Abstract
Background
Soy and red clover isoflavones are controversial due to purported estrogenic activity and possible effects on breast cancer. We conducted a systematic review of soy and red clover for efficacy in improving menopausal symptoms in women with breast cancer, and for potential impact on risk of breast cancer incidence or recurrence.
Methods
We searched MEDLINE, Embase, the Cochrane Library, and AMED from inception to March 2013 for human interventional or observational data pertaining to the safety and efficacy of soy and red clover isoflavones in patients with or at risk of breast cancer.
Results
Of 4179 records, we included a total of 131 articles: 40 RCTs, 11 uncontrolled trials, and 80 observational studies. Five RCTs reported on the efficacy of soy for hot flashes, showing no significant reductions in hot flashes compared to placebo. There is lack of evidence showing harm from use of soy with respect to risk of breast cancer or recurrence, based on long term observational data. Soy intake consistent with that of a traditional Japanese diet (2-3 servings daily, containing 25-50mg isoflavones) may be protective against breast cancer and recurrence. Human trials show that soy does not increase circulating estradiol or affect estrogen-responsive target tissues. Prospective data of soy use in women taking tamoxifen does not indicate increased risk of recurrence. Evidence on red clover is limited, however existing studies suggest that it may not possess breast cancer-promoting effects.
Conclusion
Soy consumption may be associated with reduced risk of breast cancer incidence, recurrence, and mortality. Soy does not have estrogenic effects in humans. Soy intake consistent with a traditional Japanese diet appears safe for breast cancer survivors. While there is no clear evidence of harm, better evidence confirming safety is required before use of high dose (≥100mg) isoflavones can be recommended for breast cancer patients.
Introduction
Breast cancer accounts for almost one third of cancers diagnosed among women. In the United States, there were approximately 288 thousand new cases expected for 2011 [1]. Breast cancer is also the second leading cause of cancer death among women, with nearly 40 thousand attributable deaths expected in 2011 in the US [1]. Dietary interventions are emerging as increasingly important strategies for reducing risk of developing breast cancer or recurrence [2,3]. Among breast cancer survivors, for instance, the Women’s Healthy Eating and Living (WHEL) study found that interventions with a diet high in fruits and vegetables, dietary fibre, and low in saturated fat reduced recurrence by 31% among women without hot flashes compared to the control group [4], and that higher vegetable intake, particularly cruciferous vegetables, may have enhanced the effect of tamoxifen, with a 44% reduction in recurrence [5]. If shown effective, these and other dietary strategies represent an important way for women to reduce their cancer risk, or for breast cancer patients to reduce recurrence and safely augment the effects of cancer treatment. Soy has emerged as a specific food that may reduce breast cancer risk [6], and is among the most commonly used complementary medicines utilized by breast cancer patients seeking to reduce risk of recurrence [7,8]. There remains considerable controversy, however, as to its safety, particularly in breast cancer survivors due to purported estrogenic effects [9].
Soy, also known as Glycine max, contains the class of phytoestrogens known as isoflavones, specifically, genistein, daidzein, glycitein, biochanin A, and formononetin [9]. Isoflavones resemble 17-beta-estradiol in structure, and as such are able to bind the estrogen receptor (ER) in vitro [10], behaving much as a natural selective estrogen receptor modulator (SERM) [9]. For instance, the soy isoflavones have been found to exert partial ER agonist and antagonist activity depending on local estrogen concentrations, with antagonist properties in concentrations similar to premenopausal levels, and agonist properties with postmenopausal levels [9,11-13]. Much of the present debate around soy stems from conflicting in vitro and animal evidence. Some studies show that soy isoflavones can increase tumor cell proliferation [14], while other studies show the opposite [15-18]. The effects of isoflavones in human systems promise to be equally complex [19]. Furthermore, in about 30% of the population, daidzein can be further metabolized in the gut to equol, a metabolite with higher affinity for ER-ß [20-22]. Finally, in addition to ER mediated activity, soy exerts ER-independent effects, including inhibition of vascular endothelial growth factor (VEGF), and proapoptotic effects; genistein in particular inhibits tyrosine kinase and induces the tumor suppressor protein, PTEN [23-25].
Like soy, red clover contains the isoflavones genistein, daidzein, biochanin A, and formononetin, however, soy contains higher amounts of genistein and daidzein, while the dominant isoflavones in red clover are biochanin A and formononetin [26-29]. In vivo, formononetin is metabolized to daidzein [30], which may be metabolized to equol among equol producers [31-33]. There are several commercial extracts of red clover, marketed for the treatment of menopausal symptoms including hot flashes (Promensil®), as well as for menopause related health concerns such as bone loss and dyslipidemia (Rimostil®); Trinovin® is marketed for men’s health, specifically for benign prostatic hypertrophy. Promensil® contains 26mg biochanin A, 16mg formononetin, 1mg genistein, and 0.5mg daidzein per tablet (~40mg total) [27]. Other commercial products such as Rimostil® and Trinovin® contain slightly varying amounts, but are still largely comprised of biochanin A and formononetin [27]. Red clover also contains coumestrol, a coumestan that has been less well characterized, however, it is present in very low amounts such that its net contribution to red clover’s purported estrogenic effect is questionable [30,34].
To better elucidate the effect of soy, red clover, or isoflavones from these plants on breast cancer, we conducted a systematic review of soy and red clover as used by breast cancer patients or those at risk of breast cancer, assessing their impact on the risk of primary breast cancer or risk of recurrence. We also assessed the impact of isoflavones on surrogate endpoints for predicting breast cancer risk, including circulating estradiol and effects on estrogen responsive tissues such as the breast, endometrial, and vaginal tissues. Finally, we assessed the efficacy of isoflavones in treating menopausal symptoms in patients who have undergone breast cancer treatment.
Methods
Search strategy
Electronic search strategies were developed and tested through an iterative process by an experienced medical information specialist in consultation with the review team. Using the OVID platform, we searched Ovid MEDLINE®, Ovid MEDLINE®In-Process & Other Non-Indexed Citations, EmbaseClassic+Embase, and AMED (Allied and Complementary Medicine). We also searched the Cochrane Library on Wiley (including CENTRAL, Cochrane Database of Systematic Reviews, DARE, HTA, and NHS EED). The strategy was peer reviewed prior to execution by an experienced information specialist using the PRESS Checklist [35]. No amendments were suggested.
Strategies utilized a combination of controlled vocabulary (e.g., Soybeans, Phytoestrogens, Breast Neoplasms) and keywords (soy, plant estrogen, breast cancer). Vocabulary and syntax were adjusted across databases. Searches pertaining to soy and soy isoflavones were performed in May 2011 and updated in March 2013; there were no language or date restrictions on any of the searches. Searches pertaining to red clover and red clover isoflavones were performed in October 2011 and updated in December 2012. Additional references were also sought through hand-searching the bibliographies of relevant items. Specific details regarding the searches appear in Table S1 .
Inclusion criteria
For inclusion, evidence had to come from clinical trials or observational studies in humans. Human trials had to: a) assess the safety and/ or efficacy of soy or red clover or isoflavones from these plants in breast cancer patients for the purposes of treatment or secondary prevention, or the reduction of side effects associated with chemo- or radiation- therapy; alternately, human trials had to: b) assess the effect of soy or red clover or isoflavones from these plants on risk of primary breast cancer among women without a history of previous breast cancer. Clinical surrogate studies were included if they examined endpoints directly related to breast cancer risk, pathogenesis, or objective markers assessing healthy bodily function such as hematological function in breast cancer patients. All types of breast cancers (carcinoma in situ, invasive breast cancer) were included.
Observational studies had to report on risk of primary breast cancer or breast cancer recurrence associated with soy or red clover consumption compared with non-consumption, in a prospective or retrospective design. In vitro and in vivo studies were excluded due to the high risk for confounding and previous work on natural health products (vitamin A) showing a lack of correlation between preclinical and clinical results [36]. Due to the nature of soy as a commonly consumed food and red clover as a non-dietary item, there were limited observational studies of red clover consumption expected or identified. Therefore these studies focus solely on soy.
Record screening and selection
First pass record screening was based on title review with second pass conducted on abstracts and/or full texts where uncertainty existed. Reports published in English only were included for full analysis if they met inclusion criteria.
Data extraction
We piloted data extraction forms and conducted extraction independently in duplicate to assess inter-researcher reliability (HF, RF, GF, SV). No major inconsistencies in data extraction were found. Both quality and efficacy data were extracted. Extraction sheets were prepared based on the Consolidated Standards of Reporting Trials (CONSORT) statement for clinical trials and the Newcastle-Ottawa scale (NOS) for observational studies [37-39]. RCTs were assessed for bias using the Cochrane Risk of Bias tool[40].
Outcomes
Data was collected on breast cancer incidence, recurrence, or death; impact on hot flashes in breast cancer patients; adverse events; and impact on blood or urinary hormone levels: estrone (E1), estradiol (E2), estriol (E3), progesterone (P), leutinizing hormone (LH), follicle stimulating hormone (FSH), and sex hormone binding globulin (SHBG). Data was also collected on the impact of soy on hormonally active tissues, including breast tissue, endometrial tissue, vaginal tissue, and cervical tissue, as well as on menstrual cycle length in premenopausal women.
Statistics
We were unable to pool study findings due to heterogeneity between studies, however we display individual study results graphically via forest plots. Although we did not quantitatively calculate heterogeneity, an informal assessment indicated qualitative incoherence between studies on important parameters, most importantly the type and dose of intervention or exposure, as well as study populations and endpoints used.
Results
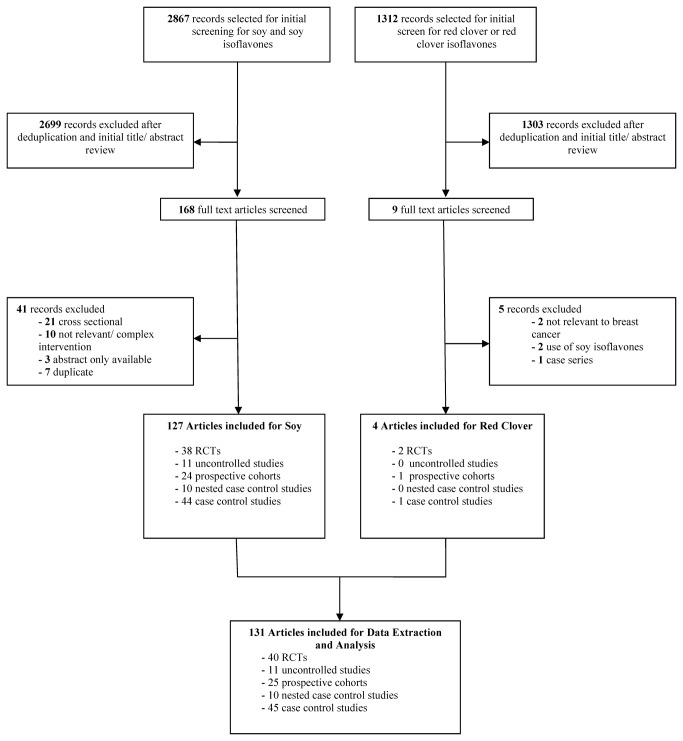
A total of 4179 records were screened, and 131 records were included: from the soy literature search, 2867 records were screened, of which 127 were included for full analysis and review. From the red clover search, 1312 records were screened, of which four were included for full review. Figure 1 shows a flowchart of the literature search and study selection. We consider the evidence pertaining to soy and red clover independently below.
Figure 1. Literature Flowchart.
