Abstract
C3 glomerulopathy is a recently introduced pathological entity whose original definition was glomerular pathology characterized by C3 accumulation with absent or scanty immunoglobulin deposition. In August 2012, an invited group of experts (comprising the authors of this document) in renal pathology, nephrology, complement biology, and complement therapeutics met to discuss C3 glomerulopathy in the first C3 Glomerulopathy Meeting. The objectives were to reach a consensus on: the definition of C3 glomerulopathy, appropriate complement investigations that should be performed in these patients, and how complement therapeutics should be explored in the condition. This meeting report represents the current consensus view of the group.
Keywords: clinical immunology, clinical nephrology, complement, glomerulonephritis, immunology and pathology
C3 glomerulopathy is a recently introduced pathological entity whose original definition was glomerular pathology characterized by C3 accumulation with absent or scanty immunoglobulin deposition.1 The term was introduced for three reasons: first, it was recognized that glomerular pathology associated with isolated C3 accumulation is far more heterogeneous than previously appreciated. Consequently, many lesions could not be satisfactorily placed within existing pathological descriptors based on morphology. For example, the absence of membranoproliferative changes on light microscopy in some cases precluded the use of descriptors like ‘membranoproliferative glomerulonephritis (MPGN) type II' or ‘MPGN type I with isolated deposits of C3'. Second, advances in our understanding of complement-mediated kidney injury have made it possible to identify the cause of renal disease through specific complement investigations. A renal biopsy report that classified a lesion as C3 glomerulopathy should prompt an investigation of the complement system. Third, the existence of a licensed complement inhibitor (eculizumab, Alexion Pharmaceuticals, Cheshire, CT) together with the many complement inhibitors in clinical development meant that it was therapeutically relevant to identify patient groups most likely to benefit from an anti-complement therapeutic approach. C3 glomerulopathy, by definition, encompassed complement-mediated renal disease, and defined a logical patient population in which to test the efficacy of complement inhibitors. C3 glomerulopathy incorporated rather than replaced existing disease entities where these terms were considered to be satisfactorily descriptive of the pathology, that is, dense deposit disease and C3 glomerulonephritis. The term C3 glomerulonephritis was coined to describe glomerular lesions in which there is glomerular accumulation of C3 with little or no immunoglobulin in the absence of the characteristic highly electron-dense transformation seen in dense deposit disease. C3 glomerulopathy also incorporates entities where the presence of a disease-associated complement mutation is causally associated with the underlying renal pathology. Examples include familial dense deposit disease with C3 mutation2 and familial C3 glomerulonephritis with mutations in the CFHR genes.3, 4
TOWARD A CONSENSUS
In August 2012, an invited group of experts (comprising the authors of this document) in renal pathology, nephrology, complement biology, and complement therapeutics met to discuss C3 glomerulopathy in the first C3 Glomerulopathy Meeting. The meeting was organized by Matthew Pickering and Terry Cook, hosted at the Wellcome Trust Conference Centre, Hinxton, Cambridge, UK, and sponsored by an unconditional educational grant from Alexion Pharmaceuticals. The objectives of this working group were: (i) through expert-based discussion, to reach a consensus on the definition of C3 glomerulopathy; (ii) through expert-based discussion, to reach a consensus on the appropriate complement investigations that should be performed in these patients; (iii) through expert-based discussion, to reach a consensus on how complement therapeutics should be explored in C3 glomerulopathy; and (iv) to garner support for an International Registry of C3 Glomerulopathy. This document represents the current consensus view of the group.
PATHOLOGY
Limitations and difficulties with the original definition
The last decade has seen increasing recognition of a spectrum of glomerular diseases in which the primary pathogenic process is abnormal control of complement activation, deposition, or degradation leading to deposition of fragments of C3 in glomeruli. The C3 fragments can be detected by immunohistochemistry (IHC)/immunofluorescence (IF) and are associated with electron-dense deposits on electron microscopy (EM). The C3 fragments are detected in routine IHC/IF by an antibody directed against C3c and positivity with this antibody is by convention said to show C3 localization (Figure 1). The term C3 glomerulopathy was suggested to encompass a range of conditions regardless of the light or electron microscopic appearances.1 C3 glomerulopathy is distinct from atypical hemolytic uremic syndrome although both diseases are due to abnormal control of the alternative pathway. In atypical hemolytic uremic syndrome, activation of complement occurs on glomerular or microvascular endothelium causing a thrombotic microangiopathy; in most cases, no electron-dense deposits are seen on EM and glomerular C3 is not detected on IHC/IF.
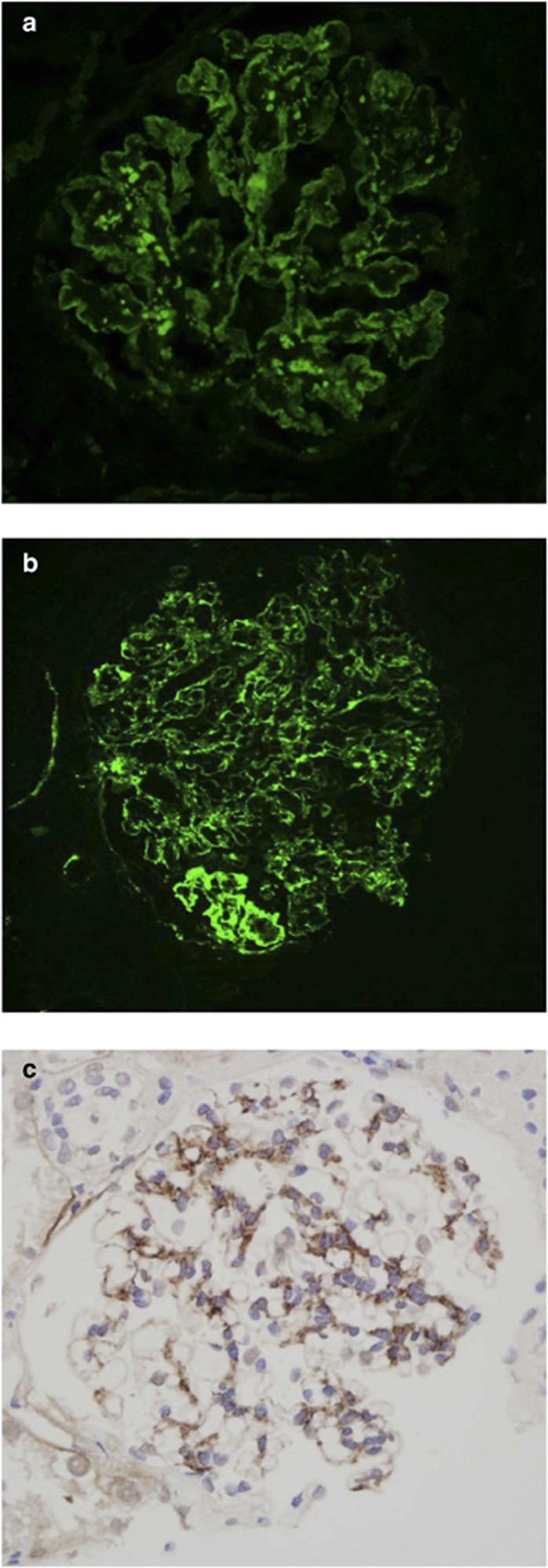
Figure 1.
