the reproductive performance before the study. The use of these animals in this study was approved by the Institutional Animal Care and Use Committee at the NICHD (IACUC animal study protocol 09-039).
Environmental parameters
The system used for this study included a 12.87 m3 recirculating aquaculture system (Aquaneering, San Diego, CA) utilizing a 0.71 m3 propeller washed bead filter for biomechanical filtration, a 6.37 m3 fluidized bed for biological filtration, and UV sterilization (90,000 μws/cm2). Dissolved oxygen (mg/L), conductivity, pH, and water temperature (°C) were monitored daily using a YSI6500 field panel (YSI, Inc., Yellow Springs, OH). Ammonia-N (mg/L), nitrite-N (mg/L), and nitrate-N (mg/L) were measured weekly using LaMotte test kits (LaMotte, Chestertown, MD). Ammonia-N, nitrite-N, nitrate-N, dissolve oxygen, water temperature, conductivity, and pH were 0.05±0.003, 0.03±0.004, 25.53±1.50, 7.48±0.12, 83.56±0.23, 1007.80±3.07, and 7.25±0.02 throughout the study, respectively. The diurnal cycle was maintained at 14 h light:10 h dark. The self-cleaning tanks used were 6L in capacity and contained a baffle at the back for solid removal. The flow rate maintained throughout the study was six turns per hour or 180 mL/min.
Feeds
Feeds evaluated in this study included Calamac (Aquafauna Bio-Marine, Inc., Hawthorne, CA), Ziegler zebrafish diet (Ziegler Brothers, Gardners, PA), and Artemia salina (San Francisco strain, Brine Shrimp Direct, Ogden, UT). Artemia nauplii were decapsulated and cultured for a 24-h period in a 10-L polycarbonate conical-bottom tank using reverse osmosis water and maintaining a salinity of 25 ppm. Aeration was provided to each tank via an air pump fitted with hard plastic tubing that was set at the bottom of the tank. Artemia nauplii were enriched during this period with Spirulina microfine powder (Argent Chemical Laboratories, Redmond, WA) as per the facility's standard operating procedure. Artemia nauppli were harvested from the bottom of the tank into a 170-μm sieve, rinsed with reverse osmosis water, and concentrated in a pitcher before morning and afternoon feedings. Aliquots of 100 mL of Artemia concentrate were collected before the remaining Artemia concentration was diluted for system-wide feeding. The concentrated aliquot of Artemia was used for study feedings. Samples of each diet were stored in nitrogen flushed containers at −80°C before being sent to the New Jersey Feed Labs (New Jersey Feed labs, Inc., Trenton, NJ) for crude proximate, amino and fatty acid analysis.
Feeding and experimental design
Before the beginning of the study, all fish were fed following NICHD's normal feeding protocol. At 7 months of age, 30 male (initial weight of 350.08±0.01 mg) and 30 female (initial weight of 550.90±0.02 mg) zebrafish were randomly placed in one of the fifteen 6-L self-cleaning tanks. Each tank was randomly assigned one of five feeding regimes (n=3) and fed to satiation three times daily (09:00, 11:30, and 15:00) during the work week, and twice daily during holidays and weekends (11:30 and 15:00), for a period of 5 weeks (Table 1). Satiation was defined as the point within a 5-min period, where zebrafish were no longer actively searching for food.15 The dietary group AAA was considered the control group, while dietary group AZA was included to evaluate the current feeding protocol at NICHD.
Table 1.
Feeding Schedule of Dietary Sources Artemia (A), Ziegler Zebrafish Diet (Z), and Calamac (C), and Combinations Fed to Adult AB Zebrafish
All feedings were fed to apparent satiation, defined as the period within 5 minutes, where zebrafish are no longer actively searching for food.
Represents weekend and holiday feeding for adults.
At the end of the fifth week, twelve male and female zebrafish from each tank were euthanized using a lethal dose of buffered tricaine methanosulfonate (Argent Chemical Laboratories), patted dry, weighed, dissected of gonads, and used to determine growth parameters. Gonads were pooled by gender and tank, stored at −80°C in containers flushed with nitrogen gas until analyzed for fatty acids. The remaining fish were set up to spawn three times in pair-wise spawning tanks using protocols previously described.15 Zebrafish were given a 1-week interspawning interval, during which time, zebrafish were fed according to their assigned feeding regime. After the third crossing, females that did not spawn were gently squeezed to ensure that females were not egg bound and that eggs were of good quality, as previously described.15 All fish were fed for one more day before being euthanized. Gonads were dissected and pooled with samples previously collected. Pooled samples were sent to New Jersey Feed Labs for fatty acid analysis. Only female gonads were analyzed due to mass limitations of male gonad tissue samples.
Weight gain (WG) was calculated using the following equation:
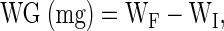 |
where WF and WI represent the final and initial weight gain, respectively. The specific growth rate (SGR) was determined using the following equation:
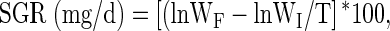 |
where T represents the length of the study in days. The gender weight ratio (GWR), defined as the ratio between the weight gain of female zebrafish (WGF) compared to male zebrafish (WGM; Gonzales, 2012), was determined using the following calculation:
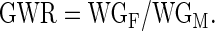 |
Gonadosomatic index (GSI;%) was calculated using the following equation:
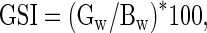 |
where Gw represents gonadal weight and Bw represents body weight.
Nutrient analysis
Feeds used in this study were subjected to proximate analysis.19 Feeds were analyzed for amino acids using AOAC methods 994.12, 985.28, and 988.15.19 Fatty acid profiles for feeds and female gonads were determined by extracting lipids using an acid hydrolysis method (AOAC 948.15).19 Fatty acid methyl esters were prepared with boron trifluoride in methanol (AOAC 969.33).19 Fatty acid methyl esters were separated and quantified using gas chromatography performed by a capillary column (AOAC 991.39).19
Data and statistical analysis
All data were analyzed as a completely randomized design with each tank acting as an experimental unit. All data were analyzed using one-way analysis of variance (ANOVA).20 The Student–Newman–Keuls test was used to separate means when significant differences were detected by ANOVA. An acceptable level of significance was 0.05. All statistical analyses were conducted using SPSS software (v.17.0; Chicago, IL). Tests for normality and constant variance were performed before all statistical analysis.
Results
Chemical compositions of feeds evaluated in this study were very similar in almost all categories analyzed with a few exceptions. Proximate analysis of diets showed that Artemia contained the greatest amount of crude fiber and the least amount of nitrogen-free energy (NFE). The Ziegler diet contained the most NFE, followed by the Calamac diet (Table 2). Crude protein and lipid values appeared similar between the three feeds. The amino acid profiles of the three feeds were very similar with the exception of arginine and glutamic acid (Table 3). In both cases, Artemia had less arginine and glutamic acid than both the Ziegler and Calamac diet.
