Abstract
Anti-Müllerian hormone (AMH) is a member of the TGF-β superfamily secreted by the gonads of both sexes. This hormone is primarily known for its role in the regression of the Müllerian ducts in male fetuses. In females, AMH is expressed in granulosa cells of developing follicles. Like other members of the TGF-β superfamily, AMH transduces its signal through two transmembrane serine/threonine kinase receptors including a well characterized type II receptor, AMHR-II. The complete signalling pathway of AMH involving Smads proteins and the type I receptor is well known in the Müllerian duct and in Sertoli and Leydig cells but not in granulosa cells. In addition, few AMH target genes have been identified in these cells. Finally, while several co-receptors have been reported for members of the TGF-β superfamily, none have been described for AMH. Here, we have shown that none of the Bone Morphogenetic Proteins (BMPs) co-receptors, Repulsive guidance molecules (RGMs), were essential for AMH signalling. We also demonstrated that the main Smad proteins used by AMH in granulosa cells were Smad 1 and Smad 5. Like for the other AMH target cells, the most important type I receptor for AMH in these cells was BMPR-IA. Finally, we have identified a new AMH target gene, Id3, which could be involved in the effects of AMH on the differentiation of granulosa cells and its other target cells.
Introduction
Anti-Müllerian hormone (AMH) also called Müllerian inhibiting substance (MIS) is a member of the TGF-β superfamily. AMH is well known for its role in Müllerian duct regression in male fetuses [1]. In female fetuses, the lack of AMH expression allows the development of the Müllerian ducts into oviduct, uterus, cervix and the upper part of the vagina. Postnatally, AMH is secreted by granulosa cells (GCs) of small growing follicles (preantral and small antral) [1]. In mice, AMH is a negative regulator of the primordial to primary follicle transition [2]. In addition it decreases FSH sensitivity of growing follicles [3]. In clinics, serum AMH is now widely used in oncology and gynecology. Indeed, it is a very useful diagnostic and prognostic tool, as an early indicator of relapse of ovarian GC tumors [4] and as a reliable marker of the ovarian follicular status. AMH assay is also an important tool to control ovarian hyperstimulation [5]. However, despite the increasing interest of ovarian AMH in clinics, little is known on its mechanism of action on GCs.
Members of the TGF-β family signal through a type II transmembrane serine/threonine kinase receptor which forms a complex with a type I serine/threonine kinase receptor [6]. The type II receptor phosphorylates serine and threonine residues of type I receptor. Once activated, the type I receptor phosphorylates the receptor-regulated Smads (R-Smad) which interact with a common partner Smad4. The Smad complex accumulates into the nucleus and regulates target gene expression [7]. TGF-β and activins activate TbetaRI and ActR-IB type I receptors and R-Smads 2 and 3, whereas Bone Morphogenetic Proteins (BMPs) mediate their effects through ActR-IA, BMPR-IA and BMPR-IB type I receptors and R-Smads 1, 5 or 8. This canonical signalling pathway is regulated at different levels, in particular by co-receptors which amplify or antagonize TGF-β family members action.
AMH has a single specific type II receptor (AMHRII, also known as MISRII) [8], [9]. Indeed, Amh and Amhr2 inactivation causes the same phenotype in males [10], a complete retention of an ectopic female reproductive tract, indicating that the AMH type II receptor is the only type II receptor that transduces AMH signal [11]. Moreover, in human, mutations of AMH or AMHRII are involved in Müllerian duct derivative persistance [12]. Regarding the other components of AMH signalling pathway, it was first shown in different cell lines of gonadal origin that AMH phosphorylates R-Smad 1/5/8, meaning that it uses ActR-IA, BMPR-IA or BMPR-IB type I receptors to transduce its effects [13]. Then the disruption of these type I receptors and R-Smads in mice led to the conclusion that BMPR-IA is the primary type I receptor required for Müllerian duct regression but that ActR-IA is capable of transducing the AMH signal in the absence of BMPR-IA, and that R-Smad 1/5/8 function redundantly [14]–[16]. Similarly, in the immature Sertoli cell line SMAT-1, AMH mediates its signal through BMPR-IA and ActR-IA [17]. In contrast, the type I receptors and R-Smads involved in AMH effects on post-natal GCs remain unknown. In addition, to date, no co-receptor has been found for AMH. Because AMH share with BMPs its type I receptors and R-Smad proteins, it could have the same co-receptors, like the Repulsive Guidance Molecule (RGM) [18] which were first shown to induce axonal guidance during neurogenesis [19] and have three isoforms: RGMa, RGMb (Dragon) and RGMc (Hemojuvelin).
To date, only few AMH target genes have been identified. In males, AMH inhibits Sertoli and Leydig cells differentiation through repression of several steroidogenic proteins like P450scc (Cyp11a1), 3β-HSD (Hsd3b1) or P450C17 (Cyp17a1) [17], [20], [21]. Little is known on the genes involved in the inhibitory effect of AMH on GCs differentiation, preventing the study of AMH mechanism of action on these cells. Aromatase (Cyp19a1) and LH receptor (Lhcgr) are down-regulated by AMH in rat and porcine GCs [22]. In human GCs, FSHR (Fshr) is also repressed by AMH [23] which could explain the inhibitory role of this hormone on follicles sensitivity to FSH. Because BMPs share their signalling pathway with AMH and regulate GCs differentiation, BMPs target genes involved in this process could also be modulated by AMH. This could be the case for the family of Inhibitor of differentiation/Deoxyribonucleic Acid-Binding (Id) genes which are BMP2 and BMP4 target genes in mice [24]. These genes have been shown recently to be related to the status of GC differentiation [25]. They lack a domain required for DNA binding and act as negative antagonists of bHLH transcription factors and gene expression in mammals [26].
In this study, we used primary GCs isolated from immature mice to identify target genes and co-receptors potentially implicated in AMH effects on GCs differentiation and to define the involvement of the different type I receptors and Smads proteins in this process. We also took advantage on conditional mutant mice for different actors of AMH signalling pathway to confirm our data. Our studies reveal that Id3 is a new AMH target gene in GCs. In addition, this work indicates that BMPR-IA and Smad 1/5 are the main components of AMH signalling pathway in GCs.
Materials and Methods
Ethics Statement
Housing and care, method of euthanasia and experimental protocols were conducted in accordance with the recommendations of the French Accreditation of Laboratory Animal Care and in compliance with the NIH Guide for Care and Use of Laboratory Animals. The animal facility is licensed by the French Ministry of Agriculture (agreement N°C92-023-01). All animal experiments were supervised by Dr. Soazik Jamin (agreement delivered by the French Ministry of Agriculture for animal experiment N°92–299). Animals were sacrificed with CO2. All efforts were made to minimize animal suffering.
Reagents
Recombinant human AMH was produced from the culture medium of Chinese hamster ovary (CHO) cells stably transfected with a human AMH cDNA, purified and cleaved by plasmin (plasmin-cleaved AMH or PC-AMH) as previously described [27]. PC-AMH subsequently called AMH was used at a concentration of 8 or 40 nM. BMP2 was a kind gift of Prof. Walter Sebald (University of Würzburg, Germany) and was used at a concentration of 10 nM [28]. TGF-β was purchased from R&D Systems (Lille, France) and used at 1 nM. Smad1-Gal4, Smad5-Gal4, Smad8-Gal4 and Gal-Luc plasmids were kindly provided by Dr. Azeddine Atfi (Inserm UMR S938, Paris).
Mice
Immature female C57BL/6JRj mice (3 weeks old) were purchased at Elevage Janvier (Le Genest-St-Isle, France).
Amhr2-cre [15], Acvr1 +/− [29], Bmpr1a +/− [30], Acvr1 fx/fx [31], Bmpr1a fx/fx [32] mice were maintained on a C57BL/6J; 129/SvEv mixed genetic background. Amhr2-cre; Acvr1 +/− or Amhr2-cre; Bmpr1a +/− males were bred to Acvr1 fx/fx or Bmpr1a fx/fx females to generate females that were conditionally null for Acvr1 or Bmpr1a respectively (designated as Acvr1 cKO and Bmpr1a cKO).
Mouse genotyping
Genomic DNA was extracted from tail biopsies using a NucleoSpin Tissue kit (Macherey-Nagel, Hoerdt, France) according to the manufacturer's instructions. The primers used to detect Acvr1 and Bmpr1a alleles are listed Table S1. The amplification conditions were 95°C for 5 min, followed by 94°C for 45 s, 58°C for 45 s, and 72°C for 45 s (35 cycles), with a final extension at 72°C for 10 min. The amplified PCR fragments were analysed on 2% agarose gels.
