Abstract
The precise quantitation of smoking during pregnancy is difficult in retrospective studies. Routinely collected blood specimens from newborns, stored as dried blood spots, may provide a low-cost method to objectively measure maternal smoking close to the time of delivery. This article compares cotinine levels in dried blood spots to those in umbilical cord blood to assess cotinine in dried blood spots as a biomarker of maternal smoking close to the time of delivery. The California Genetic Disease Screening Program provided dried blood spots from 428 newborns delivered in 2001–2003 with known umbilical cord blood cotinine levels. Cotinine in dried blood spots was measured in 6.35-mm punches by using liquid chromatography–tandem mass spectrometry (quantitation limit, 3.1 ng/mL). Repeated measures of cotinine in dried blood spots were highly correlated (R2 = 0.99, P < 0.001) among 100 dried blood spots with cotinine quantitated in 2 separate punches. Linear regression revealed that cotinine levels in dried blood spots were slightly lower than those in umbilical cord blood and predicted umbilical cord blood cotinine levels well (β = 0.95, R2 = 0.80, and P < 0.001 for both cotinine levels in log10 scale). When defining active smoking as a cotinine level of 10 ng/mL or more and using umbilical cord blood cotinine as the criterion standard, we found that measurements of cotinine in dried blood spots had high sensitivity (92.3%) and specificity (99.7%) in the prediction of maternal active smoking. Cotinine levels in dried blood spots are an accurate biomarker of maternal smoking close to the time of delivery.
Keywords: cotinine, dried blood spot, maternal smoking, newborn, pregnancy
Maternal smoking is associated with increased fetal, infant, and childhood morbidity and death (1–3). It is usually assessed by mothers' self-reports during pregnancy or after birth (3–5). Self-reported smoking data may underestimate exposures, leading to invalid associations (6) because of pregnant women's reluctance to admit active smoking (7) and because of the difficulty of recalling details of smoking (8).
Biomarkers of tobacco exposure avoid many sources of bias and error found with self-reports and may provide greater sensitivity (9). Cotinine, a principal metabolite of nicotine, is the preferred tobacco biomarker (10). Cotinine measured in blood, saliva, urine, or umbilical cord blood is a reliable marker of tobacco exposure over the previous few days (3, 11–13). Hair and meconium cotinine levels reflect exposure over longer periods of time (14–16). However, biomarker-based studies can be costly in terms of the collection and banking of large numbers of specimens, and they can be impractical for studies of rare diseases such as childhood cancers (7). These limitations have motivated the search for a more convenient and reliable biospecimen.
Blood specimens (as dried blood spots) are collected from newborns across the United States for routine screening for phenylketonuria and other disorders. In 2003, nearly all states stored residual specimens for program evaluation and development for various lengths of time. Fifteen states, including California, had policies to make them available for research purposes (17). The filter paper used by newborn screening programs is made from high-purity cotton linters and is manufactured to produce accurate and reproducible absorption of blood (18). The filter paper method of blood collection has achieved the same level of precision and reproducibility as other standard methods (e.g., vacuum tubes and capillary pipettes) for analytical and clinical testing (19). In general, analyses that can be measured from whole blood, serum, or plasma can also be measured from dried blood on filter paper.
Two studies (20, 21) to date have measured cotinine in dried blood spots. Sosnoff and Bernert (20) quantitated cotinine in blood spot extracts with spiked cotinine, and the quantitated cotinine levels corresponded to the spiked cotinine concentrations above 1 ng/mL. Spector et al. (21) found that cotinine levels in dried blood spots from newborns were, on average, higher among infants of smoking mothers (28.7 ng/mL) than among infants of nonsmoking mothers (7.2 ng/mL). Results from these studies suggest that the routinely collected and stored dried blood spots might be low-cost biospecimens for the objective measurement of maternal smoking close to the time of delivery. However, it is not yet clear the extent to which cotinine in dried blood spots can accurately measure tobacco exposure.
This study compared cotinine levels in stored, frozen dried blood spots with cotinine levels in linked umbilical cord blood samples with the aims of assessing the validity, reliability, and measurement error of cotinine levels in dried blood spots. We describe laboratory and analytical methods and approaches to maximize sensitivity and specificity and to minimize false positive rates.
MATERIALS AND METHODS
The study protocol was approved by the California Health and Human Services Agency Committee for the Protection of Human Subjects and derived a convenience sample of 428 subjects from the Project Baby's Breath Study (22). The Project Baby's Breath Study collected and banked biospecimens from multiple time points during pregnancies in Southern California in 1999–2003, including 12,505 routinely collected umbilical cord blood specimens. Mothers who participated provided written informed consent for research use of the umbilical cord blood. The umbilical cord blood specimens were stored in refrigerators before being frozen. The median time from collection to frozen storage (at −20°C) was 9 days.