Table 2.
Proximate Analysis of Diets Artemia (A), Ziegler Zebrafish Diet (Z), and Calamac (C) Fed to Adult AB Zebrafish (% Dry Matter Basis)
| A | Z | C | |
|---|---|---|---|
| Dry matter | 11.74 | 91.66 | 95.49 |
| Crude protein | 61.84 | 58.20 | 59.30 |
| Crude lipid | 16.95 | 14.91 | 17.10 |
| Crude fiber | 4.94 | 0.73 | 0.70 |
| Ash | 10.57 | 10.00 | 11.50 |
| NFE | 5.69 | 16.15 | 11.34 |
Table 3.
Amino Acid Profile of Diets Artemia (A), Ziegler Zebrafish Diet (Z), and Calamac (C) Fed to Adult AB Zebrafish (% Protein)
| Amino acid | A | Z | C |
|---|---|---|---|
| Methionine | 1.27 | 1.26 | 1.32 |
| Cystine | 0.68 | 0.72 | 0.64 |
| Lysine | 4.34 | 3.99 | 4.00 |
| Phenylalanine | 2.55 | 2.45 | 2.70 |
| Leucine | 3.57 | 4.52 | 4.78 |
| Isoleucine | 3.06 | 2.46 | 2.34 |
| Threonine | 2.47 | 2.42 | 2.33 |
| Valine | 3.23 | 2.80 | 2.71 |
| Histidine | 1.36 | 1.38 | 1.69 |
| Arginine | 1.27 | 3.67 | 3.41 |
| Glycine | 2.89 | 4.27 | 3.56 |
| Aspartic acid | 3.74 | 4.94 | 5.15 |
| Serine | 2.29 | 2.69 | 2.55 |
| Glutamic acid | 5.87 | 8.26 | 8.67 |
| Proline | 3.32 | 4.26 | 3.95 |
| Hyrdoxy proline | 0.00 | 0.74 | 0.54 |
| Alanine | 4.08 | 3.64 | 3.75 |
| Tyrosine | 2.47 | 1.93 | 2.06 |
| Trytophan | 0.76 | 0.48 | 0.52 |
The most recognizable differences in feed composition are seen in the fatty acid profile of the three diets (Table 4). Total saturates, total monoenes, linolenic, eicosapentaenoic (EPA), and docosahexaenoic (DHA) acid concentrations were greatest in the Calamac diet followed by the Ziegler diet and Artemia. Total ω-3 and ω-6 fatty acids were greatest in the Calamac diet, followed by the Ziegler diet and Artemia. Artemia contained the greatest concentration of linolenic acid, followed by the Calamac and Ziegler diets. Artemia also had the largest ω-3: ω-6 ratio among all diets, followed by the Ziegler and Calamac diets.
Table 4.
Fatty Acid Profile of Diets Artemia (A), Ziegler Zebrafish Diet (Z), and Calamac (C) Fed to Adult AB Zebrafish (% Lipid)
| Fatty acid | A | Z | C |
|---|---|---|---|
| Total saturates | 1.51 | 17.52 | 18.56 |
| Total monoenes | 2.37 | 22.49 | 21.50 |
| 16:2 | 0.00 | 0.39 | 0.23 |
| 16:3 | 0.00 | 0.30 | 0.26 |
| 16:4 | 0.06 | 0.24 | 0.45 |
| 18:2ω-6 | 0.54 | 6.54 | 13.61 |
| 18:2ω-4 | 0.00 | 0.10 | 0.10 |
| 18:3ω-6 | 0.05 | 0.10 | 0.10 |
| 18:3ω-3 | 3.36 | 0.93 | 1.72 |
| 18:4ω-3 | 0.65 | 0.93 | 1.17 |
| 20:2ω-6 | 0.01 | 0.17 | 0.11 |
| 20:3ω-6 | 0.04 | 0.08 | 0.00 |
| 20:3ω-3 | 0.15 | 0.10 | 0.04 |
| 20:4ω-6 | 0.04 | 0.53 | 0.42 |
| 20:4ω-3 | 0.13 | 0.44 | 0.33 |
| 20:5ω-3 | 0.08 | 3.99 | 5.42 |
| 21:5ω-3 | 0.00 | 0.17 | 0.24 |
| 22:4ω-6 | 0.00 | 0.17 | 0.24 |
| 22:5ω-6 | 0.00 | 0.17 | 0.15 |
| 22:5ω-3 | 0.00 | 0.78 | 0.76 |
| 22:6ω-3 | 0.00 | 4.83 | 6.52 |
| Total ω-3 | 4.37 | 12.20 | 16.24 |
| Total ω-6 | 0.68 | 7.73 | 14.41 |
| ω-3/ω-6 | 6.42 | 1.57 | 1.12 |
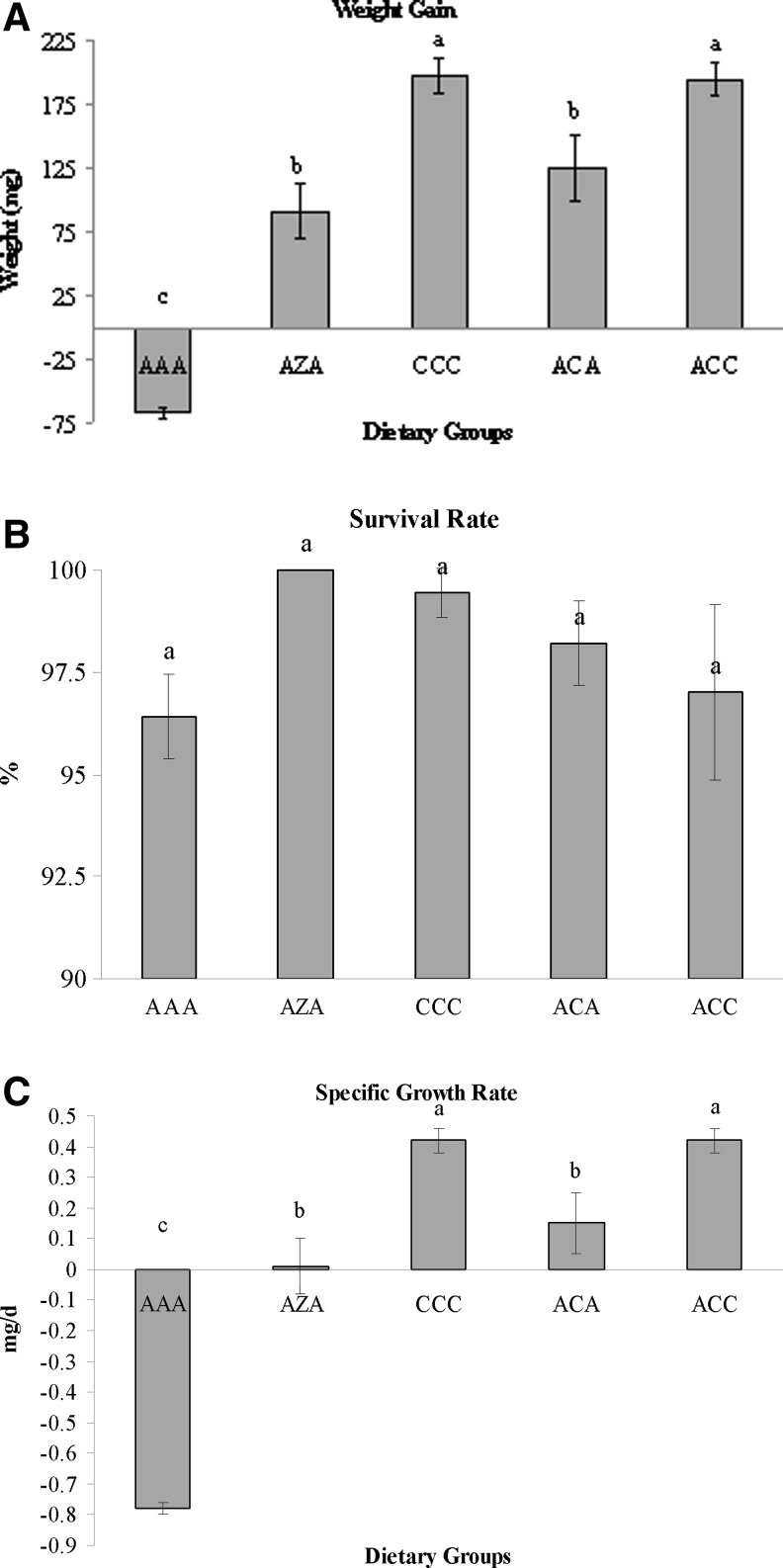
