Abstract
Chronic noncommunicable diseases (NCDs) are now prevalent in many low- and middle-income countries and confer a heightened risk of disability. It is unclear how public health programs can identify the older adults at highest risk of disability related to NCDs within diverse developing country populations. We studied nationally representative survey data from 7,150 Indian adults older than 50 years of age who participated in the World Health Organization Study on Global Aging and Adult Health (2007–2010) to identify population subgroups who are highly disabled. Using machine-learning algorithms, we identified sociodemographic correlates of disability. Although having 2 or more symptomatic NCDs was a key correlate of disability, the prevalence of symptomatic, undiagnosed NCDs was highest among the lowest 2 wealth quintiles of Indian adults, contrary to prior hypotheses of increased NCDs with wealth. Women and persons from rural populations were also disproportionately affected by nondiagnosed NCDs, with high out-of-pocket health care expenditures increasing the probability of remaining symptomatic from NCDs. These findings also indicate that NCD prevalence surveillance studies in low- and middle-income countries should expand beyond self-reported diagnoses to include more extensive symptom- and examination-based surveys, given the likely high rate of surveillance bias due to barriers to diagnosis among vulnerable populations.
Keywords: chronic disease, developing countries, disability, India, vulnerable populations
India is expected to face a higher burden of chronic disease than any other country in the world over the next decade. The rapid decline in the infant mortality rate since the early 1950s, as well as improved control of some key infectious diseases, has produced a significantly aging population in India, with over 16% of the population being older than 50 years of age as of 2010; the proportion over 50 years of age is expected to rise to 33% by the year 2050 (1). Within the next 2 decades, older adults (defined as those 50 years of age or older) are expected to bear nearly half of the total disease burden in India, mostly due to noncommunicable diseases (NCDs) (2).
To date, the data that have been used to evaluate who is most at risk for disability in developing countries like India and China has been criticized for being limited and of poor quality (3). Most assessments have used projections from small surveys conducted in isolated populations, generating criticism that many of our statistics about aging, disability, and NCDs do not reflect which subpopulations are most affected within highly heterogeneous countries (4). The dilemma of which populations should be targeted for assistance is a major problem facing public health departments with limited resources. A number of alternative theories have been put forth regarding the optimal public health response to aging, NCDs, and disability in developing countries. On one hand, it has been argued that NCDs and associated disabilities among older populations are diseases of affluence that are more common among high-income populations (5, 6). Conversely, it has been argued that populations such as the urban poor and rural women have not commonly been the subjects of NCD prevalence studies; the lack of access to health care among such groups may produce surveillance biases in surveys that rely on self-reported diagnostic history (7).
Our goal in the present study was to find and characterize which older adults in India face the highest burden of disability by identifying their sociodemographic characteristics to help public health programs target the most affected populations, particularly given the high rate of yet-undiagnosed NCDs. Our approach was to analyze individual-level longitudinal data from wave 1 of the recent World Health Organization (WHO) Study on Global Ageing and Adult Health (SAGE) to isolate the relationships between disability and chronic disease among adults older than 50 years of age in India (8). SAGE offers data on NCDs, disability, and sociodemographic characteristics in a nationally representative manner, using innovative techniques to detect previously undiagnosed NCDs (1).
MATERIALS AND METHODS
Survey methods
The SAGE survey was a standardized, validated household and individual survey conducted among adults aged 50 years or older with a comparison sample of younger adults aged 18 to 49 years. A multistage, stratified clustered sample design was used, as detailed in the Web Appendix (available at http://aje.oxfordjournals.org/); household clusters were sampled to reflect data from the Indian Census in terms of age, sex, level of wealth/local economic development, and urban/rural residency (9). Institutionalized populations were excluded. We examined the first wave of SAGE (the most current data available), which was conducted from 2007 to 2010, followed by data-checking and synthesis by WHO from 2011 through 2012 and public use data release in February 2013. Hence, our analysis is cross-sectional in nature.
The survey instrument consisted of a household roster with questions on dwelling characteristics, assets, and expenditures; an individual questionnaire on health status, risk factors, disability, work history, and chronic conditions; and a proxy questionnaire for individuals judged by the interviewer to require a proxy response from other members of the household because of sensory, motor, or cognitive limitations. Questionnaires were piloted in 2005 as part of a SAGE pretest among 469 respondents in India (8). Wealth was assessed through a tabulation of household ownership of durable goods (chairs, tables, cars, television, telephone, or washing machineor access to electricity), dwelling characteristics (type of floors, walls, and cooking stove), and access to services such as clean water, sanitation, and cooking fuel. This metric is less biased by respondent inconsistencies than simple reporting of income (10). Disability was assessed using the composite World Health Organization Disability Assessment Schedule 2.0, inverted (WHODASi) disability questionnaire, which captures 6 domains of day-to-day functioning in the last 30 days: understanding and communicating, getting around, self-care, getting along with people, life activities, and participation in society (see Web Appendix 1 for the full question list, which includes functional status questions for activities of daily living and instrumental activities of daily living). The instrument was validated in India prior to use (11).