Subject selection
Eligible subjects had frozen umbilical cord blood and dried blood specimens and had participated in California's prenatal α-fetoprotein screening program (23). A total of 428 subjects were selected on the basis of previously measured cotinine values to represent a diverse pattern of tobacco exposures; they did not represent a random sample of pregnant women in California. Cotinine levels in umbilical cord blood were measured by liquid chromatography–tandem mass spectrometry by personnel at the Division of Laboratory Science at the Centers for Disease Control and Prevention (quantitation limit, 0.018 ng of cotinine per mL of serum (ng/mL); n = 299) or at the Clinical Pharmacology Laboratory at the University of California, San Francisco (quantitation limit 1 ng/mL as applied to samples from smokers; n = 129).
Dried blood spot specimens from newborns in California
One complete dried blood specimen was retrieved from California's Newborn Screening Program for each subject. As part of the routine newborn screening program, five 14-mm diameter blood spots were collected from nearly all live newborns in California on filter paper (Schleicher & Schuell BioScience, GmbH, Keene, New Hampshire) by heel stick after 12 hours and usually no later than 6 days of age (median age at collection in 2000–2005, 29 hours). Blood spots were dried at room temperature and stored at ambient conditions for approximately 1–3 days. After routine testing, remaining specimens were packed and stored at −20°C. Prior to testing, parents were provided with a privacy notification that described the possible research use of these specimens with the option to request that specimens not be used for such purposes. The details of dried blood specimen collection and storage are described elsewhere (24).
Laboratory methods
Cotinine levels in dried blood spots were measured by using liquid chromatography–tandem mass spectrometry. The method of Jacob et al. (25) was modified for extraction of dried blood spots and determination by using electrospray ionization mass spectrometry as described below. Laboratory personnel were blinded to subjects' tobacco exposure status.
To prepare dried blood spot standards, we obtained heparinized whole blood from a healthy volunteer who was unexposed to tobacco. Whole blood aliquots of 3.00 mL (volume verified by weight) were spiked with 0.030 mL of cotinine dissolved in 0.02N HCl at concentrations of 0, 0.156, 0.313, 0.625, 1.25, 2.5, 5, and 10 µg/mL. This generated dried blood spot standards at concentrations of 0, 1.56, 3.13, 6.25, 12.5, 25, 50, and 100 ng/mL by adding 0.06 mL of each spiked whole blood standard onto Whatman 903 filter paper (Whatman, Ltd., Maidstone, United Kingdom) and drying them overnight at room temperature. A common handheld paper punch was used to punch out 6.35-mm diameter punches, which were placed in 1.5-mL microfuge tubes and stored at 4°C.
A maximum of five 6.35-mm punches could be obtained from 1 complete dried blood spot. One punch of a dried blood spot, which was estimated to contain 12 µL of whole blood on average (20) for the calculation of cotinine concentration, had a quantitation limit of 3.13 ng/mL (coefficient of variation, 20%). Increasing the number of punches to 5 could theoretically improve the quantitation limit to 0.67 ng/mL, but in practice, this would not be attainable because of a concurrent increase in background noise (including cotinine present in the environment, in solvents and reagents used in sample preparation, and in the sampling paper). Therefore, this study used only 1 punch for each cotinine determination. The single 6.35-mm punch was first sonicated in a 1.5-mL microfuge tube with 450 µL of reagent grade water and 50 µL of 100-ng/mL internal standard cotinine-d9 for 75 minutes at 55°C. Then, 50 µL of 30% perchloric acid was added, and the samples were centrifuged for 1 minute. After centrifugation, the supernatant was poured into 13×100-mm glass culture tubes, and 1 mL of 3.6 M tripotassium phosphate was added. The samples were then extracted with 4 mL of methylene chloride, and the extract was transferred to 13×100-mm culture tubes, acidified by the addition of 100 µL of 1N HCl in methanol and evaporated in a Savant SpeedVac Concentrator SC210A (ThermoQuest Corp., Holbrook, New York). The residue was dissolved in 100 µL of 0.02N HCl and transferred to a 300-µL polypropylene microvial. Forty µL was analyzed by liquid chromatography–tandem mass spectrometry as described by Jacob et al. (25) with the exception that a heated electrospray ionization source was used instead of atmospheric pressure chemical ionization.
A pilot study examined potential false positive quantitation of cotinine due to environmental contamination in 80 punches obtained from 20 dried blood spots. False negative quantitation was unlikely given the stability of cotinine in body fluids under various conditions (26). False positive quantitation (≥3.13 ng/mL) was confirmed in 6 of 59 punches taken from 15 dried blood spots with umbilical cord blood cotinine levels of less than 3.13 ng/mL. There was no false negative quantitation of cotinine (<3.13 ng/mL) in 21 punches taken from 5 dried blood spots with umbilical cord blood cotinine levels of 3.13 ng/mL or higher. To reduce false positives, we took 2 punches of specimens from each dried blood spot, allowing for a duplicate test if cotinine was quantitated in the first punch.