Significant differences were observed in the growth parameters evaluated in this study. Mean weight gain and specific growth rate were significantly greater in zebrafish fed dietary groups CCC and ACC, followed by zebrafish fed dietary groups 2 and 4, while zebrafish fed dietary group AAA lost weight throughout the study period (Fig. 1). Male zebrafish in dietary groups CCC and ACC had the most significant weight gains, followed by male zebrafish fed dietary groups AZA and ACA, while male zebrafish fed dietary group AAA lost weight throughout the study period (Table 5). It is not surprising then to see that zebrafish males in dietary groups AZA through ACA had significantly greater specific growth rates than male zebrafish fed dietary group AAA. Female zebrafish in dietary groups CCC and ACC had a significantly greater weight gain than female zebrafish fed dietary groups AAA and AZA. Not surprisingly, female zebrafish in dietary groups AZA thru ACC had significantly greater specific growth rates than female zebrafish in dietary group AAA. No significant differences were observed in the survival rate or in the gender weight ratio.
FIG. 1.
Weight gain (A), specific growth rate (B), and survival rate (C) of zebrafish fed various dietary regimes containing Artemia (A), Ziegler Zebrafish diet (Z), or Calamac (C). Values (mean±SEM) within a column with different superscripts were significantly (p<0.05, n=3) different as determined by ANOVA and the Student Neuman–Keuls test.
Table 5.
Weight Gain, Specific Growth Rate, Survival Rate, and Gender Weight Ratio Between Male and Female Zebrafish Fed Various Dietary Regimes Containing Artemia (A), Ziegler Zebrafish Diet (Z), or Calamac (C)
| Dietary group | Male weight gain (mg) | Male SGR (mg/d) | Male SR (%) | Female weight gain (mg) | Female SGR (mg/d) | Female SR (%) | GWR |
|---|---|---|---|---|---|---|---|
| AAA | −22.02±8.82c | −0.13±0.07b | 98.81±1.19a | −110.67±0.83c | −0.62±0.09b | 94.05±1.19a | 1.34±0.03a |
| AZA | 67.96±15.31b | 0.40±0.08a | 100.00±0.00a | 114.32±41.93b | 0.42±0.14a | 100.00±0.00a | 1.59±0.12a |
| CCC | 119.36±4.62a | 0.68±0.02a | 100.00±0.00a | 273.21±22.79a | 0.93±0.06a | 98.81±1.19a | 1.75±0.03a |
| ACA | 61.02±18.36b | 0.36±0.10a | 100.00±0.00a | 189.87±64.34ab | 0.67±0.19a | 96.43±2.06a | 1.81±0.22a |
| ACA | 119.91±11.79a | 0.68±0.05a | 100.00±0.00a | 268.48±14.91a | 0.92±0.04a | 94.06±4.29a | 1.74±0.02a |
Values (mean±SEM) within a column with different superscripts were significantly (p<0.05, n=3) different as determined by ANOVA and the Student–Neuman–Keuls test.
SGR, specific growth rate; SR, survival rate; GWR, gender–weight ratio.
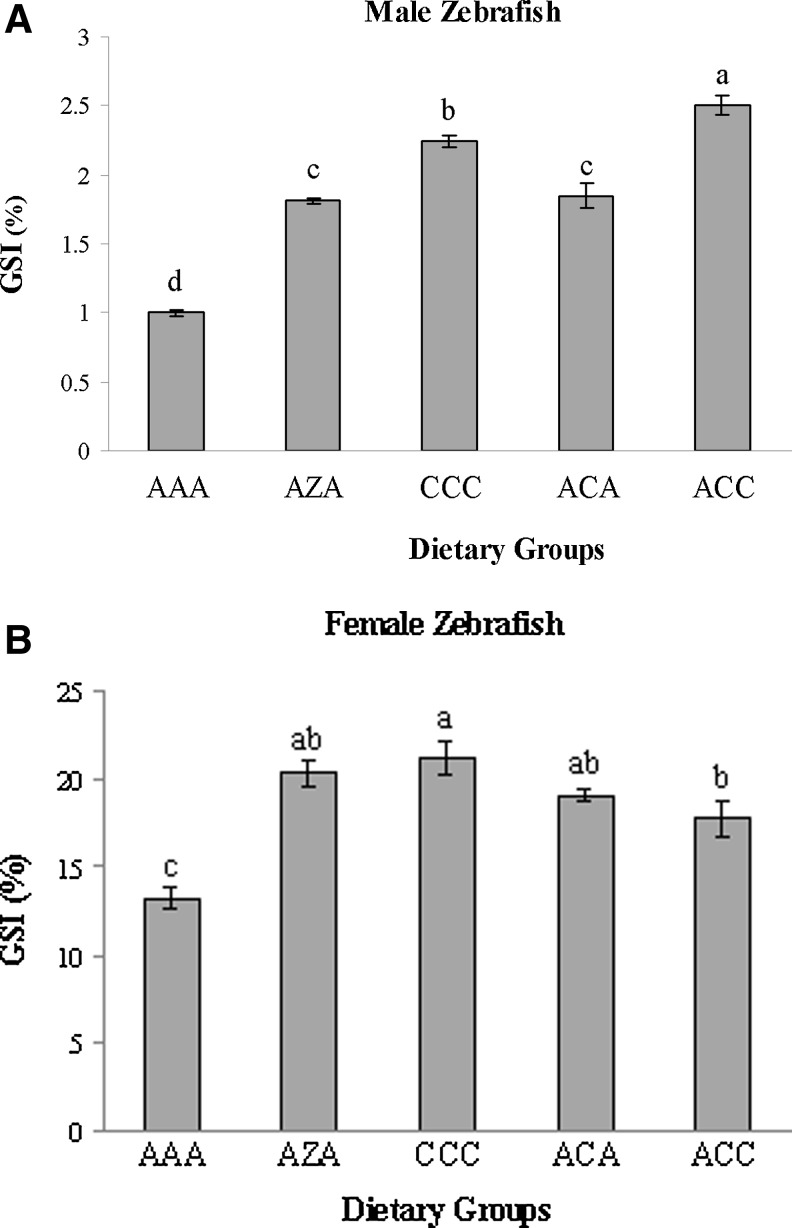
GSIs differed significantly among dietary groups (Fig. 2). The GSI was significantly greatest among male zebrafish in dietary group ACC, followed by males in dietary group CCC. Male zebrafish in dietary group AAA had the significantly lowest GSI value. Female GSI values were significantly greatest in dietary group CCC, followed by females in dietary group ACC. Females in dietary group AAA had the least significant GSI value.