To assess chronic disease status, SAGE included 3 sets of measurements. First, previously diagnosed NCDs, which are subject to diagnostic and recall biases, were assessed using the question, “Have you ever been told by a health professional that you have …?” or “Have you ever been diagnosed with …?” for each of 9 NCDs: angina, osteoarthritis, asthma/reactive airway disease, cataracts, chronic obstructive lung disease (chronic obstructive pulmonary disease/emphysema/chronic bronchitis), diabetes, depression, hypertension, and stroke. Commonly accepted terms in local languages, with explanations, were provided and validated in the pilot study against medical histories. Second, symptom-based diagnoses were assessed using validated symptom scales for angina (a Rose questionnaire (12, 13)), osteoarthritis (based on a receiver operating characteristic curve analysis what generated an algorithm for osteoarthritis diagnosis by symptoms (14)), depression (the Composite International Diagnostic Interview for depression (15)), and stroke (also through receiver operating characteristic curve analysis, (14)). Third, examination-based diagnoses were made during the survey itself using standardized protocols for visual problems using a Tumbling “E” logMAR chart (with corrective lenses on, if applicable), for chronic obstructive pulmonary disease using a series of 3 spirometry tests, and for hypertension through 3 repeated blood pressure assessments with an automated sphygmomanometer (16, 17). Web Appendix 1 contains full details of the validated symptom-based and examination-based diagnostic algorithms. The diagnosis of diabetes was not assessed by symptoms (given the absence of a validated symptomatic algorithm) or examination testing (given the absence of laboratory testing facilities). Respondents were also asked if they had ever received medical therapy for each chronic condition they reported, received therapy in the past year, and were currently taking medication or other treatment for each condition at the time of the interview. Further questions included metrics of out-of-pocket cost, as specified in Table 1.
Table 1.
Summary Sociodemographic Characteristics of the Studied Cohort, World Health Organization Study on Global Ageing and Adult Health, India, 2007–2010a
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| 50–59 | 3,492 | 48.8 |
| 60–69 | 2,183 | 30.5 |
| ≥70 | 1,475 | 20.6 |
| Sex | ||
| Male | 3,666 | 51.3 |
| Female | 3,484 | 48.7 |
| Wealth quintile | ||
| Lowest | 1,275 | 17.9 |
| Second | 1,374 | 19.3 |
| Middle | 1,335 | 18.8 |
| Fourth | 1,385 | 19.5 |
| Highest | 1,738 | 24.5 |
| Residence | ||
| Urban | 2,100 | 29.4 |
| Rural | 5,050 | 70.6 |
| Marital status | ||
| Never married | 60 | 0.8 |
| Currently married | 5,484 | 76.7 |
| Separated/divorced | 40 | 0.6 |
| Widowed | 1,566 | 21.9 |
| Health care insurance | ||
| Yes, mandatory | 143 | 2.0 |
| Yes, voluntary | 122 | 1.7 |
| Yes, both mandatory and voluntary | 14 | 0.2 |
| No, none | 6,872 | 96.1 |
| Total | 7,150 | 100.0 |
a All estimates used sample weights to correct for sampling probability and nonresponse. Web Table 5 provides the estimates disaggregated by sex. Note that wealth quintiles are imperfect when using the World Health Organization sampling weights because the weights were calculated using an older version of the Indian Census than was applicable during actual sampling; we chose not to recode the weights to force wealth quintile equality, as this recoding would generate subtle biases in the sex and residential (urban/rural) distributions rather than the more transparent skew towards higher wealth quintiles which is easier to interpret, as per World Health Organization guidelines and recent analyses (9, 23).
The questionnaire was translated from English into 8 local languages and administered face-to-face at participants’ homes via trained mobile survey staff. Standardized training, interview protocols, and quality assurance procedures were used across all participating sites (8). Each survey team comprised 1 male and 1 female interviewer and an additional person to conduct the anthropometric measurements. Site-based training for survey staff averaged 4.5 days across the sites. The median interview time was approximately 40 minutes for the household questionnaire and 120 minutes for the individual questionnaire. One household questionnaire was completed per household. A total of 12,198 individuals in 10,424 households were interviewed, including 7,150 individuals 50 years of age or older, with a 92% questionnaire response rate.
