Abstract
Bacterial enoyl-acyl carrier protein reductase (FabI) is a promising novel antibacterial target. We isolated a new class of FabI inhibitor from Penicillium chrysogenum, which produces various antibiotics, the mechanisms of some of them are unknown. The isolated FabI inhibitor was determined to be meleagrin by mass spectroscopy and nuclear magnetic resonance spectral analyses, and its more active and inactive derivatives were chemically prepared. Consistent with their selective inhibition of Staphylococcus aureus FabI, meleagrin and its more active derivatives directly bound to S. aureus FabI in a fluorescence quenching assay, inhibited intracellular fatty acid biosynthesis and growth of S. aureus, and increased the minimum inhibitory concentration for fabI-overexpressing S. aureus. The compounds that were not effective against the FabK isoform, however, inhibited the growth of Streptococcus pneumoniae that contained only the FabK isoform. Additionally no resistant mutant to the compounds was obtained. Importantly, fabK-overexpressing Escherichia coli was not resistant to these compounds, but was resistant to triclosan. These results demonstrate that the compounds inhibited another target in addition to FabI. Thus, meleagrin is a new class of FabI inhibitor with at least one additional mode of action that could have potential for treating multidrug-resistant bacteria.
Introduction
Multidrug-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococci, and vancomycin-resistant S. aureus have become an important global health concern [1], [2]. One approach to combat antibiotic resistance is to identify new drugs that can function through novel mechanisms of action. One such target is bacterial type 2 fatty acid synthesis (FASII), which is essential for bacterial cell growth [3]–[5].
FASII is conducted by a set of individual enzymes, whereas mammalian fatty acid synthesis is mediated by a single multifunctional enzyme-acyl carrier protein (ACP) complex referred to as type I. Enoyl-ACP reductase catalyzes the final and rate-limiting step of the chain-elongation process of the FASII. Four isoforms have been reported for enoyl-ACP reductase. FabI is highly conserved among most bacteria, including S. aureus and Escherichia coli. Streptococcus pneumoniae contains only FabK, whereas Enterococcus faecalis and Pseudomonas aeruginosa contain both FabI and FabK, and Bacillus subtilis contains both FabI and FabL. Recently, the FabV isoform was isolated from Vibrio cholera, Pseudomonas aeruginosa, and Burkholderia mallei [6], [7]. No analogue protein is present in mammals for similar transformation; thus, FabI inhibitors should not interfere with mammalian fatty acid synthesis. Because of these properties, FabI is an attractive target for antibacterial drug development [8], [9]. As drugs with single targets such as rifampicin and fosfomycin are particularly vulnerable to mutational resistance [10], FabI-specific inhibitors also have a tendency to develop resistance in bacteria by mutations that alter the drug-binding site. FabI is known to be the main target for triclosan and isoniazid, which have been used in consumer products and for treating tuberculosis, respectively [11], [12]. Triclosan-resistant bacteria and isoniazid-resistant M. tuberculosis are highly prevalent because of point mutations in their FabI genes [13]–[15]. In addition, rapid mutation development has been often reported in synthetic FabI inhibitors [16]. Thus, it has been recently emphasized that ideal antibiotics should bind to multiple targets [17].
Many FabI inhibitors have been reported from high-throughput screening of existing compound libraries. However, most are not suitable for the development of new antibiotics because of their lack of permeability into cell membranes and efflux in addition to their high mutational frequency [18]. The problem with such screening results lies in the compound libraries, which are systematically biased. Microorganisms produce diverse antibiotics that function in an antagonistic capacity in nature where they have competition. Most antibacterial agents in clinical use today are either microbial products or analogs [19]. A few FabI inhibitors have been reported from microorganisms [20]–[22], and most of these are phenolic compounds. Therefore, more unique FabI inhibitors need to be obtained from microorganisms.
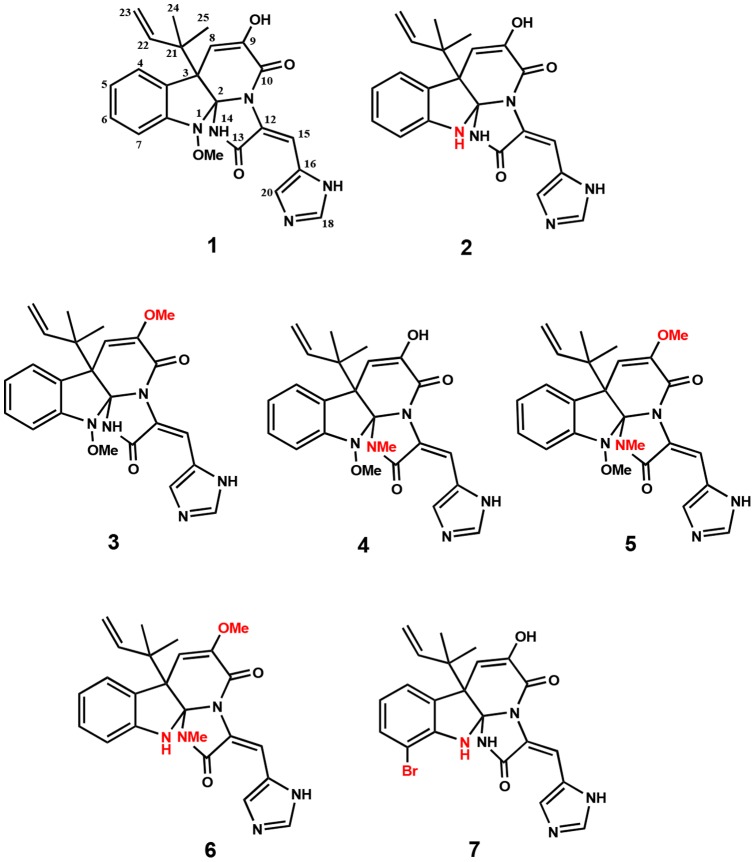
During our continued screening for FabI inhibitors from microbial metabolites, we found meleagrin (1) with a druggable structure during solid-state fermentation of a seashore slime-derived Penicillium chrysogenum, a penicillin-producing species (Figure 1). Here, we report the isolation and analog preparation of meleagrin, in addition to its inhibition of FabI isoforms and whole cells of various pathogenic bacteria, target validation, and its multitarget effect.
