Abstract
Kisspeptin (Kiss1) neurons are vital for reproduction. GnRH neurons express the kisspeptin receptor, GPR 54, and kisspeptins potently stimulate the release of GnRH by depolarising and inducing sustained action potential firing in GnRH neurons. As such Kiss1 neurons may be the pre-synaptic pacemaker neurons in the hypothalamic circuitry that controls reproduction. There are at least two different populations of Kiss1 neurons: one in the rostral periventricular area (RP3V) that is stimulated by oestrogens and the other in the arcuate nucleus that is inhibited by oestrogens. How each of these Kiss1 neuronal populations participate in the regulation of the reproductive cycle is currently under intense investigation. Based on electrophysiological studies in the guinea pig and mouse, Kiss1 neurons in general are capable of generating burst firing behavior. Essentially all Kiss1 neurons, which have been studied thus far in the arcuate nucleus, express the ion channels necessary for burst firing, which include hyperpolarization-activated, cyclic nucleotide gated cation (HCN) channels and the T-type calcium (Cav3.1) channels. Under voltage clamp conditions, these channels produce distinct currents that under current clamp conditions can generate burst firing behavior. The future challenge is to identify other key channels and synaptic inputs involved in the regulation of the firing properties of Kiss1 neurons and the physiological regulation of the expression of these channels and receptors by oestrogens and other hormones. The ultimate goal is to understand how Kiss1 neurons control the different phases of GnRH neurosecretion and hence reproduction.
Kisspeptin (encoded by the Kiss1 gene) and its cognate receptor (GPR54) are essential for gating the onset of puberty and regulating reproductive function in mammals (Seminara et al., 2003;De Roux et al., 2003). Kiss1 mRNA is expressed in the hypothalamic arcuate nucleus and rostral periventricular area (RP3V), which includes the anteroventral periventricular nucleus in rodents (AVPV) (Gottsch et al., 2004;Mikkelsen & Simonneaux, 2009;Bosch et al., 2012). Kiss1 neurons are thought to serve as relay neurons for mediating the negative and positive feedback effects of gonadal steroids on GnRH and LH secretion (Smith et al., 2005a;Smith et al., 2005b;Dungan et al., 2007;Glidewell-Kenney et al., 2008;Clarkson & Herbison, 2009;Oakley et al., 2009). Kiss1 neurons may also act as the presynaptic pacemaker neurons for driving GnRH neurons based on several lines of evidence. First, virtually all GnRH neurons express the kisspeptin receptor, GPR 54 (Parhar et al., 2004;Irwig et al., 2004;Bosch et al., 2013), which makes them a potential target of Kiss1 neuronal input (Clarkson et al., 2008;Mikkelsen & Simonneaux, 2009). Second, electrical stimulation of AVPV Kiss1 neurons increases the action potential firing of mouse GnRH neurons (Liu et al., 2011). Third, kisspeptin potently stimulates the release of GnRH (Gottsch et al., 2004) by depolarising and inducing the sustained firing of action potentials in GnRH neurons (Han et al., 2005;Liu et al., 2008;Dumalska et al., 2008;Zhang et al., 2008;Pielecka-Fortuna et al., 2008) Lastly, kisspeptin antagonists inhibit pulsatile GnRH/LH secretion (Roseweir et al., 2009). Collectively, these findings suggest that Kiss1 neurons are proximal (presynaptic) to GnRH neurons and are capable of driving GnRH neuronal activity.
Role of pacemaker current (Ih) and T-type calcium current (IT) in burst firing
If Kiss1 neurons are the presynaptic pacemaker neurons that drive GnRH neurons, then they should exhibit the biophysical properties shared by other pacemaker-type neurons in hypothalamus, thalamus, and subthalamic nucleus (Loose et al., 1990;McCormick & Huguenard, 1992;Erickson et al., 1993a;Erickson et al., 1993b;Bevan & Wilson, 1999;Lyons et al., 2010;Qiu et al., 2011). In these nuclei, burst firing is generated primarily by the hyperpolarization-acitvated, non-selective cation “h”-current (Ih) and the low threshold, transient “T”-type calcium channel current (IT) (for review, see (Zagotta & Siegelbaum, 1996;Lüthi & McCormick, 1998). Ih is mediated by the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel family, which includes channel subtypes HCN 1-4. The T-type calcium current, IT, is mediated by the low-threshold voltage-gated calcium channels, CaV3.1-3.3 (for review see (Perez-Reyes, 2003). The T-type Ca2+ subtypes are ordered according to their inactivation kinetics, where CaV3.1 has the fastest and CaV3.3 has the slowest inactivation kinetics. In a bursting model, Ih depolarises neurons from hyperpolarised potentials, raising the membrane potential into the range of IT activation (Erickson et al., 1993a;Kelly & Rønnekleiv, 1994;Lüthi & McCormick, 1998). Once activated, IT initiates a prolonged Ca2+-driven depolarisation above threshold, which has been termed the low threshold spike (Tsien et al., 1987;Llinás, 1988). This depolarisation then drives the neuron to fire an ensemble of Na+-driven action potentials, which constitutes the burst firing. It is this burst firing that facilitates peptide (kisspeptin) release that is so vital for activating GnRH neurons, pubertal development and reproductive competence.