Soy
Case Control Studies
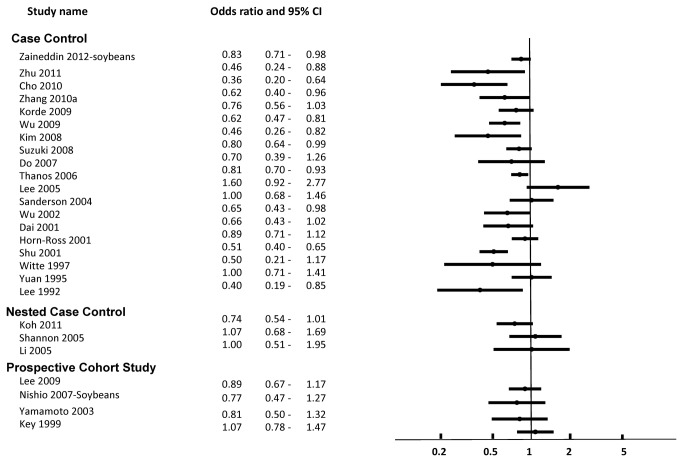
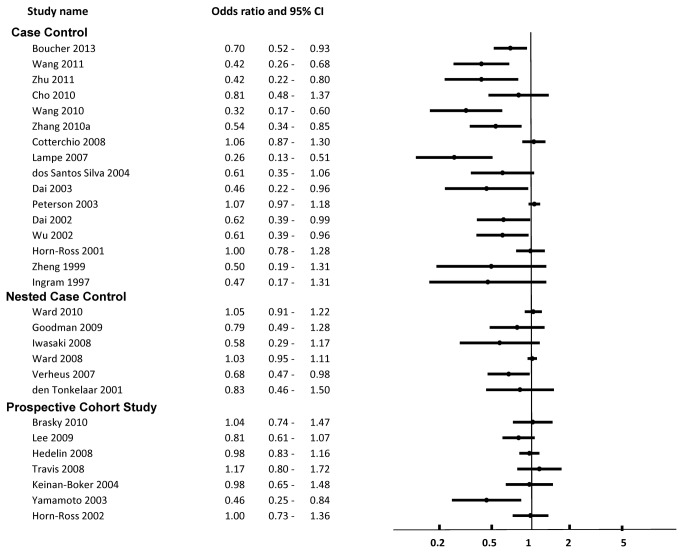
A total of 44 case control studies pertaining to soy were included. These are described in Table S2 [41-84]. Of the 44 studies, 30 reported risk ratios and confident intervals relating to the risk of breast cancer; these are displayed in Figures 2-3 [42-49,51,52,56,57,61-65,68,70-73,75,76,78,80,81,83,84]. Fourteen studies did not report associations as odds ratios with confidence for overall risk of breast cancer, and so were not included in the figures [41,53-55,58-60,66,67,69,74,79,82,83].
Figure 2. Risk of Breast Cancer Associated with Intake of Soy Food or Soy Protein.
Overall, of the 44 case control studies, 32 showed that higher consumption of soy foods and/ or soy isoflavones was associated with lower risk for primary breast cancer [42-46,49-51,53,54,56-58,61-64,66,71-73,75,77-79,81,83], breast cancer mortality [55], or improved markers of prognosis (ER+ status vs receptor negative status) [74,82] among the overall study population. None of the case control studies examined effects on recurrence or association with use of tamoxifen. None of the studies showed evidence of harm from higher consumption of soy, ie. increased risk of breast cancer or mortality.
Among studies reporting ORs with confidence intervals, 19 studies described breast cancer risk associated with soy food or soy protein intake [44-46,50,51,56,61,62,64,65,70-73,76-78,80,81], and 16 examined intake of soy isoflavones [42,43,45-49,52,56,57,63,68,75,77,81,84]. Of the 19 studies, 11 (58%) showed significant inverse relationships between soy food or protein intake and risk of breast cancer [44-46,61,64,71-73,77,78,81]. Of the 16 studies, nine (56%) showed a significant inverse relationship between soy isoflavone intake and risk of breast cancer [42,43,45,48,49,63,75,77,81]. There were no studies showing significantly increased risk of breast cancer associated with soy intake.
On subgroup analysis, there was no clear difference in effect based on the type of soy exposure (food / protein, or isoflavones), or according to study quality (NOS score), the measure of exposure (recall or measurement of blood or urinary isoflavone levels), or menopausal status (Figures S1-S2 ). There was an indication that higher soy intake, defined as greater than 1 serving of soy food or 6.25g soy protein or 12.5mg isoflavones daily, was associated with a greater protective effect compared to studies examining lower intakes (Figure S3 ). Eight of 12 (67%) studies examining high soy food or protein [45,46,61,64,72,77,78,81] and six of seven (86%) studies examining high soy isoflavones [42,43,45,75,77,81] reported inverse associations between soy and breast cancer risk. On the other hand, two of nine (22%) and zero of three studies respectively found inverse associations with low-medium intake [44,71].
Seven studies examined the effect of soy by receptor status; these findings were mixed. Three studies reported no modification of effect by ER/ PR status [46,58,81]. These studies found significant protective effects associated with the use of soy overall, but there was no modification of this effect by receptor status. Anderson et al found that soy isoflavone intake in adulthood was not associated with risk of breast cancer for any receptor type (ER+/PR+, ER-/PR-, or ER+/PR-), but did find that higher intake during adolescence was associated with lower odds of the mixed receptor type: AOR 0.77, 95%CI 0.60-0.99 [41]. Zhang et al found that soy was protective among ER+, ER-, PR+, and PR- tumor types, but the effect was strongest against both ER+/PR+ and ER-/PR- tumors, as opposed to mixed types, ER-/PR+ and ER+/ PR- [83]. Suzuki et al found that soy was significantly protective for ER+, PR+, and Her2- tumors, but not ER-, PR-, or Her2+ tumors [72]. Finally Touillard found that soy was protective for ER+ but not ER- tumors [74]. Five of six studies examining the effects of soyfood consumption in childhood found that higher soy intake, ranging from ≥1 to ≥4 servings per week, compared to lower intake, was significantly protective against breast cancer in adulthood [62,71,73,77,78]. In addition, there was some evidence that soy may be more protective among women with higher waist-to-hip ratio and/ or body mass index, or higher serum estradiol (>5.73 pg/mL) [49].
Nested Case Control Studies
A total of 10 nested case control studies were included pertaining to soy, shown in Figures 2-3 . The nested case control studies were embedded within larger cohorts, which are also reported in their entirety as prospective cohort studies below. These were the Shanghai Breast Self-Exam study, various arms of the EPIC study, and the Multiethnic cohort in Hawaii [85-92]. As with case control studies, these studies primarily assessed the risk of developing breast cancer associated with soy intake, with mixed results.
Of eight studies assessing risk of breast cancer, one showed significantly reduced risk associated with higher plasma genistein [90]. Grace et al reported a small but significant increase in risk of breast cancer associated with higher serum daidzein and serum and urinary equol levels; this was expressed as a log2 odds ratio (associated with doubling of exposure) and as a different measure was not included in the forest plots [88]. Nonetheless, these findings indicated a 30-45% increased odds of breast cancer with a doubling of daidzein (equol precursor) and equol levels. In this study, 39% of the population were equol producers. No significant associations were found for other isoflavones including genistein, glycitein, or O-MDA (O-desmethylangolensin, another daidzein metabolite).
Five studies showed no significant effects in either direction. One study examined mammographic density as a surrogate of breast cancer risk, however with mixed findings [85]. While cases were shown to have higher breast density at all ages compared with control subjects, and this was significantly associated with soy intake during adulthood (-8.6%, p=0.04), the validity of equating this association with increased risk of breast cancer was undermined by associations between body mass index and breast density. Leaner women with BMI <25 had greater percent density, but lower risk of breast cancer.
Prospective Cohort Studies
A total of 24 prospective cohort studies were included, described in Table 1 and Table S3 [93-116]. Of 11 studies reporting on risk of primary breast cancer, one (9%) showed that soy isoflavone intake was associated with overall reduced risk of breast cancer [116], while the remaining studies showed no associations [99,104,105,107-109,111,113-115,117]. A total of nine unique studies from this group reported odds ratios with confidence intervals, and are shown in Figures 2-3 [99,104,105,107-109,111,114,116]. There were no studies indicating a higher risk of breast cancer associated with soy consumption. As with case control studies, there was no clear difference in effect direction when studies were grouped according to study quality (≤5 or >5 NOS score).
Table 1. Prospective Cohort Studies of Soy and Breast Cancer Recurrence and Survival.
| Ref | Cohort Name | CohortN | Cases N | Geographic area | Menopause status | Tamoxifen Use? | Anastrozole Use? | Herceptin Use? | Exposure* | High quartile | Study duration | Years f/u** | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kang 2012 | Mongolia Medical College | 288 | 125 | China | Pre and post | Y: 206 | NR | NR | Soy protein & IF | >15.78g protein; >35.30mg IF | 2004-2011 | 5-7y | ↑Survival |
| Woo 2012 | Korean cohort | 339 | 25 | Korea | Pre and post | Y: n=195 | NR | Y: n=28 | Soyfoods& soy IF | ≥65.7g soyfood; ≥15.2mg IF | 2007-2008+ | 32.6mo | ↔Recurrence |
| Zhang 2012 | Mongolia Medical College | 616 | 79 (deaths) | China | Pre and post | 40-60% | NR | NR | Soy protein & IF | >13.03g protein; >28.83mg IF | 2004-2006+ | 52.1mo | ↑Survival |
| Caan 2011 | WHEL | 2736 | 271 | USA | Pre and post | ~66% | NR | NR | Soy IF | >16.33mg IF | 1991-2006 | 7.3 | ↔Survival ↔Recurrence |
| Kang 2010 | Harbin, China | 524 | 185 (recur) | China | Pre and post | 100% T or A | 100% T or A | NR | Soy IF | >42.3mg IF | 2002-2008 | 5.1 | ↔Survival ↓Recurrence (postM) |
| Guha 2009 | LACE | 1954 | 282 | USA | Pre and post | 20-40% | NR | NR | Genistein intake | >13.02mg genistein | 2000-2008 | 6.31 | ↔Recurrence |
| Shu 2009 | SBCSS | 5042 | 534 (recur) | Shanghai | Pre and post | Y: n=2622 | NR | NR | Soy protein & IF | >15.31g protein; >62.68mg IF | 2002-2009 | 3.9 | ↓Recurrence ↑Survival |
| Fink 2007 | Long Island BrCa Study | 1210 | 113 (deaths) | USA | Pre and post | NR | NR | NR | Soy IF | ≥7.48mg IF | 1996-2002 | ~6 | ↑Survival |
| Boyapati 2005 | Shanghai Breast Cancer study | 1459 | 216 (deaths) | Shanghai | Pre and post | NR | NR | NR | Soyfoods | NR | 1996-2002 | 5.2 | ↔Survival |
No significant effect; A anastrozole; IF isoflavones; LACE study Life After Cancer Epidemiology study; postM post-menopausal women; preM pre-menopausal women; SBCSS Shanghai Breast Cancer Survival Study; T tamoxifen; WHEL Women’s Healthy Eating & Living study
Exposure is dietary unless specified otherwise (ie. supplements)
Where (~) is used, the follow up period was not reported in the publication, but an estimate was calculated based the time between the end of the recruitment period and data censure/ end of follow-up.
Two studies reported on associations with mammographic density as a predictor of breast cancer risk [102,110]. One study reported an inverse association between soy intake and breast density [110], while the second reported an inverse association between soy intake and breast density among equol producers only [102].
Two studies reported on menopausal symptoms and quality of life among breast cancer patients [93,95]. There was a positive association between soy intake and hot flashes among premenopausal breast cancer patients in one study suggesting relative estrogen antagonism [93], and an indication that soy supplement use was associated with better physical quality of life in a second study [95].
Prospective Studies on Recurrence and Survival
Nine prospective studies reported on risk of breast cancer recurrence or mortality [94,96-98,100,101,103,106,112]. These are described in Table 1 . Five of nine reported on breast cancer recurrence [97,100,103,106,112], and seven of nine reported on mortality [94,96,98,100,101,106].