Primary cultures of mouse granulosa cells
The preparation of mouse GCs primary cultures was adapted from a rat primary GC culture protocol [33]. Immature ovaries from 3 weeks old mice were collected in RPMI medium (Invitrogen). Then, they were exposed to 6.8 mM EGTA, 0.2% BSA in Medium 199 (Invitrogen) for 15 min at 37°C. After a 5 min centrifugation at 500 rpm, ovaries were placed in a hypertonic solution (0.5 M sucrose, 1.8 mM EGTA, 0.2% BSA) in Medium 199 for 5 min at 37°C. Three volumes of Medium 199 were added to stop the reaction. After a 5 min centrifugation at 500 rpm, ovaries were placed in DMEM/F-12 medium with 1% Fetal Bovine Serum (FBS, Life technologies) and GCs were dissociated with a blunt spatula. Cells were pelleted by a 10 min centrifugation at 1300 rpm. Supernatant was discarded and cells were resuspended in DMEM/F-12 without phenol red, 10% FBS. The collected cells were counted in the presence of Trypan blue (0.07%) and seeded in DMEM/F-12 without phenol red, 10% FBS and 1% penicillin/streptomycin (Eurobio, Courtaboeuf, France) at 37°C in 5% CO2. 36 h after plating, GCs were exposed to AMH, BMP2 or TGF-β in DMEM/F-12 without phenol red, 1% FBS and 1% penicillin/streptomycin (Eurobio) for 24 h for RNA analyses. For protein analyses, GCs were starved in serum-free medium for 1 h prior to a 3 h AMH, BMP2 or TGF-β exposure.
β-galactosidase and pmaxGFP transfection
Primary GCs seeded in 6-well plates (5×105 cells/well) were transfected using Fugene6 reagent (Roche Diagnostics, Meylan, France) with 1 µg β-galactosidase reporter gene or pmaxGFP vector when cells were 80% confluent. After 24 h, β-galactosidase activity was detected with X-gal staining and GFP signal was directly visualized.
Immunofluorescence
Cells were seeded in 4-well Lab-Tek (1×105 cells/well) (Dutscher, Brumath, France) and incubated at 37°C in 5% CO2. After a quick wash in 1X phosphate-buffered saline (PBS), the cells were fixed in 4% paraformaldehyde (PFA) for 15 min. After a permeabilization step in tris-buffered saline (TBS)/0.2% Triton, cells were incubated in blocking buffer (PBS 1X, 10% BSA, 0.3% Triton) for 1 h at room temperature. Then cells were incubated with the anti-AMH (1∶500, Santa Cruz, Heidelberg, Germany) primary antibody in dilution buffer (PBS 1X, 1% BSA, 0.3% Triton) overnight at 4°C. After one wash in TBS/Triton, cells were incubated with the secondary antibody (Alexa Fluor-488 conjugated rabbit antibody) 1 h at room temperature in the dark. Cells were washed in high salt PBS and slides were covered with mounting medium containing DAPI (Vector Laboratories, Abcys, Les Ulis, France).
Immunohistochemistry
Ovaries from 3 weeks old C57BL/6J females were collected and fixed in 4% PFA for 4 h at 4°C. Samples were then washed with PBS (pH 7.4), dehydrated in graded ethanol, embedded in paraffin and cut into 5 µm thick sections. For antigen retrieval, samples were boiled in citrate buffer 10 mM pH 6.0 at 80°C for 45 min. Thereafter, slides were washed with PBS and blocked with PBS-BSA 10% for 1 h before an overnight incubation with a primary anti-RGMb antibody (1∶100 in DAKO buffer, Santa Cruz). Specimens were washed with PBS and incubated with a secondary biotinylated antibody (anti-rabbit 1∶500) 1 h at room temperature. After washing, slides were incubated 1 h in ABC reagent (ABC kit, Vectastain, Abcys) and stained with DAB reagent during 5 min.
siRNA gene knockdown
Small-interfering RNAs (siRNAs) for Acvr1 (59932), Bmpr1a (160872), Bmpr1b (60258), and a negative control siRNA #1 were obtained from Ambion (Life Technologies) and siRNA Rgmb (J05553408) was obtained from Dharmacon (Fisher Scientific, Illkirch, France). siRNAs were used at a final concentration of 100 nM. Primary GCs seeded in 6-well plates (5×105 cells/well) were transfected using Oligofectamine reagent (Life Technologies) with 100 nM siRNA when cells were 50% to 80% confluent. The medium was removed after 3 h and replaced by DMEM/F-12 1% FBS. The cells were then cultured 3 h or 24 h in the presence of 8 nM AMH.
siRNA GAPDH-cy3 (4390849, Life Technologies) used to show transfection efficiency was transfected as described above and the fluorescence was analysed by flow cytometry (FACS Calibur, Becton Dickinson).
Luciferase Assay
Primary GCs seeded in 6-well plates (5×105 cells/well) were co-transfected with a luciferase reporter (UAS-luc, 300 ng), expression construct (Smad-Gal4, 200 ng) [34] and pRLTK (1 ng, Promega) as a control for transfection efficiency. Transfection was performed using FugeneHD according to the manufacturer's instructions (Roche). Cells were subsequently treated 24 h with AMH (8 nM), washed with PBS and lysed in passive lysis buffer (Promega). All lysates were analysed for Firefly and Renilla luciferase activity according to the manufacturer (Dual Luciferase kit, Promega). Results were expressed as a percentage of stimulation of Firefly luciferase activity (after normalization to Renilla luciferase activity) in the presence of AMH compared to cells cultured in control medium.
RNA isolation and Reverse Transcription
Total RNAs were isolated using the RNeasy Minikit (QIAGEN) according to the manufacturer's instructions. Reverse transcription was performed in a total of 20 µl with the Omniscript Reverse Transcription Kit for RT-PCR (QIAGEN) using 500 ng or 1 µg total RNA, Omniscript reverse transcriptase, oligo-dT primers (1 µM) and random hexamers (10 µM) as recommended by the manufacturer. The samples were incubated 1 h at 37°C.
PCR amplification
cDNA from primary GCs or total ovary were used to amplify Inha, Lhcgr, and Fshr by PCR. The primer pairs used are shown Table S2. PCR was performed using PCR mix (Qiagen). The amplification conditions were 95°C for 5 min, followed by 35 cycles of 94°C for 45 s, 58°C for 45 s, and 72°C for 45 s, with a final extension at 72°C for 10 min. The amplified PCR fragments were analysed on 2% agarose gels.
Quantitative real-time PCR
Quantification of the content of Amhr2, Acvr1, Bmpr1a, Bmpr1b, Cyp11a1, Fshr, Id3, Inha, Rgma, Rgmb, Rgmc, Smad1, Smad4, Smad5, Smad8, Star and Hprt mRNA was performed by real-time PCR using the TaqMan PCR method. The primers and the UPL probes (Roche Diagnostics, Mannheim, Germany) used to amplify these genes are indicated Table S2. Real-time PCR was performed with one fifth dilution of the cDNAs using the Lightcycler 480 Probes Master kit (Roche Diagnostics, Mannheim, Germany). Primers were used at a concentration of 5 µM and probes at 1 µM. The PCR protocol used an initial denaturating step at 95°C for 10 min followed by 45 cycles of 95°C for 10 s, 58°C for 30 s, 72°C for 1 s. To generate standard curves, different concentrations of the purified and quantified PCR products were amplified. Relative gene expression was normalized to an endogenous control gene (Hprt).
Western blot
Mouse primary GCs were seeded into 6 wells plates at a density of 5×105 cells/well in 2ml of culture medium. Cells were then harvested, and lysed in 50 mM Tris HCL (pH 7.4)/150 mM NaCl, 1% protease inhibiting cocktail and 1% Triton. Insoluble material was removed by centrifugation at 12,000×g, for 5 min at 4°C. The supernatants were recovered, and protein concentrations were measured using the BCA protein assay kit (Pierce). Equivalent amounts of protein lysates (8 to 20 µg) were subjected to 4–20% SDS-PAGE (Biorad) and electrophoretically transferred onto nitrocellulose membranes. After the blocking of non-specific binding sites for 1 h in Tris-buffered saline (25 mM Tris and 150 mM NaCl, pH 7.6) containing 5% non-fat milk and 0.1% Tween 20, the membranes were exposed to the primary antibodies (anti-phospho-Smad 1/5/8 and anti-phospho-Smad2/3 (Cell Signalling Technology) both at 1∶500) overnight at 4°C. Reactive proteins were detected with horseradish peroxidase-conjugated secondary antibodies (1∶5000) for 1 h at room temperature and developed with West Pico Western blotting detection reagents (Pierce). The membranes were stripped with a stripping buffer (Pierce), then reprobed with a mouse monoclonal antibody to β-Actin (Sigma Aldrich) or mouse monoclonal anti-α-Tubulin (Sigma Aldrich). Western blots were quantified using ImageJ software.
Statistical analysis
All experimental data are presented as means ± SEM. Data were analyzed using t-test or one-way ANOVA followed by Tukey test for all-pair comparisons to compare respectively two or several means. A difference was considered statistically significant when the p-value was <0.05. * p<0.05, ** p<0.01, *** p<0.001. All calculations were made using GraphPad Prism 5.1 (GraphPad Software, La Jolla, CA).