To confirm the cotinine stability in dried blood spots, we stored 22 spiked dried blood spot standards, prepared as described above, with cotinine levels of 3.13, 6.25, 12.5, 25, 50, and 100 ng/mL at room temperature for 7 months in the dark and retested them along with freshly prepared standards spiked with the same range of cotinine levels.
Data analysis
The laboratory dried blood spot cotinine levels were linked to existing data for analysis, including demographic and pregnancy-related data from birth certificates, blood specimen collection date and time from the California Newborn Screening Program, gestational age from the California Prenatal Screening Program, and umbilical cord blood cotinine levels from the Project Baby's Breath Study.
The study population was described by selected maternal, pregnancy, and infant characteristics. For subjects with cotinine quantitated in 2 punches, the averaged cotinine level from both punches was assigned as the final dried blood spot cotinine level. Subjects with false positive quantitation of cotinine in the first dried blood spot punch but not the second punch were treated as nonquantitated. Scatter plots were used to visually display relationships between repeated measures of dried blood spot cotinine and the final dried blood spot cotinine in association with umbilical cord blood cotinine in log10 scale. The ratio of cord blood cotinine to the final dried blood spot cotinine was also plotted against infant age (days in log10 scale) at blood specimen collection to examine potential decline in dried blood spot cotinine due to metabolism (27) and against dried blood spot freezer storage duration (in months) to examine stability over long-term freezer storage. The association strength was measured by the R2 value from linear regression modeling. The rates of sensitivity and specificity of dried blood spot cotinine in identifying active smoking (defined by umbilical cord blood cotinine as ≥10 ng/mL) from no smoking were calculated for selected cutpoints and graphed by using a receiver operating characteristic curve. The optimum cutpoint was defined as the one that maximized the sum of sensitivity and specificity. All analyses were performed by using SAS, version 9.2, software (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Approximately 80% of the mothers were white or Hispanic, and 80% were between the ages of 20 and 34 years. One-third attained less than 12 years of education, 40% were delivering their first live birth, and one-fifth smoked close to the time of delivery based on umbilical cord blood cotinine levels (≥10 ng/mL). Approximately 6% of the babies were born preterm. Blood spot samples were collected prior to 24 hours of age for one-third of the infants and after 39 hours for one-fifth of the infants (results not shown).
A total of 123 spots from the selected 428 subjects had cotinine quantitated in the first dried blood spot punch and, therefore, had duplicate cotinine tests in the second dried blood spot punch to confirm the positive quantitation. Twenty three of them did not have quantitated cotinine levels in the second dried blood spot punch and were therefore treated as nonquantitated with false positive quantitation of cotinine in the first dried blood spot punch. Eleven of the 23 spots had cotinine levels less than 5 ng/mL in the first punch, 6 spots had levels of 5 to less than 10 ng/mL, 4 had levels of 10 to less than 15 ng/mL, and 2 had levels of 250 ng/mL or higher. None of the 23 spots showed active smoking exposure based on cord blood cotinine levels (defined as ≥10 ng/mL).
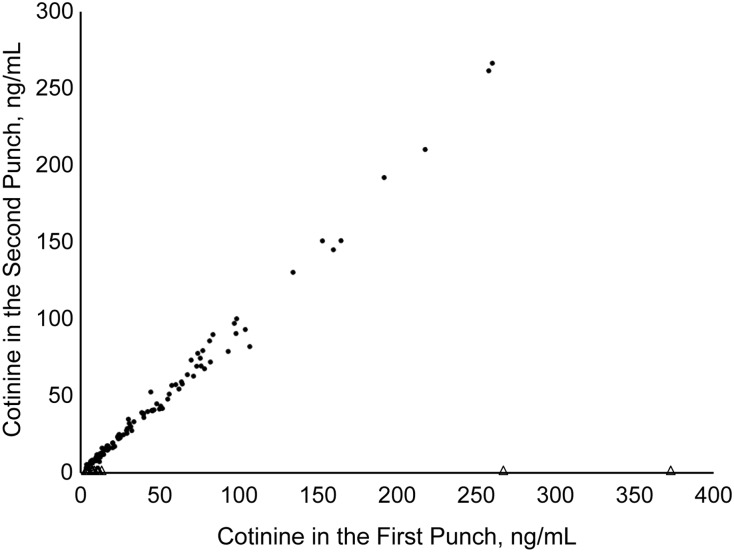
Of the 100 spots with quantitated cotinine in both punches, 89% had umbilical cord blood cotinine levels of 10 ng/mL or higher. Cotinine levels quantitated in first punches were highly correlated and close to the cotinine levels in second punches (Figure 1) (R2 = 0.99; linear regression slope = 1.01; P < 0.001). The average absolute cotinine difference between the 2 punches was 3.39 ng/mL. The final dried blood spot cotinine levels averaged from both punches ranged from 6.76 to 263.35 ng/mL for smokers with umbilical cord blood cotinine levels of 10 ng/mL or higher and from 3.33 to 13.25 ng/mL for nonsmokers with umbilical cord blood cotinine levels of less than 10 ng/mL.