Of five studies reporting on recurrence [97,100,103,106,112], two (40%) showed protective effects [106,112]. Four studies reported risk for the overall study population (Figure S4 ), of which one (25%) found significantly decreased risk of breast cancer recurrence or longer disease-free survival (defined as combined relapse or death related to breast cancer) associated with higher soy intake [112]. The fifth study reported results by pre- and post- menopausal status only; this study found lower risk of recurrence among post-menopausal patients, HR 0.67 (95%CI 0.54-0.85) only [106]. A similar association was found for ER+/ PR+ patients and for those on anastrozole [106]. The amount of soy associated with protective effects in these studies was >15.31g soy protein or >62.68mg soy isoflavones [112]; and >42.3mg soy isoflavones (post-menopausal) [106]. Three of the five studies (60%) found non-significant associations [97,100,103], and there were no studies reporting increased risk of recurrence associated with soy intake.
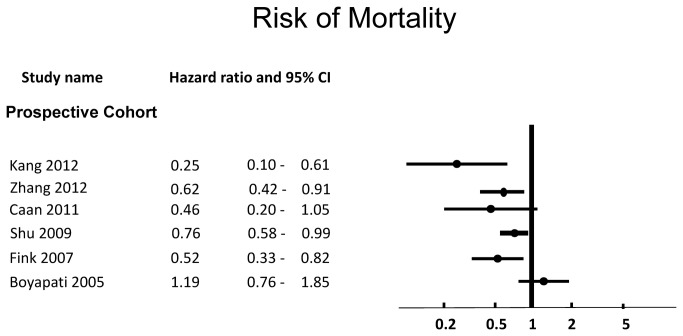
Of seven studies examining survival [94,96,98,100,101,106,112], four (57%) showed protective effects on breast cancer mortality or mortality in breast cancer patients [94,96,101,112]. Six studies reported overall results for the study population, and are shown in Figure 4 . These effects were seen at a soy intake of >35.3mg soy isoflavones or >15.78g soy protein [94]; >28.83mg soy isoflavones or >13.03g soy protein [96]; >7.48mg soy isoflavones [101]; >62.68mg soy isoflavones or 15.31g soy protein [112]. Caan et al reported a trend toward decreased risk of death among tamoxifen users with intake of total isoflavones ≥6.3mg per day (median 26.7mg), HR 0.26 (0.06-1.01, ptrend=0.05), and also among women with ER+ or PR+ status, HR 0.31 (0.10-0.98, ptrend=0.07), but this was not significant [100]. Two additional studies showed no significant effects [98,106].
Figure 4. Risk of Mortality Associated with Intake of Soy Protein or Isoflavones.
Uncontrolled Trials
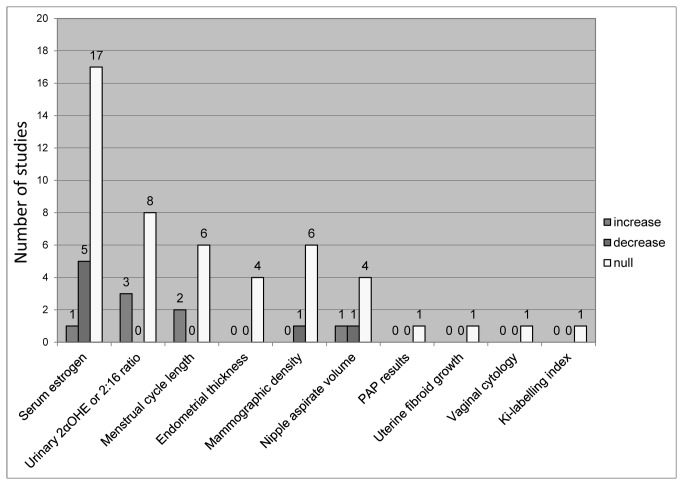
We included 11 uncontrolled trials examining the effect of soy on safety outcomes relevant to breast cancer risk and progression [118,119]. These are summarized in Figure 5 alongside the RCTs, with additional details available in Table S4 . These studies showed no evidence of harm from consumption of soy.
Figure 5. Effect of Soy on Hormonal Biomarkers and Estrogen Dependent Tissues (RCTs and Uncontrolled Trials).
In studies conducted among healthy women, there were no significant changes compared to baseline in endometrial thickness [118]; rates of normal mammogram results [118]; menstrual cycle length [120-122]; or urinary excretion of estrogen [123] or the ratio of urinary estrogen metabolites 2:16 OH-estrone (E1) [123,124]. There were varying results with regard to impact on serum estrogen and progesterone levels, with two studies showing no significant impact on serum estradiol [120,125], while two studies show significant decreases in estradiol [121,122], and three studies show decreases in progesterone/ luteal phase progesterone [120-122]. One study indicated that soy may have differential effects in equol producers compared with non-producers: soy consumption increased serum estradiol from 33.75 to 137.4 pmol/L among equol producers, but decreased it from 51.5 to 29.75 in non-producers [119]. With respect to nipple aspirate volume (NAV), a marker of breast tissue proliferative activity, one study showed no change [126] while one study found a significant increase in NAV among premenopausal women only, from 16 to 36 µL [125].
Randomized Controlled Trials
Of the 38 randomized controlled trials, five assessed the efficacy of soy for hot flashes among breast cancer survivors [127-131], while 34 assessed safety parameters in healthy women [131-164]; one study assessed both hot flashes and safety parameters and hence is counted twice [129]. The five RCTs assessing hot flashes or menopausal symptom scores all found reduced symptoms in both the treatment and the control groups, between 15% to 50% compared to baseline [129,131], however with no significant difference between them.
A total of 18 RCTs assessed circulating estrogens levels (E1, E2, and/ or E3) Fifteen of 18 studies found no significant impact from soy compared to the control group (p>0.05) [129,132,133,138,140,143,145,147,149,150,155,156,158,160,163]. Three studies found a significant reduction in circulating estrogens compared to the control group (p<0.05) [151,153,155]. One of these found that the consumption of 36oz isoflavone-free soy milk delivering approximately 38g soy protein every day for one menstrual cycle resulted in a 20% decrease in serum estradiol compared to the control group (p<0.03) across the cycle; this was accompanied by a 30% decrease in luteal phase progesterone (p=0.0002), but no change in LH or FSH [151]. One study found that consumption of soy protein (64 or 128g daily for three cycles) resulted in decreases in estrone (E1), testosterone, androstenedione, DHEA, DHEA-s, and cortisol; and increases in progesterone, progesterone: estradiol ratio, FSH and SHBG among equol producers only, compared to non-producers (p<0.05 for all) [153]. Another study by the same group found no significant effect on serum estradiol, FSH, or LH (p>0.05 for all), but a significant decrease in estrone (E1) (p=0.002) and increase in SHBG (p=0.04) compared to control subjects [155]. There were no RCTs showing a significant increase in circulating estrogens.
A total of nine RCTs assessed the impact of soy consumption on estrogen metabolism as measured through urinary excretion of urinary metabolites. Of these, five reports of six studies found no significant change in 2-hydroxyestrone, 16α-hydroxyestrone, or 2:16α hydroxyestrone ratio compared to the control group (p>0.05 for all) [148,149,156,161,164]. The remaining three studies found significant increases in 2-hydroxyestrone or 2:16α hydroxyestrone ratio [141,152,154], which has been thought to represent an anticancer shift in estrogen metabolism. Nettleton found that consumption of soy protein at a dose of 0.64mg isoflavones/kg daily had no impact on urinary estrogen metabolites in the group as a whole; however there was a significant increase in 2-hydroxyestrone (p=0.01) and 2:16α hydroxyestrone ratio (p=0.04) among subjects who were equol producers [141]. Xu reported a significant decrease in 4-hydroxyestrone (p<0.05) and an increase in 2:16α hydroxyestrone ratio (p<0.05) among subjects consuming soy protein isolate (65 or 132g daily) compared to those consuming a low-isoflavone protein isolate (control) [152]. Lu reported similar results from consumption of 36oz soymilk delivering 158mg isoflavones daily, with significant increases in 2-hydroxyestrone and 2:16α hydroxyestrone ratio compared to subjects consuming an isoflavone-free soymilk [154].
None of the studies assessing endometrial thickness [132,136,155], mammographic density or mammographic changes [66,132,136,137,144,146], nipple aspirate volume [134], PAP results and uterine fibroid growth [132], or vaginal cytology [155] found any evidence of negative impact from soy compared to the control group. Kataoka et al. reported no impact of soy on mammographic density when utilizing two of the three assessment methods; one method found a significant decrease in breast density among the soy group compared to the controls (p=0.04), but this became non significant after adjusting for baseline density [137]. Two of five studies assessing menstrual cycle length reported significant increases of between 1.8 to 3.5 days in cycle length associated with soy consumption compared to control subjects [149,150]; the remaining studies found no effects [147,156,158].
Three studies looked at molecular markers of breast cell proliferation or genetic markers of breast cancer risk. One study found no change in BRCA 1 and 2 mRNA level due to soy consumption, however there was a decrease over time in the placebo group, yielding a significant difference between groups (p<0.001) [136]. One study of women with either benign or malignant breast disease, found that consumption of 60g soy protein delivering 45mg isoflavones daily increased in vivo markers of breast epithelial proliferation: thymidine labeling index showing the number of cells in S-phase (p=0.026) and progesterone receptor expression (p=0.04), both increased after two weeks compared to baseline [157]. On the other hand, a recent study found that consumption of an isoflavone supplement (235mg daily) for six months had no effect on Ki-67 labeling index compared to placebo, although there was a significant decrease in both treatment and placebo groups compared to baseline [160]. There was also no change in the rate of atypical cytology.
Risk of bias
RCTs were assessed as having a moderate risk of bias, with inadequate reporting of random sequence generation and allocation concealment (selection bias) in 18 and 32 of 43 unique RCTs, respectively. The majority of RCTs showed low risk of performance bias, detection bias, attrition bias, and reporting bias; description of blinding of participants, blinding of outcome assessment, complete outcome data and reporting was adequate in over 88% of the studies. The risk of bias across studies is shown in Figure S5 .
Adverse Events
The most common adverse event associated with soy consumption was mild to moderate gastrointestinal discomfort, but this usually occurred with comparable frequency in both the soy and the placebo groups [128,130,131,136,142,147,150]; only in one study was there considerable difference in the frequency of GI upset, 47% in the soy group compared to 22% in the control group [130]. Amongst all the studies, there was report of only one case of breast cancer recurrence that occurred in a soy-allocated subject [142]; and two new cases of breast cancer and one case of endometrial cancer among soy allocated subjects compared to five in the control subjects [132,145,147,160]. There were no other serious adverse events.
Interactions
Seven studies included in our review investigated the effect of soy in combination with hormonal therapies: tamoxifen and aromatase inhibitors. Four cohort studies [100,103,106,112] and three RCTs [128,130,131] reported no significant differences with respect to treatment outcomes or rates of adverse effects associated with use of soy among women who were receiving tamoxifen therapy. In one RCT, vaginal spotting was reported in four subjects in the soy group compared to one in the placebo group, but with such low numbers, the statistical significance of this finding was not reported [130].
Of the cohort studies, the WHEL cohort exhibited relatively low soy isoflavone intake (≥6.3mg total isoflavones) and yielded null results: HR for recurrence among tamoxifen users was 0.59 (0.27-1.29) [100]. Guha et al found no significant effects overall associated with intake of either ~13mg genistein, ~9.5mg daidzein, or ~800mg glycitein, however there was a trend toward a protective effect among tamoxifen users, p trend =0.05 [103]. Shu et al examined 5042 breast cancer survivors, roughly half of whom were on tamoxifen (n=2262), and found that both soy protein intake (>15.31mg/d) and total isoflavone intake (>62.68mg/d) were protective against recurrence and death, HR recurrence 0.66 (0.52-0.84) for protein, and HR 0.74 (0.59-0.95) for isoflavones [112]. When analyzed by receptor status, these associations remained significant only among women with ER+ status [112]. Kang et al found decreased risk of recurrence among post-menopausal women consuming >42.3mg isoflavones in a cohort of breast cancer patients, all of whom were on tamoxifen or aromatase inhibitors, AHR 0.67 (0.54-0.85) [106].
One cohort study examined soy consumption by post-menopausal women who were on anastrozole, and found decreased risk of recurrence among those with an intake of >42mg/d isoflavones, AHR 0.65 (0.47-0.85) [106].