Immunohistology for C3c. (a) Immunofluorescence in dense deposit disease, (b) immunofluorescence in a case of C3 glomerulonephritis showing predominantly capillary wall staining, and (c) immunoperoxidase in a case of C3 glomerulonephritis showing predominantly mesangial staining.
As the primary process that leads to glomerular C3 fragment deposition in C3 glomerulopathy is complement activation via the alternative pathway, typical cases do not show any deposition of immunoglobulin or of early components of the classical or lectin pathways, specifically C1q and C4c. Therefore, a purist approach would be to restrict the term solely to cases with C3 staining in the absence of immunoglobulins, C1q and C4c. However, as discussed below, well-substantiated cases occur where the pathogenesis and histopathological features are typical for C3 glomerulopathy but variable amounts of immunoglobulin are detected on IHC/IF. The converse situation also arises. In cases of post-infectious glomerulonephritis (PIGN), there may be isolated staining for C3 on IHC/IF, but the clinical features are consistent with a self-limiting immune complex–mediated process. Therefore, the problem in clinical practice is to distinguish those patients in whom the pathological process is C3 deposition due to abnormalities of complement control from those with another pathogenesis such as immune complex deposition. We suggest that the term C3 glomerulopathy be used to designate a disease process rather than just a set of biopsy appearances. This is, for example, analogous to systemic lupus erythematosus, immunoglobulin A (IgA) nephropathy or diabetic nephropathy in which, despite variable morphological appearances on biopsy, the pathologist can confidently reach a diagnosis based on the synthesis of light, electron microscopic, IHC/IF, and clinical features.
We will now discuss the range of pathological appearances seen in C3 glomerulopathy and then make practical recommendations for terminology and future research.
Morphology
C3 glomerulopathy may show a range of features on light microscopy and EM. Light microscopic appearances include mesangial proliferative, membranoproliferative, and endocapillary proliferative; in each case, crescents may also be present. In rare cases, glomeruli may be normal by light microscopy. One of the most variable findings between cases is in the quality of the deposits seen in glomeruli on EM. In many cases, these have a distinctive highly electron-dense, osmiophilic appearance and this has been designated as dense deposit disease (Figure 2a). It is not known why the deposits develop this particular morphological appearance. In other cases, the deposits do not have this characteristic density. However, the meeting recognized that, although in typical cases there is generally good agreement among pathologists on a diagnosis of dense deposit disease, there may be cases where the decision to call the deposit ‘dense' is not clear cut. In addition, the typical dense appearance may only be present in some segments of glomeruli (Figure 2b) and therefore diagnosis may be affected by EM sampling. In dense deposit disease, the deposits are typically found within the glomerular basement membrane, as rounded deposits in the mesangium, and, in many cases, in Bowman's capsule and tubular basement membranes. The glomerular deposits often form band-like or sausage-like shapes punctuated by skip areas of more normal-appearing glomerular basement membrane. These deposits tend to thicken and transform the lamina densa, but may also involve the subendothelial region and produce hump-shaped subepithelial deposits that resemble those seen in acute PIGN. Other than the difference in the morphology of the deposits on EM, which when well established may also lead to characteristic appearances on light microscopy, there are no other specific histopathological or clinical features that allow a distinction between cases of C3 glomerulopathy with the appearance of dense deposit disease and those without. By light microscopy, cases of dense deposit disease may show a range of appearances. In some, there is a typical membranoproliferative pattern, whereas others show predominantly mesangial proliferation. There may be prominent endocapillary proliferation, leukocyte infiltration, and/or crescents.5 Glomerular and tubular basement membrane deposits are often visible by light microscopy with the help of trichrome and silver stains.
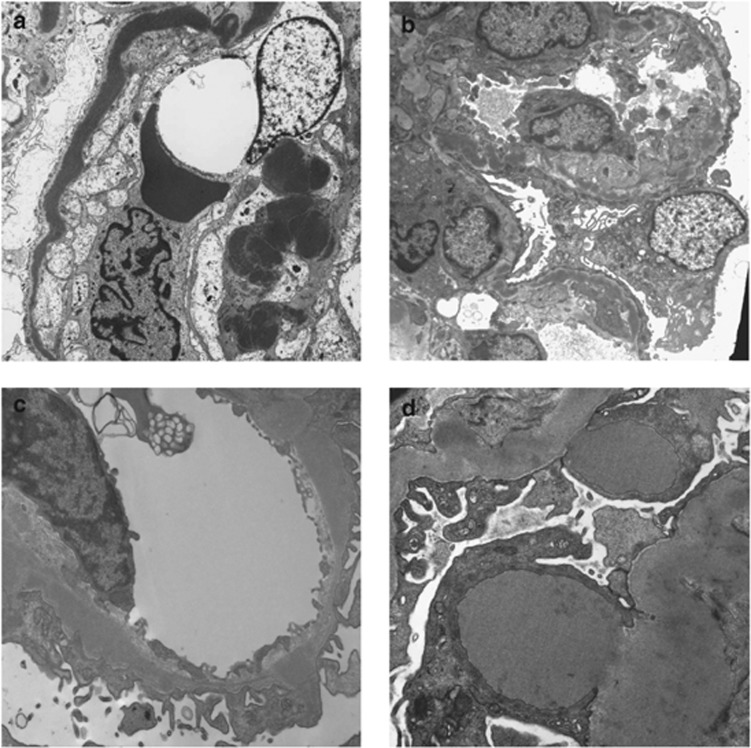
Figure 2.
Electron microscopy in C3 glomerulopathy. (a) Dense deposit disease showing very electron-dense osmiophilic deposit occupying most of the glomerular basement membrane. (b) A case of dense deposit disease in which the highly osmiophilic deposit is only seen in segments of the glomerular basement membrane. (c) A case of C3 glomerulopathy in which there is electron-dense material that is expanding the basement membrane. The material is less electron dense and less well defined than in dense deposit disease. (d) A case of C3 glomerulopathy showing two large subepithelial hump-shaped deposits.