Figure 1.
Scatter plot relating repeated measures of cotinine levels (in ng/mL) in dried blood spots from 123 newborns delivered in California in 2001–2003 with quantitated cotinine in the first dried blood spot punches. For the 100 subjects with quantitated cotinine levels in both punches, cotinine in the first punch = 1.42 + 1.01 × cotinine in the second punch (R2 = 0.99). Triangles indicate that cotinine was not quantitated in the second punch; dots indicate that cotinine was quantitated in the second punch.
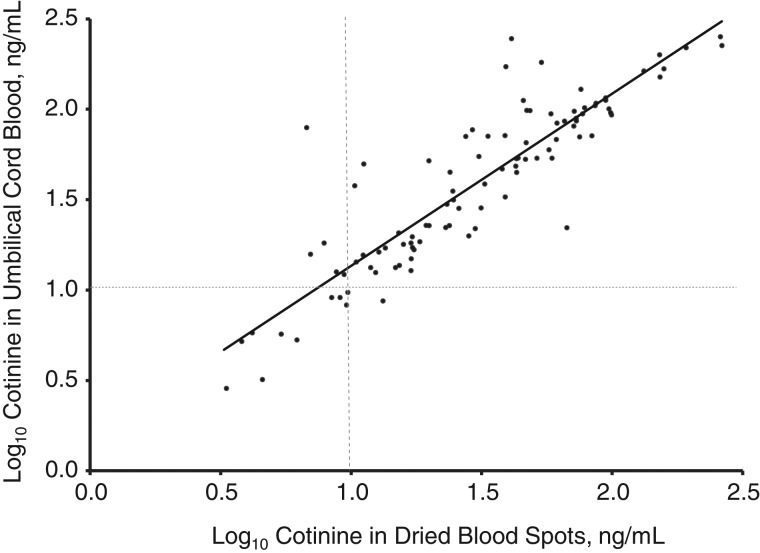
Levels of dried blood spot cotinine and umbilical cord blood cotinine were highly correlated, with dried blood spot cotinine slightly lower than cord blood cotinine (Figure 2) (R2 = 0.80; linear regression slope of log10-transformed cotinine levels = 0.95; P < 0.001). Mean cotinine in the 100 dried blood spots with quantitated cotinine in both punches was 15.5 ng/mL lower than in umbilical cord blood.
Figure 2.
Scatter plot relating cotinine levels in umbilical cord blood and dried blood spots from 100 newborns delivered in California in 2001–2003 with quantitated cotinine in both dried blood spot punches. Log10 cord blood cotinine (in ng/mL) = 0.18 + 0.95 × log10 cotinine in newborn dried blood spots (R2 = 0.80 for all 100 subjects with quantitated dried blood spot cotinine in both punches).
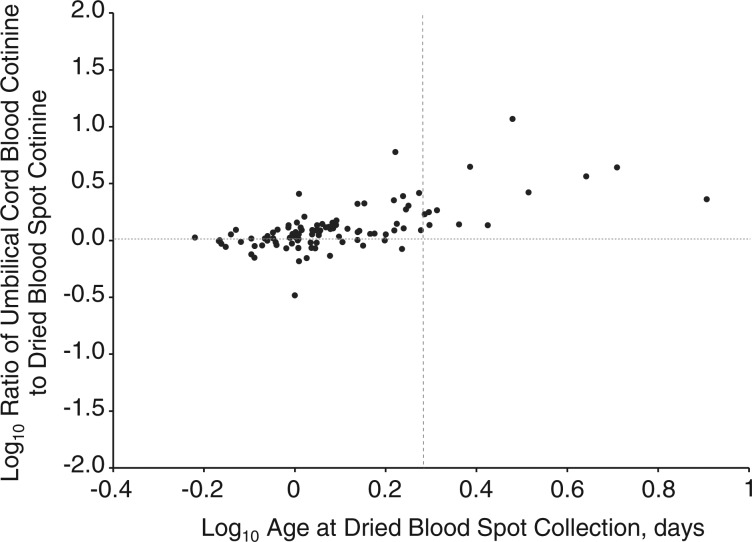
Figure 3 shows an increasing ratio of umbilical cord blood cotinine to dried blood spot cotinine with increasing infant age at dried blood spot collection. Umbilical cord blood cotinine, on average, was 1.08, 1.38, or 3.75 times higher than dried blood spot cotinine when dried blood spots were collected at infant ages of less than 1, 1–2, or more than 2 days, respectively (linear regression intercept = 1.14; slope = 3.71 for days in log10 scale; P < 0.001). This correlation between dried blood spot cotinine and umbilical cord blood cotinine became slightly stronger when adjusted for infant age at dried blood spot specimen collection; the R2 value increased from 0.80 to 0.86, and the linear regression slope of log10-transformed cotinine levels increased from 0.95 to 1.00. There was no significant ratio change of umbilical cord blood cotinine to dried blood spot cotinine as a function of duration of freezer storage (results not shown).