Red Clover
Human trials
Two RCTs pertaining to red clover were included [165,166]. These investigated the effects of the proprietary red clover extract, Promensil®, on hot flashes or estrogen-responsive tissues as a surrogate of breast cancer risk, among high risk populations.
In brief, Atkinson et al assessed the effect of red clover on mammographic density as well as a panel of other markers of estrogenic activity, in 205 women with an increased risk of breast cancer due to their breast density pattern (Wolfe P2 or DY mammographic breast patterns); there was also a secondary assessment of hot flashes [165]. Participants were randomized to receive 40 mg red clover isoflavones (Promensil®) or placebo daily for one year.
Powles et al investigated the effect of red clover in 401 women with a family history of breast cancer (at least one first degree relative affected), assessing circulating FSH, endometrial thickness, mammographic density, and bone density [166]. Participants were randomized to receive 40mg red clover isoflavones (Promensil®) or placebo for three years.
Hot flashes
Results of the study by Atkinson showed no significant changes in hot flash score (p=0.88) or mean number of daily hot flashes (p=0.41) when groups were compared [165]. Nonetheless, it should be noted that this was a secondary endpoint, and that this study included women in whom the severity of menopausal symptoms was already low at baseline; menopausal symptoms were not present in all subjects. Baseline mean number of hot flashes per day was 2.1 +/-2.7 for the treatment group and 2.5 +/-3.0 in the control group. There was a comparable decrease of -0.8 +/-2.1 at 12 months for the red clover group and -1.0 +/-1.8 for the control group (p=0.41), which however was not significant. Similar non-statistically significant results were found for the menopausal symptom score, a composite of 21 symptoms including night sweats, palpitations, tension/ nervousness, irritability, insomnia, and mood alterations scored for severity. Adverse events (AE) were not reported.
Risk of breast cancer
Neither of the RCTs reported on breast cancer incidence rates, however surrogate markers of estrogenic activity were examined. Atkinson reported no significant changes in estradiol, FSH, or LH over the one year period: estradiol increased by 14.0 pmol/L in the red clover group compared to a decrease of 0.9 in the placebo group, however this was not significant, p=0.49); FSH decreased 4.2 IU/L in the red clover group compared to a decrease of 2.9 in the placebo group, p=0.83; LH decreased 4.0 IU/L in the red clover group compared to a decrease of 4.2 IU/L in the placebo group, p=0.71; and tyrosine kinase increased 1.62 units of activity/ µg protein in the red clover group compared to an increase of 0.90 in the placebo group, p=0.16 [165].
Neither of the two RCTs reported any significant changes in mammographic density among both pre- and post-menopausal women [165,166], and Powles found no significant changes in endometrial thickness between groups [166], though neither of these markers are considered to be highly specific or sensitive predictors of breast cancer risk. Atkinson reported a significant interaction between treatment group allocation and the ESR1 polymorphism with respect to effect on mammographic breast density, with decreases of -3.4 (+/-9.7) and -5.2 (+/- 12.0) percent among the CC and CT genotypes respectively, and a 1.4 (+/-12.3%) increase in the TT group (P = 0.009) [165]. ESR1 codes for the estrogen receptor α, and variations may be associated with risk of breast cancer [167]. For instance, CC and CT genotypes may be associated with a small reduction in risk of breast cancer compared to TT: CC vs. TT: OR 0.92, 95%CI 0.86-0.99, and CC/CT vs. TT: OR 0.95, 95%CI 0.89-1.00 [167].. It is difficult to predict if or how a possible increase in breast density might affect breast cancer risk among TT carriers.
Adverse Events
Atkinson did not report adverse events. Powles reported adverse effects, which most commonly included breast abnormality, “skin related symptoms” (not described), and other minor adverse events, however these were equally distributed between red clover and placebo groups [166].
Risk of Bias
According to the Cochrane risk of bias tool, the trial reported by Powles was assessed as having low risk of bias in all of the categories, except one for which information about detection bias was not provided [166]. The trial by Atkinson was assessed as having low risk of bias in approximately half of the categories but failed to provide adequate information to assess for selection bias, detection bias, and attrition bias [165]. Overall, the RCTs were assessed as having low to moderate risk of bias.
Discussion
The results of our systematic review suggest that there is a lack of real evidence showing that soy increases risk of breast cancer or breast cancer recurrence. This is an important finding given the generally perceived controversial status of soy in relation to breast cancer [168]. Our review suggests that on the contrary, soy consumption may protect against the development of breast cancer [46,75,109,117], and less so, breast cancer recurrence and mortality [106,112], although this is based on observational data only. Larger, long-term trials are needed to better define these effects. In particular, research is needed to more clearly identify possible subgroups of women that may differentially benefit from soy or not, based on receptor status and/ or use of anti-estrogen therapy. In the meantime, since the overall effect of soy, if any, appears to be protective for both breast cancer incidence and recurrence, moderate soy consumption appears to be safe and possibly beneficial for most women.
Among studies included in our review, case control studies showed a stronger association between soy and reduced risk of breast cancer. As shown in Figures 2 and 3 , case control studies were much more likely to report significant protective associations between soy intake and risk of breast cancer, while prospective studies were less likely to do so. The reasons for this are unclear. Although not shown here, we conducted subgroup analysis according to the method of exposure assessment to assess for the possibility of recall bias. Our analysis showed no clear separation however, between studies utilizing food frequency questionnaires, structured interviews, or objective assessments of blood or urinary isoflavone concentrations. It is possible that cohort studies were not long enough in duration to prospectively capture the true effect of long term soy exposure.
Figure 3. Risk of Breast Cancer Associated with Intake of Soy Isoflavones.
The effect of soy on hot flashes in breast cancer patients is not clear. RCTs noted some improvements over time, but not in comparison to placebo [127-131]. This finding may be due to the possibility that soy simply does not possess sufficient estrogenic effects to alleviate hot flashes, or due to confounding by concomittant usage of tamoxifen in three of five of these studies [128,130,131]. There is also the possibility that lack of difference between groups may be due to a large placebo effect, since hot flashes are a subjective outcome, and up to a 40% improvement was reported by the placebo group in one study [130]. Finally, it is possible that soy may in fact possess anti-estrogen activity, as suggested by Dorjgochoo et al, who found that higher soy consumption was associated with increased prevalence of hot flashes among premenopausal breast cancer patients [93].
Several factors influence the biological activity of soy isoflavones in the body. First, soy isoflavones show selectivity toward ER-ß over ER-α [168-175]. This is important because ERß appears to be associated with antiproliferative, anticarcinogenic effects, while ERα is thought to promote carcinogenesis, and is the form measured clinically in the treatment of breast cancer patients [176,177]. In the breast, ERß is found in ductal and lobular epithelial cells as well as stromal cells, while ERα is found only in epithelial cells and not stroma of the breast [177]. Moreover, ERα is the receptor used to classify ER positive breast cancer, and the one through which tamoxifen exerts its antiproliferative effects [176,178]. Some have suggested that ERß functions as a possible tumor suppressor gene, pointing out evidence that ERß may control ERα-induced proliferation, and that expression is lost in many breast tumors [177,179,180]. If soy preferentially activates ERß, this may explain its chemopreventive effects.
Secondly, preclinical evidence has shown that under conditions of high estrogen concentration similar to premenopausal levels, soy isoflavones act as ER antagonists, while under conditions of low estrogen comparable to postmenopausal levels, they are ER agonists [12,181,182]. In a study of soy and MCF-7 breast cancer cell growth as assessed in the presence and absence of estradiol, Imhof et al observed “minor proliferation enhancing effects” [18] that occurred “only at unphysiologically low estrogen levels” [18]. According to Imhof, typical in vivo estrogen levels range from 50–400 pM, with even menopausal estrogen levels exceeding 20 pM, the concentration of genistein achieved through supplementation [18]. According to this line of reasoning, at these relative concentrations, the agonistic effects of estrogen would be expected to outweight those of genistein [18]. It would appear that an important component of interpreting these in vitro and in vivo studies is assessing how well they reflect human biological conditions.
Placed in this context, the clinical data reviewed by our study, which demonstrates a lack of any clear pro-estrogenic effects from use of soy, is quite noteworthy. We found no effects on circulating estradiol, and no measurable effects on estrogen-sensitive target tissues, such as breast tissue (density) and endometrium. In addition, our findings are in agreement with those reported by Hooper et al in a 2009 meta analysis of 47 trials [31]. Hooper found that soy did not significantly affect estradiol, estrone, or sex hormone binding globulin (SHBG), although amongst pre-menopausal women there was a significant 20% decrease in LH and FSH (p=0.01, 0.5 respectively) [31]. In post-menopausal women there were no significant effects on any hormone including estradiol and estrone, with a small nonsignificant 14% increase in estradiol based on 21 studies (p=0.07) and a nonsignificant decrease in estrone. A second meta analysis similarly found no significant effects from soy on breast density, also considered a surrogate of breast cancer risk [183].
Despite this, and despite the fact that our study failed to show any clear estrogenic effects observable in humans overall, the possibility of soy having estrogen-like effects under some circumstances in certain subgroups of women cannot be ruled out [184]. Of particular concern is the use of soy among women who are receiving antiestrogen therapy. Tamoxifen acts primarily through the ERα while soy is preferential toward ERß, which theoretically suggests minimal risk of interaction based on competitive receptor binding, however this has not been tested clinically [176,178]. Some preliminary evidence indicates that isoflavones may have synergistic effects with tamoxifen in cancer models and may reduce the development of tamoxifen resistance [185,186]. The in vitro receptor binding activity of genistein, daidzein, equol, and their metabolites is approximately 3% or less that of estradiol for ERα, and 18% or less for ERß [187], while the relative transactivation activity of these isoflavones ranged from 55 to 84% relative to estradiol, compared to 43 to 55% for tamoxifen for both ERα and ERß [187]. Nonetheless, caution should be used with using soy alongside tamoxifen until there is clinical data demonstrating the safety.
Our review revealed no clear modification of soy’s effect based on menopausal status or ER+/- status in breast cancer patients in large observational studies. However we did find variation according to geographical locale; studies conducted in Asian countries more often reported chemopreventive effects compared to studies in Western countries, which more often reported null results. We attribute this to a difference in soy consumption between these areas. The traditional Japanese diet contains between 6-11g of soy protein and 25-50mg isoflavones; top percentiles of soy intake in Asian studies consume as much as 20g soy protein or 100mg isoflavones per day [188], while the cut off for the top quartiles of intake among Western populations is in the range of a few hundred micrograms (mcg) per day [52,74].
The suggestion has been made that the effect of soy depends on genetic variations present in Asian populations. Nechuta et al conducted an analysis of the effect of soy on breast cancer recurrence, investigating the possibility of ethnic variations [189]. They reported that in cohorts of American women, after elimination of women of Asian-American descent, the inverse association between soy and breast cancer recurrence remains, undermining the suggestion that the effect of soy is dependent upon genetic difference between ethnicities [189].
Strengths
Our review is broad in its scope, assessing soy in the context of breast cancer from several perspectives, including risk of breast cancer, risk of recurrence, estrogenic effects, and risk of interactions with tamoxifen and other hormonal therapies. Our findings are generally in agreement with those of the American Cancer Society, suggesting that moderate amounts of soy intake (up to 3 servings per day) is likely safe for consumption by women with breast cancer [190].
Limitations
Although we included a large number of studies regarding soy and breast cancer risk, we were unable to pool data with respect to risk of breast cancer and risk of recurrence due to heterogeneity. There is a lack of long term interventional data assessing cancer risk. This is a particularly important shortcoming because RCTs of under 2 years duration are unlikely to reveal any serious adverse effects in breast cancer survivors, including possible interactions between soy and tamoxifen. This question deserves high priority for future research in this area. In addition, there is a need to more carefully assess the dose-response relationship between soy intake and risk of breast cancer in order to more clearly delineate the threshold of exposure needed for potential therapeutic effects.