C3 glomerulopathies in which the deposits do not fulfill the criteria for dense deposit disease have been designated as ‘C3 glomerulonephritis'.6 By definition, the deposits of C3 glomerulonephritis are less electron dense than those seen in classic dense deposit disease. On EM, there are a range of appearances. It appears that the changes that have been designated as MPGN type III of Strife and Anders often represent C3 glomerulonephritis. In these cases, there is a complex pattern of mesangial increase and glomerular basement membrane thickening with variable combinations of subendothelial, intramembranous, and subepithelial deposits associated with fraying of the lamina densa. In general, the glomerular basement membrane deposits of these examples of C3 glomerulonephritis tend to be less discrete, more ill-defined, more confluent, and more likely to blend with the extracellular matrix than those in dense deposit disease (Figure 2c). Other examples of C3 glomerulonephritis have more discrete subendothelial deposits resembling MPGN type I. Deposits in the mesangium tend to be rounded in appearance. It has been proposed by some pathologists that the appearances of the deposits on EM are highly suggestive of C3 glomerulonephritis and even of the underlying genetic defect, as, for example, in CFHR5 nephropathy. However, in the absence of larger unbiased studies, it remains to be seen whether these deposit characteristics are supportive of a given genetic defect or specific disease trigger. As described for dense deposit disease, C3 glomerulonephritis may exhibit large subepithelial hump-like deposits (Figure 2d), similar to those seen in PIGN. The significance of these humps and their relationship to intercurrent infections needs to be defined in larger studies.
Light microscopy in C3 glomerulonephritis is variable and the glomerular morphology broadly corresponds to the features seen on EM. Some show a mesangial proliferative pattern and others show a membranoproliferative pattern. There may be variable endocapillary proliferation, leukocyte infiltration, and crescent formation. The ability to detect deposits by light microscopy varies between cases. There is some evidence that the morphological patterns may relate to pathogenesis, and to underlying genetic abnormalities, but this requires confirmation in larger studies.
Immunohistochemistry/immunofluorescence
By definition, kidneys with C3 glomerulopathy show staining for complement C3 in glomeruli. In most laboratories, C3 is detected using an antibody to C3c, one of the physiological breakdown products of activated C3 (denoted C3b). These products are collectively known as the C3 fragments and comprise iC3b, C3c, and C3dg. These fragments can mediate distinct biological responses through their interactions with complement receptors. Consequently, it may be helpful in the future to use specific antibodies to different C3 fragments in order to determine their presence and relative location in glomeruli because these distinctions may reflect different pathophysiological mechanisms.
Looking for the presence of C5b-9, as a marker of complement terminal pathway activation, might be relevant when considering therapeutic C5 inhibition. However, it is important to recognize that (1) C5b-9 may be detected in glomeruli from normal kidneys,7 and (2) glomerular and tubular basement membrane deposits of C5b-9 have been shown to persist in repeat renal biopsies of C3 glomerulonephritis and dense deposit disease performed 1 year after initiation of eculizumab therapy despite the normalization of soluble C5b-9 levels in serum.7
An important question concerns the presence of immunoglobulins in bona fide cases of C3 glomerulopathy (Figure 3). In many glomerular diseases, small amounts of immunoglobulin may become trapped in areas of sclerosis or accumulate as droplets in podocytes. In dense deposit disease, approximately one-third of patients with either mesangial proliferative (8 out of 28 cases) or acute proliferative and exudative (3 out 8 cases) subtypes had glomerular IgG staining.5 Dr D'Agati and colleagues further explored the effect of applying different ‘cutoff' levels of immunoglobulin deposition to cases with typical appearance of dense deposit disease on EM.8 In summary, their data showed that only 41% of cases had C3 only (without immunoglobulin), 59% had dominant C3 with up to 1+ IgM, and 80% of cases had dominant C3 of ⩾2 orders of magnitude of intensity by IF greater than any other immune reactant (using a scale of 0 to 3, including 0, trace, 1+, 2+, 3+). However, even with this liberal interpretation of dominant C3, 20% of cases of dense deposit disease would not be classified as C3 glomerulopathy. It is likely that IgM staining has a different significance from staining with IgG or IgA and this should be a subject of further study. Thus, if criteria exclude cases with any immunoglobulin deposition, it is very likely that cases in which the pathogenesis is alternative pathway dysregulation will be overlooked.
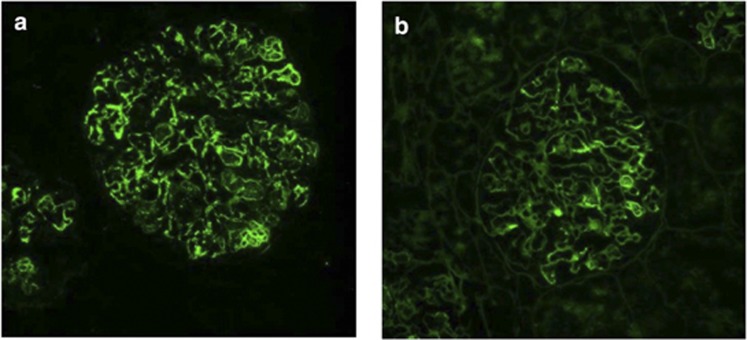
Figure 3.
Immunofluorescence in a case of C3 glomerulonephritis illustrating that a small amount of immunoglobulin G (IgG) may be present. (a) C3c and (b) IgG.
Some pathologists believe that there are characteristic electron microscopic appearances in non-dense deposit disease C3 glomerulopathy. These ultrastructural findings would support the diagnosis of C3 glomerulonephritis even when immunoglobulin is present, but this requires confirmation in further studies.
It is noteworthy that in a large series of cases classified as idiopathic MPGN type I on the basis of the presence of immunoglobulin in addition to complement, there was a significant incidence of either C3NeF or of genetic mutations of proteins involved in the alternative pathway.6 There may be several explanations for this. First, it may be that there is an interaction between immune complex deposition and complement dysregulation; it is plausible that in some cases immune complex deposition may trigger or exacerbate disease when there is genetic or acquired complement dysregulation.1 Theoretically, an initial trigger of the classical pathway might uncover a defect in the alternative pathway and then continued complement activation is sustained through the alternative pathway. This could result in an otherwise typically self-limiting illness, for example, post-infectious nephritis, entering a chronic phase. Second, it is important to remember that immunoglobulin may be seen nonspecifically in areas of scarring or in podocyte droplets. These possibilities require further study.
Post-infectious glomerulonpehritis
It is not uncommon for typical cases of PIGN, particularly those beyond the acute stage, to show deposition of C3 without immunoglobulin.9 In this case, distinction from C3 glomerulopathy will depend on the absence of atypical features on light microscopy and EM, and also on a typical clinical course with resolution. However, it is clear that C3 glomerulopathy may present following an infectious episode, often a streptococcal infection, and, as noted above, subepithelial humps are often a feature of C3 glomerulopathy. Therefore, the presence of any atypical clinical or histological features in a case of apparent PIGN should raise suspicion of C3 glomerulopathy.
RESEARCH CONSIDERATIONS
More information is required on the relationship of morphological changes in biopsies with clinical features, clinical course, and outcome. Specific questions include:
What features on biopsy are best predictive of clinical course?