Results
Primary cultures of immature mouse granulosa cells
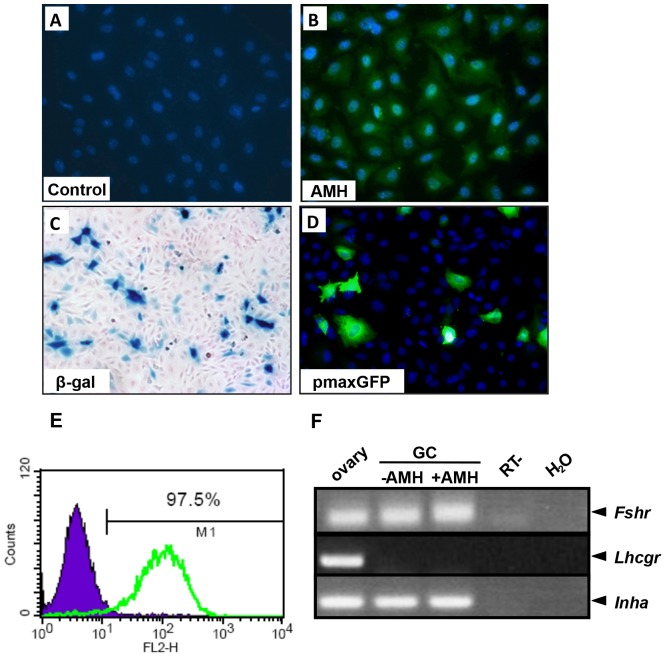
To identify the different components of the AMH signalling pathway in GCs, we first isolated immature GCs from 3 weeks old mice [33] and we characterized them for different criteria. Immature ovaries are mainly composed of small growing follicles whose GCs express AMH [35]. Therefore, we used an AMH antibody coupled to an Alexa fluor-488 secondary antibody to evaluate the purity of the primary GCs by immunofluorescence (Fig. 1A and 1B). About 90% of the cells expressed AMH which indicated an efficient enrichment of GCs (Fig. 1A and 1B). Since we had to transfect plasmid DNA in GCs, we then assayed transfection efficiency in these cells using a β-galactosidase reporter gene (Fig. 1C) or pMax-GFP vector (Fig. 1D). After X-Gal staining, we found that about 20% of the cells stained positively for β-galactosidase activity, indicating that they had been properly transfected (Fig. 1C). The transfection capacity of the primary GC culture was confirmed with pMax-GFP transfection by visualizing GFP-expressing cells (Fig. 1D). We also checked siRNA transfection efficiency. GCs were transfected with a fluorescently-labelled control siRNA (GAPDH-cy3 siRNA). The next day, the cells were trypsinized and the cell suspension was analysed by flow cytometry. We found that 97.5% of the GCs were transfected with the fluorescent siRNA, demonstrating a high siRNA transfection efficiency (Fig. 1E). We then checked for the presence of GCs markers (Fig. 1F). As in the total ovary, GCs expressed FSH receptor (Fshr) and α inhibin (Inha) (Fig. 1F). On the other hand, LH receptor (Lhcgr), a theca cell marker at this stage, was detected in the 3 weeks old ovary, as expected, but absent in the GCs. These results confirmed that the culture was at least 90% pure with little or no theca cell contamination.
Figure 1. Characterization of granulosa cells in primary culture.
Granulosa cells (GCs) were collected from 3 weeks old C57BL/6 mice ovaries and seeded at a density 1×105 cells/well. 24 h later, GCs were incubated without primary antibodies (IgG) for the control condition (A) or with an anti-AMH antibody (B). The secondary antibody was coupled to FITC and DAPI was used to visualize the nucleus. AMH expression was detected in the cytoplasm as expected. To asssay the transfection efficiency, primary GCs were transfected either with a β-galactosidase vector (C, 1 µg), pMax-GFP vector (D, 1 µg) or GAPDH-cy3 siRNA (E). After 24 h, GC were fixed, stained with X-Gal and counterstained with nuclear fast red (C) or fluorescence was visualized (D). Alternatively, siRNA transfection efficiency was assayed with a GAPDH-cy3 siRNA on isolated GCs using flow cytometry analysis (E). (F), Markers of immature GCs were analysed by RT-PCR to confirm their status.
Granulosa cells can respond to plasmin cleaved AMH
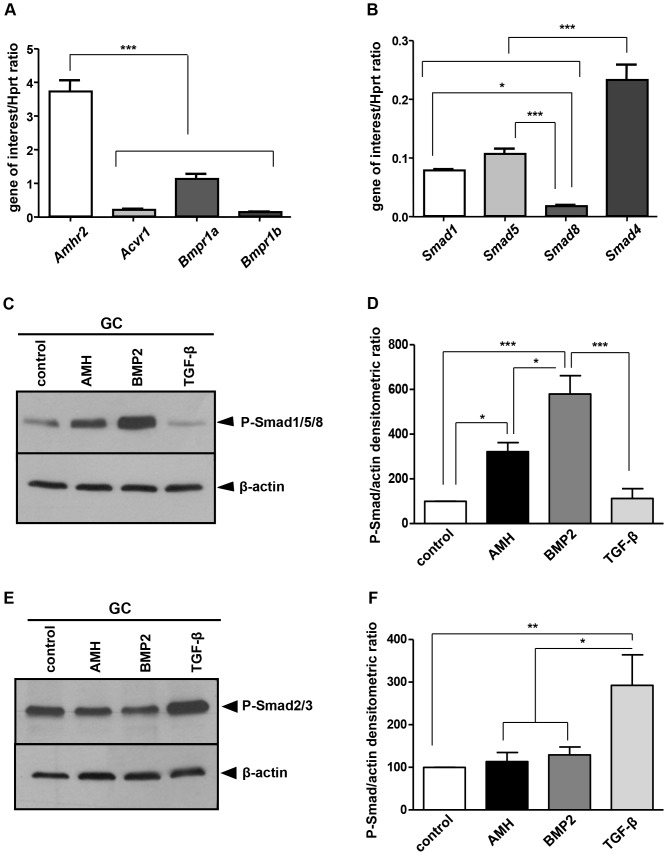
We next studied the expression of the different components of AMH signalling pathway in GCs. Using real time PCR, we observed that all of the serine/threonine kinase receptors candidate genes (Acvr1, Bmpr1a, Bmpr1b and Amhr2) (Fig. 2A) and the three candidate Smads (Fig. 2B) were expressed in primary GCs. The results showed that Amhr2 was expressed at higher levels (between 3.5 and 37 times) than the type I receptors (Fig. 2A), Bmpr1a being the most represented (Fig. 2A). Among Smads, Smad4 was the most expressed, the levels of Smad1 and Smad5 were similar and Smad8 protein was poorly represented (Fig. 2B). We then tested if primary GCs were responsive to AMH (plasmin-cleaved AMH). We analysed the response of GCs after a 3 h exposure to AMH by Western blot using a phospho-Smad 1/5/8 antibody (Fig. 2C). Both AMH and BMP2, which is the primary inducer, could increase phospho-Smad 1/5/8 levels (Fig. 2C and 2D) while TGF-β had no effect on this pathway. We then checked if AMH could also activate the alternative Smad2/3 pathway. TGF-β which is a primary inducer of the Smad 2/3 pathway was used as a positive control in parallel with AMH (Fig. 2E). As expected, in the presence of TGF-β, phospho-Smad 2/3 levels were increased in primary GCs (Fig. 2E and 2F). However, neither AMH nor BMP2 were able to activate the Smad2/3 pathway (Fig. 2E and 2F). Thus, the primary GCs displayed all the characteristics necessary for our study. They expressed all of the factors of the AMH signalling pathway, they were properly transfected and they were sensitive to AMH. These primary GCs were then used for the rest of the studies described in this paper.
Figure 2. AMH activates Smad1 pathway in granulosa cells.