Conclusions
Soy does not appear to influence levels of circulating estrogen or exert estrogen-like effects at target tissues. There is a lack of evidence showing clear effects of soy consumption or supplementation on reduction of hot flashes in breast cancer patients. Observational data suggest that higher soy intake, consistent with that of a traditional Japanese diet, may be protective against the development of breast cancer as well as breast cancer recurrence and mortality, although there is a need for clinical studies to confirm this relationship. Until there is more data supporting safety, caution is warranted with high dose isoflavone supplements in patients with breast cancer.
Supporting Information
Risk of Breast Cancer By Study Quality (Isoflavones).
(TIF)
Risk of Breast Cancer by Menopausal Status (Isoflavones).
(TIF)
Risk of Breast Cancer by Dose of Soy Isoflavones.
(TIF)
Risk of Breast Cancer Recurrence with Intake of Soy Protein or Isoflavones.
(TIF)
Risk of Bias Across Studies.
(TIFF)
Medline Search Strategy.
(DOCX)
Case Control Studies of Soy and Risk of Breast Cancer.
(DOC)
Prospective Cohort Studies of Soy and Risk of Primary Breast Cancer.
(DOC)
Trials Assessing Effect of Soy on Hormonal Biomarkers and Estrogen Dependent Tissues.
(DOC)
(DOC)
Funding Statement
This project was funded by a Knowledge Synthesis grant from the Canadian Institutes of Health Research. DAK was supported by a career grant from the Sickkids Foundation. No other sources of funding were accessed. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Desantis C, Siegel R, Bandi P, Jemal A (2011) Breast cancer statistics, 2011. CA: Cancer J Clin. [DOI] [PubMed] [Google Scholar]
- 2. Bandera EV, King M, Chandran U, Paddock LE, Rodriguez-Rodriguez L et al. (2011) Phytoestrogen consumption from foods and supplements and epithelial ovarian cancer risk: a population-based case control study. BMC Womens Health 11: 40. doi: 10.1186/1472-6874-11-40. PubMed: 21943063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barnes S, Prasain J, D'Alessandro T, Arabshahi A, Botting N et al. (2011) The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems. Food Funct 2: 235-244. doi: 10.1039/c1fo10025d. PubMed: 21779561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhanot A, Sharma R, Noolvi MN (2011) Natural sources as potential anti-cancer agents: A review. International Journal of Phytomedicine 3: 09-26. [Google Scholar]
- 5. Body JJ (2011) How to manage postmenopausal osteoporosis? Acta Clin Belg 66: 443-447. PubMed: 22338309. [DOI] [PubMed] [Google Scholar]
- 6. Wu AH, Yu MC, Tseng CC, Pike MC (2008) Epidemiology of soy exposures and breast cancer risk. [Review ] [40 refs]. Br J Cancer 98: 9-14 doi: 10.1038/sj.bjc.6604145. PubMed: 18182974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harris PF, Remington PL, Trentham-Dietz A, Allen CI, Newcomb PA (2002) Prevalence and treatment of menopausal symptoms among breast cancer survivors. J Pain Symptom Manage 23: 501-509. PubMed: 12067774. [DOI] [PubMed] [Google Scholar]
- 8. Lammersfeld CA, King J, Walker S, Vashi PG, Grutsch JF et al. (2009) Prevalence, sources, and predictors of soy consumption in breast cancer. Nutr J 8: 2. doi: 10.1186/1475-2891-8-2. PubMed: 19159489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hwang CS, Kwak HS, Lim HJ, Lee SH, Kang YS et al. (2006) Isoflavone metabolites and their in vitro dual functions: they can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J Steroid Biochem Mol Biol 101: 246-253. doi: 10.1016/j.jsbmb.2006.06.020. PubMed: 16965913. [DOI] [PubMed] [Google Scholar]
- 10. Kostelac D, Rechkemmer G, Briviba K (2003) Phytoestrogens modulate binding response of estrogen receptors alpha and beta to the estrogen response element. J Agric Food Chem 51: 7632-7635. doi: 10.1021/jf034427b. PubMed: 14664520. [DOI] [PubMed] [Google Scholar]
- 11. Ju YH, Allred KF, Allred CD, Helferich WG (2006) Genistein stimulates growth of human breast cancer cells in a novel, postmenopausal animal model, with low plasma estradiol concentrations. Carcinogenesis 27: 1292-1299. doi: 10.1093/carcin/bgi370. PubMed: 16537557. [DOI] [PubMed] [Google Scholar]
- 12. Velders M, Solzbacher M, Schleipen B, Laudenbach U, Fritzemeier KH et al. (2010) Estradiol and genistein antagonize the ovariectomy effects on skeletal muscle myosin heavy chain expression via ER-beta mediated pathways. J Steroid Biochem Mol Biol 120: 53-59. doi: 10.1016/j.jsbmb.2010.03.059. PubMed: 20347979. [DOI] [PubMed] [Google Scholar]
- 13. Qin W, Zhu W, Shi H, Hewett JE, Ruhlen RL et al. (2009) Soy isoflavones have an antiestrogenic effect and alter mammary promoter hypermethylation in healthy premenopausal women. Nutr Cancer 61: 238-244. doi: 10.1080/01635580802404196. PubMed: 19235040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Duursen MB, Nijmeijer SM, de Morree ES, de Jong PC, van den Berg M (2011) Genistein induces breast cancer-associated aromatase and stimulates estrogen-dependent tumor cell growth in in vitro breast cancer model. Toxicology 289: 67-73. doi: 10.1016/j.tox.2011.07.005. PubMed: 21854827. [DOI] [PubMed] [Google Scholar]
- 15. Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M et al. (2001) Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull 24: 351-356. PubMed: 11305594. [DOI] [PubMed] [Google Scholar]
- 16. Chen J, Zeng J, Xin M, Huang W, Chen X (2011) Formononetin induces cell cycle arrest of human breast cancer cells via IGF1/PI3K/Akt pathways in vitro and in vivo. Horm Metab Res 43: 681-686. doi: 10.1055/s-0031-1286306. PubMed: 21932171. [DOI] [PubMed] [Google Scholar]
- 17. Rajah TT, Du N, Drews N, Cohn R (2009) Genistein in the presence of 17beta-estradiol inhibits proliferation of ERbeta breast cancer cells. Pharmacology 84: 68-73. doi: 10.1159/000226123. PubMed: 19556829. [DOI] [PubMed] [Google Scholar]
- 18. Imhof M, Molzer S (2008) Effects of soy isoflavones on 17beta-estradiol-induced proliferation of MCF-7 breast cancer cells. Toxicol In Vitro 22: 1452-1460. doi: 10.1016/j.tiv.2008.04.018. PubMed: 18554862. [DOI] [PubMed] [Google Scholar]
- 19. An G, Morris ME (2011) The sulfated conjugate of biochanin A is a substrate of breast cancer resistant protein (ABCG2). Biopharm Drug Dispos 32: 446-457. doi: 10.1002/bdd.772. PubMed: 21910126. [DOI] [PubMed] [Google Scholar]
- 20. Boni V, Dominguez I, Garcia Velloso MJ, Lopez-Vega JM, Martinez P, et al. (2011) Bevacizumab changes in patients with naive, stage II-III breast cancer assessed by 18F-fluoromisonidazole and 18F-fluorotymidine PET-CT. Journal of Clinical Oncology. Conference: ASCO Annual Meeting 2011 Chicago, IL United States. Conference Start: 20110603. Conference End: 20110607. Conference Publication: (varpagings) 29: 15 Suppl 1. [Google Scholar]
- 21. Boucher BA, Cotterchio M, Anderson LN, Kirsh VA, Kreiger N, et al. (2011) Use of supplements containing isoflavones (phytoestrogens) and breast cancer risk: Case control study among women in ontario, Canada. American Journal of Epidemiology Conference: 3rd North American Congress of Epidemiology Montreal, QC Canada. Conference Start: 20110621. Conference End: 20110624. Conference Publication: (var.pagings) 173: S87. [Google Scholar]
- 22. Boucher BA, Cotterchio M, Harris SA, Kirsh VA, Kreiger N, et al. (2011) Phytoestrogen food and supplement intake among women newly diagnosed with breast cancer. American Journal of Epidemiology Conference: 3rd North American Congress of Epidemiology Montreal, QC Canada. Conference Start: 20110621. Conference End: 20110624. Conference Publication: (var.pagings) 173: S87. [Google Scholar]
- 23. Park K, Choi K, Kim H, Kim K, Lee MH et al. (2009) Isoflavone-deprived soy peptide suppresses mammary tumorigenesis by inducing apoptosis. Exp Mol Med 41: 371-381. PubMed: 19322027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dave B, Eason RR, Till SR, Geng Y, Velarde MC et al. (2005) The soy isoflavone genistein promotes apoptosis in mammary epithelial cells by inducing the tumor suppressor PTEN. Carcinogenesis 26: 1793-1803. doi: 10.1093/carcin/bgi131. PubMed: 15905199. [DOI] [PubMed] [Google Scholar]
- 25. Ravindranath MH, Muthugounder S, Presser N, Viswanathan S (2004) Anticancer therapeutic potential of soy isoflavone, genistein. Adv Exp Med Biol 546: 121-165. doi: 10.1007/978-1-4757-4820-8_11. PubMed: 15584372. [DOI] [PubMed] [Google Scholar]
- 26. Saviranta NM, Julkunen-Tiitto R, Oksanen E, Karjalainen RO (2010) Red clover (Trifolium pratense L.) isoflavones: root phenolic compounds affected by biotic and abiotic stress factors. J Sci Food Agric 90: 418-423. [DOI] [PubMed] [Google Scholar]
- 27. Booth NL, Piersen CE, Banuvar S, Geller SE, Shulman LP et al. (2006) Clinical studies of red clover (Trifolium pratense) dietary supplements in menopause: a literature review. Menopause 13: 251-264. doi: 10.1097/01.gme.0000198297.40269.f7. PubMed: 16645539. [DOI] [PubMed] [Google Scholar]
- 28. Saviranta NM, Julkunen-Tiitto R, Oksanen E, Karjalainen RO (2010) Leaf phenolic compounds in red clover (Trifolium pratense L.) induced by exposure to moderately elevated ozone. Environ Pollut 158: 440-446. doi: 10.1016/j.envpol.2009.08.029. PubMed: 19766367. [DOI] [PubMed] [Google Scholar]
- 29. Booth NL, Overk CR, Yao P, Totura S, Deng Y et al. (2006) Seasonal variation of red clover (Trifolium pratense L., Fabaceae) isoflavones and estrogenic activity. J Agric Food Chem 54: 1277-1282. doi: 10.1021/jf052927u. PubMed: 16478248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Booth NL, Overk CR, Yao P, Burdette JE, Nikolic D et al. (2006) The chemical and biologic profile of a red clover (Trifolium pratense L.) phase II clinical extract. J Altern Complement Med 12: 133-139. doi: 10.1089/acm.2006.12.133. PubMed: 16566672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wong JM, Kendall CW, Marchie A, Liu Z, Vidgen E et al. (2012) Equol status and blood lipid profile in hyperlipidemia after consumption of diets containing soy foods. Am J Clin Nutr 95: 564-571. doi: 10.3945/ajcn.111.017418. PubMed: 22301925. [DOI] [PubMed] [Google Scholar]
- 32. Jou HJ, Wu SC, Chang FW, Ling PY, Chu KS et al. (2008) Effect of intestinal production of equol on menopausal symptoms in women treated with soy isoflavones. Int J Gynaecol Obstet 102: 44-49. doi: 10.1016/j.ijgo.2008.01.028. PubMed: 18395723. [DOI] [PubMed] [Google Scholar]