Although single action potential-generated calcium influx is sufficient to spark the release of classical neurotransmitters, burst firing or tetanic stimulation is required for the release of neuropeptides (Bicknell, 1988;Shakiryanova et al., 2005;Masterson et al., 2010). Twenty years ago we showed that Ih and IT contribute to the phasic burst firing of guinea pig supraoptic vasopressin neurons (Erickson et al., 1993a;Erickson et al., 1993b). These findings were not limited to the magnocelluar neurosecretory neurons; guinea pig tuberoinfundibular (A12) dopamine (parvocellular neurosecretory) neurons were also found to express these critical pacemaker conductances (Loose et al., 1990). Recently, these findings were corroborated by Swedish researchers who determined that rat tuberoinfundibular dopamine neurons (identified post hoc with dual neurobiotin/avidin-immunoctyochemical staining for tyrosine hydroxylase) also exhibit rhythmic burst firing (Lyons et al., 2010). One would predict that rat tuberoinfundibular dopamine neurons also express Ih and IT, but Lyons and colleagues did not do voltage clamp experiments to determine if the rat dopamine neurons express Ih and IT. It is well known that the parvocellular neurosecretory GnRH neurons exhibit burst firing and express both Ih and IT (Suter et al., 2000;Zhang et al., 2007;Zhang et al., 2009;Chu et al., 2010). Therefore, expression of Ih and IT may be endogenous properties of hypothalamic “bursting” neurons. Most importantly, 17β-oestradiol (E2) treatment (a paradigm that produces a LH surge in mice) up-regulates the expression of T-type calcium channel (Cav3.1, Cav3.2 and Cav3.3) transcripts in GnRH neurons, which translates into a greater whole-cell T-type current (Zang et al., 2009) (Zhang et al., 2009). In all of these above mentioned studies, the T-type calcium channels are blocked with low concentrations of Ni 2+, which is a property of these low-voltage activated Ca2+ channels (Catterall et al., 2005).
To characterize the conductances in guinea pig and mouse kisspeptin neurons, a combination of whole-cell voltage clamp recordings and single-cell RT-PCR (scRT-PCR) were used in the hypothalamic slice preparation to determine whether Kiss1 neurons in arcuate nucleus exhibit burst firing and express the channels and currents necessary for generating this activity (Qiu et al., 2011;Gottsch et al., 2011).
Biophysical properties of guinea pig Kiss1 neurons and response to glutamate
The initial studies were done in female guinea pigs in late follicular phase by targeting arcuate neurons using “blind patch” recording, and subsequently identifying the neurons post hoc using scRT-PCR or immunocytochemistry (Qiu et al., 2011). Of the 190 recorded neurons, 69 (36%) of these were identified as Kiss1 neurons. Guinea pig Kiss1 neurons in the arcuate nucleus exhibited a resting membrane potential of −59 mV and Rin = 770 MΩ, and the vast majority (80%) of these cells expressed both Ih and IT. Although adjacent POMC and NPY/AgRP neurons express one or both of these pacemaker conductances, neither express such large amplitude currents (Kelly et al., 1990;Van den Top et al., 2004). The T-type calcium current was blocked with mibefradil (10 μM) and the h-current was blocked with ZD 7288 (50 μM) to pharmacologically verify the channel types. Since the vast majority of Kiss1 neurons expressed these pacemaker currents, it was of interest to see if these critical “relay” neurons in the reproductive circuit would exhibit burst firing behavior. Indeed, the glutamate receptor agonist N-methyl-D-aspartate (NMDA, 20 μM) reliably induced burst firing activity in 78% of guinea pig Kiss1 neurons, essentially the same percentage that express Ih and IT. The NMDA-induced bursting activity was similar to that reported for midbrain dopamine neurons (Seutin et al., 1994). Therefore, Kiss1 neurons in the guinea pig arcuate nucleus express the endogenous pacemaker conductances (T-type calcium and h-currents) and can be driven via glutamatergic inputs to generate burst firing activity.