Figure 1. Meleagrin (1) and its chemically prepared derivatives.
Materials and Methods
Bacterial strains
The bacterial strains used in the antibacterial activity assays were obtained from the Culture Collection of Antimicrobial Resistant Microbes of Korea and the Korean Collection for Type Cultures. The pump-negative (tolC) E. coli EW1b was obtained from the E. coli Genetic Stock Center of Yale University.
Screening and isolation of compound 1
Over 25,000 microbial extracts composed of actinomycetes and fungi were screened against S. aureus FabI and confirmed through a target-based whole cell assay by using fabI-overexpressing S. aureus. This analysis led to the identification of compound 1 from fungal strain F717 (Fig. 1). Compound 1 was isolated from the fermented whole medium of the fungal strain F717, which was isolated from seashore slime collected at Daechun beach, Chungcheongnam-do, Korea. The strain was identified as Penicillium chrysogenum based on standard biological and physiological tests and taxonomic determination. Seed culture was conducted in a liquid culture medium containing 2% glucose, 0.2% yeast extract, 0.5% peptone, 0.05% MgSO4, and 0.1% KH2PO4 (pH 5.7 before sterilization). A sample of the strain from a mature plate culture was inoculated into a 500-mL Erlenmeyer flask containing 80 mL of the above sterile seed liquid medium and cultured on a rotary shaker (150 rpm) at 28°C for 3 days. Subsequently, 5 mL of the seed culture was transferred into 500-mL Erlenmeyer flasks (54 flasks) containing 80 g of bran medium, which was cultivated for 7 days at 28°C to produce the active compound. The culture solid state was extracted with 80% acetone, and the extract was concentrated in vacuo to an aqueous solution. The aqueous solution was then extracted 3 times with an equal volume of ethyl acetate (EtOAc). The EtOAc extract was concentrated in vacuo to dryness. The crude extract was subjected to SiO2 (Merck Art No. 7734.9025) column chromatography followed by stepwise elution with CHCl3-MeOH (100∶1, 50∶1, and 10∶1). The active fractions eluted with CHCl3-MeOH (50∶1) were pooled and concentrated in vacuo to give an oily residue. The residue was applied again to a Sephadex LH-20 and then eluted with CHCl3-MeOH (1∶1). The active fraction was dissolved in MeOH and was further purified by reverse-phase high-performance liquid chromatography (20×150 mm; YMC C18) by using a photodiode array detector. The column was eluted using MeOH: H2O (75∶25) at a flow rate of 5 mL/min to afford compound 1 with >99% purity at a retention time of 19.4 min. The chemical structure of compound 1 was determined to be meleagrin [23] by mass spectroscopy (MS) and nuclear magnetic resonance (NMR) spectra as follows: [α]D = −96.7° (c = 0.04, MeOH); HRESI-MS: m/z 434.18463 (M+H)+, C23H23N5O4 requires 434.18228; 1H-NMR (600 MHz, DMSO-d6): 8.30 (1H, s, NH-19), 8.17 (1H, s, H-15), 7.77 (1H, s, H-20), 7.53 (1H, d, J = 7.5, H-4), 7.34 (1H, s, H-18), 7.25 (1H, t, J = 7.5, H-6), 7.03 (1H, t, J = 7.5, H-5), 6.96 (1H, d, J = 7.5, H-7), 6.00 (1H, brs, H-22), 5.25 (1H, s, H-8), 5.01 (1H, d, J = 17.1, Ha-23), 4.98 (1H, d, J = 9.0, Hb-23), 3.66 (3H, s, 1-OCH3), 1.19 (6H, s, CH3-24 and 25), 13C-NMR (150 MHz, DMSO-d6): 165.0 (C-13), 158.6 (C-10), 146.2 (C-7a), 143.3 (C-22), 142.7 (C-9), 137.6 (C-20), 134.1 (C-18), 127.8 (C-6), 126.0 (C-3a), 125.9 (C-16), 124.7 (C-4), 123.7 (C-12), 123.1 (C-5), 112.8 (C-23), 111.6 (C-7), 109.2 (C-8), 106.7 (C-15), 101.5 (C-2), 64.8 (1-OCH3), 52.2 (C-3), 41.8 (C-21), and 23.0 (C-24 and 25).
Preparation of derivatives of compound 1
Several derivatives of 1 were obtained by chemical modification of functional groups such as hydroxyl and amine groups (Fig. 1). Demethoxylation of compound 1 afforded glandicolin A (2) together with compound 7 as a byproduct. Methylation of compound 1 produced oxaline (3), N14-methylmeleagrin (4), and O,N14-dimethylmeleagrin (5). O,N14-dimethylglandicolin (6) was obtained by methylation of compound 2. Details regarding the preparation procedures and spectral data of compounds 2–7 are presented in Information S1.