Statistical analysis
The goal of the present analysis was to identify sociodemographic correlates of disability as measured on the WHODASi scale. We first performed standard multivariate regressions on disability against the metrics in Appendix Table 1, which included metrics of sociodemographic status, chronic disease, and health care access/utilization. Furthermore, we adopted a machine-learning/data-mining algorithm known as regression tree analysis that does not become potentially biased in the presence of multiple collinear variables (e.g., wealth and education) (18). The algorithm tests multiple complex combinations of interactions among variables to identify a logical sequence of sociodemographic and health variables that are associated with higher or lower disability scores. As detailed in the prior literature (19), the algorithm sequentially partitions the sampled population to identify subgroups with significantly higher or lower disability scores than others using recursive partitioning to find an optimal factor that can divide the population into higher or lower disability score values with at least 50% sensitivity and 50% specificity. After choosing and splitting on the first optimal factor, the algorithm then separately searches each subgroup or branch of the first split for the next most optimal factor on which to divide the population, using all factors as potential candidates. This procedure is repeated to build subsequent branches to the tree until the subgroup samples become small (n < 100) or no further significant discriminating variables are found (at a conservative 2-sided P < 0.001 threshold, using a Bonferroni correction to prevent multiple testing error). The analysis was performed in R, version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria), and further details and associated statistical code for replication is provided in Web Appendix 1.
For the analysis, we used all metrics listed in Appendix Table 1 as regressors (including metrics). Participants with missing data were excluded, but SAGE survey sampling weights were applied to construct a nationally representative sample, taking into account unequal probabilities of sample selection resulting from the survey design, nonresponse, and noncoverage. Reported prevalence rates for the studied NCDs were age-standardized using the direct method against United Nations population estimates (20).
RESULTS
Table 1 provides summary statistics of sociodemographic characteristics among the analyzed cohort of Indian adults 50 years of age or older (see Web Table 1 for disaggregation by sex). The table reveals a relatively even split among age, sex, and wealth categories, a higher sampling of rural (71%) than urban respondents, a large (>20%) number of widows, and the majority of the population (>95%) without health care insurance coverage, all of which are consistent with Indian Census estimates of the country's population characteristics among adults 50 years of age or older (21).
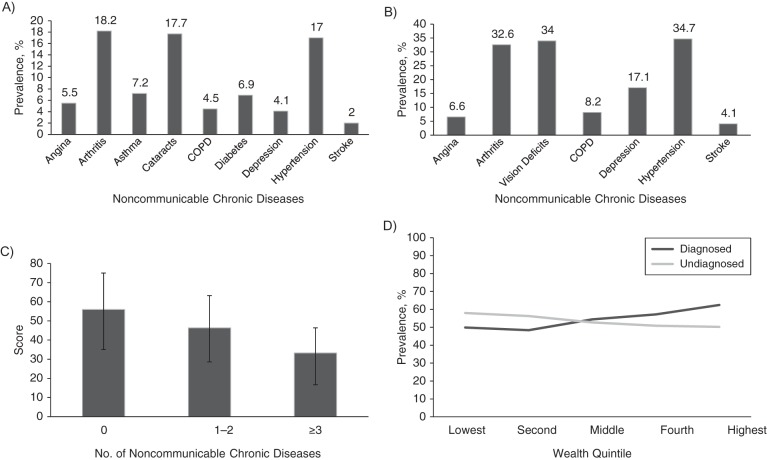
Figure 1 provides the data on NCD prevalence (previously diagnosed and symptom/examination-based) in the studied cohort, as well as levels of disability and details on the socioeconomic distribution of NCD prevalence. Arthritis was the most common previously diagnosed NCD (18.2% prevalence in adults aged 50 years or older), followed by cataracts (17.7%) and hypertension (17.0%). The prevalence of NCDs based on the survey's symptom-based and examination-based algorithms was, expectedly, higher than previously diagnosed NCD prevalence, given limited health care access in India. As shown in Figure 1, among the diagnoses for which symptom- or examination-based metrics were directly comparable to previous diagnoses (angina, arthritis, depression, hypertension, and stroke), the symptom/examination-based and previous-diagnosis metrics were most different for hypertension (17.0% of the sample with a previous diagnosis versus 34.7% hypertensive on examination; the examination did not detect those diagnosed with hypertension that was adequately controlled via medical therapy at the time of the examination) and osteoarthritis (18.2% of the sample with a previous diagnosis versus 32.6% who met osteoarthritis criteria by the validated symptom-based diagnostic algorithm).
Figure 1.
Noncommunicable diseases and disability among Indian adults aged 50 years or older, World Health Organization Study on Global Ageing and Adult Health, India, 2007–2010. A) Participants previously diagnosed angina, arthritis, asthma, cataracts, chronic obstructive pulmonary disease (COPD), diabetes, depression, hypertension, and stroke. B) Results of a symptom-based algorithm suggesting the presence of angina, arthritis, depression, or stroke and examination-based diagnosis of visual deficits, chronic lung disease, and hypertension. C) Rates of disability on transformed World Health Organization Disability Assessment Schedule 2.0 scale (0 indicates maximum disability/worst functioning ability and 100 indicated minimum disability/best functioning ability) among those who reported 0 noncommunicable diseases, 1–2 noncommunicable diseases, or 3 or more noncommunicable diseases (based on symptom/examination-based diagnosis), with error bars reflecting the interquartile range. D) Wealth disparities in previously diagnosed versus symptom/examination-based previously undiagnosed noncommunicable disease prevalence (% of population aged 50 years or older with at least 1 of the measured noncommunicable diseases).