Biophysical properties of mouse Kiss1 neurons in the arcuate nucleus
A substantial step forward was the development of a of Kiss1-CreGFP knock-in mouse by Robert Steiner and Richard Palmiter at U. Washington (Gottsch et al., 2011) making it possible to target GFP-expressing Kiss1 neurons. As we would have predicted based on the guinea pig studies, both Ih and IT were prominent in Kiss1 neurons in the mouse arcuate nucleus, and these neurons also expressed the critical transcripts (HCN and Cav3). Based on quantitative PCR of pooled Kiss1 neurons, HCN 2, which is the most sensitive to cyclic nucleotide gating (Craven & Zagotta, 2006), was expressed at 2.5 fold higher levels than HCN 1 and about 50% higher levels than HCN 3. Moreover, Cav3.1 channel transcripts were expressed at higher levels than Cav3.2, and Cav3.3 was not detected in the mouse Kiss1 neurons. The mouse Kiss1 neurons in the arcuate nucleus of ovariectomized females exhibit a resting membrane potential of −64 mV (Gottsch et al., 2011) and −75 mV for castrated males (Navarro et al., 2011). The relatively more negative resting membrane potential for mouse versus guinea pig Kiss1 neurons in the arcuate nucleus (−59 mV) probably contributes to the higher percentage (~50%) of silent (no spontaneous activity) neurons in this species (Gottsch et al., 2011;de Croft et al., 2012).
Although mouse Kiss1 neurons in the arcuate nucleus are silent or display low irregular firing patterns (Gottsch et al., 2011;de Croft et al., 2012), they are essentially “one synapse” away from generating burst firing activity. The glutamate agonist NMDA induced burst firing behaviour in all of Kiss1 neurons in the arcuate nucleus. This was demonstrated in both the loose-cell attached mode, in which there was no disruption of the intracellular constituents, and in whole-cell current clamp recordings (Figure 1). In the presence of tetrodotoxin (TTX) to block the fast Na+ spikes, the membrane oscillations (up and down states) induced by NMDA were clearly visible. The oscillatory behaviour that underlies the burst of Na+ action potentials appears to be an endogenous property of these Kiss1 neurons. A recent report has shown that neurokinin B (NKB) will induce burst firing behavior in male Kiss1 (Kiss1-Cre) neurons in the arcuate nucleus (Navarro et al., 2011), and the actions of NKB were antagonized by a neurokinin 3 receptor antagonist (Alreja, 2013). Kiss1 neurons co-localize NKB and dynorphin; hence, the origin of the “KNDy” acronym (Lehman et al., 2010). The autoregulatory excitation by NKB may be a mechanism by which Kiss1 neurons synchronise to excite GnRH neurons. Certainly in vivo multiunit recordings in the arcuate nucleus region of KNDy neurons in the goat would suggest that there is some synchronous burst firing behaviour (Ohkura et al., 2009).
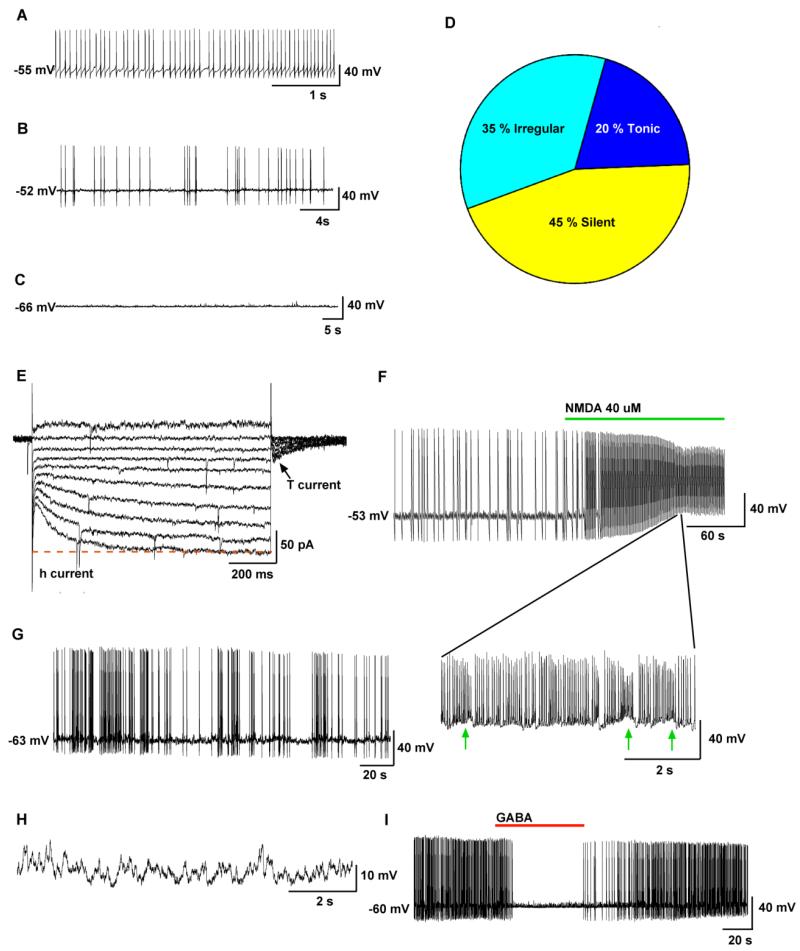
Figure 1.