Are there correlations between biopsy appearances and underlying pathogenic processes particularly specific genetic mutations?
Are there characteristic electron microscopic features that are suggestive of C3 glomerulonephritis?
Which C3 fragments are deposited in glomeruli in C3 glomerulopathy and is there any significance to their relative locations? Are these C3 fragments different from those seen in either ‘MPGN type I with immunoglobulin deposits' or those seen in typical post-infectious GN?
What is the etiologic and clinical significance of subepithelial hump-shaped deposits in cases of C3 glomerulopathy?
How can we refine the diagnosis of C3 glomerulopathy to deal with those cases that also have immunoglobulin deposits
PATHOLOGY—RECOMMENDATIONS
The term C3 glomerulopathy should be used to designate a disease process due to abnormal control of complement activation, deposition, or degradation and characterized by predominant glomerular C3 fragment deposition with electron-dense deposits on EM.
The level of suspicion on a renal biopsy for the disease process of C3 glomerulopathy will depend on the interpretation of light microscopy, IHC, EM, and clinical history.
It is suggested that in renal biopsy diagnosis the use of the descriptive morphological term ‘glomerulonephritis with dominant C3' is useful to indicate the likelihood that the case represents the disease process of C3 glomerulopathy (Figure 4).
We suggest that in practice ‘glomerulonephritis with dominant C3' should be used as a morphological term for those cases with dominant staining for C3c. Dominant is defined as C3c intensity⩾2 orders of magnitude more than any other immune reactant on a scale of 0 to 3 (including 0, trace, 1+, 2+, 3+).
We believe that the use of ‘glomerulonephritis with dominant C3' in this way will identify most cases of C3 glomerulopathy and exclude most cases of immune complex disease, but attention must also be paid to the other histological features and clinical history. In particular, there may be EM appearances that are also very helpful in making a diagnosis of C3 glomerulopathy—particularly, the presence of features of dense deposit disease. As discussed above, some cases of typical PIGN may show C3 dominance on IHC/IF.
As with all biopsies, interpretation of individual cases depends on integration of information from the biopsy together with clinical, serological, and genetic features and, at present, no single algorithm can correctly identify all cases of C3 glomerulopathy. The role of the pathologist must be to draw attention to cases in which there is likely to be an underlying defect in the complement system.
We suggest that the term dense deposit disease be applied to those cases of C3 glomerulopathy in which characteristic very dense osmiophilic deposits are present and that other cases should be called C3 glomerulonephritis. However, we recognize that there will be borderline cases. Although the presence of dense deposit disease may be strongly suspected on the basis of light microscopy, the gold standard for diagnosis is EM.
It should be emphasized that in many cases of glomerulonephritis with subepithelial humps on EM and isolated or dominant C3 deposits, including some formerly classified as persistent or resolving PIGN and even some with a documented infection history, the differentiation of true PIGN from C3 glomerulonephritis often cannot be made on the basis of morphology and clinical and laboratory data available at the time of biopsy. According to our recommendation, a PIGN patient's biopsy may be read as ‘glomerulonephritis with dominant C3 (infection-associated)'. However, this does not mean that the patient has C3 glomerulopathy. In these cases, refining the differential diagnosis will require following the patient clinically and serologically over several months to determine the course of urinary abnormalities and serum C3 levels. If these parameters do not follow a typical course of PIGN (i.e., normalization of the decreased peripheral C3 level in 8–12 weeks), a diagnosis of C3 glomerulopathy should be reconsidered and additional investigations performed as outlined below.
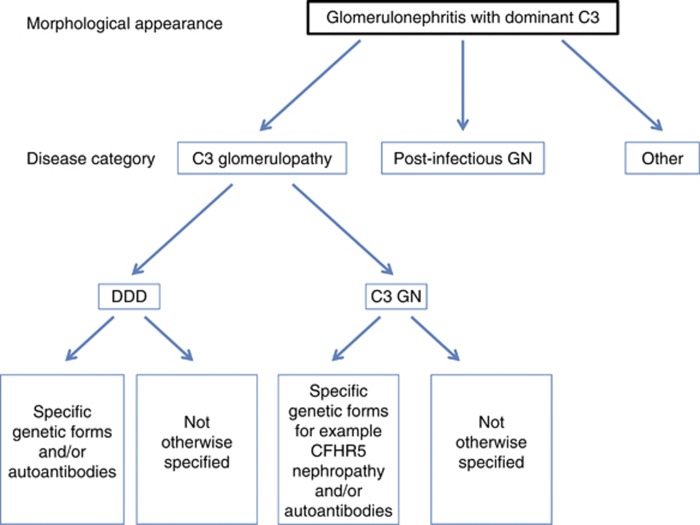
Figure 4.
A schematic diagram showing an approach to the classification of disease in a biopsy showing the morphological changes of a glomerulonephritis with dominant C3. DDD, dense deposit disease; Post-infectious GN, post-infectious glomerulonephritis.
COMPLEMENT INVESTIGATIONS IN C3 GLOMERULOPATHY
Complement genetic screening
Genetic factors have been reported in cohorts of patients with dense deposit disease, C3 glomerulonephritis, and MPGN type 1. These include, but are not limited to, mutations in the complement regulatory protein factor H, factor I, and CD46 (also termed membrane cofactor protein).6
In a series of 134 patients with idiopathic MPGN type I (n=48), dense deposit disease (n=29), or C3 glomerulonephritis (n=56), mutation screening of the CFH, CFI, and CD46 genes (encoding factor H, factor I, and CD46, respectively) was performed. Out of the 134 patients screened, 17 (12.7%), 6 (4.5%), and 1 (0.7%) had mutations in the CFH, CFI, and CD46 genes, respectively.6 Conversely, 110 out of 134 (82.1%) did not have a mutation in these genes, demonstrating that in the majority of patients mutations in these genes are not present.
Gain-of-function changes in the complement activation proteins, factor B10 and C3,2 are rare but provide important information. For example, one gain-of-function C3 mutation associated with familial C3 glomerulopathy (reported as dense deposit disease) is resistant to inhibition by factor H.2 Hence, factor H-based therapy would be predicted to be ineffective in this instance.
Genomic rearrangements within the complement factor H-related genes, which do not affect the CFH gene, have been reported in familial C3 glomerulopathy. There are five complement factor H-related genes: CFHR1, CFHR2, CFHR3, CFHR4, and CFHR5.