There was surprisingly little overlap between individuals who reported previously diagnosed NCDs and those who screened positive on the symptom/examination-based NCD diagnostic metrics. For example, only 88 individuals who reported previously diagnosed angina met the clinical criteria for angina on symptom screening, whereas 274 others met symptom/examination-based criteria but denied being diagnosed previously (see Web Table 2 for correspondence tables by diagnosis). Less surprisingly, there was a significant wealth gradient between the 2 metrics, as shown in Figure 1 (P < 0.001 in the wealth-by-diagnosis interaction). Although previously diagnosed NCDs were most prevalent in the top 2 wealth quintiles, previously undiagnosed NCDs that were first diagnosed through the SAGE symptom/examination-based algorithms were most prevalent in the bottom 2 wealth quintiles.
Both previously diagnosed and previously undiagnosed persons had significantly greater disability scores than did those without any NCDs. Having 1 or more previously diagnosed NCD (48.9% of the studied population) was associated with a 9.6-point lower WHODASi score (worse disability) than having no diagnosed NCDs (P < 0.001) after incorporation of the sociodemographic control variables from Table 1 into the regression (see Table 2 for regression results). An approximately 5-point difference in WHODASi score corresponded to clinically meaningful variations in disability (22). Persons with more than 2 previously diagnosed NCDs (19.8% of the studied population) had a 10.6-point worse WHODASi score than did those with 1 or no NCDs after incorporation of the sociodemographic control variables (P < 0.001). Upon analysis of individual NCDs, stroke was associated with the greatest degree of disability (−12.6 points) and cataracts was associated with the lowest degree of disability (−4.5 points), although all individual NCDs were associated with significantly worse disability scores (see Web Tables 3 and 4 for disaggregated tables by individual NCD). When re-running the regressions using the symptom/examination-based metrics rather than previous diagnosis as a metric, the key differences in the results were higher prevalences of NCDs in persons with lower wealth and in more rural populations, but there was still a significant correlation between disability and having symptom/examination-based NCDs (Table 2 and Web Table 4).
Table 2.
Multivariate Regression on Disability (n = 6,521 Observations), World Health Organization Study on Global Ageing and Adult Health, India, 2007–2010a
| Model 1b |
Model 2c |
|||
|---|---|---|---|---|
| β | 95% CI | β | 95% CI | |
| Presence of >1 NCD vs. none | −9.78*** | −11.6, −7.99 | −8.64*** | −10.8, −6.48 |
| Age | −0.71*** | −0.80, −0.62 | −0.73*** | −0.83, −0.63 |
| Sexd | −10.9*** | −12.8, −8.95 | −10.6*** | −12.6, −8.58 |
| Wealth quintilee | 3.19*** | 2.57, 3.81 | 2.76*** | 2.13, 3.39 |
| Urban/rural residencef | −3.73** | −6.30, −1.17 | −3.15* | −5.88, −0.43 |
| Marital statusg | −2.53* | −4.74, −0.32 | −2.64* | −4.93, −0.34 |
| Health insurance statush | −5.04* | −9.36, −0.72 | −3.52 | −7.86, 0.82 |
| R2 | 0.215 | 0.200 | ||
Abbreviations: CI, confidence interval; NCD, noncommunicable diseases; WHODASi, World Health Organization Disability Assessment Schedule 2.0, inverted.
* P < 0.05, **P < 0.01, ***P < 0.001.
a Difference in WHODASi disability scale scores among those with and without NCDs, where lower values of the WHODASi indicate worse disability. Regression coefficients for age and wealth quintiles represent differences in disability score per category change. Significant interactions between sex and age were not observed in further analysis; using Akaike's Information Criterion, the addition of such interactions did not explain a significantly higher portion of the variance.
b Based on previous diagnosis of NCDs.
c Using symptom/examination-based diagnosis of NCDs.
d Dichotomized as 0 for male, 1 for female.
e Higher quintiles indicated wealthier subjects.
f Dichotomized as 0 for urban, 1 for rural.
g Dichotomized as 0 for married, 1 for not married.
h Dichotomized as 0 for insured, 1for uninsured.