Electrophysiological characteristics of arcuate Kiss1 neurons in oil-treated, ovariectomised Kiss1-CreGFP mice using whole-cell patch recording. Kiss1 neurons in the ARC (of the female) rest at −63.8 ± 2.3 mV (n=20) (A-C) Representative traces of action potentials recorded from arcuate Kiss1 neurons showing tonic (A), irregular (B) and silent (C) firing patterns. (D) Summary pie chart of the firing pattern distribution in Kiss1 neurons of the arcuate nucleus (n=20). (E-H) Endogenous conductances and NMDA-induced burst firing of an ARC Kiss1 neuron. (E) Ensemble of currents in response to depolarising/hyperpolarising steps from −50 to −140 mV illustrating the expression of a hyperpolarisation-activated cyclic nucleotide-gated cation current (h-current) and a T-type Ca2+ current (arrow) in a representative Kiss1 neuron. Vhold = −60 mV. (F) Current clamp recording in a Kiss1 neuron of the arcuate nucleus showing the response to NMDA (40 μM). The action potential firing was expanded to illustrate the pronounced effects of NMDA on burst firing activity of Kiss1neurons highlighting the ensemble of Na+ spikes riding on top of low-threshold spikes (arrows). (G) In a current clamped state close to the resting membrane potential, one can see the bursting activity in the presence of NMDA. (H) In the presence of tetrodotoxin (TTX) to block the fast Na+ spikes, one can clearly see the membrane oscillations (up and down states) induced by NMDA. Eighty percent of ARC Kiss1 neurons expressed the endogenous conductances (h-current, T-current) that are critical for generating burst firing activity. Although Kiss1 neurons in the arcuate nucleus did not exhibit spontaneous burst firing activity, glutamate (NMDA) was able to induce burst firing activity in all of the cells. (I) GABA (100 μM) inhibited firing in another arcuate kisspeptin neuron. Drugs were rapidly perfused into the bath as a 100μl bolus.
[From Gottsch et al., Molecular Properties of Kiss1 Neurons in the Arcuate Nucleus of the Mouse, Endocrinology 152(11): 4298-4309, Copyright 2011, The Endocrine Society]
Kiss1 neurons in the arcuate nucleus exhibit a relatively negative resting membrane potential (−64 mV in mouse, −59 mV in guinea pig), but this membrane potential is not hyperpolarised enough to recruit a critical fraction of T-type calcium channels for generating burst firing (Zhang et al., 2009). The vast majority of the h-current is activated at even more negative potentials (Chu et al., 2010). Thus, some sort of inhibitory synaptic input is necessary for reaching these nadirs in the membrane potentials,and γ-amino butyric acid (GABA) hyperpolarises Kiss1 neurons (Qiu et al., 2011;Gottsch et al., 2011), thereby providing a critical inhibitory feedback circuit for generating rhythmic burst firing (McCormick & Huguenard, 1992;Bevan & Wilson, 1999). GABA neurons are abundant in the arcuate nucleus (Wagner et al., 1999), and a subset of Kiss1 neurons expresses GAD (Cravo et al., 2011). As such, Kiss1 neurons may themselves be an endogenous source of GABA, whose action could be autoinhibitory and perhaps be responsible for hyperpolarising Kiss1 neurons via GABAB receptors in addition to the actions of dynorphin via kappa opioid receptors (Navarro et al., 2009;Wakabayashi et al., 2010). Therefore, Kiss1 neurons in the Kiss1-Cre mouse express the same pacemaker conductances as the Kiss1 neurons in the female guinea pig (identified by scRT-PCR) and under the appropriate inputs have the capacity to rhythmically burst fire.
Properties of mouse Kiss1 neurons in the AVPV/RP3V
Kiss1 neurons in the AVPV project to GnRH neurons in the preoptic area (POA) (Clarkson & Herbison, 2006) and have been identified as excitatory afferents to the GnRH neurons (Liu et al., 2011). Kisspeptins are the most potent agonist to induce GnRH neuronal firing (Han et al., 2005;Zhang et al., 2008;Pielecka-Fortuna et al., 2008). Although cell-attached recordings of Kiss1 neurons in the AVPV have been reported (Ducret et al., 2010;de Croft et al., 2012), studies are forthcoming characterising the biophysical and molecular properties of these neurons. One recent study characterised some of the elementary properties, limited to the resting membrane potential, input resistance and cell capacitance, but did not elucidate any of the critical endogenous conductances for generating burst firing activity (Frazão et al., 2013).