FIG. 2.
Gonadosomatic index (GSI) of zebrafish males (A) and females (B) fed various dietary regimes containing Artemia (A), Ziegler Zebrafish diet (Z), or Calamac (C). Values (mean±SEM) within a column with different superscripts were significantly (p<0.05, n=3) different as determined by ANOVA and the Student Neuman–Keuls test.
The fatty acid composition of female gonads did not differ significantly among dietary groups (Table 6). The most abundantly found fatty acid in all treatments was DHA, followed by linoleic, EPA, and linolenic acid. The ARA content was surprisingly consistent among all dietary groups. The ω-3/ω-6 ratio ranged between 2.55±0.25 and 3.52±0.71. Additionally, the DHA/EPA and EPA/ARA ratio ranged from 2.18±0.06 to 2.46±0.10 and 3.58±0.25 to 5.31±0.14, respectively, between dietary groups.
Table 6.
Fatty Acid Profile of Female Zebrafish Gonads Fed Various Dietary Regimes Contianing Artemia (A), Ziegler Zebrafish Diet (Z), or Calamac (C) (mg/g Sample)
|
Dietary groups | |||||
|---|---|---|---|---|---|
| AAA | AZA | CCC | ACA | ACC | |
| 18:0 | 2.25±0.08 | 2.56±0.13 | 2.18±0.17 | 2.47±0.27 | 2.33±0.12 |
| 18:1ω-9 | 6.14±0.37 | 7.58±1.03 | 6.08±0.43 | 7.05±1.06 | 6.94±0.71 |
| 18:1ω-7 | 0.85±0.03 | 1.09±0.12 | 0.90±0.07 | 1.08±0.09 | 0.91±0.04 |
| 18:2ω-6 | 1.96±0.62 | 2.95±1.07 | 2.12±0.30 | 2.49±0.97 | 3.21±0.47 |
| 18:3ω-3 | 1.24±0.57 | 1.69±0.42 | 0.77±0.23 | 1.62±0.59 | 0.81±0.16 |
| 18:4ω-3 | 0.17±0.04 | 0.20±0.04 | 0.13±0.03 | 0.19±0.03 | 0.13±0.01 |
| 20:2ω-6 | 0.12±0.02 | 0.18±0.06 | 0.17±0.02 | 0.18±0.04 | 0.20±0.01 |
| 20:3ω-6 | 0.16±0.02 | 0.23±0.07 | 0.19±0.02 | 0.19±0.02 | 0.17±0.00 |
| 20:3ω-3 | 0.20±0.08 | 0.29±0.06 | 0.14±0.03 | 0.27±0.05 | 0.14±0.02 |
| 20:4ω-6 | 0.53±0.05 | 0.56±0.05 | 0.50±0.06 | 0.56±0.03 | 0.44±0.02 |
| 20:4ω-3 | 0.37±0.11 | 0.48±0.08 | 0.25±0.05 | 0.50±0.08 | 0.31±0.03 |
| 20:5ω-3 | 1.90±0.25 | 2.24±0.36 | 2.05±0.08 | 2.12±0.43 | 2.34±0.10 |
| 22:5ω-6 | 0.10±0.04 | 0.09±0.02 | 0.11±0.01 | 0.14±0.03 | 0.14±0.10 |
| 22:5ω-3 | 0.87±0.06 | 1.00±0.14 | 0.95±0.16 | 0.97±0.16 | 0.98±0.03 |
| 22:6ω-3 | 4.40±0.75 | 4.93±0.90 | 5.06±0.04 | 4.94±1.14 | 5.69±0.19 |
| Σ Saturates | 11.83±1.06 | 13.72±1.76 | 12.07±0.67 | 12.92±1.94 | 13.63±1.16 |
| Σ Monoenes | 8.37±0.66 | 10.59±1.79 | 8.70±0.59 | 10.29±1.75 | 9.99±1.12 |
| Σω-3 | 9.18±0.35 | 10.86±1.21 | 9.39±0.47 | 10.64±1.32 | 10.46±0.40 |
| Σω-6 | 2.88±0.72 | 4.01±1.23 | 3.17±0.20 | 3.59±1.06 | 4.18±0.51 |
| Σ PUFA | 12.09±1.09 | 15.01±2.53 | 12.74±0.48 | 14.37±2.38 | 14.79±0.92 |
| ω-3/ω-6 | 3.52±0.71 | 3.05±0.63 | 2.97±0.17 | 3.22±0.47 | 2.55±0.25 |
| DHA/EPA | 2.30±0.22 | 2.18±0.06 | 2.46±0.10 | 2.30±0.25 | 2.43±0.02 |
| EPA/AA | 3.58±0.25 | 4.10±0.77 | 4.27±0.63 | 3.68±0.52 | 5.31±0.14 |
Values (mean±SEM) within a column with different superscripts were significantly (p<0.05, n=3) different as determined by ANOVA and the Student Neuman–Keuls test.
Fatty acids with concentrations below 0.05 mg/g sample were not included.
Discussion
Nutrition has been well documented as an important factor affecting fish health,21,22 fish reproductive performance,7,22–24 labor and facility operating costs,17 even research outcomes.25 Yet, as zebrafish increase in popularity, our fundamental understanding of essentiality of nutrients remains elusive, although there is an increase in effort toward this end.26 In this study, we attempted to elucidate the effects of several commercially available feeds and a range of feeding regimes on growth, GSI, and fatty acid composition of gonads in an effort to optimize feeding practices at the NICHD.