In CFHR5 nephropathy, familial C3 glomerulopathy of the C3 glomerulonephritis subtype, there is an internal duplication within the CFHR5 gene.3 This specific mutation can be screened by PCR using genomic DNA.3 In another familial C3 glomerulopathy (originally described as MPGN type III subtype), another rearrangement within the CFHR locus was detected in affected individuals. This was a hybrid CFHR3-1 gene, which can also be detected by PCR using genomic DNA.4 We are aware of other rearrangements published in abstracts that include CFHR2–CFHR5 hybrid gene in familial dense deposit disease,11 an internal duplication in CFHR1 associated with dense deposit disease,12 and CFHR5 nephropathy in a family without Cypriot ancestry.13 To detect rearrangements within the CFH-CFHR locus, copy number assays such as multiplex ligation–dependent probe assay, TaqMan qPCR, or genomic hybridization assays are needed.
Outside of specifically testing for an established disease-associated variant (e.g., the internal duplication in the CFHR5 gene), complement genetic screening in these patients is de facto a candidate gene approach. It is therefore critical that the detected changes are rigorously analyzed to determine whether they represent disease-associated changes. Conditions for this could include the following: (i) the demonstration that the novel variant segregates with disease in familial cases. The lack of segregation would normally indicate that the variant is not disease associated; (ii) the demonstration of rarity of the disease-associated variant/mutation in large population databases in addition to ethnic-specific databases; and (iii) the demonstration that the mutation affects protein function. Although this has been considered the gold standard, functional testing will be impractical for all variants as more and more variants are identified. In addition, functional experiments are often contrived and do not reflect the true biology. A finding suggestive of pathogenicity is the identification of variants in the same protein domain in other families/probands with the disease.
In addition to rare variants (genetic changes <1% frequency in ethnically matched control groups), polymorphic variation (genetic changes >1% frequency in ethnically matched control groups) has been linked to C3 glomerulopathy susceptibility.14 As previously, these associations have been determined using a candidate gene approach and, in some cases, verified with functional studies. Expanding these studies to define a more extensive disease-associated complotype requires both appropriately designed and powered association studies and functional studies.
Complement serological tests
C3 glomerulopathy is associated with uncontrolled complement alternative pathway activation, and serological complement assays may be informative in these patients. These include markers that demonstrate (i) specific activation of the alternative pathway (reduced C3, normal C4, reduced factor B), (ii) C3 turnover (low C3, increased C3 breakdown product, e.g., C3d), and (iii) C5 turnover (low C5, increased soluble C5b-9 and C5a). These abnormalities may vary over the disease course.
The reported acquired causes of C3 glomerulopathy include (i) C3 nephritic factors (C3NeF, autoantibodies that stabilize the alternative pathway C3 convertase, C3bBb), (ii) anti-factor B autoantibodies, and (iii) anti-factor H autoantibodies.
Detection of C3NeF can be done in many different ways.15, 16 Some assays use patient-purified immunoglobulins to screen for autoantibodies that stabilize the alternative pathway C3 convertase.16 Other assays infer the presence of C3 convertase-stabilizing autoantibodies by detection of C3 breakdown products.16 It is possible that patients may be positive in some but not all of these assays.
More in-depth studies are required to define the significance of C3NeF in both the pathophysiology of C3 glomerulopathy and their relationship to disease course and treatment.
Monoclonal gammopathy in serum has been reported in C3 glomerulopathy without clonal deposits in the kidney tissue. In some cases, the monoclonal protein mediates complement dysregulation.17, 18 Therefore, it is recommended that paraproteinemia be looked for in these patients.19, 20 If detected, referral to specialist laboratories to determine whether the paraprotein is contributing to complement dysregulation should be considered.
COMPLEMENT INVESTIGATIONS—RECOMMENDATIONS
It is presently recommended that serological investigations in all patients should include measurement of serum C3, C4, and factor H levels; screening for paraprotein; and screening for C3 nephritic factor because these have diagnostic value (Table 1).
It is presently recommended that screening for CFHR5 nephropathy be performed, as this is an established disease-associated mutation where comprehensive descriptions of the clinical course have been reported.3, 21 Therefore, the presence or absence of this mutation is clinically informative (Table 1).
Other investigations can be considered on a case-by-case basis as they require expert interpretation and/or clinical validation (Table 1).
The above investigations should be performed regardless of whether the diagnosis has been made in the native kidney or in the kidney transplant.
The working group recognized that accessing complement assays may require referral to specialist laboratories. Within Europe, examples of laboratories offering some or all of the complement assays are listed on both the International Complement Society (www.complement.org) and European Complement Network website (www.ecomplement.org). In North America, such laboratories include the Molecular Otolaryngology and Renal Research Laboratories (www.healthcare.uiowa.edu/labs/morl/) and National Jewish Health Advanced Diagnostic Laboratories (www.nationaljewish.org/professionals/clinical-services/diagnostics/adx/about-us/lab-expertise/complement/).
The working group recognized that the interpretation of the significance of these tests requires expertise and recommends that the results be discussed with clinicians experienced in genetic and serological complement assays.
The underlying cause of C3 glomerulopathy in many individuals is not known and it is recommended that all patients be offered the informed opportunity to participate in research studies exploring both causation and response to novel therapies.
Table 1. Complement investigations in C3 glomerulopathy.
| Tests recommended in all patients | |
|---|---|
| Comment | |
| Measurement of serum C3 and C4 | Low C3 with normal C4 indicates alternative pathway activation |
| Measurement of C3 nephritic factor | C3 nephritic factors are associated with C3 glomerulopathy; their correlation with disease course is unclear |
| Measurement of serum factor H | Factor H deficiency is associated with C3 glomerulopathy and is invariably associated with reduction in serum C3 |
| Serum paraprotein detection | Paraproteinemia associated with C3 glomerulopathy, specialist tests required to determine whether paraprotein is a cause of uncontrolled C3 activation |
| Screening for CFHR5 mutation | CFHR5 nephropathy is a well-characterized cause of C3 glomerulopathy,3, 21 and thus screening for this mutation is clinically informative |
| Tests that should be considered on a case-by-case basis as they require expert interpretation and/or clinical validation | |
| Comment | |
| Measurement of serum factor B | Uncontrolled alternative pathway activation may be associated with reduced factor B levels |
| Measurement of serum C5 | May be reduced in terminal pathway activation and could indicate group most likely to benefit from therapeutic C5 inhibition |
| Measurement of markers of C3 activation, e.g., C3d, C3c, C3adesArg | Activated C3 components are more sensitive markers of C3 activation than antigenic levels of intact C3 |
| Measurement of markers of C5 activation, e.g., C5adesArg, soluble C5b-9 | Activated C5 components are more sensitive markers of C5 activation than antigenic levels of intact C5 |
| Measurement of anti-factor H autoantibodies | Anti-factor H autoantibodies are associated with C3 glomerulopathy; correlation with disease course is unclear; especially important to measure in patients with low C3 and negative C3 nephritic factor |
| Anti-factor B autoantibodies | Anti-factor B autoantibodies are associated with C3 glomerulopathy; correlation with disease course is unclear |
| Mutation screening of complement regulatory genes (e.g., CFH, CFI, CD46), activation protein genes (C3, CFB) and assessment of copy number variation across the CFH-CFHR locus | Mutations in these genes associated with C3 glomerulopathy; especially important to screen for CFH mutations in patients with low C3 and negative C3 nephritic factor |
THERAPEUTIC CONSIDERATIONS IN C3 GLOMERULOPATHY
Experience with anti-cellular immune suppression
Despite the recent designation of G3 glomerulopathy, the assumption must be that it has always existed (either in the form of dense deposit disease or C3 glomerulonephritis). Identifying potential treatment successes is complicated by terminology, and we have included in our search idiopathic MPGN types I and III (the terms historically used for many cases of C3 glomerulopathy) and MPGN type II, which refers to dense deposit disease. This task is complicated by the heterogeneity of the reported pathology (i.e., often including immune complex-mediated disease) and influenced by publication bias. Publication bias alone, particularly in the rare diseases, is likely to color substantially the perception of the relative effectiveness of any given agent. Although it is reasonable to review historical cases, they have only limited use for supporting future treatment strategies. Before 2012, treatment has invariably included some type of anti-cellular immune suppression targeting T and/or B cells (e.g., cyclophosphamide, mycophenolate, or rituximab) with or without plasma therapy.22 More recently, treatment plans have sometimes included anti-complement C5 therapy.