GCs were isolated from 3 weeks old mice ovaries and seeded at 5×105 cells/well in 6 wells plates. RNA was extracted to test AMH signalling pathway actors expression. The main known actors were analysed by real time PCR, including AMH type II and type I receptors (A, n = 6) and Smads proteins (B, n = 6). Data were analyzed using one-way ANOVA followed by Tukey test for all-pair comparisons. * p<0.05, ** p<0.01, *** p<0.001. GCs were exposed or not to 8 nM AMH, 10 nM BMP2 and 1 nM TGF-β. Proteins were extracted and analysed by Western blot using a phospho-Smad1/5/8 antibody (C, n = 4) or a phospho-Smad2/3 antibody (E, n = 4). Western blots were quantified and normalized to actin levels (D, F, n = 4). AMH could only activate the Smad1/5/8 pathway in GCs (C, D) but not the Smad2/3 one's (E, F). As controls, BMP2 only phosphorylated the Smad1/5/8 proteins (C, D) while TGF-β activated exclusively the Smad2/3 pathway (E, F). Data were analyzed using one-way ANOVA followed by Tukey test for all-pair comparisons. * p<0.05, ** p<0.01, *** p<0.001
AMH target genes in granulosa cells
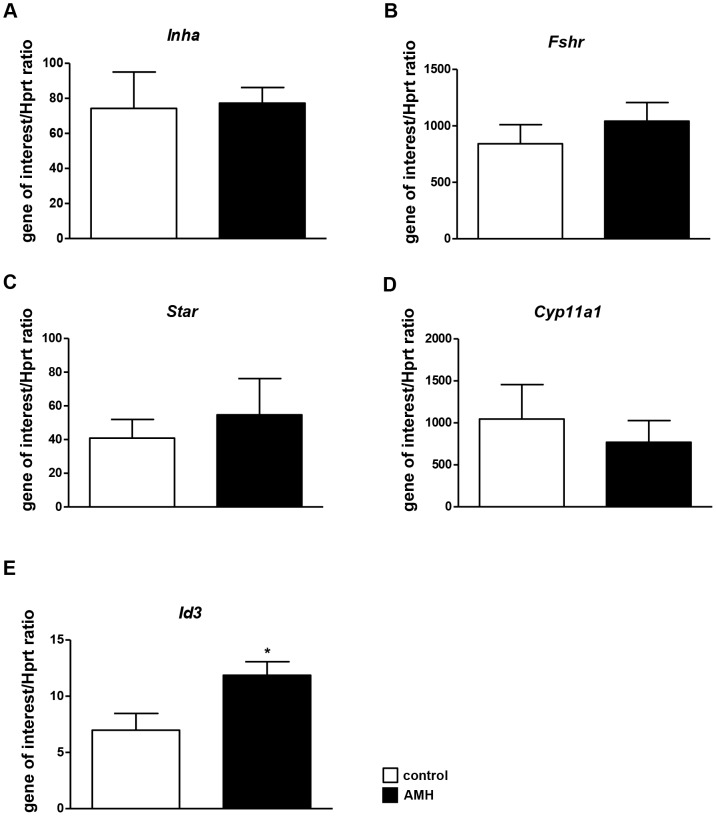
To monitor AMH signalling, we then screened for downstream target genes. GCs were treated with AMH (8 nM) during 24 h, and the expression of candidate genes was analysed by real time PCR. We first tested AMH target genes previously identified in the ovary. Aromatase (Cyp19a1) and LH receptor (Lhcgr) were expressed at very low levels and we were not able to detect any variation of their expression after AMH exposure (data not shown). We then studied α inhibin (Inha) (Fig. 3A) and FSH receptor (Fshr) (Fig. 3B) whose expression was not modulated by AMH in GCs. Because AMH regulates genes encoding steroidogenic enzymes in Sertoli and Leydig cells, we tested whether their expression was also affected by AMH in GCs. 3 β-HSD (Hsd3b1) expression was too low to detect any variation (data not shown). Star (Fig. 3C) and P450scc (Cyp11a1) (Fig. 3D) were not regulated by AMH. Finally, we tested the effect of AMH on Id genes expression which are known to be up regulated by BMPs. Id1, 2 and 3 were up-regulated by BMP2 in GCs but not by TGF-β (data not shown). Similarly to BMP2, AMH increased all Id genes expression but only Id3 gene was significantly up-regulated (Fig. 3E). After 24 h in the presence of AMH, Id3 expression in GCs is increased by 50%. Therefore, Id3 gene was used in this study to assay siRNA knockdown experiments.
Figure 3. AMH target genes in granulosa cells.
After collecting and seeding, GCs were exposed (▪) or not (□) to 8 nM AMH (A–E) for 24 h. The effect of AMH stimulation on different potential target genes was examined by real time PCR (A–D, n = 4; E, n = 5). Hprt expression was used to normalize the results. Data were analyzed using paired t-test. * p<0.05. Id3 expression is significantly increased after AMH exposure.
Involvement of the different serine/threonine kinase receptors in AMH signalling
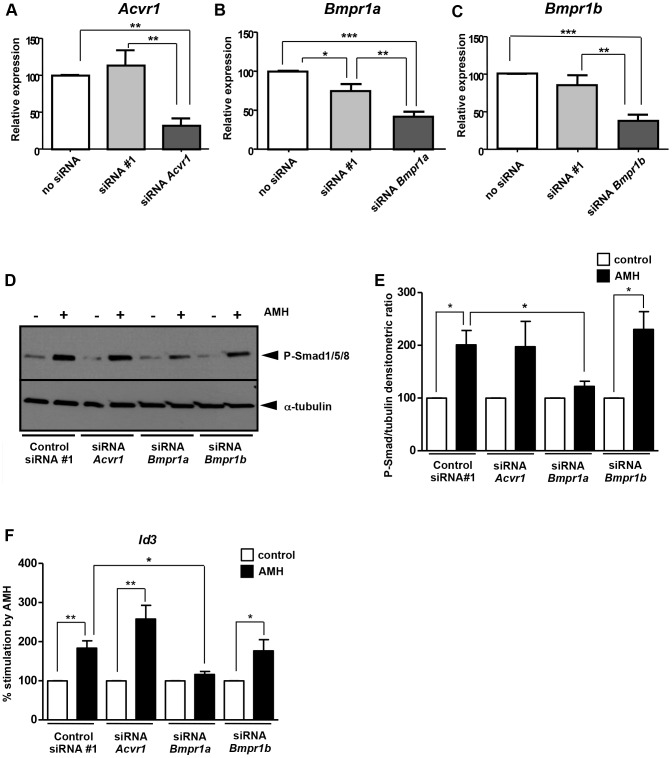
To determine which type I serine/threonine kinase receptor(s) was important for AMH signalling pathway, we used siRNA for gene knockdown (Fig. 4). Primary GCs were isolated from immature ovaries and transfected when 50 to 80% confluence was reached with different siRNAs against Acvr1, Bmpr1a and Bmpr1b. After another 24 h of culture, the cells were exposed to AMH and total RNA were extracted 24 h later. We first checked the expression of each serine/threonine kinase type I receptor gene after siRNA transfection using real time PCR (Fig. 4A–C). For each type I receptor gene, we tested three different siRNAs (data not shown) and selected the most efficient one for the rest of the study. siRNA transfection led to a 80% decrease of Acvr1 mRNA levels (Fig. 4A), a 70% decrease for Bmpr1a (Fig. 4B) and a 70% decrease for Bmpr1b (Fig. 4C). Since the down regulation was significant for each of the genes, we then analysed the responsiveness of these knocked-down GCs to AMH by Western blot with a phospho-Smad1/5/8 antibody (Fig. 4D). In parallel, the GCs were transfected with a negative control siRNA which does not interfere with any of the targeted RNAs. As expected, the negative control siRNA had no effect since the transfected cells could respond to AMH through the activation of the Smad1 pathway (Fig. 4D and 4E). Similarly, the Acvr1 and Bmpr1b knockdown cells were sensitive to AMH. However, the effect was not significant for Acvr1 because of the large standard deviation. On the other hand, in presence of siRNA against Bmpr1a, the effect of AMH on phospho-Smad1/5/8 levels was reduced significantly compared to GC transfected with the control siRNA. This result indicates that BMPR-IA is important for AMH signalling in GCs (Fig. 4D and 4E). We then analysed the effect of AMH on Id3 expression in GCs transfected with the different siRNAs (Fig. 4F). Id3 expression was up-regulated by 83% after AMH exposure in GCs transfected with control siRNA. Id3 was also up-regulated by 158% in GCs transfected with siRNA against Acvr1 and by 76% in GCs transfected with Bmpr1b siRNA. In contrast, in GCs transfected with siRNA against Bmpr1a, AMH was unable to up-regulate the expression of Id3. siRNA technology allowed us to show that BMPR-IA is important to transduce AMH signal in GCs (Fig. 4D, 4E and 4F).
Figure 4. Involvement of serine/threonine kinase type I receptors.
siRNA transfection for each type I receptor gene was performed when cells were 50% to 80% confluent. 24 h later GCs were exposed (▪) or not (□) to 8 nM AMH during another 24 h. The effect of siRNA on target gene expression was determined by real time PCR (A–C, n = 4). Data were analyzed using ANOVA followed by Tukey test for all-pair comparisons. The effect of Acvr1, Bmpr1a and Bmpr1b knockdown on AMH sensitivity was analysed by Western blot using a phospho-Smad1/5/8 antibody (D, n = 4) and was quantified and normalized (E). The effect of Acvr1, Bmpr1a and Bmpr1b knockdown on Id3 expression was analyzed by real-time PCR (F, n = 3). Data were analyzed using paired t-test. * p<0.05, ** p<0.01, *** p<0.001. Only GCs transfected with siRNA against Bmpr1a present a significant decrease of AMH response.