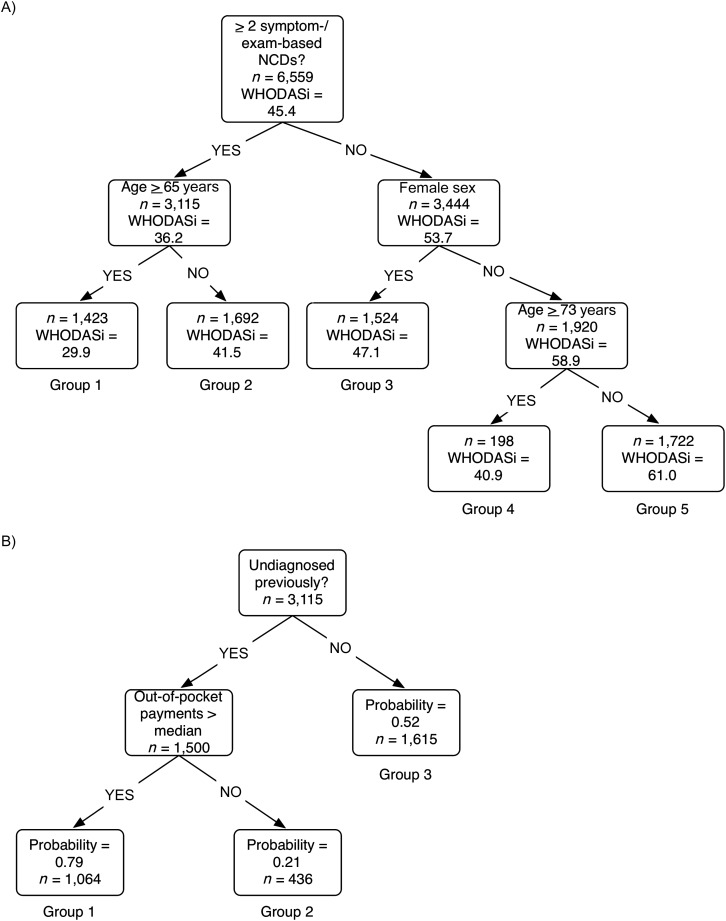
To further identify the best correlates of disability and their interactions, we performed a regression tree analysis on WHODASi disability scores (Figure 2A and Web Table 5). This data-mining approach revealed that the largest subdivision within the studied cohort using the regressors in Table 1 was between people experiencing more than 2 symptom/examination-based NCD diagnoses versus those experiencing fewer than 2, as depicted in Figure 2A. This metric separated the cohort into persons with a more severe mean disability score (36.2 or less for those with 2 or more symptom/examination-based diagnoses) and those with a less severe score (53.7 or higher). A second branch of the tree (shown on the left of Figure 2A) further separated individuals by age (greater or less than 65 years), such that the older group with more than 2 symptom/examination-based NCD diagnoses had the highest levels of overall disability (lowest mean WHODASi score of 29.9; labeled group 1 in Figure 2A). Those who had more than 2 NCD diagnoses but were less than 65 years of age were significantly less disabled (mean WHODASi score = 41.5; group 2). On the right side of the tree were the less disabled groups. Sex was a key branching correlate of disability among those with fewer than 2 symptom/examination-based NCD diagnoses. Women were significantly more disabled than were men (among women with less than 2 NCDs (group 3), WHODASi score = 47.1; among men, mean WHODASi score = 58.9), as shown in the second branch on the right in Figure 2A. Among men with fewer than 2 NCDs, age was the final correlate of disability, with respondents more than 73 years of having significantly more disability (WHODASi score = 40.9; group 4) than younger men (WHODASi score = 61.0; group 5). We further characterized the major sociodemographic characteristics of the groups having highest and lowest disability scores. As shown in Table 3, both groups were disproportionately rural, although the higher disability group was also older and predominantly female.
Figure 2.
Regression tree analysis of disability among older Indian adults (A) and probability of having 2 or more symptom/examination-based noncommunicable diseases (B), World Health Organization Study on Global Ageing and Adult Health, India, 2007–2010. Note that in the regression tree, the age cut points were determined by the algorithm. Age was entered as a continuous variable and the algorithm found optimal points for separation of the population into subgroups. A lower World Health Organization Disability Assessment Schedule 2.0, inverted (WHODASi) score reflects worse disability.
Table 3.