Figure 3.
Ratio (in log10 scale) of cotinine levels (in ng/mL) in umbilical cord blood to cotinine levels (in ng/mL) in dried blood spots by infant age (days in log10 scale) at blood spot collection among 99 subjects with known infant age at blood spot collection who were delivered in California in 2001–2003. The dotted vertical line in the plot area represents an age of 2 days (0.30 in log10 scale) at dried blood spot collection.
Laboratory testing did not detect significant cotinine changes in the 22 spiked blood spot standards after storage at room temperature. Cotinine levels in the 7-month-old standards were, on average, 0.5% (range, −15.6–16.0) lower than the freshly prepared standards (results not shown).
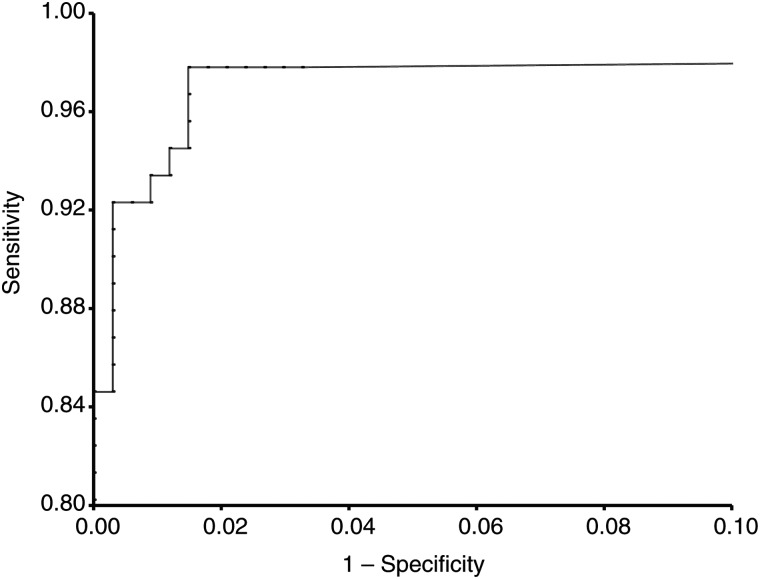
The receiver operating characteristic curve (Figure 4) indicated that dried blood spot cotinine was a nearly perfect test to distinguish maternal active smoking defined by umbilical cord blood cotinine levels of 10 ng/mL or higher. The area under the curve was 0.99. The performance characteristics of dried blood spot cotinine are listed in Table 1. At a cutpoint of 10 ng/mL, dried blood spot cotinine had a sensitivity of 92.3% and a specificity of 99.1% in prediction of maternal active smoking close to the time of delivery. The sensitivity and specificity of dried blood spot cotinine were maximized at 97.8% and 98.2%, respectively, at the optimum dried blood spot cutpoint of 6 ng/mL.
Figure 4.
Receiver operating characteristic curve of cotinine levels in dried blood spots from newborns in measurement of maternal active smoking exposure close to the time of delivery (defined as cord blood cotinine ≥10 ng/mL) among 428 study subjects delivered in California in 2001–2003.
Table 1.
Test Performance Characteristics of Cotinine Level Cutpoints in Dried Blood Spots From Newborns in Measurement of Maternal Active Smoking Exposurea Close to the Time of Delivery Among 428 Subjects Delivered in California, 2001–2003
| Cutpoints, ng/mL | Sensitivity, % | Specificity, % | No. Confirmed Positive | No. False Positive | No. Confirmed Negative | No. False Negative |
|---|---|---|---|---|---|---|
| 3.1 | 97.8 | 96.7 | 89 | 11 | 326 | 2 |
| 4 | 97.8 | 97.3 | 89 | 9 | 328 | 2 |
| 5 | 97.8 | 97.9 | 89 | 7 | 330 | 2 |
| 6 | 97.8 | 98.2 | 89 | 6 | 331 | 2 |
| 7 | 95.6 | 98.5 | 87 | 5 | 332 | 4 |
| 8 | 94.5 | 98.5 | 86 | 5 | 332 | 5 |
| 9 | 93.4 | 98.8 | 85 | 4 | 333 | 6 |
| 10 | 92.3 | 99.7 | 84 | 1 | 336 | 7 |
| 11 | 90.1 | 99.7 | 82 | 1 | 336 | 9 |
| 12 | 86.8 | 99.7 | 79 | 1 | 336 | 12 |
| 13 | 84.6 | 99.7 | 77 | 1 | 336 | 14 |
| 14 | 83.5 | 100.0 | 76 | 0 | 337 | 15 |
a Active smoking was defined as cord blood cotinine levels of 10 ng/mL or more.