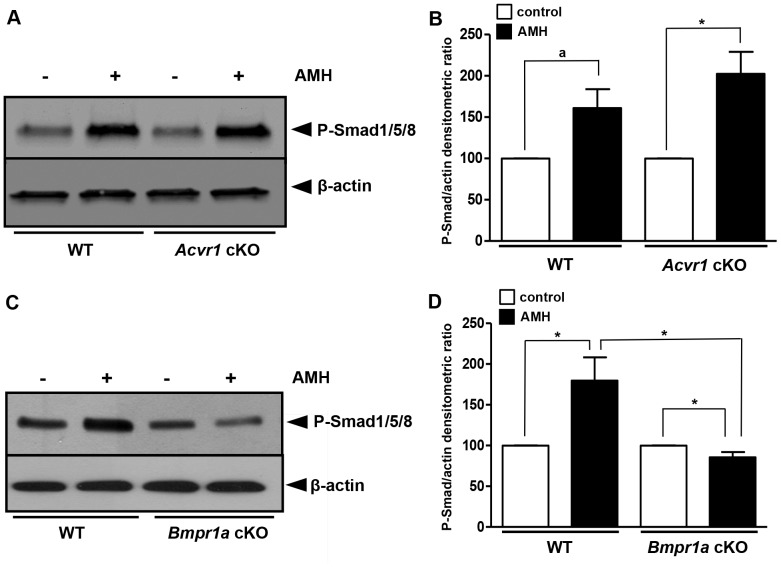
Granulosa cells from Bmpr1a cKO mice no longer transduce AMH signalling
To confirm the siRNA results, we generated Acvr1 and Bmpr1a conditional knockout (cKO) mice, using Amhr2-cre line to delete these genes in GCs. Amhr2 +/cre; Acvr1 +/− or Amhr2 +/cre; Bmpr1a +/− males were bred to Acvr1 fx/fx or Bmpr1a fx/fx females to generate females that were conditionally null either for Acvr1 or Bmpr1a in GCs. Amhr2 +/cre; Acvr1 fx/− and Amhr2 +/cre; Bmpr1a fx/− are designated Acvr1 cKO and Bmpr1a cKO, respectively. GCs were isolated from these cKO mice ovaries and we tested the response of these cells to AMH by Western blot using a phospho-Smad 1/5/8 antibody. GCs from Acvr1 cKO mice were as sensitive to AMH as GCs from control mice (Fig. 5A and 5B). In contrast, GCs from Bmpr1a cKO mice had lost their capacity to respond to AMH (Fig. 5C and 5D). The effect of AMH on phospho-Smad1/5/8 level was reduced significantly in GCs from Bmpr1a cKO compared to WT GCs (Fig. 5D). These results were consistent with the siRNA experiments and indicated that BMPR-IA was essential for AMH signalling in GCs.
Figure 5. Granulosa cells from Bmpr1a cKO mice do not respond to AMH.
Amhr2-Cre; Acvr1 +/− or Amhr2-Cre; Bmpr1a +/− males were bred to Acvr1 fx/fx or Bmpr1a fx/fx females to generate females that were conditionally null for Acvr1 (A, n = 3) or Bmpr1a (B, n = 7) in GCs. GCs were exposed (▪) or not (□) to 8 nM AMH. The AMH response was tested in GCs from these cKO mice by Western blot using a phospho-Smad1/5/8 antibody (A,C). Western blots were quantified and normalized to actin levels (B n = 3, D n = 7). AMH induced the phosphorylation of Smad1/5/8 in GCs from Acvr1 cKO (A, B) mice but not in those from Bmpr1a cKO mice (C, D). Data were analyzed using paired t-test. * p<0.05, a: p = 0.058. Only Bmpr1a conditional mutant GCs present a significant decrease of AMH response.
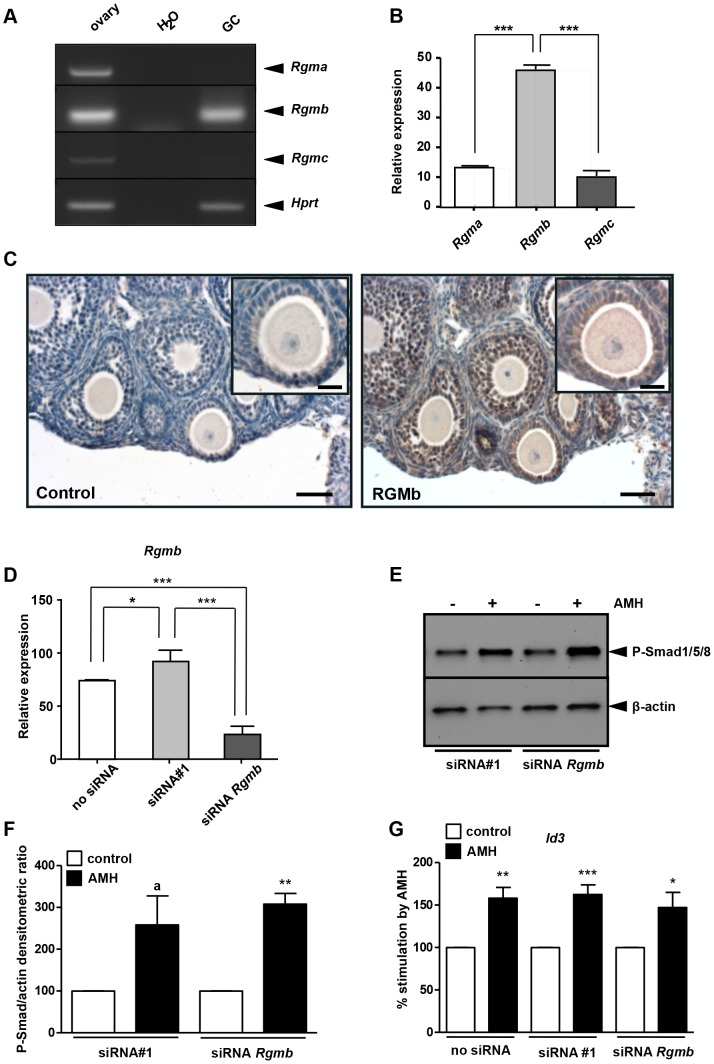
RGMb is not required for AMH signalling in granulosa cells
We then tested whether the BMP co-receptors RGMs could also be AMH co-receptors. We analysed the expression of Rgma, Rgmb and Rgmc in the ovary and primary GCs by RT-PCR (Fig. 6A) and q-PCR (Fig. 6B). Rgma and Rgmc expression was detected in total ovary but not in GCs (Fig. 6A and 6B). In GCs, we only detected Rgmb (Dragon) (Fig. 6A and 6B). In parallel, we confirmed the localization of RGMb in the ovary by immunohistochemistry where it is mainly present in GCs (Fig. 6C). To determine the potential role of RGMb in these cells, we transfected them with a siRNA directed against this co-receptor. We first checked whether this gene was down-regulated by q-PCR (Fig. 6D) and then analysed the sensitivity of these cells to AMH by Western blot using a phospho-Smad 1/5/8 antibody (Fig. 6E). siRNA transfection led to a 90% decrease of Rgmb mRNAs (Fig. 6D). The Rgmb knockdown GCs were as sensitive to AMH as control GCs (Fig. 6E and 6F). We then analysed the effect of AMH on Id3 expression in GCs transfected with siRNA against Rgmb (Fig. 6G). AMH stimulation led to a 60% increased in Id3 expression in GCs either transfected with control siRNA or not transfected. Id3 also remained up-regulated by AMH at the same level in GCs transfected with siRNA against Rgmb. Altogether, these results showed that AMH can transduce its signal in the absence of RGMb indicating that this co-receptor was not essential for AMH signalling pathway in GCs.
Figure 6. RGMb is not essential for AMH signalling in granulosa cells.
Rgma, b and c expression was analysed by RT-PCR (A) or real time PCR (B, n = 6). (A) RT-PCR showed that all three Rgm were expressed in the mouse immature ovary (left lane) while Rgmb seemed predominantly expressed in GCs (right lane) (A). Real-time PCR confirmed that Rgmb was more expressed than Rgma and Rgmc in GCs (B). Data were analyzed using one-way ANOVA followed by Tukey test for all-pair comparisons. Mouse immature ovary was subjected to immunohistochemistry using an RGMb antibody (C). The left panel was the control without the primary antibody. The right panel shows that RGMb is expressed in the cytoplasm of granulosa cells (scale bar = 50 µm; insert, scale bar = 20 µm). siRNA transfection targeting Rgmb was performed when cells were 50% to 80% confluent (D–G). GCs were exposed (▪) or not (□) to 8 nM AMH. Real-time PCR was used to quantify the decrease in Rgmb expression. Rgmb expression dropped about 75% in GC transfected with the siRNA targeting Rgmb when compared to a control siRNA (D, n = 6). Western blot with a phospho-Smad1/5/8 antibody (E, n = 4) and real-time PCR on Id3 gene (G, n = 6) showed that the knowkdown of Rgmb does not affect AMH signalling pathway. Western blots were quantified and normalized from 4 experiments (F, n = 4). Data were analyzed using paired t-test. * p<0.05, ** p<0.01, *** p<0.001, a: p = 0.074. AMH response was not significantly different between control siRNA and Rgmb siRNA transfected GCs.