Our results clearly demonstrated that the feeding regime and feed used affected the growth parameters evaluated. The use of Calamac as a feed for zebrafish culture had positive impacts on the growth and GSI of zebrafish. (Fig. 1 and Table 6). As the number of daily Calamac feedings increased, so did the growth of the zebrafish in those tanks. Conversely, zebrafish had better growth and GSI values as daily Artemia feedings were reduced. Zebrafish fed Calamac once daily had numerically greater weight gain and GSI than when fed the Ziegler diet. The most notable difference between the Ziegler and Calamac diet was the fatty acid content of the diets. The Calamac diet had a lower ω-3/ω-6 ratio and twice as much total ω-6 fatty acids as compared to the Zeigler diet. It has been reported that a lower ω-3/ω-6 ratio was an important characteristic in the feed fed to zebrafish for improved reproductive performance.8 Whereas reproductive performance was not evaluated in this study, the ω-3/ω-6 ratio of Calamac, in addition to the reported weight gain and GSI values, suggest that Calamac may be a suitable feed for rearing zebrafish.
The weight loss by zebrafish fed Artemia in this study is presumed to be as a result of the low concentrations of polyunsaturated fatty acids such as linolenic acid, ARA, EPA, and DHA reported in the Artemia. Studies have shown that the incorporation of adequate levels of nonprotein energy in the diet influences the requirement for protein and overall growth performance of fish.27 Success in sparing protein by increasing dietary lipid levels has been previously reported in Atlantic salmon,28 rainbow trout,29 tilapia,30 Jade perch,31 rohu,32 and hybrid catfish.33 Our data suggest that zebrafish fed only Artemia relied on protein energy to meet a portion of its energy demands not met by the lipid component of the diet. As a result, this dietary group did not have enough dietary protein to allocate toward growth and gonadal development, and lost weight throughout the study as a result. An improvement in the polyunsaturated fatty acid content of Artemia fed to zebrafish would most likely result in improved weight gain. Thus, we believe we are presenting preliminary evidence supporting protein sparing in zebrafish. Further research in this area is required to confirm our belief.
Artemia was selected as a control diet in this study largely due to the fact that it is routinely used as a feed for rearing zebrafish in many facilities worldwide.18 Its efficacy as a sole feed seems controversial, however. One study evaluating nonenriched Artemia found that zebrafish fed a flake diet produced larger larvae than zebrafish fed Artemia.16 Another found that Artemia enriched with spirulina was sufficient to raise juvenile zebrafish into early adult stage and did not impair early reproductive performance.15 In this study, we provide data suggesting that the use of Artemia enriched with spirulina powder following NICHD protocol is inadequate for zebrafish as fish fed the control diet lost weight throughout the study and had the lowest GSI of all dietary groups. It is likely that a different enrichment protocol would enhance the use of Artemia as a food source for zebrafish.34–36 Artemia enrichment is standard in many freshwater and marine aquaculture facilities due to the fact that Artemia are deficient in many highly unsaturated fatty acids.34 When enriched for 24 h with Algamac 200, Artemia were reported to contain ARA, EPA, and DHA concentrations of 2.3%, 5.0%, and 8.9% crude lipid content, respectively,36 which would be a significant improvement in the nutritional value of Artemia fed to zebrafish as compared to the Artemia used in this study. The use of spirulina enrichment for enhancing the nutritional value of Artemia had reported greater fatty acid values than what is reported in this study, with linoleic and DHA values of 5.72 and 0.71% total fatty acids, respectively. The use of 0.5 mg spirulina per mL enrichment water was capable of providing more ω-6 fatty acids then what we report here.37 However, in both, this study and Cho et al., EPA and DHA are not present in Artemia. It is clear that the enrichment protocol used in this study would benefit from changes in protocol and enrichment media used.
Whereas the enrichment protocol at the NICHD had not changed between the previous study15 and this study, the systems used to rear zebrafish have. We believe this affected the efficacy of Artemia as a food source under the enrichment protocol used in this study. In a previous study, the system used was not self-cleaning, requiring siphoning once every couple of days as needed, meanwhile organic materials would accumulate in the tank.15 This accumulation presumably served as breeding grounds for bacterial growth, which may have served as supplemental nutrition for zebrafish. Previous research has documented that zebrafish consume bacteria in the wild.38 It would stand to reason then that microflora growing on feed and other organic waste would serve as supplemental nutrition for laboratory-reared zebrafish. The system used in this study incorporated self-cleaning tanks containing baffles at the back end of the tank for facilitating solids removal. This cleaner environment would make the nutritional content of the feed fed to zebrafish more important as this environment would not be able to support the growth of bacteria capable of providing supplemental nutrition for zebrafish. Additional research would be needed to confirm this hypothesis, although the use of bacterial sources as feed supplements39–41 or feed replacements42–45 has been documented. It does appear, however, that Artemia enriched with spirulina powder was inadequate as a sole food source for rearing zebrafish. It is unclear what growth improvements would be realized if zebrafish were fed the commercial diets twice daily without Artemia enriched with spirulina powder.
Differences in growth between male and female zebrafish were previously reported.15 In that study, GWR values of 1.1 to 2.9 were reported. Results from this study are not as extreme, but confirm that zebrafish females outgrow their male counterparts. The GSI values reported in this study provide possible reasoning for this disparity in growth. Female zebrafish in this study are observed to have at least 10 times larger GSI than their male counterparts. This has been reported in other species such as yellow perch,46 African catfish,47,48 South American catfish,49 and Japanese flounder.50 This larger allocation of nutrients toward gonadal development and reproductive performance in female zebrafish seems a likely reason for the large GWR data reported among the dietary groups in this study. Additionally, the larger gonads of female fish, and larger GSI values of female zebrafish, would seem appropriate given the reported reproductive capabilities of female zebrafish.51
This is the first study to report the GSI of male zebrafish. The GSI data presented in this study compliments the growth parameter data. Male zebrafish fed the Calamac diet had significantly larger GSI than zebrafish fed Artemia, with males from dietary group 5 having the significantly largest GSI followed by males from dietary group 3. In comparing GSI values with other species, zebrafish males have similar GSI as shrimp,51 and much larger GSI than larger fish species such as catfish.47,52,53 Female zebrafish in this study also had larger GSI than other fish species, such as rohu54 or swordtail.55 It is surprising and indicative of the importance reproduction plays in zebrafish that so much energy and nutrient allocation is reserved for reproduction compared to other larger species. It is interesting to note that male zebrafish in dietary group 5 (Calamac ×2, Artemia ×1) and females in dietary group 3 (Calamac ×3) had significantly larger GSI values among all other dietary groups. We are not sure if this is a result of the feeding competition in the rearing tanks or preliminary indications of gender-specific nutrient requirements.