- 33. Setchell KD, Clerici C, Lephart ED, Cole SJ, Heenan C et al. (2005) S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr 81: 1072-1079. PubMed: 15883431. [DOI] [PubMed] [Google Scholar]
- 34. Zgórka G (2011) Studies on phytoestrogenic and nonphytoestrogenic compounds in Trifolium incarnatum L. and other clover species using pressurized liquid extraction and high performance column liquid chromatography with photodiode-array and fluorescence detection. J AOAC Int 94: 22-31. PubMed: 21391478. [PubMed] [Google Scholar]
- 35. Sampson M, McGowan J, Cogo E, Grimshaw J, Moher, Lefebvre C (2009) An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol;62(9): 944-952. PubMed: 19230612. [DOI] [PubMed] [Google Scholar]
- 36. Fritz H, Kennedy D, Fergusson D, Fernandes R, Doucette S et al. (2011) Vitamin A and retinoid derivatives for lung cancer: a systematic review and meta analysis. PLOS ONE 6: e21107. doi: 10.1371/journal.pone.0021107. PubMed: 21738614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Altman DG, Schulz KF, Moher D, Egger M, Davidoff F et al. (2001) The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 134: 663-694. doi: 10.7326/0003-4819-134-8-200104170-00012. PubMed: 11304107. [DOI] [PubMed] [Google Scholar]
- 38. Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J et al. (2006) Recommendations for reporting randomized controlled trials of herbal interventions: Explanation and elaboration. J Clin Epidemiol 59: 1134-1149. doi: 10.1016/j.jclinepi.2005.12.020. PubMed: 17027423. [DOI] [PubMed] [Google Scholar]
- 39. Institute OHR. The Newcastle-Ottawa Scale (NOS). Available: http://www.ohrica/programs/clinical_epidemiology/oxfordhtm. Accessed 22 November 2013
- 40. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D et al. (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343: d5928. doi: 10.1136/bmj.d5928. PubMed: 22008217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Anderson LN, Cotterchio M, Boucher BA, Kreiger N (2013) Phytoestrogen intake from foods, during adolescence and adulthood, and risk of breast cancer by estrogen and progesterone receptor tumor subgroup among Ontario women. Int J Cancer 132: 1683-1692. doi: 10.1002/ijc.27788. PubMed: 22907507. [DOI] [PubMed] [Google Scholar]
- 42. Boucher BA, Cotterchio M, Anderson LN, Kreiger N, Kirsh VA et al. (2013) Use of isoflavone supplements is associated with reduced postmenopausal breast cancer risk. Int J Cancer 132: 1439-1450. doi: 10.1002/ijc.27769. PubMed: 22886851. [DOI] [PubMed] [Google Scholar]
- 43. Wang Q, Li H, Tao P, Wang YP, Yuan P et al. (2011) Soy isoflavones, CYP1A1, CYP1B1, and COMT polymorphisms, and breast cancer: a case-control study in southwestern China. DNA Cell Biol 30: 585-595. doi: 10.1089/dna.2010.1195. PubMed: 21438753. [DOI] [PubMed] [Google Scholar]
- 44. Zaineddin AK, Buck K, Vrieling A, Heinz J, Flesch-Janys D et al. (2012) The association between dietary lignans, phytoestrogen-rich foods, and fiber intake and postmenopausal breast cancer risk: a German case-control study. Nutr Cancer 64: 652-665. doi: 10.1080/01635581.2012.683227. PubMed: 22591208. [DOI] [PubMed] [Google Scholar]
- 45. Zhu YY, Zhou L, Jiao SC, Xu LZ (2011) Relationship between soy food intake and breast cancer in China. Asian Pac J Cancer Prev 12: 2837-2840. PubMed: 22393950. [PubMed] [Google Scholar]
- 46. Cho YA, Kim J, Park KS, Lim SY, Shin A et al. (2010) Effect of dietary soy intake on breast cancer risk according to menopause and hormone receptor status. Eur J Clin Nutr 64: 924-932. doi: 10.1038/ejcn.2010.95. PubMed: 20571498. [DOI] [PubMed] [Google Scholar]
- 47. Cotterchio M, Boucher BA, Kreiger N, Mills CA, Thompson LU (2008) Dietary phytoestrogen intake--lignans and isoflavones--and breast cancer risk (Canada). Cancer Causes Control 19: 259-272. doi: 10.1007/s10552-007-9089-2. PubMed: 17992574. [DOI] [PubMed] [Google Scholar]
- 48. Dai Q, Franke AA, Jin F, Shu XO, Hebert JR et al. (2002) Urinary excretion of phytoestrogens and risk of breast cancer among Chinese women in Shanghai. Cancer Epidemiol Biomarkers Prev 11: 815-821. PubMed: 12223424. [PubMed] [Google Scholar]
- 49. Dai Q, Franke AA, Yu H, Shu XO, Jin F et al. (2003) Urinary phytoestrogen excretion and breast cancer risk: evaluating potential effect modifiers endogenous estrogens and anthropometrics. Cancer Epidemiol Biomarkers Prev 12: 497-502. PubMed: 12814993. [PubMed] [Google Scholar]
- 50. Dai Q, Shu XO, Jin F, Potter JD, Kushi LH et al. (2001) Population-based case-control study of soyfood intake and breast cancer risk in Shanghai. Br J Cancer 85: 372-378. doi: 10.1054/bjoc.2001.1873. PubMed: 11487268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Do MH, Lee SS, Jung PJ, Lee MH (2007) Intake of fruits, vegetables, and soy foods in relation to breast cancer risk in Korean women: a case-control study. Nutr Cancer 57: 20-27. PubMed: 17516859. [DOI] [PubMed] [Google Scholar]
- 52. dos SSI, Mangtani P, McCormack V, Bhakta D, McMichael AJ et al. (2004) Phyto-oestrogen intake and breast cancer risk in South Asian women in England: findings from a population-based case-control study. Cancer Causes Control 15: 805-818. doi: 10.1023/B:CACO.0000043431.85706.d8. PubMed: 15456994. [DOI] [PubMed] [Google Scholar]
- 53. Hirose K, Tajima K, Hamajima N, Inoue M, Takezaki T et al. (1995) A large-scale, hospital-based case-control study of risk factors of breast cancer according to menopausal status. Jpn J Cancer Res 86: 146-154. doi: 10.1111/j.1349-7006.1995.tb03032.x. PubMed: 7730137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hirose K, Takezaki T, Hamajima N, Miura S, Tajima K (2003) Dietary factors protective against breast cancer in Japanese premenopausal and postmenopausal women. Int J Cancer 107: 276-282. doi: 10.1002/ijc.11373. PubMed: 12949807. [DOI] [PubMed] [Google Scholar]
- 55. Ho SY, Schooling M, Hui LL, McGhee SM, Mak KH et al. (2006) Soy consumption and mortality in Hong Kong: proxy-reported case-control study of all older adult deaths in 1998. Prev Med 43: 20-26. doi: 10.1016/j.ypmed.2006.03.007. PubMed: 16631248. [DOI] [PubMed] [Google Scholar]
- 56. Horn-Ross PL, John EM, Lee M, Stewart SL, Koo J et al. (2001) Phytoestrogen consumption and breast cancer risk in a multiethnic population: the Bay Area Breast Cancer Study. Am J Epidemiol 154: 434-441. doi: 10.1093/aje/154.5.434. PubMed: 11532785. [DOI] [PubMed] [Google Scholar]
- 57. Ingram D, Sanders K, Kolybaba M, Lopez D (1997) Case-control study of phyto-oestrogens and breast cancer. Lancet 350: 990-994. doi: 10.1016/S0140-6736(97)01339-1. PubMed: 9329514. [DOI] [PubMed] [Google Scholar]
- 58. Iwasaki M, Hamada GS, Nishimoto IN, Netto MM, Motola J Jr. et al. (2009) Dietary isoflavone intake and breast cancer risk in case-control studies in Japanese, Japanese Brazilians, and non-Japanese Brazilians. Breast Cancer Res Treat 116: 401-411. doi: 10.1007/s10549-008-0168-1. PubMed: 18777206. [DOI] [PubMed] [Google Scholar]
- 59. Iwasaki M, Hamada GS, Nishimoto IN, Netto MM, Motola J Jr. et al. (2009) Isoflavone, polymorphisms in estrogen receptor genes and breast cancer risk in case-control studies in Japanese, Japanese Brazilians and non-Japanese Brazilians. Cancer Sci 100: 927-933. doi: 10.1111/j.1349-7006.2009.01118.x. PubMed: 19298602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Iwasaki M, Hamada GS, Nishimoto IN, Netto MM, Motola J Jr. et al. (2010) Dietary isoflavone intake, polymorphisms in the CYP17, CYP19, 17beta-HSD1, and SHBG genes, and risk of breast cancer in case-control studies in Japanese, Japanese Brazilians, and non-Japanese Brazilians. Nutr Cancer 62: 466-475. PubMed: 20432167. [DOI] [PubMed] [Google Scholar]
- 61. Kim MK, Kim JH, Nam SJ, Ryu S, Kong G (2008) Dietary intake of soy protein and tofu in association with breast cancer risk based on a case-control study. Nutr Cancer 60: 568-576. PubMed: 18791919. [DOI] [PubMed] [Google Scholar]
- 62. Korde LA, Wu AH, Fears T, Nomura AM, West DW et al. (2009) Childhood soy intake and breast cancer risk in Asian American women. Cancer Epidemiol Biomarkers Prev 18: 1050-1059. doi: 10.1158/1055-9965.EPI-08-0405. PubMed: 19318430. [DOI] [PubMed] [Google Scholar]
- 63. Lampe JW, Nishino Y, Ray RM, Wu C, Li W et al. (2007) Plasma isoflavones and fibrocystic breast conditions and breast cancer among women in Shanghai, China. Cancer Epidemiol Biomarkers Prev 16: 2579-2586. doi: 10.1158/1055-9965.EPI-07-0368. PubMed: 18086761. [DOI] [PubMed] [Google Scholar]
- 64. Lee HP, Gourley L, Duffy SW, Estève J, Lee J et al. (1992) Risk factors for breast cancer by age and menopausal status: a case-control study in Singapore. Cancer Causes and Control 3: 313-322. doi: 10.1007/BF00146884. PubMed: 1617118. [DOI] [PubMed] [Google Scholar]
- 65. Lee MM, Chang IY, Horng CF, Chang JS, Cheng SH et al. (2005) Breast cancer and dietary factors in Taiwanese women. Cancer Causes Control 16: 929-937. doi: 10.1007/s10552-005-4932-9. PubMed: 16132802. [DOI] [PubMed] [Google Scholar]
- 66. Maskarinec G, Erber E, Verheus M, Hernandez BY, Killeen J et al. (2009) Soy consumption and histopathologic markers in breast tissue using tissue microarrays. Nutr Cancer 61: 708-716. PubMed: 19838945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Murkies A, Dalais FS, Briganti EM, Burger HG, Healy DL et al. (2000) Phytoestrogens and breast cancer in postmenopausal women: a case control study. Menopause 7: 289-296. doi: 10.1097/00042192-200007050-00003. PubMed: 10993028. [DOI] [PubMed] [Google Scholar]
- 68. Peterson J, Lagiou P, Samoli E, Lagiou A, Katsouyanni K et al. (2003) Flavonoid intake and breast cancer risk: a case--control study in Greece. Br J Cancer 89: 1255-1259. doi: 10.1038/sj.bjc.6601271. PubMed: 14520456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Piller R, Chang-Claude J, Linseisen J (2006) Plasma enterolactone and genistein and the risk of premenopausal breast cancer. Eur J Cancer Prev 15: 225-232. doi: 10.1097/01.cej.0000197449.56862.75. PubMed: 16679865. [DOI] [PubMed] [Google Scholar]
- 70. Sanderson M, Shu XO, Yu H, Dai Q, Malin AS et al. (2004) Insulin-like growth factor-I, soy protein intake, and breast cancer risk. Nutr Cancer 50: 8-15. PubMed: 15572292. [DOI] [PubMed] [Google Scholar]
- 71. Shu XO, Jin F, Dai Q, Wen W, Potter JD et al. (2001) Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev 10: 483-488. PubMed: 11352858. [PubMed] [Google Scholar]