FabI and FabK assay
S. aureus FabI and E. coli FabI enzymes were cloned, overexpressed, and purified as described previously [24]. The wild-type fabK gene was amplified by PCR from genomic DNA obtained from Streptococcus pneumoniae KCTC 5412 by using the primers 5′-GGAAACCATATGAAAACGCGTATTACGAA-3′ and 5′-CCGCTCGAGGTCATTTCTTACAACTCCTGT-3′, which contained NdeI and XhoI restriction sites, respectively. After the DNA sequence was confirmed, the gene was cloned into the pET22b vector (Novagen, Gibbstown, NJ, USA). The construct was transformed into E. coli BL21 (DE3) for expression following induction with isopropylthiogalactoside. The C-terminal His-tagged protein was purified as described previously [24]. Assays were conducted in half-area, 96-well microtiter plates. The compounds were dissolved in DMSO and evaluated in 100-μL assay mixtures containing components specific for each enzyme (see below). Reduction of the trans-2-octenoyl N-acetylcysteamine (t-o-NAC thioester) substrate analog was measured spectrophotometrically following the utilization of NADH or NADPH at 340 nm at 30°C for the linear period of the assay. S. aureus FabI assays contained 50 mM sodium acetate (pH 6.5), 200 μM t-o-NAC thioester, 200 μM NADPH, and 150 nM S. aureus FabI.
NADH was used as a cofactor rather than NADPH for the E. coli FabI assay. Substrate concentrations used for the Lineweaver–Burk plot were 100, 200, 300, and 400 μM, whereas the concentrations of the cofactor were 100, 200, 400, and 600 μM. The rate of decrease in the amount of NADPH in each reaction was measured with a microtiter enzyme-linked immunosorbent assay (ELISA) reader by using the SOFTmax PRO software (Molecular Devices, Sunnyvale, CA, USA). The inhibitory activity was calculated according to the following formula: % of inhibition = 100× [1− (rate in the presence of compound/rate in the untreated control)]. IC50 values were calculated by fitting the data to a sigmoid equation. An equal volume of DMSO solvent was used for the untreated control. FabK assays contained 100 mM sodium acetate (pH 6.5), 2% glycerol, 200 mM NH4Cl, 50 µM t-o-NAC thioester, 200 µM NADH, and 150 nM S. pneumoniae FabK.
Fluorescence quenching assay
Fluorescence spectra were measured using a SHIMADZU fluorescence spectrophotometer (model RF-5310PC). S. aureus FabI (15 ng/μl) was incubated with different concentrations of triclosan (1, 2, 4, 8, and 16 nM in PBS buffer) and compounds 1, 5, or 7 (10, 20, 40, 80, and 160 nM in PBS buffer). Protein quenching was monitored at 25°C by using 5-nm excitation and 5-nm emission wavelength. The excitation wavelength was 280 nm, and the emission spectra were measured between 290 and 430 nm.
Determination of minimum inhibitory concentrations (MICs)
Whole-cell antimicrobial activity was determined by broth microdilution as described previously [21]. The test strains except for S. pneumoniae were grown to mid-log phase in Mueller–Hinton broth and diluted 1,000-fold in the same medium. Cells (105/mL) were inoculated into Mueller–Hinton broth and dispensed at 0.2 mL/well into a 96-well microtiter plate. S. pneumoniae was grown in tryptic soy broth supplemented with 5% sheep blood. MICs were determined in triplicate by serial 2-fold dilutions of test compounds. The MIC was defined as the concentration of a test compound that completely inhibited cell growth during a 24-h incubation at 30°C. Bacterial growth was determined by measuring the absorption at 650 nm by using a microtiter ELISA reader.
Measurement of the inhibition of macromolecular biosynthesis
To monitor the effects of compound 1 on lipid, DNA, RNA, protein, and cell wall biosynthesis, its effects on the incorporation of [1-14C] acetate (50 mCi/mmol), [2-14C] thymidine (59.8 mCi/mmol), [U-14C] uridine (539 mCi/mmol), L-[U-14C] leucine (306 mCi/mmol) or L-[U-14C] isoleucine (329 mCi/mmol), and N-acetyl-d-[1-14C] glucosamine (58.1 mCi/mmol) into S. aureus and S. pneumoniae were measured as described previously [21]. S. aureus was exponentially grown to an A650 of 0.2 in Mueller–Hinton broth. S. pneumoniae was grown in tryptic soy broth supplemented with 5% sheep blood. Each 1-mL culture was treated with drugs at 2 times the MIC for 10 min. An equal volume of DMSO solvent was added to the untreated control. After incubation with the radiolabeled precursors at 37°C for 1 h, followed by centrifugation, the cell pellets were washed twice with PBS buffer. After acetate incorporation, the total cellular lipids were extracted with chloroform-methanol-water. The incorporated radioactivity in the chloroform phase was measured by scintillation counting. For the other precursors, incorporation was terminated by adding 10% (w/v) TCA and cooling on ice for 20 min. The precipitated material was collected on Whatman GF/C glass microfiber filters, washed with TCA and ethanol, dried, and counted using a scintillation counter. The total counts incorporated at 1 h of incubation without inhibitors ranged from >7,000 for [U-14C] uridine to <13,000 for [1-14C] acetate. The inhibition of radiolabeled precursor incorporation was calculated using the following formula: % inhibition = 100× [1− (radioactivity values of the treated samples/control (no antibacterial) values)]. In all experiments, known antibacterial agents were included as positive controls.
Frequency of the spontaneously resistant mutant
The frequency of spontaneous resistance was determined for S. aureus RN4220, S. aureus KCTC 1916, and E. coli KCTC 1942. E. coli KCTC 1942 is highly sensitive to antibiotics. The organisms were grown to log-phase by dilution of an overnight culture in fresh media and re-incubation at 35°C until the cultures reached a cell density of approximately 109 CFU/mL. A volume of 100 μl of the bacterial suspension was then applied to solid media containing 4× MIC of 1, 5, or triclosan. Inocula were determined by applying 100 μl of 10-fold dilutions on solid media without drug. Colony-forming units were counted after 48 h incubation at 35°C. The ratio of the number of colonies on drug-containing plates to that on control plates was calculated as the in vitro frequency of isolation of CFU.