Zebrafish, like other freshwater species, are capable of elongating and desaturating unsaturated fatty acids into longer polyunsaturated fatty acids,7,9,21 even in the light of limited fatty acid intake.47 This helps explain the result of zebrafish fed Artemia. Female zebrafish fed Artemia in this study allocated similar amounts of DHA, EPA, ARA, linoleic and linolenic acid into gonad tissue as female zebrafish in other dietary groups. Yet, the Artemia fed in this study contained lower concentrations of polyunsaturated fatty acids than the Ziegler and Calamac feed. Whereas the enzymatic activity was not determined during this study, the reported concentrations of polyunsaturated fatty acids in the ovaries of female zebrafish fed Artemia appear difficult to explain otherwise. Surprisingly, this occurred as the female zebrafish were losing weight. We believe this data not only supports selective accumulation of polyunsaturated fatty acids in gonadal tissue, but highlights the importance of gonadal development in zebrafish even during periods of starvation.
ARA plays an important role in the reproductive development and performance. The importance of ARA in the zebrafish reproductive performance has been reported, although neither author mentions optimum levels or tissue-specific ARA concentrations.7–9 Better quality eggs were reported from female sea bass fed dietary ARA as compared to females fed other dietary lipids.47 Substantial evidence has been provided demonstrating that ARA plays a role in regulating oocyte meiosis and steroidogenesis in female sea bass and goldfish.56–58 In Penaeus monodon, ARA levels in ovaries reflected dietary ARA levels fed to females.58 The enzymatic mechanisms for converting linoleic into ARA in freshwater fish species is known.59,60 In a previous study, the dietary ARA content ranged between 0.3% and 0.7% of the dietary crude lipid, yet the ARA content of the eggs was recorded as 1.3% for all treatments.8 In another study, ARA concentrations of ovaries ranged between 75% and threefold greater than dietary ARA concentrations, although in this particular study, there were differences in ovarian ARA concentrations between treatments.9 It is not surprising then that in this study, zebrafish fed solely Artemia had the same levels of ARA as zebrafish in all other dietary groups. These three studies support the idea that while the fatty acid composition of fish eggs generally reflects the fatty acid composition of the diet, zebrafish will selectively synthesize certain polyunsaturated fatty acids denovo in spite of dietary availability. Despite the similarities in findings among these studies, it is still uncertain what the optimum level of ARA should be in ovaries or fertilized eggs.
We are uncertain why zebrafish did not spawn during the three attempts provided during this study. Mixed sex tanks were used throughout the study to optimize social interactions in the hopes of ensuring reproductive performance.61 All spawns were set up using pairwise spawning tanks to remove female pheromonal regulation of reproductive performance.62 A small spawning period was maintained to ensure any reproductive data obtained could correlate with GSI and gonadal fatty acid composition data. Whereas reproductive data would be important in determining the optimal feed and feeding regime to implement in a zebrafish facility, we feel that the data presented provide a new insight into the effects of commercially available feeds, feeding protocols, and feeding regimes on zebrafish culture. We recognize that additional studies are needed to evaluate the effects of such feeding regimes on the reproductive performance and egg composition.
Limitations in the fish facility factored into the execution of this study. In facilities used by numerous laboratories, resources such as access to space, tanks, and husbandry effort determine the size and scope of projects. This holds true in large facilities such as the facility at NICHD. We realize that the incorporation of two more dietary groups (AZZ and ZZZ) would have made for a more complete analysis, yet the increased requirement for more fish, tank space, and tanks was more than what could be afforded. We felt that a more thorough analysis of a novel diet, C, would provide more value to the community and justified the truncation of dietary groups investigated. The satiation feeding duration used in this study ensures the practicality of implementing results in large and small facilities, where even 5 min of feeding may seem impractical when facilities are short staffed. Given the nutrient content of the diets used in this study, we do not feel that an extension in feedings times, and therefore an increase in the amount of food fed, would have resulted in improved growth of fish in dietary group AAA. The deficiencies in essential fatty acids, on a percent lipid basis, would remain, requiring the fish to utilize the same biochemical processes to alleviate the shortfall in essential fatty acids, and ultimately resulting in weight loss. Additionally, the results would not be practical in implementation due to constraints in labor and time allotted for feeding in large fish facilities. Any perceived variability associated with satiation feeding was removed by having the authors conduct all feedings except weekends and holidays. In those few feedings, only a select few, trained staff were allowed to participate in the study. Thus, the decisions made in the design and implementation of this study was based on the daily realities of large facilities whose mission it is to support the use of zebrafish as an animal model for research in other fields.
Conclusion
Here we present data confirming that the use of commercial feeds and various feeding regimes does effect the growth and gonadal development of zebrafish. It appears that Artemia enriched with spirulina powder was not suitable as a sole feed source for zebrafish. Evidence is provided to support the use of Calamac as feed for zebrafish. We offer preliminary evidence for protein sparing in zebrafish and selective accumulation of fatty acids in gonadal tissue. Uncertainty still remains concerning the impact these commercially available feeds have on the reproductive performance of zebrafish. More work will be needed to improve our understanding of these impacts until a formulated diet containing proven quantities of specific nutrients is available.
Acknowledgments
We would like to thank Dr. Igor Dawid for the use of fish, laboratory space, manuscript review, and other resources made available during the course of this study. We would also like to thank the Charles River aquatics husbandry staff at the NICHD for their involvement with this study. We would like to thank Mr. Dennis Barnard for his input and review of the manuscript. We also appreciate all the comments provided by the reviewers in completing this manuscript. This work was supported by grants for Laboratory Animal Science (GLAS) from the American Association for the Laboratory Animal Science. This research was party supported by the Intramural Research Program of the NIH, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Disclosure Statement
No competing financial interests exist.