Smad1 and 5 are the main Smads used by AMH in GCs
To investigate which Smad was important for AMH signalling in GCs, we first used siRNA for gene knockdown but we were not able to decrease Smad expression more than 50% (data not shown). We then used a reporter gene assay. 24 h after the preparation of GCs they were transfected with two plasmids: an expression plasmid which encodes a fusion protein (Smad1-Gal4-DBD/Smad5-Gal4-DBD or Smad8-Gal4-DBD) and a reporter plasmid which codes for a luciferase gene placed under the control of a promoter which contains UAS sequences (UAS-luc). These sequences are known to specifically bind Gal4 [36]. 24 h after transfection, the cells were treated during another 24 h with AMH (8 nM) and luciferase activity was measured (Fig. 7). As a control, we transfected Gal4-DBD with the reporter plasmid. As shown on Figure 7, AMH significantly increased Smad1 and Smad5 activity (110% and 80%, respectively) while it had no effect on Smad8. These results indicated that Smad1 and Smad5 were equally important for AMH signalling in GCs.
Figure 7. Smad1 and 5 are the main Smads activated by AMH in granulosa cells.
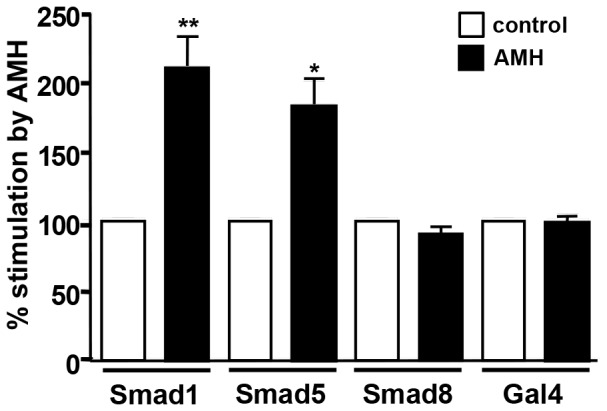
GCs were co-transfected with a luciferase reporter construct (UAS-luc) and different expression constructs (Smad-Gal4). Four different expression constructs were transfected in combination with the reporter construct: Smad1-Gal4, Smad5-Gal4, Smad8-Gal4 and Gal4 as a control. Cells were stimulated (▪) or not (□) with 8 nM AMH. In the absence of AMH, the fusion protein Smad-Gal4 remained in the cytoplasm which is reflected by a basal expression level of luciferase. After 24h of treatment with AMH, the Smad-Gal4 protein was phosphorylated and could translocate into the nucleus and increase luciferase expression. Firefly luciferase activity measured in control medium was set to 100 arbitrary units. The results are expressed as a percentage of stimulation of Firefly luciferase activity measured in the presence of AMH (n = 3). Data were analyzed using paired t-test. ** p<0.01.
Discussion
The aim of this study was to define the different actors of AMH signalling pathway including new target genes in immature GCs. We report that AMH up-regulates Id3, through BMPR-IA and that only Smad 1/5 are activated by AMH in these cells. We used in this study primary culture of mouse GCs and when available, we checked our results on GCs from conditional knockout mice for type I receptors.
GCs were prepared from 3 weeks old mouse immature ovaries. At this stage, ovaries are mainly composed of growing follicles expressing AMH and very few theca cells [22], [33]. As expected, immunocytochemistry showed that more than 90% of the cells express AMH, indicating that the culture predominantly contains GCs. Furthermore, these cultures expressed Fshr and Inha but not Lhcgr, a theca cell marker at this stage. The expression of Fshr is compatible with the presence of antral follicles at 3 weeks and with the fact that Fshr is detectable before the follicles become sensitive to FSH. These GCs were also properly transfected by siRNAs or plasmids allowing quantitative studies of the AMH signalling pathway. Finally, they expressed Amhr2, the AMH specific type II receptor, the three type I receptors and the R-Smads (Smad1 pathway) necessary to respond to AMH [37]. In keeping with this result, GCs in primary culture maintained their ability to respond to AMH by activating the Smad1 pathway.
We also addressed whether a new family of BMP co-receptors [18] the RGMs, RGMa, b (DRAGON) and c (Hemojuvelin), were expressed by GCs and could be AMH co-receptors. This hypothesis was based on the fact that BMPs and AMH share their signalling pathways, and that both RGMs and AMH are involved in anti-proliferative effects [38]–[43]. Indeed, Rgmb knockdown results in enhanced proliferation, adhesion, and migration in breast cancer cells [42] and in increased migration and invasion in PC-3 cells [43]. On the other hand, AMH has been described as a tumor suppressor gene in the mouse testis [41]. Moreover, in vitro studies have shown that AMH inhibits the growth of ovarian, endometrial and breast cancer cell lines [38]–[40]. We report that RGMb is the only RGM expressed in GCs but that the decrease of its expression using siRNA does not alter AMH responsiveness, indicating that RGMb is not essential for AMH signalling in GCs. Unfortunately, Rgmb knockout mice die within 2 to 3 weeks after birth suffering from immune and inflammatory disorders, precluding the study of the female reproductive tract [44].
We had previously shown in the adult mouse GC line AT29C-U493 that AMH activated the Smad1/5/8 signalling pathway [45]. Here we seek to identify which one of these Smad was preferentially phosphorylated and activated by AMH in GCs. We demonstrated, using reporter genes specific for each Smad, that only Smad1 and 5 are activated by AMH. Our results are in agreement with the phenotype of the transgenic models for Smad1/5/8. Indeed Smad8 knockout mice are viable and fertile [46]–[48] and Smad8 is the least expressed Smad in GCs indicating that this Smad is not important for ovarian physiology. In contrast, Smad1 and Smad5 function together in the ovary to suppress ovarian tumorigenesis [49]. Regarding the involvement of Smad proteins in AMH signalling, partial retention of Müllerian ducts is observed when Smad5 is inactivated with or without another Smad, suggesting that Smad5 is the most important Smad required for Müllerian ducts regression [16]. However, complete Müllerian duct retention in males occurs only when the three genes Smad1/Smad5/Smad8 are conditionally inactivated [16]. Consistently, using siRNA against the different Smads, we were not able to decrease Smad expression more than 50% (data not shown).
We then studied which type I receptor was important in the AMH signalling pathway testing the ability of AMH to phosphorylate R-Smad 1/5/8 and to stimulate Id3 expression in GCs either transfected with siRNA against Acvr1, Bmpr1a and Bmpr1b, or extracted from conditional KO mice for Acvr1 or Bmpr1a. We did not consider Acvrl1, another type I receptor involved in R-Smad 1/5/8 pathway whose functional ligands are BMP9 and BMP10 [50]. Indeed, previous studies on AMH signalling in different cell types did not show the involvement of this receptor [13]–[15], [17], [51]–[53]. We showed that transfection of GCs with siRNA against Bmpr1a, prevents AMH to induce Smad1/5/8 phosphorylation and to regulate Id3 expression. The use of corresponding conditional knockout mice, support these results. GCs isolated from Bmpr1a cKO mice ovaries do not transduce the AMH signal. These results indicate that BMPR-IA is the main AMH type I receptor in GCs. This is also the case for other AMH target cells. Indeed, BMPR-IA is necessary for AMH to mediate Müllerian duct regression since only Bmpr1a disruption induces Müllerian duct retention in mice [15]. Similarly, BMPR-IA is essential for AMH to activate Smad 1 in the Sertoli cell line SMAT-1 [17] and to induce Leydig cells differentiation in mice [53]. In keeping with a role of BMPR-IA in folliculogenesis, the majority of Bmpr1a cKO female mice are infertile due to a decrease of spontaneous ovulations and an inhibition of follicular development [24]. Concomitantly, 9 month-old Bmpr1a cKO mutant females exhibited increased follicular atresia [24]. Interestingly, the Amhr2 KO mice also display a follicular atresia [54].