Sociodemographic Characteristics of Groups 1 (Greatest Disability) and 5 (Least Disability),a World Health Organization Study on Global Ageing and Adult Health, India, 2007–2010
| Characteristic | Group 1 |
Group 5 |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Presence of >1 NCD vs. none | ||||
| Previously diagnosed | 406 | 28.5 | 697 | 40.5 |
| On symptom/examination- based screening | 1,423 | 100.0 | 956 | 55.5 |
| Age, years | ||||
| 50–59 | 0 | 0 | 950 | 55.2 |
| 60–69 | 534 | 37.5 | 489 | 28.4 |
| ≥70 | 889 | 62.5 | 283 | 16.4 |
| Sex | ||||
| Male | 636 | 44.7 | 1,722 | 100.0 |
| Female | 787 | 55.3 | 0 | 0 |
| Wealth quintile | ||||
| Lowest | 338 | 23.8 | 246 | 14.3 |
| Second | 304 | 21.4 | 288 | 16.7 |
| Middle | 234 | 16.4 | 325 | 18.8 |
| Fourth | 241 | 16.9 | 367 | 21.3 |
| Highest | 299 | 21.0 | 484 | 28.1 |
| Residence | ||||
| Urban | 345 | 24.2 | 516 | 30.0 |
| Rural | 1,078 | 75.8 | 1,206 | 70.0 |
| Marital status | ||||
| Never married | 9 | 0.6 | 19 | 1.1 |
| Currently married | 801 | 56.3 | 1,589 | 92.3 |
| Separated/divorced | 13 | 0.9 | 3 | 0.2 |
| Widowed | 600 | 42.1 | 111 | 6.4 |
| Health care insurance | ||||
| Yes, mandatory | 12 | 0.8 | 54 | 3.2 |
| Yes, voluntary | 25 | 1.7 | 45 | 2.6 |
| Yes, both mandatory and voluntary | 2 | 0.1 | 2 | 0.1 |
| No, none | 1,385 | 97.3 | 1,621 | 94.1 |
Abbreviation: NCD, noncommunicable disease.
a Groups are from Figure 2.
Given that the regression tree analysis revealed that having at least 2 symptom/examination-based NCDs was a key correlate of disability, we further examined which sociodemographic factors would best predict who would fall into this category. Running the data-mining algorithm against the presence of 2 or more symptom/examination-based NCDs (Figure 2B), we found that not having been diagnosed with an NCD in the past was a significant determinant of the probability of having 2 or more symptom/examination-based NCD diagnoses. Among persons with undiagnosed NCDs, out-of-pocket expenditures comprised the next most explanatory correlate, with higher-than-median expenditures increasing the probability of having at least 2 symptom/examination-based NCDs to 0.79 (group 1 in Figure 2B), compared with a probability of 0.52 among those previously diagnosed (group 3) and 0.21 among those undiagnosed but having low out of pocket expenditures (group 2 in Figure 2B). The most susceptible group (group 1) was predominantly rural (74%) and in the lowest wealth quintile, with a 47% higher chance of being undiagnosed and having high out-of-pocket expenditures than the top wealth quintile even though all groups were almost universally uninsured.
DISCUSSION
In the present study, we detected and characterized which older adults in India face the highest burden of disability and identified their sociodemographic characteristics to help public health programs target the most affected populations. In addition to performing standard multivariate regressions on disability against metrics of sociodemographic and health variables, we used a machine-learning/data-mining algorithm, which does not become potentially biased in the presence of multiple collinear variables, to test all complex combinations of interactions among variables and to identify a logical sequence of variables that are associated with higher or lower disability scores. We found high levels of NCDs in the population of Indian adults who were 50 years of age or older, with previously diagnosed NCDs most prevalent in the top 2 wealth quintiles and previously undiagnosed NCDs most prevalent in the bottom 2 wealth quintiles. Having at least 2 symptom/examination-based NCDs was a key correlate of disability. Among those with previously undiagnosed NCDs, out of pocket expenditures were also an important explanatory factor for having symptomatic/examination-detectable NCDs. Women and rural populations were particularly affected by disability, underscoring the importance of including them in this research area. These groups have been excluded or undersampled in many NCD studies to date (4).
These results contribute significant new insights to the literature on disability in low- and middle-income countries such as India. Although there are notable variations among low- and middle-income countries, India serves as a health policy model for several other developing countries because of its rapid development and the extensive internal heterogeneity, which make it a natural country for understanding aging in low- and middle-income countries. Although prior studies in such countries have found that disability is common among older adults with chronic diseases, they did not clarify which subpopulations were particularly affected and required further public health investigations. Our use of a nationally representative survey adds detailed, comparable data across a broad spectrum of social variables to simultaneously analyze large, heterogeneous populations. In fact, our results suggest that surveys based on self-reported diagnoses of NCDs alone may be biased towards finding NCDs among wealthier groups in a manner that misses key lower-income groups with significant undiagnosed NCD burdens. The fact that the disease–disability relationship was similar whether NCDs were self-reported or symptom/examination-based, however, suggests that collection of less expensive self-reports (without symptom/examination) may be adequate for understanding correlates of disability, even though prevalence estimates are biased. Furthermore, the prior literature on disability has acknowledged that numerous social factors interact to produce chronic disability. Here, by applying the novel machine-learning approaches to regression to identify key correlates and their interactions, we were able to characterize complex interactions further and examine which commonly collected variables from sociodemographic and health surveys may be optimal for Indian public health officials to note as they conduct wider-scale screening to identify targets for disability support programs.