References
- 1.Dawid I. Developmental biology of zebrafish. Ann NY Acad Sci. 2004;1038:88–93. doi: 10.1196/annals.1315.015. [DOI] [PubMed] [Google Scholar]
- 2.Bretaud S. MacRaild S. Ingham P. Bandmann O. The influence of the zebrafish genetic background on Parkinson's disease-related aspects. Zebrafish. 2011;8:103–108. doi: 10.1089/zeb.2011.0697. [DOI] [PubMed] [Google Scholar]
- 3.Craig P. Moon T. Fasted zebrafish mimic genetic and physiological responses in mammals: a model for obesity and diabetes. Zebrafish. 2011;8:109–117. doi: 10.1089/zeb.2011.0702. [DOI] [PubMed] [Google Scholar]
- 4.Pereira T. Rico E. Rosemberg D. Schirmer H. Dias R. Souto A, et al. Zebrafish as a model organism to evaluate drugs potentially able to modulate sirtuin expression. Zebrafish. 2011;8:9–16. doi: 10.1089/zeb.2010.0677. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence C. The husbandry of zebrafish (Danio rerio): a review. Aquaculture. 2007;269:1–20. [Google Scholar]
- 6.Izquierdo M. Fernandez-Palacios H. Tacon A. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture. 2001;198:25–42. [Google Scholar]
- 7.Kaushnik S. Georga I. Koumoundouros G. Growth and body composition of zebrafish (Danio rerio) larvae fed a compound feed from first feeding onward: toward implications on nutrient requirements. Zebrafish. 2011;2:87–95. doi: 10.1089/zeb.2011.0696. [DOI] [PubMed] [Google Scholar]
- 8.Meinelt T. Schulz C. Wirth M. Kurzinger H. Steinberg C. Dietary fatty acid composition influences the fertilization rate of zebrafish (Danio rerio Hamilton-Buchanan) J Appl Ichthyol. 1999;15:19–23. [Google Scholar]
- 9.Jaya-Ram A. Kuah M-K. Lim P-S. Kolkovoski S. Shu-Chien A. Influence of dietary HUFA levels on reproductive performance, tissue fatty acid profile and desaturase and elongase mRNAs expression in female zebrafish (Danio rerio) Aquaculture. 2008;277:275–281. [Google Scholar]
- 10.Siccardi A. Garris H. Jones W. Moseley D. D'Abramo L. Watts S. Growth and survival of zebrafish (Danio rerio) fed different commercial and laboratory diets. Zebrafish. 2009;6:1–6. doi: 10.1089/zeb.2008.0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawkyard M. Saele O. Nordgreen A. Langdon C. Hamre K. Effect of iodine enrichment of Artemia sp. on their nutritional value for larval zebrafish (Danio rerio) Aquaculture. 2011;316:37–43. [Google Scholar]
- 12.Yossa R. Sarker P. Vanderberg G. Preliminary evidence of the contribution of the intestinal mircoflora to biotin supply in zebrafish Danio rerio (Hamilton-Buchanan) Zebrafish. 2011;8:221–227. doi: 10.1089/zeb.2011.0706. [DOI] [PubMed] [Google Scholar]
- 13.Goolish E. Okutake K. Lesure S. Growth and survivorship of larval zebrafish Danio rerio on processed diets. N Am J Aquacult. 1999;61:189–198. [Google Scholar]
- 14.Carvalho A. Araujo L. Santos M. Rearing zebrafish (Danio rerio) larvae without live food: evaluation of a commercial, a practical and a purified starter diet on larval performance. Aqua Res. 2006;37:1107–1111. [Google Scholar]
- 15.Gonzales J. Preliminary evaluations on the growth and early reproductive performance of zebrafish (Danio rerio) JAALAS. 2012;51:412–417. [PMC free article] [PubMed] [Google Scholar]
- 16.Markovich M. Rizzuto N. Brown P. Diet affects spawning in zebrafish. Zebrafish. 2007;4:69–74. doi: 10.1089/zeb.2006.9993. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence C. Best J. James A. Maloney K. The effects of feeding frequency on growth and reproduction in zebrafish (Danio rerio) Aquaculture. 2012;369:103–108. [Google Scholar]
- 18.Castranova D. Lawton A. Lawrence C. Baumann D. Best J. Coscolla J, et al. The effect of stocking densities on reproductive performance in laboratory zebrafish (Danio rerio) Zebrafish. 2011;8:141–146. doi: 10.1089/zeb.2011.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Association of Official Analytical Chemists (AOAC) 16th. Arlington, VA: OAC International; 1997. Official Methods of Analysis of AOAC International. [Google Scholar]
- 20.Zar J. 2nd. Prentice Hall; Englewood Cliffs, NJ; 1984. Biostatistical Analysis. [Google Scholar]
- 21.Glencross B. Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev Aqua. 2009;1:71–124. [Google Scholar]
- 22.Gunasekera R. Shim K. Lam T. Effect of dietary protein level on puberty, oocyte growth, and egg chemical composition in the tilapia, Oreochromis niloticus (L.) Aquaculture. 1995;134:169–183. [Google Scholar]
- 23.Wouters R. Laves P. Nieto J. Sorgeloos P. Penaid shrimp broodstock nutrition: an updated review on research and development. Aquaculture. 2001;202:1–21. [Google Scholar]
- 24.Chong S. Ishak S. Osman Z. Hashim R. Effect of dietary protein level on the reproductive performance of female swordtails Xiphophorus helleri (Poeciliidae) Aquaculture. 2004;234:381–392. [Google Scholar]
- 25.Barnard D. Lewis S. Teter B. Thigpen J. Open- and closed-formula laboratory diets and their importance to research. JAALAS. 2009;48:709–713. [PMC free article] [PubMed] [Google Scholar]
- 26.Watts S. Powell M. D'Abramo L. Fundamental approaches to the study of zebrafish nutrition. ILAR. 2012;53:145–160. doi: 10.1093/ilar.53.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho C. Kaushik S. Nutrition energetic in fish: energy and protein utilization in rainbow trout (Salmo gairdneri) World Rev Nutr Diet. 1990;61:132–172. doi: 10.1159/000417529. [DOI] [PubMed] [Google Scholar]
- 28.Codabaccus B. Carter C. Bridle A. Nichols P. The “n-3 LC-PUFA sparing effect” of modified dietary n-3 LC-PUFA content and DHA to EPA ratio in Atlantic salmon smolt. Aquaculture. 2012;356–357:135–140. [Google Scholar]
- 29.Beamish F. Medland T. Protein sparing effects in large rainbow trout, Salmo gairdneri. Aquaculture. 1986;55:35–42. [Google Scholar]
- 30.De Silva S. Gunasekera R. Shim K. Interactions of varying dietary protein and lipid levels in young red tilapia: evidence of protein sparing. Aquaculture. 1991;95:305–318. [Google Scholar]
- 31.Song L. An L. Zhu Y. Li X. Wang A. Effects of dietary lipids on growth and feed utilization of jade perch, Scortum barcoo. J World Aquac Soc. 2009;40:266–273. [Google Scholar]
- 32.Erfanullah Jafri A. Protein sparing effect of dietary carbohydrate in diets for fingerling Labeo rohita. Aquaculture. 1995;136:331–339. [Google Scholar]
- 33.Jantrarotai W. Sitasit P. Jantrarotai P. Viputhanumas T. Srabua P. Protein and energy levels for maximum growth, diet utilization, yield of edible flesh and protein sparing of hybrid Clarias catfish (Clarias macrocephalus×Clarias gariepinus) J World Aquac Soc. 1998;29:281–289. [Google Scholar]