However, there is a redundancy among the type I receptors to transduce AMH effects. Indeed, Müllerian duct regression is blocked in about 50% of the conditional mutant males for Bmpr1a and occurs normally in 100% of the conditional mutant males for Acvr1a, but 100% of the males generated completely retained Müllerian duct derivatives only when both Acvr1a and Bmpr1a are conditionally inactivated, [16]. These findings indicate that BMPR-IA is the primary type I receptor required for Müllerian duct regression but that ActR-IA is capable of transducing the AMH signal in the absence of BMPR-IA [16]. Similarly, ActR-IA can compensate for the lack of BMPR-IA in SMAT-1 Sertoli cells [17]. Here we show that both GCs transfected with siRNA against Acvr1 or isolated from Acvr1 cKO mice ovaries are able to respond to AMH. Therefore, in contrast to Müllerian duct, Sertoli and Leydig cells, ActR-IA does not act as a secondary type I receptor for AMH in GCs. In addition, BMPR-IB does not have any compensatory effect in the absence of BMPR-IA. Interestingly, Bmpr1b KO mice are viable but females are infertile [55]. These females develop severe defects in cumulus expansion and insufficient uterine endometrial gland development. To complete our siRNA results, it would be interesting to study GCs isolated from Bmpr1b KO mice.
We needed some AMH target genes to validate the siRNA knockdown experiments. Cyp19a1 and Lhcgr have been described as AMH target genes in a previous study [22]. However, we were unable to use them in the current study because their expression levels were too low in our GCs culture (data not shown), which is consistent with their stage of differentiation. Because AMH regulates genes encoding steroidogenic enzymes in Leydig and Sertoli cells namely Hsd3b1 and Cyp11a1 [20], [56], we have assayed the expression level of these genes after AMH exposure and could not detect any changes. Finally, since BMPs share some of their signalling pathway components with AMH, we tested some proven BMPs target genes. Id genes are BMP2 target genes in osteoblastic cells [57] and in the breast cancer cell line MCF-7 [58]. These proteins function as dominant negative basic helix loop helix (bHLH) transcription factors. They regulate many genes required for growth and differentiation, through the binding to E-box sequences located in the promoter of target genes [59]. Id genes are selectively up or down regulated depending on cell conditions [60]–[62]. In the ovine and hen GCs, the exposure to respectively BMP6 or BMP2 leads to an increase in all Id genes expression [60], [61]. Conversely, activin A has an inhibitory effect on Id gene expression [61]. Similarly, in porcine GCs expression levels of Id2 and Id3 are regulated by FSH and cumulus-oocyte-complex in an opposite way [62]. A recent study has demonstrated a functional relationship between the expression of all Id isoforms and the status of GC differentiation [25]. In our study, Id3 gene expression is upregulated by AMH in immature GCs. Interestingly, Lhcgr expression is inversely correlated with Id3 transcript level in hen undifferentiated GCs [25]. Because knockout studies have led to the conclusion that AMH plays an inhibitory role on follicle maturation [3], Id3 might be one of the genes involved in this complex process.
In conclusion, using siRNA and transgenic mice for the different components of the AMH signalling pathway, we have shown that, like for the other AMH target cells, the most important type I receptor for AMH in GCs is BMPR-IA. Moreover, the main Smad proteins used by AMH in these cells are Smad 1 and Smad 5. Finally, we have identified a new AMH target gene in these cells, Id3, which could be involved in the effects of AMH on the differentiation of GCs and its other target cells.
Supporting Information
Primers sequences used for mouse genotyping (Wt:wild-type allele, Mt:mutant allele).
(DOC)
Primers sequences used for RT-PCR and real-time PCR.
(DOC)
Acknowledgments
We thank Richard Behringer for mice and helpful comments on the manuscript and Dominique Mahé for helping us with the flow cytometry analysis. We acknowledge Plateforme AnimEx Clamart for taking care of our mouse colony.
Funding Statement
This work was supported by INSERM, Université Paris-Sud XI and the French Agence Nationale de la Recherche (ANR) (grant under reference [ANR-08-JCJC-0059] to Soazik P. Jamin). Lauriane Sèdes holds a PhD studentship from Université Paris-Sud XI. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Josso N, Picard JY, Rey R, di Clemente N (2006) Testicular anti-Mullerian hormone: history, genetics, regulation and clinical applications. Pediatr Endocrinol Rev 3: 347–358. [PubMed] [Google Scholar]
- 2. Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, et al. (2002) Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 143: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 3. Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, et al. (2001) Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 142: 4891–4899. [DOI] [PubMed] [Google Scholar]
- 4. Rey R, Sabourin JC, Venara M, Long WQ, Jaubert F, et al. (2000) Anti-Mullerian hormone is a specific marker of sertoli- and granulosa-cell origin in gonadal tumors. Hum Pathol 31: 1202–1208. [DOI] [PubMed] [Google Scholar]
- 5. La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, et al. (2010) Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update 16: 113–130. [DOI] [PubMed] [Google Scholar]
- 6. Massagué J, Gomis RR (2006) The logic of TGFbeta signaling. FEBS Lett 580: 2811–2820. [DOI] [PubMed] [Google Scholar]
- 7. Massagué J, Seoane J, Wotton D (2005) Smad transcription factors. Genes Dev 19: 2783–2810. [DOI] [PubMed] [Google Scholar]
- 8. Baarends WM, van Helmond MJ, Post M, van der Schoot PJ, Hoogerbrugge JW, et al. (1994) A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the mullerian duct. Development 120: 189–197. [DOI] [PubMed] [Google Scholar]
- 9. di Clemente N, Wilson C, Faure E, Boussin L, Carmillo P, et al. (1994) Cloning, expression, and alternative splicing of the receptor for anti-Mullerian hormone. Mol Endocrinol 8: 1006–1020. [DOI] [PubMed] [Google Scholar]
- 10. Mishina Y, Rey R, Finegold MJ, Matzuk MM, Josso N, et al. (1996) Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev 10: 2577–2587. [DOI] [PubMed] [Google Scholar]
- 11. Mishina Y, Whitworth DJ, Racine C, Behringer RR (1999) High specificity of Mullerian-inhibiting substance signaling in vivo. Endocrinology 140: 2084–2088. [DOI] [PubMed] [Google Scholar]
- 12. Belville C, Josso N, Picard JY (1999) Persistence of Mullerian derivatives in males. Am J Med Genet 89: 218–223. [DOI] [PubMed] [Google Scholar]
- 13. Gouédard L, Chen YG, Thevenet L, Racine C, Borie S, et al. (2000) Engagement of bone morphogenetic protein type IB receptor and Smad1 signaling by anti-Mullerian hormone and its type II receptor. J Biol Chem 275: 27973–27978. [DOI] [PubMed] [Google Scholar]
- 14. Clarke TR, Hoshiya Y, Yi SE, Liu X, Lyons KM, et al. (2001) Mullerian inhibiting substance signaling uses a bone morphogenetic protein (BMP)-like pathway mediated by ALK2 and induces SMAD6 expression. Mol Endocrinol 15: 946–959. [DOI] [PubMed] [Google Scholar]
- 15. Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR (2002) Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet 32: 408–410. [DOI] [PubMed] [Google Scholar]
- 16. Orvis GD, Jamin SP, Kwan KM, Mishina Y, Kaartinen VM, et al. (2008) Functional redundancy of TGF-ß family Type I receptors and receptor-Smads in mediating AMH-induced Müllerian duct regression in the mouse. Biol Reprod 78: 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Belville C, Jamin SP, Picard JY, Josso N, di Clemente N (2005) Role of type I receptors for anti-Mullerian hormone in the SMAT-1 Sertoli cell line. Oncogene 24: 4984–4992. [DOI] [PubMed] [Google Scholar]
- 18. Halbrooks PJ, Ding R, Wozney JM, Bain G (2007) Role of RGM coreceptors in bone morphogenetic protein signaling. J Mol Signal 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Monnier PP, Sierra A, Macchi P, Deitinghoff L, Andersen JS, et al. (2002) RGM is a repulsive guidance molecule for retinal axons. Nature 419: 392–395. [DOI] [PubMed] [Google Scholar]
- 20. Racine C, Rey R, Forest MG, Louis F, Ferre A, et al. (1998) Receptors for anti-Mullerian hormone on Leydig cells are responsible for its effects on steroidogenesis and cell differentiation. Proc Natl Acad Sci U S A 95: 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fynn-Thompson E, Cheng H, Teixeira J (2003) Inhibition of steroidogenesis in Leydig cells by Mullerian-inhibiting substance. Mol Cell Endocrinol 211: 99–104. [DOI] [PubMed] [Google Scholar]
- 22. di Clemente N, Goxe B, Remy JJ, Cate RL, Josso N, et al. (1994) Inhibitory effect of AMH upon the expression of aromatase and LH receptors by cultured granulosa cells of rat and porcine immature ovaries. Endocrine 2: 553–558. [Google Scholar]