Using a Kiss1-Cre/GFP mouse to target Kiss1 neurons in the AVPV/RP3V (Cravo et al., 2011), Frazao and colleagues described two distinct populations of AVPV/RP3V Kiss1 neurons in males and females: Type I neurons fired irregularly and exhibited a RMP of −50 mV; Type II neurons were silent and rested at −67 mV (Frazão et al., 2013). The negative RMP of Type II neurons appeared to depend on the expression of the KATP channel since blockade of the channel activity with the sulfonylurea drug tolbutamide depolarised the Type II neurons to a membrane potential equivalent to the RMP of Type I neurons. However, Frazao and colleague did not report that any of the Kiss1-Cre/GFP neurons in the AVPV/RP3V exhibited spontaneous burst firing activity in the presence of the KATP channel blocker. For comparison, Frazao et al. (Frazão et al., 2013) measured the firing activity of Kiss1 neurons in the arcuate nucleus and found comparable firing rates as the AVPV/RP3V, but the Kiss1 neurons in the arcuate nucleus did not exhibit a bimodal distribution of resting membrane potentials. In contrast in loose cell attached recording studies, the Herbison lab reported that 20-25% of AVPV/RP3V Kiss 1 neurons in wild type (C57BL/6J) mice, identified post hoc by immunocytochemical staining, exhibited burst firing with a small increase in burst firing activity during proestrous (Ducret et al., 2010). The differences between the Frazao et al. study and the Ducret et al. study could be due the experimental approach (whole-cell versus cell-attached recording) or the fact that some of the recordings in the Kiss1-Cre/GFP mouse were done from “ectopic” Kiss1-expressing neurons as originally indicated (Cravo et al., 2011). 17β-oestradiol treatment of ovariectomised females shifted the firing pattern of AVPV/RP3V Kiss1 neurons from quiescent (Type II) to spontaneously firing (Type I). However, steroid treatment also increased the “IPSC” amplitude, which is difficult to reconcile with an increase in firing, unless the GABAA input is excitatory as has been reported for the actions of GABAA agonists on GnRH neurons (Herbison & Moenter, 2011). In support of the excitatory GABAA input is the fact that female prepubertal AVPV/RP3V Kiss1 neurons also exhibited more “IPSCs,” yet all of these neurons were spontaneously active (Frazão et al., 2013). Again, no spontaneous burst firing was reported in these initial recordings, but clearly additional voltage-clamp studies are warranted.
Kiss1 neurons respond to hormones
It has been hypothesised that mouse Kiss1 neurons in the arcuate neurons are a target for the actions of leptin based on the findings that these neurons express the leptin receptor (LRb) (Smith et al., 2006). However, studies had not shown a direct action of leptin to activate (excite) Kiss1 neurons. We hypothesised that leptin would depolarize kisspeptin neurons via activation of a nonselective cationic current as previously described for proopiomelanocortin (POMC) neurons (Qiu et al., 2010). Indeed, leptin (100 nM) depolarizes and increases the firing frequency of Kiss1 neurons by two fold (Qiu et al., 2011). Similar to mouse POMC neurons (Qiu et al., 2010), the leptin-activated current (measured under voltage-clamp conditions) reverses near −15 mV, indicative that leptin activates a non-selective cationic current. Since in POMC neurons leptin activates canonical transient receptor potential (TRPC) channels, it follows that these channels were also conducting the cation current in Kiss1 neurons.
In heterologous expression systems micromolar concentrations of lanthanum (La3+) potentiate TRPC4 and TRPC5 channels (Strübing et al., 2001).In arcuate Kiss1 neurons, La3+ (100 μM) potentiates the leptin-induced current in Kiss1 neurons by about two-fold. Also, the relatively selective TRPC channel blocker 2-aminoethyldiphenylborinate (2-APB, 100 μM) (Clapham et al., 2005) completely abrogates the effects of leptin. Therefore, the physiological and pharmacological characterisation indicates that TRPC4 and 5 channels are involved in the leptin-mediated depolarisation of Kiss1 neurons. The pharmacological experiments were followed by molecular studies in which the expression of TRPC1, 4, 5 channel transcripts were measured in guinea pig Kiss1 neurons using scRT-PCR. In the analysis of 114 guinea pig Kiss1 neurons, identified by Kiss1 mRNA expression, it was found that TRPC1 mRNA was expressed in 60 % of the cells, TRPC4 mRNA in 20% of the cells and TRPC5 mRNA in 64% of the cells, indicating that TRPC1 and 5 are the primary channel transcripts in Kiss 1 neurons. Moreover, dual immunocytochemical analysis of tissue sections through the arcuate nucleus of the guinea pig revealed that TRPC 5 protein was expressed in Kiss1 neurons (Qiu et al., 2011). Collectively, these findings suggest that arcuate Kiss1 neurons are sensitive to leptin activation via TRPC 5 channels.