As with any survey-based study, however, the results of our study have several notable limitations. First, although symptom- and examination-based screenings are validated approaches for the detection of some NCDs, they were not possible for other NCDs, such as diabetes, and may not be directly comparable to office-based diagnostic approaches supplemented by laboratory study. Furthermore, low language or health literacy in some Indian populations may mean that self-reported NCD metrics may be biased downwards; this would serve to strengthen our concern that self-reported surveys alone may miss much of the NCD burden, artifactually producing the wealth disparities we noted between previously diagnosed and previously undiagnosed disease. Nevertheless, it is logical that differential health care access may also be playing a role.
Another limitation is that, although SAGE is a longitudinal study, here we only have data from symptom- and examination-based metrics that were performed in wave 1 of the study, as wave 2 and later waves remain to be completed and data from them have not yet been publicly released. Hence, we cannot attribute unidirectional causality between variables; we can only identify sociodemographic and health variables as associations and indicators of disability. There is likely a reverse causal pathway by which disability also contributes to higher NCD risk (e.g., disability increasing the risk of sedentary lifestyles, which in turn could increase the risk of additional NCDs), producing a vicious cycle between NCDs and disability. From a public health perspective, the “chicken or egg” issue of unidirectional causality can be viewed separately from the issue of how to identify and intervene among populations most vulnerable to chronic disability. Hence, our focus was on identifying and characterizing the indicators of disability that are commonly available in national health surveys that omit detailed functional assessments but include basic symptom and sociodemographic data. Commonly used theoretical frameworks for disability assume the main association is in the direction we have tested in this article (i.e., NCDs to disability) (10).
Despite these limitations, SAGE offers novel opportunities to investigate pathways of interaction between social variables, health states, and disability. As data from the longitudinal component of SAGE become available in the future, it will be possible to answer a few key hypotheses through further research. A key dimension for future research, given our results, is to understand what variations in social and health care circumstances, in addition to health behavior practices, may lead to variability between men and women in disability, as well as what factors particularly affect rural groups and determine out-of-pocket health care expenditures. This may require further ethnographic analysis and understanding of local health systems, which is not possible through the survey framework we adopted here.
In summary, the present study provides critical insights into the vast heterogeneity of disability within India, which is likely also relevant to other low- and middle-income countries in which disability and NCD prevalence studies to-date have typically focused on national average statistics without distinguishing the causes of varied vulnerability. As SAGE and other studies take place, we can identify factors that contribute to vulnerability and resilience, with the aim of targeting public health programs toward those most likely to suffer from chronic disabling states.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Stanford Prevention Research Center, Department of Medicine, School of Medicine, Stanford University, Stanford, California (Sanjay Basu, Abby King); and Department of Health Research & Policy, School of Medicine, Stanford University, Stanford, California (Abby King)
This work was supported by the National Institute on Aging (grant P30-AG017253) through the Stanford Center on the Demography and Economics of Health and Aging. The World Health Organization Study on Global Ageing and Adult Health was supported by the National Institute on Aging Division of Behavioral and Social Research through Interagency Agreements (OGHA 04034785, YA1323-08-CN-0020, and Y1-AG-1005-01) and grants (R01-AG034479 and R21-AG034263-0182) and by the World Health Organization's Department of Health Statistics and Information Systems. S.B. is also supported by the International Development Research Center of Canada and the Stanford Department of Medicine.
Conflict of interest: none declared.
Appendix Table 1.
Parameters Used in the Analysis, World Health Organization Study on Global Ageing and Adult Health, India, 2007–2010a
| Parameter Type | Metrics |
|---|---|
| Sociodemographic characteristics | Age (50–59, 60–69, or ≥70 years), sex, wealth quintile, urban/rural residence, educational level, and marital status |
| Noncommunicable diseases | (1) Self-reported prior diagnosis of angina, arthritis, asthma, cataracts, chronic lung disease (chronic obstructive pulmonary disease), diabetes, depression, hypertension, or stroke and (2) symptom-based prevalence of angina, arthritis, depression, and stroke supplemented by examination-based diagnosis of visual deficits, chronic lung disease, and hypertension. Further indicator variables were used to describe these diagnoses as undiagnosed but symptomatic on interview or evident on examination or diagnosed but untreated. |
| Disability | WHODAS 2.0, which evaluates 6 domains (2 items per domain) of day-to-day functioning in the last 30 days: understanding and communicating, getting around, self-care, getting along with people, life activities, and participation in society. Results from the 12 items were summed to get an overall WHODAS score, which was then transformed to a 0–100 scale, 0 indicating maximum disability/worst functioning ability and 100 indicating minimum disability/best functioning ability. |
| Health care | Whether the respondent had received outpatient care and/or inpatient care and how frequently, how often care was needed and how often was it sought, reasons for not going to health care providers despite perceived need, amount of out-of-pocket payment for last hospitalizations, and sources for health care payment, including self, spouse/partner, son/daughter, other family member, nonfamily member, insurance, and free of charge |
Abbreviation: WHODAS, World Health Organization Disability Assessment Schedule.
a Further details of the parameters shown and their definitions are presented in Web Appendix 1.