- 23.Pellatt L, Rice S, Dilaver N, Heshri A, Galea R, et al.. (2011) Anti-Mullerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil Steril 96: 1246–1251 e1241. [DOI] [PubMed]
- 24. Edson MA, Nalam RL, Clementi C, Franco HL, Demayo FJ, et al. (2010) Granulosa cell-expressed BMPR1A and BMPR1B have unique functions in regulating fertility but act redundantly to suppress ovarian tumor development. Mol Endocrinol 24: 1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson AL, Haugen MJ, Woods DC (2008) Role for inhibitor of differentiation/deoxyribonucleic acid-binding (Id) proteins in granulosa cell differentiation. Endocrinology 149: 3187–3195. [DOI] [PubMed] [Google Scholar]
- 26. Ruzinova MB, Benezra R (2003) Id proteins in development, cell cycle and cancer. Trends Cell Biol 13: 410–418. [DOI] [PubMed] [Google Scholar]
- 27. di Clemente N, Jamin SP, Lugovskoy A, Carmillo P, Ehrenfels C, et al. (2010) Processing of anti-mullerian hormone regulates receptor activation by a mechanism distinct from TGF-{beta}. Mol Endocrinol 24: 2193–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kubler NR, Reuther JF, Faller G, Kirchner T, Ruppert R, et al. (1998) Inductive properties of recombinant human BMP-2 produced in a bacterial expression system. Int J Oral Maxillofac Surg 27: 305–309. [DOI] [PubMed] [Google Scholar]
- 29. Mishina Y, Crombie R, Bradley A, Behringer RR (1999) Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev Biol 213: 314–326. [DOI] [PubMed] [Google Scholar]
- 30. Mishina Y, Suzuki A, Ueno N, Behringer RR (1995) Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev 9: 3027–3037. [DOI] [PubMed] [Google Scholar]
- 31. Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V (2004) Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev 121: 173–182. [DOI] [PubMed] [Google Scholar]
- 32. Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR (2002) Generation of Bmpr/Alk3 conditional knockout mice. Genesis 32: 69–72. [DOI] [PubMed] [Google Scholar]
- 33. Campbell KL (1979) Ovarian granulosa cells isolated with EGTA and hypertonic sucrose: cellular integrity and function. Biol Reprod 21: 773–786. [DOI] [PubMed] [Google Scholar]
- 34. Liu F, Hata A, Baker JC, Doody J, Carcamo J, et al. (1996) A human Mad protein acting as a BMP-regulated transcriptional activator. Nature 381: 620–623. [DOI] [PubMed] [Google Scholar]
- 35. Vigier B, Picard JY, Tran D, Legeai L, Josso N (1984) Production of anti-Mullerian hormone: another homology between Sertoli and granulosa cells. Endocrinology 114: 1315–1320. [DOI] [PubMed] [Google Scholar]
- 36. Vashee S, Kodadek T (1995) The activation domain of GAL4 protein mediates cooperative promoter binding with general transcription factors in vivo. Proc Natl Acad Sci U S A 92: 10683–10687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. di Clemente N, Josso N, Gouédard L, Belville C (2003) Components of the anti-Mullerian hormone signaling pathway in gonads. Mol Cell Endocrinol 211: 9–14. [DOI] [PubMed] [Google Scholar]
- 38. Masiakos PT, MacLaughlin DT, Maheswaran S, Teixeira J, Fuller AF Jr, et al. (1999) Human ovarian cancer, cell lines, and primary ascites cells express the human Mullerian inhibiting substance (MIS) type II receptor, bind, and are responsive to MIS. Clin Cancer Res 5: 3488–3499. [PubMed] [Google Scholar]
- 39. Renaud EJ, MacLaughlin DT, Oliva E, Rueda BR, Donahoe PK (2005) Endometrial cancer is a receptor-mediated target for Mullerian Inhibiting Substance. Proc Natl Acad Sci U S A 102: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Segev DL, Ha TU, Tran TT, Kenneally M, Harkin P, et al. (2000) Mullerian inhibiting substance inhibits breast cancer cell growth through an NFkappa B-mediated pathway. J Biol Chem 275: 28371–28379. [DOI] [PubMed] [Google Scholar]
- 41. Matzuk MM, Finegold MJ, Mishina Y, Bradley A, Behringer RR (1995) Synergistic effects of inhibins and Mullerian-inhibiting substance on testicular tumorigenesis. Mol Endocrinol 9: 1337–1345. [DOI] [PubMed] [Google Scholar]
- 42. Li J, Ye L, Sanders AJ, Jiang WG (2012) Repulsive guidance molecule B (RGMB) plays negative roles in breast cancer by coordinating BMP signaling. J Cell Biochem 113: 2523–2531. [DOI] [PubMed] [Google Scholar]
- 43. Li J, Ye L, Kynaston HG, Jiang WG (2012) Repulsive guidance molecules, novel bone morphogenetic protein co-receptors, are key regulators of the growth and aggressiveness of prostate cancer cells. Int J Oncol 40: 544–550. [DOI] [PubMed] [Google Scholar]
- 44. Xia Y, Cortez-Retamozo V, Niederkofler V, Salie R, Chen S, et al. (2011) Dragon (repulsive guidance molecule b) inhibits IL-6 expression in macrophages. J Immunol 186: 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dutertre M, Gouédard L, Xavier F, Long WQ, di Clemente N, et al. (2001) Ovarian granulosa cell tumors express a functional membrane receptor for anti-Mullerian hormone in transgenic mice. Endocrinology 142: 4040–4046. [DOI] [PubMed] [Google Scholar]
- 46. Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk MM, et al. (1999) Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development 126: 1631–1642. [DOI] [PubMed] [Google Scholar]
- 47. Huang Z, Wang D, Ihida-Stansbury K, Jones PL, Martin JF (2009) Defective pulmonary vascular remodeling in Smad8 mutant mice. Hum Mol Genet 18: 2791–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lechleider RJ, Ryan JL, Garrett L, Eng C, Deng C, et al. (2001) Targeted mutagenesis of Smad1 reveals an essential role in chorioallantoic fusion. Dev Biol 240: 157–167. [DOI] [PubMed] [Google Scholar]
- 49. Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, et al. (2008) Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol 28: 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S (2007) Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 109: 1953–1961. [DOI] [PubMed] [Google Scholar]
- 51. Lebeurrier N, Launay S, Macrez R, Maubert E, Legros H, et al. (2008) Anti-Mullerian-hormone-dependent regulation of the brain serine-protease inhibitor neuroserpin. J Cell Sci 121: 3357–3365. [DOI] [PubMed] [Google Scholar]
- 52. Visser JA, Olaso R, Verhoef-Post M, Kramer P, Themmen AP, et al. (2001) The serine/threonine transmembrane receptor ALK2 mediates Mullerian inhibiting substance signaling. Mol Endocrinol 15: 936–945. [DOI] [PubMed] [Google Scholar]
- 53. Wu X, Zhang N, Lee MM (2012) Mullerian inhibiting substance recruits ALK3 to regulate Leydig cell differentiation. Endocrinology 153: 4929–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Visser JA, Durlinger AL, Peters IJ, van den Heuvel ER, Rose UM, et al. (2007) Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Mullerian hormone null mice. Endocrinology 148: 2301–2308. [DOI] [PubMed] [Google Scholar]
- 55. Yi SE, LaPolt PS, Yoon BS, Chen JY, Lu JK, et al. (2001) The type I BMP receptor BmprIB is essential for female reproductive function. Proc Natl Acad Sci U S A 98: 7994–7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Messika-Zeitoun L, Gouédard L, Belville C, Dutertre M, Lins L, et al. (2001) Autosomal recessive segregation of a truncating mutation of anti-Mullerian type II receptor in a family affected by the persistent Mullerian duct syndrome contrasts with its dominant negative activity in vitro. J Clin Endocrinol Metab 86: 4390–4397. [DOI] [PubMed] [Google Scholar]
- 57. Ogata T, Wozney JM, Benezra R, Noda M (1993) Bone morphogenetic protein 2 transiently enhances expression of a gene, Id (inhibitor of differentiation), encoding a helix-loop-helix molecule in osteoblast-like cells. Proc Natl Acad Sci U S A 90: 9219–9222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Clement JH, Marr N, Meissner A, Schwalbe M, Sebald W, et al. (2000) Bone morphogenetic protein 2 (BMP-2) induces sequential changes of Id gene expression in the breast cancer cell line MCF-7. J Cancer Res Clin Oncol 126: 271–279. [DOI] [PubMed] [Google Scholar]
- 59. Massari ME, Murre C (2000) Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol 20: 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Haugen MJ, Johnson AL (2011) Bone morphogenetic protein 2 inhibits FSH responsiveness in hen granulosa cells. Reproduction 140: 551–558. [DOI] [PubMed] [Google Scholar]
- 61. Hogg K, Etherington SL, Young JM, McNeilly AS, Duncan WC (2010) Inhibitor of differentiation (Id) genes are expressed in the steroidogenic cells of the ovine ovary and are differentially regulated by members of the transforming growth factor-beta family. Endocrinology 151: 1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Verbraak EJ, van 't Veld EM, Groot Koerkamp M, Roelen BA, van Haeften T, et al. (2011) Identification of genes targeted by FSH and oocytes in porcine granulosa cells. Theriogenology 75: 362–376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers sequences used for mouse genotyping (Wt:wild-type allele, Mt:mutant allele).
(DOC)
Primers sequences used for RT-PCR and real-time PCR.
(DOC)