There are no controlled trials to support the use of anti-cellular immune therapy in C3 glomerulopathy. Mycophenolate mofetil or rituximab did not alter renal survival in one retrospective cohort.6 Steroid therapy has not been effective in dense deposit disease23 and variably so for MPGN.22 Recent KDIGO (Kidney Disease: Improving Global Outcomes) clinical guidelines suggest that ‘adults and children with presumed idiopathic MPGN accompanied by the nephritic syndrome and progressive decline in kidney function receive oral cyclophosphamide or MMF plus low dose daily or alternate day corticosteroids with initial therapy limited to less than 6 months.' They note that this recommendation is based on very low-quality evidence.24 Recently, glucocorticoids failed to establish remission in a dense deposit disease patient despite a 5-year treatment period.25 Strategies to reduce C3Nef with either mycophenolate mofetil or rituximab have not been studied formally, and there are reports of both response and nonresponse in the published literature. The most recent reports suggest that the anti-cellular immunosuppressive approach remains wholly unsatisfying: McCaughan et al.26 reported a failure to respond to glucocorticoid, mycophenolate mofetil, and rituximab therapy and Bomback et al.27 reveal the failure of both prednisone and mycophenolate mofetil treatment despite appropriate escalation of both agents.
Experience with plasma therapy
There are no data to support the use of plasma therapy in C3 glomerulonephritis. As with anti-cellular therapy, the support for plasma therapy in dense deposit disease relies on case reports. Licht et al.28 reported efficacy of plasma therapy in a sibling pair with dense deposit disease and factor H deficiency. There are reports of recovery of acute kidney injury in dense deposit disease patients with plasmapheresis.29, 30 Conversely, McCaughan et al.26 reported an inability to establish remission in dense deposit disease despite the documented removal of C3Nef via plasmapheresis. As a result of limited successes and the absence of definitive therapy, it is likely that plasma therapy will continue to be used on a case-by-case basis in C3 glomerulopathy.
Experience with eculizumab
As data have begun to accumulate supporting the causal relationship between alternative pathway dysregulation and C3 glomerulopathy, it was appropriate to turn to anti-complement C5 therapy as a potential definitive treatment approach. This approach has been supported by data from animal models,31 and anti-C5 therapy, although not currently licensed for use in C3 glomerulopathy, has been used. Specifically, eculizumab was seen to mitigate disease in three case reports25, 26, 32 and in one small trial.27
Vivarelli et al.32 presented the case of a 17-year-old patient with a 7-year history of dense deposit disease. The patient had normal renal function, normal blood pressure, and no complement gene mutations. Renal biopsy revealed 40% glomerular sclerosis on renal biopsy. Following worsening of nephrotic-range proteinuria, eculizumab was commenced, and during treatment there was a remarkable improvement in proteinuria. When eculizumab was stopped 18 months later, she had a relapse of proteinuria that again remitted with the restart of eculizumab. Sequential post-treatment renal biopsies showed a progressive reduction of C3 and C5b-9 by IF and a progressive reduction in mesangial proliferation and glomerular capillary wall thickness.32
Similarly, Daina et al.25 reported the case of a 22-year-old patient with a long-standing history of dense deposit disease and nephrotic syndrome, nonresponsive to 5 years of steroids. The patient had two dense deposit disease ‘at-risk' CFH alleles,14 but no complement gene mutations, low C3, positive C3Nef, elevated soluble C5b-9 (sC5b-9) levels, and normal renal function. Rituximab treatment was associated with reduction in C3Nef but there was no disease response, and 5 months after rituximab the creatinine began to rise. She was then treated with eculizumab for 48 weeks, during which her serum albumin normalized and her creatinine decreased. No post-treatment renal biopsy was reported in this case.
Finally, McCaughan et al.26 reported the efficacy of eculizumab in a case of recurrent dense deposit disease post-renal transplant. A 29-year-old patient with dense deposit disease developed a recurrence of the disease 4 weeks post transplant, heralded by 6 g of urine protein, despite prednisone, mycophenolate mofetil, and tacrolimus. The patient had a low C3, positive C3Nef, and no complement gene mutation. Despite rituximab and plasmapheresis (with a subsequent normalization of C3Nef), she continued to progress, and 13 weeks after transplant eculizumab was started (length of therapy is not reported). Creatinine recovered to 1.9 mg/dl from 4.93 mg/dl. As in the preceding case,26 a post-treatment biopsy was not reported.
A single trial of eculizumab in C3 glomerulopathy exists.27 This was an open-label, proof of concept, efficacy, and safety study in which three dense deposit disease patients (one with a renal transplant) and three C3 glomerulonephritis patients (two with a renal transplant) received eculizumab every other week for 1 year. All had proteinuria >1 g/day and/or acute kidney injury at enrollment. Genetic and complement function testing revealed a mutation in CFH and CD46 in one subject each and C3NeF in three subjects. After 12 months of therapy, two subjects showed significantly reduced serum creatinine (dense deposit disease patient 1 and C3 glomerulonephritis patient 3), one subject achieved marked reduction in proteinuria (dense deposit disease patient 3), and one subject had stable laboratory parameters but histopathological improvement (C3 glomerulonephritis patient 3). Not surprising, given the mechanism of action of eculizumab, elevated sC5b-9 levels normalized in all assessed patients while on therapy. The authors concluded that there was a response to eculizumab in some but not all subjects, and that an elevation of sC5b-9 was potentially a marker of responsiveness. Follow-up for these patients is ongoing.