It is known that LRb is coupled to the janus kinase (Jak2)- phosphotidylinositide 3 kinase (PI3K) signaling pathway, and this pathway will activate TRPC channels in mouse POMC neurons (Qiu et al., 2010). Subsequently it was shown in guinea pig Kiss1 neurons, that the Jak inhibitor AG490 (10 μM) potently blocks the effects of leptin. In addition, PI3K is essential for leptin-induced neuronal activation (Hill et al., 2008; Qiu et al. 2010) (Hill et al., 2008;Qiu et al., 2010), and it is also critical for the membrane insertion of TRPC channels (Bezzerides et al., 2004). In fact, the selective PI3K inhibitor wortmannin (100 nM) completely abrogates the TRPC current in Kiss1 neurons. This indicates that LRb couples to Jak2 to activate PI3K in guinea pig Kiss1 neurons. Moreover, the selective phospholipase C-gamma (PLCγ) inhibitor ET-18-OCH3 (15 μM) potently blocks the effects of leptin (Qiu et al., 2011), similar to its actions in POMC neurons (Qiu et al., 2010). Again, molecular studies followed the physiological/pharmacological analysis, and scRT-PCR revealed that LRb and PLCγ1 transcripts are expressed in guinea pig Kiss1 neurons. Therefore, it appears that Kiss1 neurons in the arcuate nucleus of the guinea pig are a target for the actions of leptin, and suggests that this population of Kiss1 neurons are critical relay neurons in conveying energy status to GnRH neurons and are vital for pubertal development (Dungan et al., 2006).
Kiss1-GnRH neuronal communication—from channels to circuits
A recent publication by Catterall et al. (Catterall et al., 2012) reminded readers of the Hodgkin-Huxley heritage and the fact that investigators must move from studying channels to exploring circuits. What is critical for the control of GnRH neuronal excitability and ultimately the control of fertility is the hypothalamic circuitry. This “circuitry” not only includes the excitatory and inhibitory synaptic input onto Kiss1 and GnRH neurons, but also the effects of circulating hormones like 17β-oestradiol and leptin that convey vital feedback information about reproductive and energy states, respectively. Over twenty years ago we identified the conductances (before the channels were cloned) first in the tuberoinfundibular dopamine neurons and then in vasopressin neurons that were critical for generating burst firing behaviour (see Kelly & Rønnekleiv, 1994). These conductances (Ih, IT) were also described in thalamic neurons about the same time (McCormick & Huguenard, 1992). Although GnRH neurons express both of these endogenous pacemaker conductances (Zhang et al., 2007;Zhang et al., 2009;Chu et al., 2010), GnRH neurons are not the sole pacemaker neurons in the circuitry. It is apparent that Kiss 1 neurons, which highly express both Ih and IT, will generate burst firing with very little provocation—i.e., excitatory glutamate input (Figure 1). Therefore, the future challenge is to not only identify all of key players (channels) and how they are regulated—e.g., HCN and Cav3 channels are highly up-regulated by 17β-oestradiol treatment (Zhang et al., 2009;Bosch et al., 2013)—but also how these channels and perhaps other channels fit into the Kiss1-GnRH neuronal circuitry for generation of burst firing.
New findings.
What is the topic for this review?
Analysis of the electrophysiological properties of hypothalamic kisspeptin neurons.
What advances does it highlight?
Molecular and cellular electrophysiological analysis of kisspeptin neurons reveals that they express the critical pacemaker channels for generating burst firing activity.
Acknowledgements
The work in the authors’ laboratories was supported by the National Institutes Health R01 grants NS 38809, NS 43330 and DK 68098. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. The authors would like to acknowledge the excellent technical support of Ms. Martha A. Bosch.