REFERENCES
- 1.He W, Muenchrath MN, Kowal P. Geneva, Switzerland: World Health Organization; 2012. Shades of Gray: A Cross-Country Study of Health and Well-Being of the Older Populations in SAGE Countries, 2007–2010. [Google Scholar]
- 2.Chatterji S, Kowal P, Mathers C, et al. The health of aging populations in China and India. Health Aff (Millwood) 2008;27(4):1052–1063. doi: 10.1377/hlthaff.27.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebrahim S, Pearce N, Smeeth L, et al. Tackling non-communicable diseases in low- and middle-income countries: is the evidence from high-income countries all we need? Plos Med. 2013;10(1):e1001377. doi: 10.1371/journal.pmed.1001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stuckler D, Siegel K. Sick Societies: Responding to the Global Challenge of Chronic Disease. Oxford, UK: Oxford University Press; 2011. [Google Scholar]
- 5.McKeown T. The Origins of Human Disease Continued. Basel, Switzerland: Blackwell Press; 1988. [Google Scholar]
- 6.Trowell HC, Burkitt DP. Western Diseases: Their Emergence and Prevention. Cambridge, MA: Harvard University Press; 1981. [Google Scholar]
- 7.Ezzati M, Vander Hoorn S, Lawes CM, et al. Rethinking the “diseases of affluence” paradigm: global patterns of nutritional risks in relation to economic development. Plos Med. 2005;2(5):e133. doi: 10.1371/journal.pmed.0020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Geneva, Switzerland: World Health Organization; 2011. Study on Global Ageing and Adult Health (SAGE) [Google Scholar]
- 9.Kowal P, Chatterji S, Naidoo N, et al. Data resource profile: the World Health Organization Study on Global Ageing and Adult Health (SAGE) Int J Epidemiol. 2012;41(6):1639–1649. doi: 10.1093/ije/dys210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography. 2001;38(1):115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 11.Sousa RM, Dewey ME, Acosta D, et al. Measuring disability across cultures—the psychometric properties of the WHODAS II in older people from seven low and middle income countries. The 10/66 Dementia Research Group population based survey. Int J Methods Psychiatr Res. 2010;19(1):1–17. doi: 10.1002/mpr.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oei HH, Vliegenthart R, Deckers JW, et al. The association of Rose questionnaire angina pectoris and coronary calcification in a general population: the Rotterdam Coronary Calcification Study. Ann Epidemiol. 2004;14(6):431. doi: 10.1016/j.annepidem.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Sorlie PD, Cooper L, Schreiner PJ, et al. Repeatability and validity of the Rose questionnaire for angina pectoris in the Atherosclerosis Risk in Communities Study. J Clin Epidemiol. 1996;49(7):719–725. doi: 10.1016/0895-4356(96)00022-4. [DOI] [PubMed] [Google Scholar]
- 14.Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 15.Kessler RC, Üstün TB. The World Mental Health (WMH) survey initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13(2):93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu S, Millett C. Social epidemiology of hypertension in middle-income countries: determinants of prevalence, diagnosis, treatment and control in the WHO SAGE study. Hypertension. 2013;62(1):18–26. doi: 10.1161/HYPERTENSIONAHA.113.01374. [DOI] [PubMed] [Google Scholar]
- 17.Halbert RJ, Natoli JL, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 18.Howard-Pitney B, Winkleby MA. Chewing tobacco: who uses and who quits? Findings from NHANES III, 1988–1994. National Health and Nutrition Examination Survey III. Am J Public Health. 2002;92(2):250–256. doi: 10.2105/ajph.92.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Therneau TM, Atkinson EJ. Rochester, MN: Mayo Foundation; 1997. An introduction to recursive partitioning using the RPART routines. Technical Report 61, Section of Biostatistics, Mayo Clinic http://www.mayo.edu/hsr/techrpt/61.pdf. Accessed July 12, 2013. [Google Scholar]
- 20.United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects, the 2010 Revision: Population by Age Groups and Sex. Geneva, Switzerland: United Nations; 2011. http://esa.un.org/wpp/excel-data/population.htm. Accessed March 26, 2013. [Google Scholar]
- 21.Registrar General & Census Commissioner. Census of India. Delhi, India: Ministry of Home Affairs; 2011. [Google Scholar]
- 22.Garin O, Ayuso-Mateos JL, Almansa J, et al. Validation of the “World Health Organization Disability Assessment Schedule, WHODAS-2” in patients with chronic diseases. Health Qual Life Outcomes. 2010;8(1):51. doi: 10.1186/1477-7525-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vellakkal S, Subramania SV, Millett C, et al. Socioeconomic inequalities in non-communicable diseases prevalence in India: disparities between self-reported diagnoses and standardized measures. Plos One. 2013;8(7):e68219. doi: 10.1371/journal.pone.0068219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.