Overexpression assay
An overexpression assay using S. aureus RN4220, S. aureus RN4220 (pE194), and S. aureus RN4220 (pE194-fabI) was conducted to perform target validation of FabI inhibitors as described previously [21]. Additionally, both fabI- and fabK-overexpressing E. coli were constructed to test a multitarget effect of the compounds. The wild-type fabI gene from the genomic DNA of E. coli W3110 was amplified by PCR by using the primers 5′-ATGGGTTTTCTTTCCGGTAAGCGCA-3′ and 5′-TTTCAGTTCGAGTTCGTTCATT-3′. The wild-type fabK gene from the genomic DNA of S. pneumoniae KCTC 5412 was amplified by PCR by using the primers 5′-ATGAAAACGCGTATTACA-3′ and 5′-GTCATTTCTTAC AACTCCTGTCCA-3′. The resulting products were cloned into the pBAD-TOPO TA expression vector (Invitrogen, Carlsbad, CA, USA) to yield the pBAD-fabI and pBAD-fabK recombinant plasmids, which placed the expression of the genes fabI and fabK, respectively, under the control of the arabinose promoter [25]. Recombinant pBAD-fabI and pBAD-fabK were then introduced into the pump-negative (tolC) E. coli EW1b via electroporation to generate E. coli EW1b (pBAD-fabI) and E. coli EW1b (pBAD-fabK), respectively.
Results
Isolation of meleagrin as a new FabI inhibitor
A FabI inhibitor was isolated from Penicillium chrysogenum F717, which is known as a penicillin-producing species. MS and NMR spectral analyses of the inhibitor revealed that it was meleagrin (1) (Fig. 1). Compound 1 inhibited both E. coli and S. aureus FabI with IC50 values of 33.2 and 40.1 μM, respectively (Table 1). To determine whether compound 1 selectively inhibited FabI, its effect on FabK, which is the enoyl-ACP reductase of S. pneumoniae, was examined. Compound 1 did not inhibit S. pneumoniae FabK even at 200 μM, which indicates that it is selective for FabI.
Table 1. Comparison of the inhibitory effects of meleagrin (1) and its derivatives against Staphylococcus aureus and E. coli FabI, bacterial growth, and [14C] acetate and [14C] leucine incorporation into membrane fatty acids.
| Compounds | IC50 (μM) | MIC (μg/mL) | IC50 (μM) | |||||
| saFabI | ecFabI | spFabK | S. aureus a | E. coli b | S. pneumoniae c | [14C] acetate incorporation | [14C] leucine incorporation | |
| 1 | 40.1 | 33.2 | >200 | 64 | 32 | 64 | 40.3 | >200 |
| 2 | 54.6 | 49.7 | >200 | 64 | 64 | 64 | - | - |
| 3 | 38.7 | 38.8 | >200 | 64 | 32 | 64 | 33.5 | >200 |
| 4 | 48.0 | 33.1 | >200 | 64 | 32 | 64 | 35.7 | >200 |
| 5 | 13.5 | 15.6 | >200 | 16 | 8 | 16 | 16.3 | >200 |
| 6 | 13.1 | 15.4 | >200 | 16 | 8 | 16 | 19.8 | >200 |
| 7 | >200 | >200 | >200 | >128 | >128 | >128 | >200 | >200 |
| Triclosan | 0.66 | 0.98 | >200 | 0.01 | 0.01 | 64 | 0.04 | >10 |
S. aureus RN4220; b E. coli KCTC 1924; c S. pneumoniae KCTC 3932.
Mode of FabI inhibition
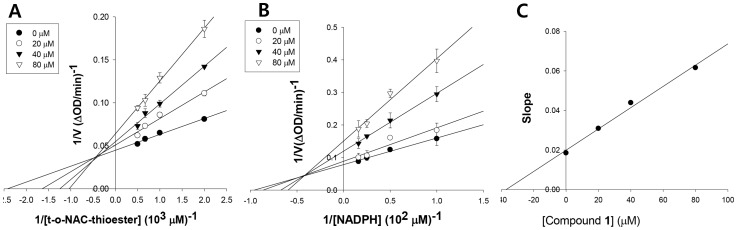
The FabI reaction mechanism requires the nucleotide cofactors NADH or NADPH as the first substrates [26]. The FabI inhibitor could bind to the free enzyme, the enzyme-substrate complex, or both to prevent catalysis. In the first case, the inhibition pattern with respect to the cofactor would be competitive; in the second, the inhibition pattern would be non-competitive; and in the third case, mixed-type inhibition would occur. Inhibition of S. aureus FabI by compound 1 was mixed with respect to trans-2-octenoyl N-acetylcysteamine, with a Ki value of 39.8 μM (Fig. 2A and 2C). In addition, compound 1 exhibited mixed inhibition with respect to NADPH, with a Ki value of 32.3 μM (Fig. 2B). Thus, compound 1 must bind to both the free enzyme and the FabI-NADPH complex to prevent binding of the nucleotide cofactor and the substrate, respectively.
Figure 2. The mechanism of inhibition of Staphylococcus aureus FabI by meleagrin respective to t-o-NAC thioester (A) and NADPH (B), and Ki determination of meleagrin (C).
Effects of structural changes in compound 1 on FabI and related activity
To determine whether structural changes in compound 1 influence its effects on FabI, compound 1 and its derivatives were tested against S. aureus and E. coli FabI and bacterial growth (Table 1). Compounds 5 and 6, which were modified at both the 9-OH and 14-NH groups, produced a significant increase in S. aureus and E. coli FabI-inhibitory activity, and they enhanced antibacterial activity against S. aureus and E. coli. In contrast, compounds 2, 3, and 4, which were modified at the 1-NH, 9-OH, and 14-NH groups, respectively, did not affect activity. Compound 7, which was brominated at the benzene ring of compound 2, totally lost its activity.