DISCUSSION
This study evaluated dried blood spot cotinine as a biomarker of maternal smoking close to the time of delivery. According to Florescu et al. (7), an adequate measurement method should have good validity and reliability and acceptable measurement error. Dried blood spot cotinine was found to predict umbilical cord blood cotinine well (Figure 2), indicating that dried blood spot cotinine is another valid biomarker of maternal smoking close to the time of delivery and is as good as umbilical cord blood cotinine. The strong linear correlation between the repeated measures of dried blood spot cotinine and the evidence of dried blood spot cotinine stability over short-term storage at room temperature in spiked dried blood spots and over long-term freezer storage in study samples indicates that dried blood spot cotinine is a reliable biomarker. The high rates of sensitivity and specificity reveal that dried blood spot cotinine is a biomarker with minimal measurement error. Collectively, these findings suggest that dried blood spot cotinine is an adequate measure of maternal smoking close to the time of delivery.
The optimal cutpoint to distinguish smokers from nonsmokers in a given population varies by smoking prevalence and behavior, racial/ethnic and gender distribution, and smoke-free regulations (28). The observed optimal cutpoint of dried blood spot cotinine in this study was 6 ng/mL, which is slightly lower than the widely used cutpoint of 10 ng/mL for maternal smoking during pregnancy (29). However, the sensitivity and specificity changed little when the cutpoint increased from 3.13 ng/mL to 10 ng/mL (Table 1) (sensitivity >90%, specificity >96%). We would therefore recommend 3.13–10 ng/mL as the optimal cutpoint range of dried blood spot cotinine to distinguish smokers from nonsmokers in our study population and possibly others.
This study indicates the occurrence of occasional false positive quantitation of cotinine in dried blood spot punches, potentially due to environmental contamination during specimen collection, handling, and/or testing. Duplicate tests are therefore recommended to minimize false positive quantitation. The priority of duplicated tests should be given to dried blood spots with quantitated cotinine levels less than 10 ng/mL, because the false positive quantitation is more likely to occur among those specimens. If only 1 punch of a dried blood spot could be used at a researcher's discretion, the false positive quantitation from unduplicated tests would lead to a contaminated exposed group, though the misclassification would be limited because cotinine levels from the first punch had only slightly worse specificity rates (89.9%–99.1% vs. 96.7%–100.0% for cutpoint range 3.13–14 ng/mL) and similar sensitivity rates (82.4%–97.8% vs. 83.5%–97.8% for cutpoint range 3.13–14 ng/mL) (results not presented) in prediction of maternal smoking close to the time of delivery compared with the averaged cotinine levels from both punches.
This study could not examine dried blood spot cotinine as a biomarker of maternal secondhand smoke because of the limited size of a dried blood spot. The quantitation limit of 3.13 ng/mL for a 6.35-mm dried blood spot punch is not sufficient to measure secondhand smoke. To reach a detection limit of 0.02 ng/mL by using liquid chromatography–tandem mass spectrometry for the measurement of a wide range of secondhand smoke exposure, approximately 17 dried blood spots would be required (based on the following: 12 µL blood can be extracted from one 6.35-mm punch (20); a maximum of 5 punches can be derived from a complete dried blood spot; and 17 dried blood spots are equal to 1 mL of whole blood). Currently, the newborn screening programs in the United States collect only 5 or 6 dried blood spots from newborns, and some of those spots are needed for routine newborn screening. It is therefore not feasible to use dried blood spot cotinine to measure maternal secondhand smoke because of insufficient quantity, even though the dried blood spot medium itself can be used to measure secondhand smoke given sufficient quantity.
There are limitations of dried blood spot cotinine as a measure of maternal smoking. First, dried blood spot cotinine can measure only recent maternal smoking because of the short half-life of cotinine (about 9 hours) in blood during pregnancy (30). It would certainly underestimate maternal smoking in earlier pregnancy and possibly close to the time of delivery if the mother does not smoke during a prolonged labor. Second, the accuracy of dried blood spot cotinine in measurement of maternal smoking is expected to be lower with increasing time of collection after delivery because of cotinine metabolism in the newborn (27). This study finds a statistically significant increase in the ratio of umbilical cord blood cotinine to dried blood spot cotinine with infant age at the time of dried blood spot specimen collection, which is consistent with the rate of cotinine elimination in newborns reported by Dempsey et al. (27). Third, the accuracy of dried blood spot cotinine levels could vary among racial/ethnic groups because of potential differences in cotinine metabolism in newborns by racial/ethnic group. The correlation between dried blood spot cotinine and umbilical cord blood cotinine was stronger in blacks (R2 = 0.88) compared with whites (R2 = 0.78), supporting this hypothesis. The higher correlation between dried blood spot and umbilical cord cotinine levels in blacks than in whites could be explained by a slower cotinine metabolism and longer half-life in black newborns, as observed in adults (28, 30). Fourth, this study could not prove the cotinine stability in dried blood spots over long-term storage at room or refrigerator temperatures. Many states do not store residual dried blood spots in a frozen state. According to a national survey in 2003, only 12 states banked dried blood spots in frozen storage (17). Fifth, adjacency contamination is a theoretical concern because dried blood spots on filter paper cards are stored next to each other (31), though evidence of contamination has never been documented for any analyte quantitated from residual dried blood spots. Blood on the filter paper was dried thoroughly before transportation and freezer storage, and the retrieved study samples did not present any moisture contamination. It is almost impossible for cotinine to migrate from a dried blood spot to an adjacent paper card.