References
- Alreja M. Electrophysiology of kisspeptin neurons. In: Kauffman AS, Smith JT, editors. Adv Exp Med Biol. Springer; New York: 2013. pp. 349–362. [DOI] [PubMed] [Google Scholar]
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- Bicknell RJ. Optimizing release from peptide hormone secretory nerve terminals. J exp Biol. 1988;139:51–65. doi: 10.1242/jeb.139.1.51. [DOI] [PubMed] [Google Scholar]
- Bosch MA, Tonsfeldt KJ, Ronnekleiv OK. mRNA expression of ion channels in GnRH neurons: subtype-specific regulation by 17β-Estradiol. J Mol Cell Endocrinol. 2013;367:85–97. doi: 10.1016/j.mce.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch MA, Xue C, Ronnekleiv OK. Kisspeptin expression in guinea pig hypothalamus: Effects of 17β-estradiol. J Comp Neurol. 2012;520:2143–2162. doi: 10.1002/cne.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International union of pharmacology. XLVIII. nomenclature and structure-function relationship of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Raman IM, Robinson HPC, Sejnowski TJ, Paulsen O. The hodgkin-huxley heritage: from channels to circuits. J Neurosci. 2012;32:14064–14073. doi: 10.1523/JNEUROSCI.3403-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Takagi H, Moenter SM. Hyperpolarization-activated currents in gonadotropin-releasing hormones (GnRH) neurons contribute to intrinsic excitability and are regulated by gonadal steroid feedback. The Journal of Neuroscience. 2010;30:13373–13383. doi: 10.1523/JNEUROSCI.1687-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D, Julius D, Montell C, Schultz G. International union of pharmacology. XLIX. nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endo. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinizing surge. J Neuroendocrinol. 2009;21:305–311. doi: 10.1111/j.1365-2826.2009.01835.x. [DOI] [PubMed] [Google Scholar]
- Craven KB, Zagotta WN. CNG and HCN channels: two peas, one pod. Annu Rev Phys. 2006;68:375–401. doi: 10.1146/annurev.physiol.68.040104.134728. [DOI] [PubMed] [Google Scholar]
- Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, Rovinsky S, Frazão R, Lee CE, Gautron L, Zigman JM, Elias CF. Characterization of Kiss1 neurons using transgenic mouse models. Neurosci. 2011;173:37–56. doi: 10.1016/j.neuroscience.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Croft S, Piet R, Mayer C, Mai O, Boehm U, Herbison AE. Spontaneous kisspeptin neuron firing in the adult mouse reveals marked sex and brain region differences but no support for a direct role in negative feedback. Endo. 2012;153:1–10. doi: 10.1210/en.2012-1616. [DOI] [PubMed] [Google Scholar]
- De Roux N, Genin E, Carel J-C, Matsuda F, Chaussain J-L, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS 1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducret E, Gaidamaka G, Herbison AE. Electrical and morophological characteristics of anteroventral periventricular nucleus kisspeptin and other neurons in the female mouse. Endocrinolgy. 2010;151:2223–2232. doi: 10.1210/en.2009-1480. [DOI] [PubMed] [Google Scholar]
- Dumalska I, Wu M, Morozova E, Liu R, Van den Pol AN, Alreja M. Excitatory effects of the puberty-initiating peptide kisspeptin and group I metabotropic glutamate receptor agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J Neurosci. 2008;28:8003–8013. doi: 10.1523/JNEUROSCI.1225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endo. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci. 2007;27:12088–12095. doi: 10.1523/JNEUROSCI.2748-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KR, Rønnekleiv OK, Kelly MJ. Electrophysiology of guinea-pig supraoptic neurones: Role of a hyperpolarization-activated cation current in phasic firing. J Physiol (Lond) 1993a;460:407–425. doi: 10.1113/jphysiol.1993.sp019478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KR, Rønnekleiv OK, Kelly MJ. Role of a T-type calcium current in supporting a depolarizing potential, damped oscillations, and phasic firing in vasopressinergic guinea pig supraoptic neurons. Neuroendo. 1993b;57:789–800. doi: 10.1159/000126438. [DOI] [PubMed] [Google Scholar]
- Frazão R, Cravo RM, Donato J, Jr, Ratra DV, Clegg DJ, Elmquist JK, Zigman JM, Williams KW, Elias CF. Shift in kiss1 cell activity requires estrogen receptor α. J Neurosci. 2013;33:2807–2820. doi: 10.1523/JNEUROSCI.1610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Weiss J, Hurley LA, Levine JE, Jameson JL. Estrogen receptor α signaling pathways differentially regulate gonadrotropin subunit gene expression and serum follicle-stimulating hormone in the female mouse. Endo. 2008;149:4168–4176. doi: 10.1210/en.2007-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endo. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, Kelly MJ, Ronnekleiv OK, Sanz E, McKnight GS, Clifton DK, Palmiter RD, Steiner RA. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinol. 2011;152:4298–4309. doi: 10.1210/en.2011-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S-K, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABAA receptor activation on gonadotrophin-releasing hormone neurons: towards an emerging consensus. Endocrinology. 2011;23:557–569. doi: 10.1111/j.1365-2826.2011.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signalng in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuronendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Loose MD, Rønnekleiv OK. Opioids hyperpolarize β-endorphin neurons via μ-receptor activation of a potassium conductance. Neuroendo. 1990;52:268–275. doi: 10.1159/000125597. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Rønnekleiv OK. Electrophysiological analysis of neuroendocrine neuronal activity in hypothalamic slices. In: Levine JE, editor. Methods in Neurosciences: Pulsatility in Neuroendocrine Systems. Academic Press, Inc; San Diego: 1994. pp. 47–67. [Google Scholar]