Effects on fluorescence quenching of S. aureus FabI
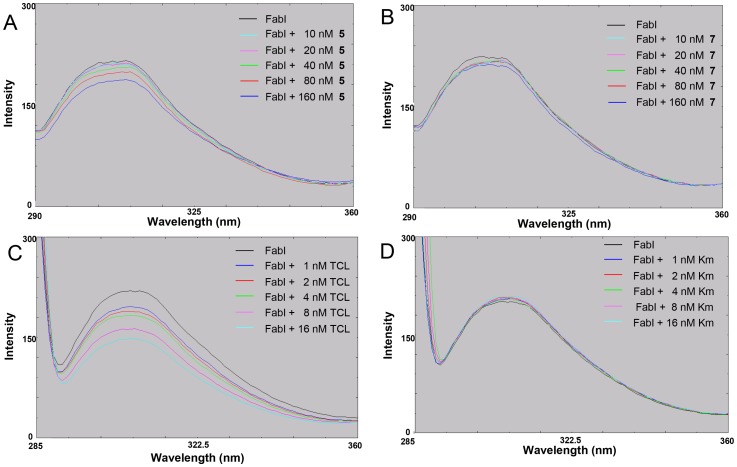
We examined whether active compounds directly bind with FabI by fluorescence quenching analysis. S. aureus FabI displayed strong maximal fluorescence at 307 nm after excitation at 270 nm (Fig. 3), whereas triclosan, kanamycin, 5, and 7 had no fluorescence at this wavelength (data not shown). When S. aureus FabI was incubated with increasing amounts of active compound 5, its fluorescence intensity decreased gradually (Fig. 3A), whereas the inactive compound 7 did not exhibit such an effect (Fig. 3B). Compound 1 showed the same pattern as compound 5 (data not shown). As a positive control, triclosan binding resulted in fluorescence quenching of S. aureus FabI (Fig. 3C), whereas kanamycin as a negative control did not (Fig. 3D). These data indicate that the active compounds 1 and 5 directly interact with S. aureus FabI, whereas compound 7 does not, thus explaining their effects on FabI.
Figure 3. Direct binding of the derivatives of meleagrin with Staphylococcus aureus FabI by fluorescence quenching assay.
(A) The more active derivative (5), (B) The inactive derivative (7), (C) triclosan (TCL) as a positive control, and (D) kanamycin (Km) as a negative control.
Inhibition of cellular fatty acid synthesis
To evaluate whether the active compounds inhibit cellular fatty acid synthesis, we determined whether the compounds inhibited the incorporation of acetate into membrane fatty acids in vivo. We measured their effects on the incorporation of [1-14C] acetate into membrane fatty acids in S. aureus. In agreement with their antibacterial activity and FabI-inhibitory activity, the more active compounds 5 and 6 indeed blocked incorporation of radioactively-labeled acetate into chloroform/methanol-extractable phospholipids in vivo in a concentration-dependent manner, with approximately 2-fold higher activity than the less active compounds 1, 3, and 4 (Table 1). The inactive compound 7 did not exhibit such fatty acid synthesis inhibition even at 200 μM, as expected. As a positive control, triclosan inhibited fatty acid synthesis in a concentration-dependent manner (data not shown). In contrast, the incorporation of leucine into proteins was not inhibited by the active compounds (Table 1), whereas the protein synthesis inhibitor, chloramphenicol, inhibited incorporation (data not shown).
Antibacterial activity
Consistent with their FabI-inhibitory activity, compounds 5 and 6 showed 2–4 times higher antibacterial activity than compound 1 against S. aureus RN4220 and the highly sensitive strain E. coli KCTC 1924 (Table 1), as expected. Interestingly, compounds that were inactive against the FabK isoform exhibited antibacterial activity against S. pneumoniae KCTC 3932, which contains only the FabK isoform. This finding suggests that the compounds inhibit not only FabI but also another target. Compounds 5 and 6 also showed antibacterial activity against other gram-positive bacteria, including S. aureus 503, S. aureus KCTC 1916, MRSA CCARM 3167, MRSA CCARM 3506, QRSA CCARM 3505, QRSA CCARM 3519, Staphylococcus epidermis KCTC 3958, B. subtilis KCTC 1021, and Micrococcus luteus KCTC 1056 with MIC values of 8–16 μg/mL.
Effects on fabI-overexpressing S. aureus
The increase in the MIC for the fabI-overexpressing strain relative to the wild type is indicative of FabI being the mode of antibacterial action [27]. The antibacterial activity of the active compounds for the fabI-overexpressing strain was investigated to determine whether overexpression of fabI shifted the MIC for S. aureus. The MICs for the fabI-overexpressing strain S. aureus RN4220 (pE194-fabI) were 4–8-fold higher than those of the wild-type strain S. aureus RN4220, or the vector-containing strain S. aureus RN4220 (pE194) (Table 2). The MIC for triclosan in the fabI-overexpressing strain increased, which was used as a positive control. Erythromycin, the selection marker for the vector pE194, increased the MICs for both the fabI-overexpressing strain and the vector-containing strain, which indicated that the engineered constructs functioned as expected. Antibiotics with different modes of action such as oxacillin and norfloxacin were applied as negative controls and did not change the MICs of the 3 strains, which indicates that altered expression of fabI does not alter the sensitivity of cells to antibiotics in general. These results indicate that the active compounds inhibited the growth of S. aureus by inhibiting the fabI-encoded ENR.