This is the first study to investigate the validity, reliability, and measurement error of cotinine levels in frozen stored dried blood spots from newborns. The strengths of this study include the use of umbilical cord blood cotinine as the criterion standard instead of self-reported smoking as an approximation, the use of real samples collected and freezer-stored for about 10 years, and a sufficient sample size with varying cotinine levels. Results from this study provide instruction for the use of dried blood spot cotinine in future studies.
In conclusion, dried blood spot cotinine appears to be an adequate and accurate biomarker to objectively measure maternal active smoking close to the time of delivery. The major advantage of measuring cotinine in dried blood spots is their nearly universal collection and fairly wide-scale banking by newborn screening programs, allowing the conduct of large studies, as well as studies of rare diseases. California has banked more than 16 million newborn specimens from nearly all births in the state dating back to 1982. The large supply and ease of collection, handling, and transport of dried blood spots make these specimens a valuable and cost-efficient source and support the extensive use of dried blood spot cotinine as a biomarker of maternal tobacco exposure despite some limitations.
ACKNOWLEDGMENTS
Author affiliations: Sequoia Foundation, Richmond, California (Juan Yang, Michelle Pearl); Division of Clinical Pharmacology and Experimental Therapeutics, Departments of Medicine and Bioengineering and Therapeutic Sciences, University of California at San Francisco, San Francisco, California (Peyton Jacob III, Neal L. Benowitz, Lisa Yu, Christopher Havel); Division of Research, Kaiser Permanente Northern California, Oakland, California (Gerald N. DeLorenze); and Program Research and Demonstration Section, Genetic Disease Screening Program, California Department of Public Health, Richmond, California (Martin Kharrazi).
This study was funded by the California Tobacco-Related Disease Research Program (grant 17RT-0138). Project Baby's Breath and Effectiveness of a Large Prenatal Tobacco Reduction Program, which provided eligible subjects for this study, were also funded by the California Tobacco-Related Disease Research Program (grant 8RT-0115 to M.K. and G.N.D. and grant RT-0070 to M.P.). Laboratory staff and infrastructure at the University of California, San Francisco, were supported by the National Institutes of Health (grant P30 DA012393).
Dr. Steven Myers contributed to the study. Steve Graham and Oren Bergman conducted record linkage of screening, umbilical cord blood, and vital records data files. Angela DiLaura assisted with proposal preparation. Ester Nith and Deshante Carmichael-Lucas helped pull and ship dried blood spot and umbilical cord blood specimens.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the California Department of Public Health.
Conflict of interest: none declared.
REFERENCES
- 1.U.S. Department of Health and Human Services. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. [Google Scholar]
- 2.U.S. Department of Health and Human Services. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. The Health Consequences of Smoking: A Report of the Surgeon General. [Google Scholar]
- 3.Pichini S, Basagana X, Pacifici R, et al. Cord serum cotinine as a biomarker of fetal exposure to cigarette smoke close to delivery. Environ Health Perspect. 2000;108(11):1079–1083. doi: 10.1289/ehp.001081079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florescu A, Ferrence R, Einarson TR, et al. Reference values for hair cotinine as biomarker of active and passive smoking in women of reproductive age, pregnant women, children, and neonates: systematic review and meta-analysis. Ther Drug Monit. 2007;29(4):437–446. doi: 10.1097/FTD.0b013e318074df6e. [DOI] [PubMed] [Google Scholar]
- 5.Patrick DL, Cheadle A, Thompson DC, et al. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994;84(7):1086–1093. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Delaimy WK, Woodward JA. Is the hair nicotine level a more accurate biomarker of environmental tobacco smoke exposure than urine cotinine? J Epidemiol Community Health. 2002;56(1):66–71. doi: 10.1136/jech.56.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Florescu A, Ferrence R, Einarson T, et al. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology. Ther Drug Monit. 2009;31(1):14–30. doi: 10.1097/FTD.0b013e3181957a3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etter JF, Duc TV, Perneger TV. Saliva cotinine levels in smokers and nonsmokers. Am J Epidemiol. 2000;151(3):251–258. doi: 10.1093/oxfordjournals.aje.a010200. [DOI] [PubMed] [Google Scholar]