- 34.Watanabe T. Oowa F. Kitajima C. Fujita S. Nutritional quality of brine shrimp Artemia salina as a living feed from the viewpoint of essential fatty acids for fish. Bull Jpn Soc Sci Fish. 1978;40:1115–1122. [Google Scholar]
- 35.Barclay W. Zeller S. Nutritional enhancement of n-3 and n-6 fatty acids in rotifers and artemia nauplii by feeding spray dried schizochytrium sp. J World Aquac Soc. 1996;27:314–322. [Google Scholar]
- 36.Palmtag M. Faulk C. Holt G. Highly unsaturated fatty acid composition of rotifers (Brachionus plicatilis) and artemia fed various enrichments. J World Aquac Soc. 2006;37:126–131. [Google Scholar]
- 37.Cho S. Hur S. Jo J. Effect of enriched live feeds on survival and growth rates of larval Korean rockfish, Sebastes schlegeli Hilgendorf. Aqua Res. 2001;32:199–208. [Google Scholar]
- 38.Spence R. Fatema M. Ellis S. Ahmed Z. Smith C. The diet growth and recruitment of wild zebrafish (Danio rerio) in Bangladesh. J Fish Bio. 2007;71:304–309. [Google Scholar]
- 39.Kiessling A. Askbrandt S. Nutritive valueof two bacterial strains of single-cell protein for rainbow trout (Oncorhynchus mykiss) Aquaculture. 1993;109:119–130. [Google Scholar]
- 40.Eya J. Ashame M. Pomeroy C. Nutritive value of proteinfrom anerobically digested poultry wastes as a dietary ingredient replacer for channel catfish, Ictalurus punctatus. J World Aquac Soc. 2010;41:179–190. [Google Scholar]
- 41.Berge G. Baeverfjord G. Skrede A. Storebakken T. Bacterial protein grown on natural gas as protein source in diets for Atlantic salmon, Salmo salar, in saltwater. Aquaculture. 2005;224:233–240. [Google Scholar]
- 42.El-Sayed A. Alternative protein sources for farmed tilapia, Oreochomris spp. Aquaculture. 1999;179:149–168. [Google Scholar]
- 43.Zerai D. Fitzsimmons K. Collier R. Duff G. Evaluation of brewers waste as partial replacement of fish meal protein in Nile tilapia, Oreochromis niloticus, diets. J World Aquac Soc. 2008;39:556–564. [Google Scholar]
- 44.Gonzales J. Brown P. Nutrient retention capabilities of Nile tilapia (Oreochromis niloticus) fed bio-regenerative life support system (BLSS) waste residue. Adv Space Res. 2007;40:1725–1734. [Google Scholar]
- 45.Gonzales J. Lowry B. Brown P. Beyl C. Nyochemberg L. The effects of composting on the nutritional composition of fibrous bio-regenerative life support systems (BLSS) plant waste residues and its impact on the growth of Nile tilapia (Oreochromis niloticus) Adv Space Res. 2009;43:1243–1249. [Google Scholar]
- 46.Dabrowski K. Ciereszko A. The dynamics of gonad growth and ascorbate status in yellow perch, Perca flavescens (Mitchill) Aqua Res. 1996;27:539–542. [Google Scholar]
- 47.Henken A. Boon J. Cattel B. Lobee H. Differences in growth rate and feed utilization between male and female African catfish Clarias gariepinus (Burchell 1822) Aquaculture. 1987;63:221–232. [Google Scholar]
- 48.Adewumi A. The growth and gonadal maturation of the African catfish, Clarias gariupinus (Burchell) broodstock fed differently heated soybean-based diets. Aqua Nutr. 2006;12:267–274. [Google Scholar]
- 49.Dabrowski K. Arslan M. Rinchard J. Palacios M. Growth, maturation, induced spawning, and production of the first generation of South American catfish, Pseudoplatystoma sp., in North America. J World Aquac Soc. 2008;39:174–183. [Google Scholar]
- 50.Furuita H. Tanaka H. Yamamoto T. Suzuki N. Takeuchi T. Supplemental effect of vitamin A in diet on the reproductive performance and egg quality of the Japanese flounder Paralichthys olivaceus (T&S) Aqua Res. 2003;34:461–467. [Google Scholar]
- 51.Westerfield M. Eugene, OR: University of Oregon Press; 2007. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio) [Google Scholar]
- 52.Pascual C. Valeria E. Regis C. Gaxiola G. Sanchez A. Amos L, et al. Effect of water temperature on reproductive tract condition of Penaeus setiferus adult males. J World Aquac Soc. 1998;29:477–484. [Google Scholar]
- 53.Oteme Z. Rodriguez J. Kouassi C. Hem S. Agnese J-F. Testicular structure, spermatogenesis and sperm cryopreservation in the African clariid catfish Heterobranchus longifilis (Valenciennes, 1840) Aqua Res. 1996;27:805–813. [Google Scholar]
- 54.Khan M. Jafri A. Chadha N. Effects of varying dietary protein levels on growth, reproductive performance, body and egg composition of rohu, Labeo rohita (Hamilton) Aqua Nutr. 2005;11:11–17. [Google Scholar]
- 55.Ling S. Hashim R. Kolkovski S. Shu-Chien A. Effect of varying dietary lipid andprotein levels on growth and reproductive performance of female swordtails Xiphophorus helleri (Poeciliidae) Aqua Res. 2006;37:1267–1275. [Google Scholar]
- 56.Bell J. Farndale B. Bruce M. Navas J. Carrillo M. Effecs of broodstock dietary lipid on fatty acid compositions of eggs from sea bass (Dicentrarchus labrax) Aquaculture. 1997;149:107–119. [Google Scholar]
- 57.Sorbera L. Asturiano J. Carrillo M. Zanuy S. Effects of polyunsaturated fatty acids and prostaglandins on oocyte maturation in a marine teleost, the European sea bass (Dicentrarchus labrax) Bio Repro. 2001;64:382–389. doi: 10.1095/biolreprod64.1.382. [DOI] [PubMed] [Google Scholar]
- 58.Pati D. Habibi H. Involvement of protein kinase C and arachidonic acid pathways in the gonadotropin-releasing hormone regulation of oocyte meiosis and follicular steriodogenesis in the goldfish ovary. Bio Repro. 2002;66:813–822. doi: 10.1095/biolreprod66.3.813. [DOI] [PubMed] [Google Scholar]
- 59.Coman G. Arnold S. Barclay M. Smith D. Effect of arachidonic acid supplementation on reproductive performance of tank domesticated Penaeus monodon. Aqua Nutr. 2009;15:1–11. [Google Scholar]
- 60.Sargent J. Bell J. Bell M. Henderson R. Tocher D. The metabolism of phospholipids and polyunsaturated fatty acids in fish. In: Lahlou B, editor; Vitiello P, editor. Coastal and Estuarine Studies-Aquaculture: Fundamental and Applied Research. Vol. 43. Washington, DC: AGU; 1993. pp. 103–124. [Google Scholar]
- 61.Moretz J. Martins E. Robinson B. The effects of early and adult social environment on zebrafish (Danio rerio) behavior. Environ Biol Fish. 2007;80:91–101. [Google Scholar]
- 62.Gerlach G. Pheromonal regulation of reproductive success in female zebrafish: female suppression and male enhancement. Anim Behav. 2006;72:1119–1124. [Google Scholar]