Table 2. Reduced susceptibility of fabI-overexpressing Staphylococcus aureus to meleagrin (1) and its derivatives.
| Compounds | IC50 (μM) | MIC (μg/mL) | Mode of action | ||
| saFabI | Wild type | S. aureus (pE194) | S. aureus (pE194-fabI) | ||
| 1 | 40.1 | 64 | 64 | 256 | FabI |
| 3 | 38.7 | 64 | 64 | 256 | FabI |
| 4 | 48.0 | 64 | 64 | 256 | FabI |
| 5 | 13.5 | 16 | 16 | 128 | FabI |
| 6 | 13.1 | 16 | 16 | 128 | FabI |
| Triclosan | 0.6 | 0.01 | 0.01 | 1.6 | FabI |
| Erythromycin | >100 | 0.5 | 64 | 64 | Protein synthesis |
| Oxacillin | >100 | 0.25 | 0.25 | 0.25 | Cell wall |
| Norfloxacin | >100 | 1 | 1 | 1 | DNA synthesis |
Frequency of spontaneously resistant mutants
We isolated resistant mutants to determine which other gene or genes were targeted by the active compounds (Table 3). As a control, triclosan-resistant mutants were isolated at a frequency of 3.30±0.13×10−8, 2.58±0.04×10−9, and 9.07±0.08×10−8 from S. aureus RN4220, S. aureus KCTC 1916, and the antibiotic-sensitive E. coli KCTC 1942, respectively. However, no mutants resistant to compounds 1 and 5 were detected from the strains tested. These results suggest that compounds 1 and 5 inhibit multiple targets.
Table 3. Frequency of resistance to meleagrin (1) and its more active derivative.
| Strains | MIC (μg/mL) | Exposure | Frequency of resistance | ||||
| 1 | 5 | Triclosan | 1 | 5 | Triclosan | ||
| S. aureus RN4220 | 64 | 16 | 0.01 | 4× MIC | <1.62×10−10 | <1.62×10−10 | 3.30±0.13×10−8 |
| S. aureus KCTC 1916 | 32 | 16 | 0.01 | 4× MIC | <1.03×10−10 | <1.03×10−10 | 2.58±0.04×10−9 |
| E. coli KCTC 1924 | 32 | 8 | 0.02 | 4× MIC | <6.7×10−9 | <6.7×10−9 | 9.07±0.08×10−8 |
Effects on macromolecular biosynthesis
To identify other pathways inhibited by compound 1, the effects of compound 1 on the incorporation of radiolabeled precursors of macromolecular synthesis in S. pneumoniae and in S. aureus were investigated. All reference antibacterial agents selectively inhibited the macromolecular synthesis pathway, which is consistent with their known mechanism of action (Table 4). Compound 1 inhibited the incorporation of acetate into lipids in both S. aureus and S. pneumoniae by 62% and 65%, respectively, whereas the incorporation of thymidine, uridine, isoleucine, and N-acetylglucosamine, into DNA, RNA, protein, and the cell wall, respectively, was not inhibited. Because compound 1 is inactive against the FabK isoform, these data suggest that compound 1 inhibits at least one additional target in addition to FabI in the fatty acid pathway.
Table 4. Effects of meleagrin (1) on incorporation of radiolabeled precursors into S. aureus and S. pneumoniae.
| Strains | Compounds | Inhibition of precursor incorporation (%) | ||||
| [1-14C] Acetate | [2-14C] Thymidine | [U-14C] Uridine | L-[U-14C] Isoleucine | N-Acetyl-D-[1-14C] Glucosamine | ||
| S. aureus a | Reference antibacterialc | 87 | 79 | 69 | 74 | 79 |
| 1 | 62 | 13 | 17 | 6 | 25 | |
| S. pneumoniae b | Reference antibacteriald | 95 | 83 | 92 | 85 | 88 |
| 1 | 65 | 15 | 2 | 9 | 3 | |
S. aureus RN4220; b S. pneumoniae KCTC 3932. cReference antibacterials used for inhibition of acetate, thymidine, uridine, isoleucine, and N-acetyl-d-glucosamine incorporation are triclosan, norfloxacin, rifampin, chlorampenicol, and vancomycin, respectively. dReference antibacterials in S. pneumoniae were the same as in S. aureus, except cerulenin was used instead of triclosan for acetate inhibition.
Effects on fabK-overexpressing E. coli
To demonstrate that active compounds 1 and 5 inhibit not only FabI but also an additional target, we cloned fabK and fabI into an arabinose-inducible expression system, vector pBAD TOPO, and placed this plasmid in a TolC-negative E. coli host. Because FabK is resistant to compounds 1 and 5, if the compounds inhibited only FabI, expression of FabK in E. coli would lead to resistance to compounds 1 and 5 because the expressed FabK can compensate for the inhibited FabI. As expected, the MICs of compounds 1 and 5 for fabI-overexpressing E. coli EW1b (pBAD-fabI) were 4-fold higher than those for wild-type E. coli EW1b and vector-containing E. coli EW1b (pBAD) in the presence of arabinose (Table 5). However, the MICs for the fabK-overexpressing E. coli EW1b (pBAD-fabK) did not change. As a positive control, triclosan, which does not inhibit FabK, showed inducer-dependent higher MICs for fabK-overexpressing E. coli and fabI-overexpressing E. coli. Therefore, S. pneumoniae FabK replaced E. coli FabI for fatty acid synthesis, which, in turn, indicates that FabI is the only target of triclosan in this system. Ampicillin, which is the selection marker for the pBAD vector, increased the MICs for all vector-containing strains, thereby demonstrating normal functioning of the constructs. Actinonin, which is a PDF inhibitor applied as a negative control, did not change the MICs of any of the tested strains. This result clearly indicates that active compounds 1 and 5 inhibit an additional target as well as FabI, unlike triclosan.