- 9.Al-Delaimy WK, Crane J, Woodward A. Questionnaire and hair measurement of exposure to tobacco smoke. J Expo Anal Environ Epidemiol. 2000;10(4):378–384. doi: 10.1038/sj.jea.7500102. [DOI] [PubMed] [Google Scholar]
- 10.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Stable EJ, Benowitz NL, Marin G. Is serum cotinine a better measure of cigarette smoking than self-report? Prev Med. 1995;24(2):171–179. doi: 10.1006/pmed.1995.1031. [DOI] [PubMed] [Google Scholar]
- 12.Abrams DB, Follick MJ, Biener L, et al. Saliva cotinine as a measure of smoking status in field settings. Am J Public Health. 1987;77(7):846–848. doi: 10.2105/ajph.77.7.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Britton GR, Brinthaupt J, Stehle JM, et al. Comparison of self-reported smoking and urinary cotinine levels in a rural pregnant population. J Obstet Gynecol Neonatal Nurs. 2004;33(3):306–311. doi: 10.1177/0884217504264866. [DOI] [PubMed] [Google Scholar]
- 14.Chan D, Caprara D, Blanchette P, et al. Recent developments in meconium and hair testing methods for the confirmation of gestational exposures to alcohol and tobacco smoke. Clin Biochem. 2004;37(6):429–438. doi: 10.1016/j.clinbiochem.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Eliopoulos C, Klein J, Phan MK, et al. Hair concentrations of nicotine and cotinine in women and their newborn infants. JAMA. 1994;271(8):621–623. [PubMed] [Google Scholar]
- 16.Ostrea EM, Jr, Knapp DK, Romero A, et al. Meconium analysis to assess fetal exposure to nicotine by active and passive maternal smoking. J Pediatr. 1994;124(3):471–476. doi: 10.1016/s0022-3476(94)70378-7. [DOI] [PubMed] [Google Scholar]
- 17.Olney RS, Moore CA, Ojodu JA, et al. Storage and use of residual dried blood spots from state newborn screening programs. J Pediatr. 2006;148:618–622. doi: 10.1016/j.jpeds.2005.12.053. [DOI] [PubMed] [Google Scholar]
- 18.Hannon WH, Boyle J, Davin B, et al. Wayne, PA: National Committee for Clinical Laboratory Standards; 1997. Blood Collection on Filter Paper for Neonatal Screening Erograms, 3rd Edition, Approved Standard, National Committee for Clinical Laboratory Standards Document A4A3. [Google Scholar]
- 19.Mei JV, Alexander JR, Adam BW, et al. Use of filter paper for the collection and analysis of human whole blood specimens. J Nutr. 2001;131(5 suppl):1631S–1636S. doi: 10.1093/jn/131.5.1631S. [DOI] [PubMed] [Google Scholar]
- 20.Sosnoff CS, Bernert JT. Analysis of cotinine in dried blood spots by LC APCI tandem mass spectrometry. Clin Chim Acta. 2008;388(1-2):228–229. doi: 10.1016/j.cca.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 21.Spector LG, Hecht SS, Ognjanovic S, et al. Detection of cotinine in newborn dried blood spots. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1902–1905. doi: 10.1158/1055-9965.EPI-07-0230. [DOI] [PubMed] [Google Scholar]
- 22.Kharrazi M, Pearl M, Yang J, et al. Evaluation of two birth certificate smoking questions [abstract] Am J Epidemiol. 2008;167(11 suppl):S35. [Google Scholar]
- 23.Cunningham GC, Tompkinson DG. Cost and effectiveness of the California Triple Marker Prenatal Screening Program. Genet Med. 1999;1(5):199–206. doi: 10.1097/00125817-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Kharrazi M, Pearl M, Yang J, et al. California Very Preterm Birth Study: design and characteristics of a population- and biospecimen bank–based nested case-control study. Paediatr Perinat Epidemiol. 2012;26(3):250–263. doi: 10.1111/j.1365-3016.2011.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob P, 3rd, Yu L, Duan M, et al. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(3-4):267–276. doi: 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watts RR, Langone JJ, Knight GJ, et al. Cotinine Analytical Workshop report: consideration of analytical methods for determining cotinine in human body fluids as a measure of passive exposure to tobacco smoke. Environ Health Perspect. 1990;84:173–182. doi: 10.1289/ehp.9084173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dempsey D, Jacob P, III, Benowitz NL. Nicotine metabolism and elimination kinetics in newborns. Clin Pharmacol Ther. 2000;67(5):458–465. doi: 10.1067/mcp.2000.106129. [DOI] [PubMed] [Google Scholar]
- 28.Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 29.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 30.Dempsey D, Jacob P, III, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301(2):594–598. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- 31.Olshan AF. Meeting report: the use of newborn blood spots in environmental research: opportunities and challenges. Environ Health Perspect. 2007;115(12):1767–1779. doi: 10.1289/ehp.10511. [DOI] [PMC free article] [PubMed] [Google Scholar]