These case reports and the single trial, coupled with our current understanding of the pathophysiologic underpinnings of C3 glomerulopathy, suggest that a formal trial of eculizumab comprising a greater number of well-characterized patients is warranted (see below).
Transplantation
The risk for recurrence of C3 glomerulopathy is derived from small data sets. In a study that included 18 transplants in dense deposit disease, recurrence was reported in 11 kidneys (61%), and there was a trend toward greater likelihood of transplant recurrence in dense deposit disease compared with either MPGN type 1 or MPGN type 3 groups.33 In a recent study, recurrence in C3 glomerulonephritis (6 out of 10 (60%)) and dense deposit disease (6 out of 11 (54.5%)) was similar.6 It is not known whether de novo disease in the transplant kidney behaves in a similar manner to recurrent disease. Given our current understanding, the definition and evaluation of C3 glomerulopathy should be applied regardless of whether this pathology is first identified in the native or the transplant kidney. Notably, some patients have developed thrombotic microangiopathy after renal transplantation.6
Future therapeutic approaches
The future of therapeutics revolves around four major issues. The first is simply who should be treated and who will have a relatively benign disease course. A related question is whether a particular clinical phenotype or histopathological pattern predicts response to therapy. Alternatively, is it the pathology that will predict response (i.e., will dense deposit disease respond differently to a given therapy than C3 glomerulonephritis) or will either disease respond similarly depending on given associated clinical findings or complement laboratory findings? Given the presumption that the C3 glomerulopathies are alternative pathway diseases, is it clear that anti-complement therapy is the only treatment option or is it possible that some patients can achieve remission with standard anti-cellular immunosuppressive therapy (with the assumption that anti-cellular therapy may have a role in mitigating the effect of C3Nef and other disease-causing autoantibodies or in inhibiting the effect of the anaphylatoxins)? Finally, once therapy commences, what should the length of the treatment course be? The corollary to this question is whether there are stable phases of disease that will allow drug-free periods. To answer the first two issues, expanded phenotypes, including robust laboratory characterization for the individual patient cohorts, must be obtained. The answer to the later questions will in part come from already established cohorts. However, it is imperative that answering these questions should guide the development of future treatment trials. On the basis of the pathology of disease, anti-complement therapy warrants consideration. This could include (1) C3 convertase inhibition, which may have its greatest utility in limiting C3 breakdown product deposition on basement membranes; and, (2) C5 or terminal complement pathway inhibition.
Optimizing trial design
Before embarking upon a treatment trial, it would be ideal if the association between disease characteristics (clinical presentation, laboratory assessments, genetic background, and pathology) and prognosis was well understood. However, this information is not yet available for C3 glomerulopathy. Furthermore, it would be ideal if homogeneous cohorts, for example, those defined by genetic or antibody-mediated mechanisms, could be studied. This ideal is also unlikely to be achieved easily given the rarity of C3 glomerulopathy and the hitherto heterogeneous case reports. In lieu of achieving these two ideal precepts, the combination of clinically well-defined cases (i.e., duration of disease, level of renal dysfunction, proteinuria, and pathology) coupled with a robust assessment of complement levels and activity may allow a post hoc assessment of predictors of not only disease severity but also of response to therapy. It follows then that any treatment trial will require a broad array of biomarker assessments (both standard and research).
The potential information that may be obtained from an examination of renal tissue under various conditions suggests that treatment response may also be supported by protocolized biopsy. In the end, given the rarity of C3 glomerulopathy, the ultimate goal of initial trials would be to determine not only whether a given therapy was effective but also the precise clinical indicators and biomarkers of disease that may predict the likely therapeutic response.
Armed with trial biomarker results, it should be possible to assess for stable disease (a time when no therapy will be required), times of flare (a time when ideally a marker would support either early or late complement pathway treatment), or when progressive disease suggests the need for long-term therapy.
RECOMMENDATIONS ON THERAPEUTIC APPROACHES
It is timely and necessary to determine the pathological spectrum of C3 glomerulopathy. The working group aims to establish an International Registry of cases of C3 glomerulopathy. This will enable us not only to define the spectrum of glomerular changes, but also allow us to devise appropriate subgroupings and severity scores that could have prognostic and therapeutic utility.
A second aim of the pathology registry would be to link specific pathological parameters with laboratory parameters, treatment history, and clinical response. This would potentially enable us to identify pathologic lesions that correlate with response (or non-response) to certain therapeutic agents or regimens, much as an association between endocapillary hypercellularity and response to corticosteroid therapy was identified in the process of developing the Oxford classification of IgA nephropathy.34
Consideration should be given to specifically investigating anti-C5 therapy in patients with C3 glomerulopathy with evidence of C5 activation either in plasma or within the kidney (see ‘Therapeutic considerations' above).
Emerging therapies that target C3 convertase activity are clearly of major interest in this condition. Optimal trial design with these agents will depend on our understanding of the heterogeneity of this condition. The collective effort of an International Pathology Registry will be invaluable toward this goal.
GBA is a consultant to Alexion Pharmaceuticals and Genentech and a Principal Investigator for an Alexion-funded research grant. FF has received fees from Alexion Pharmaceuticals for invited lectures and is member of an expert board supported by Alexion Pharmaceuticals. VF-B has received fees from Alexion Pharmaceuticals for invited lectures and is member of an expert board supported by Alexion Pharmaceuticals. VMH is a consultant to Alexion Pharmaceuticals, has received sponsored research from Taligen Therapeutics and Alexion, and has received licensing and royalty fees from Taligen and Alexion. SJ is a member of a scientific advisory board for Alexion Phamaceuticals. BPM is a consultant for Baxter International. CMN is a participant on the C3 Glomerulopathy Advisory Board, sponsored by Alexion Pharmaceuticals. DKP is a consultant for GlaxoSmithKline. MCP has received fees from Alexion Pharmaceuticals for invited lectures and funding for pre-clinical studies on experimental complement reagents. JMT is a paid consultant for Alexion Pharmaceuticals. CB, MD, GG, and PT are employees of Alexion Pharmaceuticals and own stock or stock options. SRdC has received fees from Alexion Pharmaceuticals for invited lectures and consultancy. CLH has an employment contract with GlaxoSmithKline. The remaining authors declared no competing interests.