- 72. Suzuki T, Matsuo K, Tsunoda N, Hirose K, Hiraki A et al. (2008) Effect of soybean on breast cancer according to receptor status: a case-control study in Japan. Int J Cancer 123: 1674-1680. doi: 10.1002/ijc.23644. PubMed: 18623079. [DOI] [PubMed] [Google Scholar]
- 73. Thanos J, Cotterchio M, Boucher BA, Kreiger N, Thompson LU (2006) Adolescent dietary phytoestrogen intake and breast cancer risk (Canada). Cancer Causes Control 17: 1253-1261. doi: 10.1007/s10552-006-0062-2. PubMed: 17111256. [DOI] [PubMed] [Google Scholar]
- 74. Touillaud MS, Pillow PC, Jakovljevic J, Bondy ML, Singletary SE et al. (2005) Effect of dietary intake of phytoestrogens on estrogen receptor status in premenopausal women with breast cancer. Nutr Cancer 51: 162-169. PubMed: 15860438. [DOI] [PubMed] [Google Scholar]
- 75. Wang Q, Wang YP, Li JY, Yuan P, Yang F et al. (2010) Polymorphic catechol-O-methyltransferase gene, soy isoflavone intake and breast cancer in postmenopausal women: a case-control study. Chin J Cancer 29: 683-688. doi: 10.5732/cjc.009.10700. PubMed: 20591221. [DOI] [PubMed] [Google Scholar]
- 76. Witte JS, Ursin G, Siemiatycki J, Thompson WD, Paganini-Hill A et al. (1997) Diet and premenopausal bilateral breast cancer: a case-control study. Breast Cancer Res Treat 42: 243-251. doi: 10.1023/A:1005710211184. PubMed: 9065608. [DOI] [PubMed] [Google Scholar]
- 77. Wu AH, Wan P, Hankin J, Tseng CC, Yu MC et al. (2002) Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis 23: 1491-1496. doi: 10.1093/carcin/23.9.1491. PubMed: 12189192. [DOI] [PubMed] [Google Scholar]
- 78. Wu AH, Yu MC, Tseng CC, Stanczyk FZ, Pike MC (2009) Dietary patterns and breast cancer risk in Asian American women. Am J Clin Nutr 89: 1145-1154. doi: 10.3945/ajcn.2008.26915. PubMed: 19211822. [DOI] [PubMed] [Google Scholar]
- 79. Wu AH, Ziegler RG, Horn-Ross PL, Nomura AM, West DW et al. (1996) Tofu and risk of breast cancer in Asian-Americans. Cancer Epidemiol Biomarkers Prev 5: 901-906. PubMed: 8922298. [PubMed] [Google Scholar]
- 80. Yuan JM, Wang QS, Ross RK, Henderson BE, Yu MC (1995) Diet and breast cancer in Shanghai and Tianjin, China. Br J Cancer 71: 1353-1358. doi: 10.1038/bjc.1995.263. PubMed: 7779738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang C, Ho SC, Lin F, Cheng S, Fu J et al. (2010) Soy product and isoflavone intake and breast cancer risk defined by hormone receptor status. Cancer Sci 101: 501-507. doi: 10.1111/j.1349-7006.2009.01376.x. PubMed: 19860847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhang M, Liu X, Holman CD (2010) Effect of dietary intake of isoflavones on the estrogen and progesterone receptor status of breast cancer. Nutr Cancer 62: 765-773. PubMed: 20661825. [DOI] [PubMed] [Google Scholar]
- 83. Zhang M, Yang H, Holman CD (2009) Dietary intake of isoflavones and breast cancer risk by estrogen and progesterone receptor status. Breast Cancer Res Treat 118: 553-563. doi: 10.1007/s10549-009-0354-9. PubMed: 19252980. [DOI] [PubMed] [Google Scholar]
- 84. Zheng W, Dai Q, Custer LJ, Shu XO, Wen WQ et al. (1999) Urinary excretion of isoflavonoids and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev 8: 35-40. PubMed: 9950237. [PubMed] [Google Scholar]
- 85. Maskarinec G, Pagano I, Lurie G, Kolonel LN (2006) A longitudinal investigation of mammographic density: the multiethnic cohort. Cancer Epidemiology, Biomarkers and Prevention 15: 732-739. doi: 10.1158/1055-9965.EPI-05-0798. [DOI] [PubMed] [Google Scholar]
- 86. Li M, Zhang Z, Hill DL, Chen X, Wang H et al. (2005) Genistein, a dietary isoflavone, down-regulates the MDM2 oncogene at both transcriptional and posttranslational levels. Cancer Res 65: 8200-8208. doi: 10.1158/0008-5472.CAN-05-1302. PubMed: 16166295. [DOI] [PubMed] [Google Scholar]
- 87. Shannon J, Ray R, Wu C, Nelson Z, Gao DL et al. (2005) Food and botanical groupings and risk of breast cancer: a case-control study in Shanghai, China. Cancer Epidemiol Biomarkers Prev 14: 81-90. PubMed: 15668480. [PubMed] [Google Scholar]
- 88. Grace PB, Taylor JI, Low YL, Luben RN, Mulligan AA et al. (2004) Phytoestrogen concentrations in serum and spot urine as biomarkers for dietary phytoestrogen intake and their relation to breast cancer risk in European prospective investigation of cancer and nutrition-norfolk. Cancer Epidemiol Biomarkers Prev 13: 698-708. PubMed: 15159299. [PubMed] [Google Scholar]
- 89. Goodman MT, Shvetsov YB, Wilkens LR, Franke AA, Le ML et al. (2009) Urinary phytoestrogen excretion and postmenopausal breast cancer risk: the multiethnic cohort study. Cancer Prevention Research 2: 887-894. doi: 10.1158/1940-6207.CAPR-09-0039. PubMed: 19789300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Verheus M, van Gils CH, Keinan-Boker L, Grace PB, Bingham SA et al. (2007) Plasma phytoestrogens and subsequent breast cancer risk. J Clin Oncol 25: 648-655. doi: 10.1200/JCO.2006.06.0244. PubMed: 17200150. [DOI] [PubMed] [Google Scholar]
- 91. Ward H, Chapelais G, Kuhnle GG, Luben R, Khaw KT et al. (2008) Breast cancer risk in relation to urinary and serum biomarkers of phytoestrogen exposure in the European Prospective into Cancer-Norfolk cohort study. Breast Cancer Res 10: R32. doi: 10.1186/bcr1916. PubMed: 18419813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ward HA, Kuhnle GG (2010) Phytoestrogen consumption and association with breast, prostate and colorectal cancer in EPIC Norfolk. [Review ] [71 refs]. Archives of Biochemistry and Biophysics 501: 170-175 doi: 10.1016/j.abb.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 93. Dorjgochoo T, Gu K, Zheng Y, Kallianpur A, Chen Z et al. (2011) Soy intake in association with menopausal symptoms during the first 6 and 36 months after breast cancer diagnosis. Breast Cancer Res Treat 130: 879-889. doi: 10.1007/s10549-010-1096-4. PubMed: 20703939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kang HB, Zhang YF, Yang JD, Lu KL (2012) Study on soy isoflavone consumption and risk of breast cancer and survival. Asian Pac J Cancer Prev 13: 995-998. doi: 10.7314/APJCP.2012.13.3.995. PubMed: 22631686. [DOI] [PubMed] [Google Scholar]
- 95. Ma H, Sullivan-Halley J, Smith AW, Neuhouser ML, Alfano CM et al. (2011) Estrogenic botanical supplements, health-related quality of life, fatigue, and hormone-related symptoms in breast cancer survivors: a HEAL study report. BMC Complement Altern Med 11: 109. doi: 10.1186/1472-6882-11-109. PubMed: 22067368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang YF, Kang HB, Li BL, Zhang RM (2012) Positive effects of soy isoflavone food on survival of breast cancer patients in China. Asian Pac J Cancer Prev 13: 479-482. doi: 10.7314/APJCP.2012.13.2.479. PubMed: 22524810. [DOI] [PubMed] [Google Scholar]
- 97. Woo HD, Park KS, Ro J, Kim J (2012) Differential influence of dietary soy intake on the risk of breast cancer recurrence related to HER2 status. Nutr Cancer 64: 198-205. doi: 10.1080/01635581.2012.635261. PubMed: 22211813. [DOI] [PubMed] [Google Scholar]
- 98. Boyapati SM, Shu XO, Ruan ZX, Dai Q, Cai Q et al. (2005) Soyfood intake and breast cancer survival: a followup of the Shanghai Breast Cancer Study. Breast Cancer Res Treat 92: 11-17. doi: 10.1007/s10549-004-6019-9. PubMed: 15980986. [DOI] [PubMed] [Google Scholar]
- 99. Brasky TM, Lampe JW, Potter JD, Patterson RE, White E (2010) Specialty supplements and breast cancer risk in the VITamins And Lifestyle (VITAL) Cohort. Cancer Epidemiol Biomarkers Prev 19: 1696-1708. doi: 10.1158/1055-9965.EPI-10-0318. PubMed: 20615886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Caan BJ, Natarajan L, Parker B, Gold EB, Thomson C et al. (2011) Soy food consumption and breast cancer prognosis. Cancer Epidemiol Biomarkers Prev 20: 854-858. doi: 10.1158/1055-9965.EPI-10-1041. PubMed: 21357380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fink BN, Steck SE, Wolff MS, Britton JA, Kabat GC et al. (2007) Dietary flavonoid intake and breast cancer survival among women on Long Island. Cancer Epidemiol Biomarkers Prev 16: 2285-2292. doi: 10.1158/1055-9965.EPI-07-0245. PubMed: 18006917. [DOI] [PubMed] [Google Scholar]
- 102. Frankenfeld CL, McTiernan A, Aiello EJ, Thomas WK, LaCroix K et al. (2004) Mammographic density in relation to daidzein-metabolizing phenotypes in overweight, postmenopausal women. Cancer Epidemiol Biomarkers Prev 13: 1156-1162. PubMed: 15247126. [PubMed] [Google Scholar]
- 103. Guha N, Kwan ML, Quesenberry CP Jr., Weltzien EK, Castillo AL et al. (2009) Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: the Life After Cancer Epidemiology study. Breast Cancer Research and Treatment 118: 395-405. doi: 10.1007/s10549-009-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hedelin M, Löf M, Olsson M, Adlercreutz H, Sandin S et al. (2008) Dietary phytoestrogens are not associated with risk of overall breast cancer but diets rich in coumestrol are inversely associated with risk of estrogen receptor and progesterone receptor negative breast tumors in Swedish women. J Nutr 138: 938-945. PubMed: 18424605. [DOI] [PubMed] [Google Scholar]
- 105. Horn-Ross PL, Hoggatt KJ, West DW, Krone MR, Stewart SL et al. (2002) Recent diet and breast cancer risk: the California Teachers Study (USA). Cancer Causes Control 13: 407-415. doi: 10.1023/A:1015786030864. PubMed: 12146845. [DOI] [PubMed] [Google Scholar]
- 106. Kang X, Zhang Q, Wang S, Huang X, Jin S (2010) Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy. CMAJ 182: 1857-1862. doi: 10.1503/cmaj.091298. PubMed: 20956506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Keinan-Boker L, van der Schouw YT, Grobbee DE, Peeters PH (2004) Dietary phytoestrogens and breast cancer risk. Am J Clin Nutr 79: 282-288. PubMed: 14749235. [DOI] [PubMed] [Google Scholar]
- 108. Key TJ, Sharp GB, Appleby PN, Beral V, Goodman MT et al. (1999) Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br J Cancer 81: 1248-1256. doi: 10.1038/sj.bjc.6690837. PubMed: 10584890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lee SA, Shu XO, Li H, Yang G, Cai H et al. (2009) Adolescent and adult soy food intake and breast cancer risk: results from the Shanghai Women's Health Study. Am J Clin Nutr 89: 1920-1926. doi: 10.3945/ajcn.2008.27361. PubMed: 19403632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nagel G, Mack U, von FD, Linseisen J (2005) Dietary phytoestrogen intake and mammographic density -- results of a pilot study. Eur J Med Res 10: 389-394. PubMed: 16183551. [PubMed] [Google Scholar]