- Lehman MN, Coolen LM, Goodman RL. Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endo. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone (GnRH) neurons through a phospholipase C / calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008;149:4605–4614. doi: 10.1210/en.2008-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Porteous R, d’Anglemont de Tassigny X, Colledge WH, Millar R, Petersen SL, Herbison AE. Frequency-dependent recruitment of fast amino acid and slow neuropeptide neurotransmitter release controls gonadotropin-releasing hormone neuron excitability. The Journal of Neuroscience. 2011;31:2421–2430. doi: 10.1523/JNEUROSCI.5759-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Loose MD, Rønnekleiv OK, Kelly MJ. Membrane properties and response to opioids of identified dopamine neurons in the guinea pig hypothalamus. J Neurosci. 1990;10:3627–3634. doi: 10.1523/JNEUROSCI.10-11-03627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi A, McCormick DA. H-current: properties of a neuronal and network pacemaker. Neuron. 1998;21:9–12. doi: 10.1016/s0896-6273(00)80509-7. [DOI] [PubMed] [Google Scholar]
- Lyons DJ, Horjales-Araujo E, Broberger C. Synchronized network oscillations in rat tuberoinfundibular dopamine neurons: swith to tonic discharge by thyrotropin-releasing hormone. Neuron. 2010;65:217–229. doi: 10.1016/j.neuron.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Masterson SP, Li J, Bickford ME. Frequencey-dependent release of substance P mediates heterosynaptic potentiation of glutamatergic synaptic responses in the rat visual thalamus. J Neurophysiol. 2010;104:1758–1767. doi: 10.1152/jn.00010.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Huguenard JR. A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol. 1992;68:1384–1400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Simonneaux V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides. 2009;30:26–33. doi: 10.1016/j.peptides.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Wu M, García-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Rønnekleiv OK, Braun RE, Plamiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrionolgy. 2011;152:4265–4275. doi: 10.1210/en.2011-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda K-I, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinology. 2009;21:813–821. doi: 10.1111/j.1365-2826.2009.01909.x. [DOI] [PubMed] [Google Scholar]
- Parhar IS, Ogawa S, Sakuma Y. Laser-captured single digoxigenin-labeled neurons of gonadotropin-releasing hormone types reveal a novel G protein-coupled receptor (Gpr54) during maturation in cichlid fish. Endocrinlogy. 2004;145:3613–3618. doi: 10.1210/en.2004-0395. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Fang Y, Bosch MA, Rønnekleiv OK, Kelly MJ. Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology. 2011;152:1503–1514. doi: 10.1210/en.2010-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. Leptin exceites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci. 2010;30:1560–1565. doi: 10.1523/JNEUROSCI.4816-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29:3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MBL, Crowley WF, Aparicio SAJR, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Seutin V, Johnson SW, North RA. Effect of dopamine and baclofen on N-methyl-D-aspartate-induced burst firing in rat ventral tegmental neurons. Neuroscience. 1994;58:201–206. doi: 10.1016/0306-4522(94)90167-8. [DOI] [PubMed] [Google Scholar]
- Shakiryanova D, Tully A, Hewes RS, Deitcher DL, Levitan ES. Activity-dependent liberation of synaptic neuropeptide vesicles. Nat Neurosci. 2005;8:173–178. doi: 10.1038/nn1377. [DOI] [PubMed] [Google Scholar]
- Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005a;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005b;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Suter KJ, Wuarin JP, Smith BN, Dudek FE, Moenter SM. Whole-cell recordings from preoptic/hypothalamic slices reveal burst firing in gonadotropin-releasing hormone neurons identified with green fluorescent protein in transgenic mice. Endocrinology. 2000;141:3731–3736. doi: 10.1210/endo.141.10.7690. [DOI] [PubMed] [Google Scholar]
- Tsien RW, Hess P, McCleskey EW, Rosenberg RL. Calcium channels: mechanisms of selectivity, permeation, and block. Ann Rev Biophys Biophys Chem. 1987;16:265–290. doi: 10.1146/annurev.bb.16.060187.001405. [DOI] [PubMed] [Google Scholar]
- Van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Bosch MA, Kelly MJ, Rønnekleiv OK. A powerful GABAB receptor-mediated inhibition of GABAergic neurons in the arcuate nucleus. NeuroReport. 1999;10:2681–2687. doi: 10.1097/00001756-199908200-00045. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K-I, Steiner RA, Okamura H. Nuerokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. The Journal of Neuroscience. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta WN, Siegelbaum SA. Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express KATP channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci. 2007;27:10153–10164. doi: 10.1523/JNEUROSCI.1657-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK. 17β-estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2009;29:10552–10562. doi: 10.1523/JNEUROSCI.2962-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008;28:4423–4434. doi: 10.1523/JNEUROSCI.5352-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]