References
- Fakhouri F, Fremeaux-Bacchi V, Noel LH, et al. C3 glomerulopathy: a new classification. Nat Rev Nephrol. 2010;6:494–499. doi: 10.1038/nrneph.2010.85. [DOI] [PubMed] [Google Scholar]
- Martinez-Barricarte R, Heurich M, Valdes-Canedo F, et al. Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest. 2010;120:3702–3712. doi: 10.1172/JCI43343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale DP, de Jorge EG, Cook HT, et al. Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet. 2010;376:794–801. doi: 10.1016/S0140-6736(10)60670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik TH, Lavin PJ, Goicoechea de Jorge E, et al. A hybrid CFHR3-1 gene causes familial C3 glomerulopathy. J Am Soc Nephrol. 2012;23:1155–1160. doi: 10.1681/ASN.2012020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker PD, Ferrario F, Joh K, et al. Dense deposit disease is not a membranoproliferative glomerulonephritis. Mod Pathol. 2007;20:605–616. doi: 10.1038/modpathol.3800773. [DOI] [PubMed] [Google Scholar]
- Servais A, Noel LH, Roumenina LT, et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454–464. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- Herlitz LC, Bomback AS, Markowitz GS, et al. Pathology after eculizumab in dense deposit disease and C3 GN. J Am Soc Nephrol. 2012;23:1229–1237. doi: 10.1681/ASN.2011121186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Markowitz GS, Herlitz LC, et al. Toward a working definition of C3 Glomerulopathy by immunofluorescence Kidney Int 2013(this issue). doi: 10.1038/ki.2013.340 [DOI] [PubMed]
- Sethi S, Fervenza FC, Zhang Y, et al. Atypical postinfectious glomerulonephritis is associated with abnormalities in the alternative pathway of complement. Kidney Int. 2012;83:293–299. doi: 10.1038/ki.2012.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel S, Zimmering M, Papp K, et al. Anti-factor B autoantibody in dense deposit disease. Mol Immunol. 2010;47:1476–1483. doi: 10.1016/j.molimm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Chen Q, Wiesner M, Eberhardt H, et al. A novel hybrid CFHR2/CFHR5 gene develops MPGN II and provides insights into disease mechanism and therapeutic implications. Immunobiology. 2012;217:1131. [Google Scholar]
- Tortajada A, Yébenes H, Abarrategui-Garrido C, et al. C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation. J Clin Invest. 2013;123:2434–2446. doi: 10.1172/JCI68280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medjeral-Thomas N, Malik TH, Patel MP, et al. A novel CFHR5 fusion protein causes C3 glomerulopathy in a family without Cypriot ancestry Kidney Int 2013(this issue). [DOI] [PMC free article] [PubMed]
- Abrera-Abeleda MA, Nishimura C, Frees K, et al. Allelic variants of complement genes associated with dense deposit disease. J Am Soc Nephrol. 2011;22:1551–1559. doi: 10.1681/ASN.2010080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paixao-Cavalcante D, Lopez-Trascasa M, Skattum L, et al. Sensitive and specific assays for C3 nephritic factors clarify mechanisms underlying complement dysregulation. Kidney Int. 2012;82:1084–1092. doi: 10.1038/ki.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Meyer NC, Wang K, et al. Causes of alternative pathway dysregulation in dense deposit disease. Clin J Am Soc Nephrol. 2012;7:265–274. doi: 10.2215/CJN.07900811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokiranta TS, Solomon A, Pangburn MK, et al. Nephritogenic lambda light chain dimer: a unique human miniautoantibody against complement factor H. J Immunol. 1999;163:4590–4596. [PubMed] [Google Scholar]
- Nozal P, Strobel S, Ibernon M, et al. Anti-factor H antibody affecting factor H cofactor activity in a patient with dense deposit disease. Clin Kidney J. 2012;5:133–136. doi: 10.1093/ckj/sfs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Sukov WR, Zhang Y, et al. Dense deposit disease associated with monoclonal gammopathy of undetermined significance. Am J Kidney Dis. 2010;56:977–982. doi: 10.1053/j.ajkd.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Zand L, Leung N, et al. Membranoproliferative glomerulonephritis secondary to monoclonal gammopathy. Clin J Am Soc Nephrol. 2010;5:770–782. doi: 10.2215/CJN.06760909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiou Y, Voskarides K, Gale DP, et al. Familial C3 glomerulopathy associated with CFHR5 mutations: clinical characteristics of 91 patients in 16 pedigrees. Clin J Am Soc Nephrol. 2011;6:1436–1446. doi: 10.2215/CJN.09541010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester CM, Smith RJ. Treatment options for C3 glomerulopathy. Curr Opin Nephrol Hypertens. 2013;22:231–237. doi: 10.1097/MNH.0b013e32835da24c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel GB, Cook HT, Hageman G, et al. Membranoproliferative glomerulonephritis type II (dense deposit disease): an update. J Am Soc Nephrol. 2005;16:1392–1403. doi: 10.1681/ASN.2005010078. [DOI] [PubMed] [Google Scholar]
- Group KDIGOKGW KDIGO Clinical Practice Guideline for Glomerulonphritis. Kidney Int (Suppl) 2012;2:198–199. [Google Scholar]
- Daina E, Noris M, Remuzzi G. Eculizumab in a patient with dense-deposit disease. N Engl J Med. 2012;366:1161–1163. doi: 10.1056/NEJMc1112273. [DOI] [PubMed] [Google Scholar]
- McCaughan JA, O'Rourke DM, Courtney AE. Recurrent dense deposit disease after renal transplantation: an emerging role for complementary therapies. Am J Transplant. 2012;12:1046–1051. doi: 10.1111/j.1600-6143.2011.03923.x. [DOI] [PubMed] [Google Scholar]
- Bomback AS, Smith RJ, Barile GR, et al. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol. 2012;7:748–756. doi: 10.2215/CJN.12901211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht C, Weyersberg A, Heinen S, et al. Successful plasma therapy for atypical hemolytic uremic syndrome caused by factor H deficiency owing to a novel mutation in the complement cofactor protein domain 15. Am J Kidney Dis. 2005;45:415–421. doi: 10.1053/j.ajkd.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Banks RA, May S, Wallington T. Acute renal failure in dense deposit disease: recovery after plasmapheresis. Br Med J (Clin Res Ed) 1982;284:1874–1875. doi: 10.1136/bmj.284.6332.1874-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krmar RT, Holtback U, Linne T, et al. Acute renal failure in dense deposit disease: complete recovery after combination therapy with immunosuppressant and plasma exchange. Clin Nephrol. 2011;75 (Suppl 1:4–10. [PubMed] [Google Scholar]
- Pickering MC, Warren J, Rose KL, et al. Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc Natl Acad Sci USA. 2006;103:9649–9654. doi: 10.1073/pnas.0601094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivarelli M, Pasini A, Emma F. Eculizumab for the treatment of dense-deposit disease. N Engl J Med. 2012;366:1163–1165. doi: 10.1056/NEJMc1111953. [DOI] [PubMed] [Google Scholar]
- Little MA, Dupont P, Campbell E, et al. Severity of primary MPGN, rather than MPGN type, determines renal survival and post-transplantation recurrence risk. Kidney Int. 2006;69:504–511. doi: 10.1038/sj.ki.5000084. [DOI] [PubMed] [Google Scholar]
- Cattran DC, Coppo R, Cook HT, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]