Table 5. Unchanged susceptibility of fabK-overexpressing E. coli to meleagrin (1) and its derivative (MIC, μg/mL).
| Compounds | E. coli EW1b | E. coli EW1b(pBAD) | E. coli EW1b(pBAD-fabI) | E. coli EW1b(pBAD-fabK) | ||||
| (−) Ara | (+) Araa | (−) Ara | (+) Ara | (−) Ara | (+) Ara | (−) Ara | (+) Ara | |
| 1 | 32 | 32 | 32 | 32 | 32 | 128 | 32 | 32 |
| 5 | 16 | 16 | 16 | 16 | 16 | 64 | 16 | 16 |
| Triclosan | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | >0.08 | 0.002 | >0.08 |
| Ampicillin | 1 | 1 | >125 | >125 | >125 | >125 | >125 | >125 |
| Actinonin | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
3% arabinose was treated.
Discussion
We screened 25,000 microbial extracts consisting of actinomycetes and fungi to identify new FabI inhibitors. Meleagrin was isolated from the solid-state fermentation of the fungal strain P. chrysogenum F717. Meleagrin was previously isolated from P. meleagrinum [28] and P. chrysogenum [29], but its biological activity, including antimicrobial activity, has not been reported. Although its activity was weak, meleagrin clearly showed inhibition selective for S. aureus FabI over S. pneumoniae FabK. Importantly, the binding of meleagrin with S. aureus FabI was demonstrated by the fluorescence quenching assay. Furthermore, its inhibition of FabI was supported by results obtained using its chemical derivatives, the intracellular fatty acid synthesis assay, and the fabI-overexpressing assay. Interestingly, meleagrin and its more active derivatives showed antibacterial activity against S. pneumoniae, in which FabK is the sole enoyl-ACP reductase, and it did not produce spontaneously resistant mutants of S. aureus or E. coli, in contrast to triclosan, which suggests that meleagrin inhibits multiple targets. Meleagrin inhibited the incorporation of radiolabeled acetate into lipids in S. pneumoniae and S. aureus, whereas incorporation of thymidine (DNA), uridine (RNA), isoleucine (protein), and N-acetylglucosamine (cell wall) was not inhibited, which indicates that these compounds inhibit fatty acid synthesis through one or more modes of action in addition to FabI inhibition. The multitarget effect was confirmed by the fabK-overexpression assay in E. coli. The multitarget effect is very important from the point of view of drug development because a single point mutation in one gene for a drug with a single target renders the strain resistant and the drug useless. Thus, when considering that one of the advantages of antibacterial agents having multiple targets is the reduced development of drug resistance [10], meleagrin and its derivatives hold promise for the development of new antibiotics that can treat infections caused by multidrug-resistant pathogens.
Several FabI inhibitors have been reported, and most were derived from compound libraries and were synthetically developed using structure-based approaches, including 1,4-disubstituted imidazoles, aminopyridines, naphthyridinones, and thiopyridines [30]. Although synthetic inhibitors are potent, they have a disadvantage, as resistant mutants occur at relatively high frequency [16], A few natural FabI inhibitors have been reported, such as vinaxanthone [21], cephalochromin [31], kalimantacin/batumin [22], EGCG, and flavonoids [32]. EGCG and flavonoids inhibit several targets such as FabG, FabZ, and FabI. The mode of action of vinaxanthone, cephalochromin, and kalimantacin/batumin was demonstrated by FabI-overexpressing strains. To our knowledge, this is the first study on a multitarget effect of FabI inhibitors.
In summary, meleagrin is a new class of FabI inhibitor with antibacterial activity against multidrug-resistant bacteria such as MRSA and QRSA. Meleagrin is structurally unique, and it inhibits at least one more target in addition to FabI, thereby resulting in a no resistance mutant; thus, meleagrin may have potential as a useful lead compound for the development of a new anti-MRSA agent.
Supporting Information
Preparation and spectral data of compounds 2–7.
(DOCX)
Acknowledgments
We thank the Culture Collection of Antimicrobial Resistant Microbes of Korea and the E. coli Genetic Stock Center of Yale University for providing the bacterial strains used in this study.
Also we express our thanks to Korea Basic Science Institute for the NMR measurements.
Funding Statement
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A2A2A01014821) and the Intelligent Synthetic Biology Center of Global Frontier Project funded by the Ministry of Education, Science and Technology (2011-0031944). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Klein E, Smith DL, Laxminarayan R (2007) Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis 13: 1840–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levy SB, Marshall B (2004) Antibacterial resistance worldwide: causes, challenges and responses. Nat Med 10: S122–129. [DOI] [PubMed] [Google Scholar]
- 3. Miesel L, Greene J, Black TA (2003) Genetic strategies for antibacterial drug discovery. Nat Rev Genet 4: 442–456. [DOI] [PubMed] [Google Scholar]
- 4. Heath RJ, Rock CO (2004) Fatty acid biosynthesis as a target for novel antibacterials. Curr Opin Investig Drugs 5: 146–153. [PMC free article] [PubMed] [Google Scholar]
- 5. Wang J, Soisson SM, Young K, Shoop W, Kodali S, et al. (2006) Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature 441: 358–361. [DOI] [PubMed] [Google Scholar]
- 6. Massengo-Tiasse RP, Cronan JE (2008) Vibrio cholerae FabV defines a new class of enoyl-acyl carrier protein reductase. Journal of Biological Chemistry 283: 1308–1316. [DOI] [PubMed] [Google Scholar]
- 7. Zhu L, Lin J, Ma J, Cronan JE, Wang H (2010) Triclosan resistance of Pseudomonas aeruginosa PAO1 is due to FabV, a triclosan-resistant enoyl-acyl carrier protein reductase. Antimicrobial Agents and Chemotherapy 54: 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang YM, White SW, Rock CO (2006) Inhibiting bacterial fatty acid synthesis. J Biol Chem 281: 17541–17544. [DOI] [PubMed] [Google Scholar]
- 9. Lu H, Tonge PJ (2008) Inhibitors of FabI, an enzyme drug target in the bacterial fatty acid biosynthesis pathway. Acc Chem Res 41: 11–20. [DOI] [PubMed] [Google Scholar]
- 10. Silver LL (2007) Multi-targeting by monotherapeutic antibacterials. Nat Rev Drug Discov 6: 41–55. [DOI] [PubMed] [Google Scholar]
- 11. McMurry LM, Oethinger M, Levy SB (1998) Triclosan targets lipid synthesis. Nature 394: 531–532. [DOI] [PubMed] [Google Scholar]
- 12. Rozwarski DA, Grant GA, Barton DH, Jacobs WR Jr, Sacchettini JC (1998) Modification of the NADH of the isoniazid target (InhA) from Mycobacterium tuberculosis. Science 279: 98–102. [DOI] [PubMed] [Google Scholar]
- 13. Cardoso RF, Cooksey RC, Morlock GP, Barco P, Cecon L, et al. (2004) Screening and characterization of mutations in isoniazid-resistant Mycobacterium tuberculosis isolates obtained in Brazil. Antimicrob Agents Chemother 48: 3373–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hazbon MH, Brimacombe M, Bobadilla del Valle M, Cavatore M, Guerrero MI, et al. (2006) Population genetics study of isoniazid resistance mutations and evolution of multidrug-resistant Mycobacterium tuberculosis. Antimicrobial Agents and Chemotherapy 50: 2640–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Y, Pi B, Zhou H, Yu Y, Li L (2009) Triclosan resistance in clinical isolates of Acinetobacter baumannii. Journal of Medical Microbiology 58: 1086–1091. [DOI] [PubMed] [Google Scholar]
- 16. Escaich S, Prouvensier L, Saccomani M, Durant L, Oxoby M, et al. (2011) The MUT056399 Inhibitor of FabI Is a New Antistaphylococcal Compound. Antimicrob Agents Chemother 55: 4692–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silver LL (2011) Challenges of antibacterial discovery. Clin Microbiol Rev 24: 71–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Livermore DM (2011) Discovery research: the scientific challenge of finding new antibiotics. Journal of Antimicrobial Chemotherapy 66: 1941–1944. [DOI] [PubMed] [Google Scholar]
- 19. Singh MP, Greenstein M (2000) Antibacterial leads from microbial natural products discovery. Curr Opin Drug Discov Devel 3: 167–176. [PubMed] [Google Scholar]
- 20. Zheng CJ, Sohn MJ, Lee S, Hong YS, Kwak JH, et al. (2007) Cephalochromin, a FabI-directed antibacterial of microbial origin. Biochemical and Biophysical Research Communications 362: 1107–1112. [DOI] [PubMed] [Google Scholar]
- 21. Zheng CJ, Sohn MJ, Kim WG (2009) Vinaxanthone, a new FabI inhibitor from Penicillium sp. Journal of Antimicrobial Chemotherapy 63: 949–953. [DOI] [PubMed] [Google Scholar]
- 22. Mattheus W, Masschelein J, Gao LJ, Herdewijn P, Landuyt B, et al. (2010) The kalimantacin/batumin biosynthesis operon encodes a self-resistance isoform of the FabI bacterial target. Chemistry and Biology 17: 1067–1071. [DOI] [PubMed] [Google Scholar]
- 23. Kawai K, Nozawa K, Nakajima S, Iitaka Y (1984) Studies on fungal products. VII. The structures of meleagrin and 9-O-p-bromobenzoylmeleagrin. Chemical and Pharmaceutical Bulletin 32: 94–98. [Google Scholar]
- 24. Zheng CJ, Yoo JS, Lee TG, Cho HY, Kim YH, et al. (2005) Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Letters 579: 5157–5162. [DOI] [PubMed] [Google Scholar]
- 25. Guzman LM, Belin D, Carson MJ, Beckwith J (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. Journal of Bacteriology 177: 4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sivaraman S, Zwahlen J, Bell AF, Hedstrom L, Tonge PJ (2003) Structure-activity studies of the inhibition of FabI, the enoyl reductase from Escherichia coli, by triclosan: kinetic analysis of mutant FabIs. Biochemistry 42: 4406–4413. [DOI] [PubMed] [Google Scholar]
- 27. Slater-Radosti C, Van Aller G, Greenwood R, Nicholas R, Keller PM, et al. (2001) Biochemical and genetic characterization of the action of triclosan on Staphylococcus aureus. J Antimicrob Chemother 48: 1–6. [DOI] [PubMed] [Google Scholar]
- 28. Nozawa K, Nakajima S (1979) Isolation of radicicol from Penicillium Luteo-Aurantium, and Meleagrin, a new metabolite, from Penicillium Meleagrinum. Journal of Natural Products 42: 374–377. [Google Scholar]
- 29. Bringmann G, Lang G, Gulder TAM, Tsuruta H, Mühlbacher J, et al. (2005) The first sorbicillinoid alkaloids, the antileukemic sorbicillactones A and B, from a sponge-derived Penicillium chrysogenum strain. Tetrahedron 61: 7252–7265. [Google Scholar]
- 30. Moir DT (2005) Identification of inhibitors of bacterial enoyl-acyl carrier protein reductase. Curr Drug Targets Infect Disord 5: 297–305. [DOI] [PubMed] [Google Scholar]
- 31. Zheng CJ, Sohn MJ, Lee S, Hong YS, Kwak JH, et al. (2007) Cephalochromin, a FabI-directed antibacterial of microbial origin. Biochem Biophys Res Commun 362: 1107–1112. [DOI] [PubMed] [Google Scholar]
- 32. Zhang YM, Rock CO (2004) Evaluation of epigallocatechin gallate and related plant polyphenols as inhibitors of the FabG and FabI reductases of bacterial type II fatty-acid synthase. J Biol Chem 279: 30994–31001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preparation and spectral data of compounds 2–7.
(DOCX)