- 111. Nishio K, Niwa Y, Toyoshima H, Tamakoshi K, Kondo T et al. (2007) Consumption of soy foods and the risk of breast cancer: findings from the Japan Collaborative Cohort (JACC) Study. Cancer Causes Control 18: 801-808. doi: 10.1007/s10552-007-9023-7. PubMed: 17619154. [DOI] [PubMed] [Google Scholar]
- 112. Shu XO, Zheng Y, Cai H, Gu K, Chen Z et al. (2009) Soy food intake and breast cancer survival. JAMA 302: 2437-2443. doi: 10.1001/jama.2009.1783. PubMed: 19996398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Touillaud MS, Thiebaut AC, Niravong M, Boutron-Ruault MC, Clavel-Chapelon F (2006) No association between dietary phytoestrogens and risk of premenopausal breast cancer in a French cohort study. Cancer Epidemiology, Biomarkers and Prevention 15: 2574-2576. doi: 10.1158/1055-9965.EPI-06-0543. [DOI] [PubMed] [Google Scholar]
- 114. Travis RC, Allen NE, Appleby PN, Spencer EA, Roddam AW et al. (2008) A prospective study of vegetarianism and isoflavone intake in relation to breast cancer risk in British women. International Journal of Cancer 122: 705-710. doi: 10.1002/ijc.23141. [DOI] [PubMed] [Google Scholar]
- 115. Wang L, Lee IM, Zhang SM, Blumberg JB, Buring JE et al. (2009) Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am J Clin Nutr 89: 905-912. doi: 10.3945/ajcn.2008.26913. PubMed: 19158208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yamamoto S, Sobue T, Kobayashi M, Sasaki S, Tsugane S et al. (2003) Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst 95: 906-913. doi: 10.1093/jnci/95.12.906. PubMed: 12813174. [DOI] [PubMed] [Google Scholar]
- 117. Iwasaki M, Inoue M, Otani T, Sasazuki S, Kurahashi N et al. (2008) Plasma isoflavone level and subsequent risk of breast cancer among Japanese women: a nested case-control study from the Japan Public Health Center-based prospective study group. J Clin Oncol 26: 1677-1683. doi: 10.1200/JCO.2007.13.9964. PubMed: 18316793. [DOI] [PubMed] [Google Scholar]
- 118. Palacios S, Pornel B, Vázquez F, Aubert L, Chantre P et al. (2010) Long-term endometrial and breast safety of a specific, standardized soy extract. Climacteric 13: 368-375. doi: 10.3109/13697131003660585. PubMed: 20380569. [DOI] [PubMed] [Google Scholar]
- 119. Hall MC, O'Brien B, McCormack T (2007) Equol producer status, salivary estradiol profile and urinary excretion of isoflavones in Irish Caucasian women, following ingestion of soymilk.[Erratum appears in Steroids (2009 Feb);74(2): 283]. Steroids 72: 64-70. PubMed: 17157887. [DOI] [PubMed] [Google Scholar]
- 120. Wu AH, Stanczyk FZ, Hendrich S, Murphy PA, Zhang C et al. (2000) Effects of soy foods on ovarian function in premenopausal women. Br J Cancer 82: 1879-1886. doi: 10.1054/bjoc.1999.1218. PubMed: 10839307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lu LJ, Anderson KE, Grady JJ, Kohen F, Nagamani M (2000) Decreased ovarian hormones during a soya diet: implications for breast cancer prevention. Cancer Res 60: 4112-4121. PubMed: 10945618. [PubMed] [Google Scholar]
- 122. Lu LJ, Anderson KE, Grady JJ, Nagamani M (1996) Effects of soya consumption for one month on steroid hormones in premenopausal women: implications for breast cancer risk reduction. Cancer Epidemiol Biomarkers Prev 5: 63-70. PubMed: 8770469. [PubMed] [Google Scholar]
- 123. Maskarinec G, Morimoto Y, Novotny R, Nordt FJ, Stanczyk FZ et al. (2005) Urinary sex steroid excretion levels during a soy intervention among young girls: a pilot study. Nutr Cancer 52: 22-28. PubMed: 16091000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cohen LA, Crespin JS, Wolper C, Zang EA, Pittman B et al. (2007) Soy isoflavone intake and estrogen excretion patterns in young women: effect of probiotic administration. In Vivo 21: 507-512. PubMed: 17591361. [PubMed] [Google Scholar]
- 125. Petrakis NL, Barnes S, King EB, Lowenstein J, Wiencke J et al. (1996) Stimulatory influence of soy protein isolate on breast secretion in pre- and postmenopausal women. Cancer Epidemiol Biomarkers Prev 5: 785-794. PubMed: 8896889. [PubMed] [Google Scholar]
- 126. Maskarinec G, Hebshi S, Custer L, Franke AA (2008) The relation of soy intake and isoflavone levels in nipple aspirate fluid. Eur J Cancer Prev 17: 67-70. doi: 10.1097/CEJ.0b013e3281108101. PubMed: 18090913. [DOI] [PubMed] [Google Scholar]
- 127. Dooley WC, Hendricks C, Gusev Y, Shockney L (2006) Internet based double-blind cross-over clinical trial to test efficacy of high dose isoflavone soy in controlling breast cancer survivor hot flashes. Journal of Clinical Oncology 24: 36s. doi: 10.1200/JCO.2004.00.7617. [DOI] [Google Scholar]
- 128. MacGregor CA, Canney PA, Patterson G, McDonald R, Paul J (2005) A randomised double-blind controlled trial of oral soy supplements versus placebo for treatment of menopausal symptoms in patients with early breast cancer. Eur J Cancer 41: 708-714. doi: 10.1016/j.ejca.2005.01.005. PubMed: 15763646. [DOI] [PubMed] [Google Scholar]
- 129. Nikander E, Kilkkinen A, Metsä-Heikkilä M, Adlercreutz H, Pietinen P et al. (2003) A randomized placebo-controlled crossover trial with phytoestrogens in treatment of menopause in breast cancer patients. Obstet Gynecol 101: 1213-1220. doi: 10.1016/S0029-7844(03)00232-1. PubMed: 12798527. [DOI] [PubMed] [Google Scholar]
- 130. Van Patten CL, Olivotto IA, Chambers GK, Gelmon KA, Hislop TG et al. (2002) Effect of soy phytoestrogens on hot flashes in postmenopausal women with breast cancer: a randomized. Controlled Clinical Trials - Journal of Clinical Oncology 20: 1449-1455. [DOI] [PubMed] [Google Scholar]
- 131. Quella SK, Loprinzi CL, Barton DL, Knost JA, Sloan JA et al. (2000) Evaluation of soy phytoestrogens for the treatment of hot flashes in breast cancer survivors: A North Central Cancer Treatment Group Trial. J Clin Oncol 18: 1068-1074. PubMed: 10694559. [DOI] [PubMed] [Google Scholar]
- 132. Steinberg FM, Murray MJ, Lewis RD, Cramer MA, Amato P et al. (2011) Clinical outcomes of a 2-y soy isoflavone supplementation in menopausal women. Am J Clin Nutr 93: 356-367. doi: 10.3945/ajcn.110.008359. PubMed: 21177797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. McLaughlin JM, Olivo-Marston S, Vitolins MZ, Bittoni M, Reeves KW et al. (2011) Effects of tomato- and soy-rich diets on the igf-I hormonal network: a crossover study of postmenopausal women at high risk for breast cancer. Cancer Prev Res (Phila) 4: 702-710. doi: 10.1158/1940-6207.CAPR-10-0329. PubMed: 21430071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Maskarinec G, Morimoto Y, Conroy SM, Pagano IS, Franke AA (2011) The volume of nipple aspirate fluid is not affected by 6 months of treatment with soy foods in premenopausal women. J Nutr 141: 626-630. doi: 10.3945/jn.110.133769. PubMed: 21325473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Maskarinec G, Verheus M, Steinberg FM, Amato P, Cramer MK et al. (2009) Various doses of soy isoflavones do not modify mammographic density in postmenopausal women. J Nutr 139: 981-986. doi: 10.3945/jn.108.102913. PubMed: 19321587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Marini H, Bitto A, Altavilla D, Burnett BP, Polito F et al. (2008) Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow-up study. J Clin Endocrinol Metab 93: 4787-4796. doi: 10.1210/jc.2008-1087. PubMed: 18796517. [DOI] [PubMed] [Google Scholar]
- 137. Kataoka M, Atkinson C, Warren R, Sala E, Day NE et al. (2008) Mammographic density using two computer-based methods in an isoflavone trial. Maturitas 59: 350-357. doi: 10.1016/j.maturitas.2008.03.005. PubMed: 18495387. [DOI] [PubMed] [Google Scholar]
- 138. Pop EA, Fischer LM, Coan AD, Gitzinger M, Nakamura J et al. (2008) Effects of a high daily dose of soy isoflavones on DNA damage, apoptosis, and estrogenic outcomes in healthy postmenopausal women: A phase I clinical trial. Menopause 15: 684-692. doi: 10.1097/gme.0b013e318167b8f2. PubMed: 18446090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. DiSilvestro RA, Goodman J, Dy E, Lavalle G (2005) Soy isoflavone supplementation elevates erythrocyte superoxide dismutase, but not plasma ceruloplasmin in postmenopausal breast cancer survivors. Breast Cancer Research and Treatment 89: 251-255. doi: 10.1007/s10549-004-2227-6. [DOI] [PubMed] [Google Scholar]
- 140. Wu AH, Stanczyk FZ, Martinez C, Tseng CC, Hendrich S et al. (2005) A controlled 2-mo dietary fat reduction and soy food supplementation study in postmenopausal women. Am J Clin Nutr 81: 1133-1141. PubMed: 15883439. [DOI] [PubMed] [Google Scholar]
- 141. Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H et al. (2005) The effect of soy consumption on the urinary 2:16-hydroxyestrone ratio in postmenopausal women depends on equol production status but is not influenced by probiotic consumption. J Nutr 135: 603-608. PubMed: 15735101. [DOI] [PubMed] [Google Scholar]
- 142. Nikander E, Metsa-Heikkila M, Ylikorkala O, Tiitinen A (2004) Effects of phytoestrogens on bone turnover in postmenopausal women with a history of breast cancer. Journal of Clinical Endocrinology and Metabolism 89: 1207-1212. doi: 10.1210/jc.2003-031166. [DOI] [PubMed] [Google Scholar]
- 143. Zittermann A, Geppert J, Baier S, Zehn N, Gouni-Berthold I et al. (2004) Short-term effects of high soy supplementation on sex hormones, bone markers, and lipid parameters in young female adults. Eur J Nutr 43: 100-108. doi: 10.1007/s00394-004-0447-5. PubMed: 15083317. [DOI] [PubMed] [Google Scholar]
- 144. Maskarinec G, Takata Y, Franke AA, Williams AE, Murphy SP (2004) A 2-year soy intervention in premenopausal women does not change mammographic densities. J Nutr 134: 3089-3094. PubMed: 15514280. [DOI] [PubMed] [Google Scholar]
- 145. Maskarinec G, Franke AA, Williams AE, Hebshi S, Oshiro C et al. (2004) Effects of a 2-year randomized soy intervention on sex hormone levels in premenopausal women. Cancer Epidemiol Biomarkers Prev 13: 1736-1744. PubMed: 15533901. [PubMed] [Google Scholar]
- 146. Maskarinec G, Williams AE, Carlin L (2003) Mammographic densities in a one-year isoflavone intervention. Eur J Cancer Prev 12: 165-169. doi: 10.1097/00008469-200304000-00011. PubMed: 12671541. [DOI] [PubMed] [Google Scholar]
- 147. Maskarinec G, Williams AE, Inouye JS, Stanczyk FZ, Franke AA (2002) A randomized isoflavone intervention among premenopausal women. Cancer Epidemiology, Biomarkers and Prevention 11: 195-201. [PubMed] [Google Scholar]
- 148. Maskarinec G, Franke AA, Williams AE, Stanczyk FC (2002) The effects of an isoflavone intervention on the urinary excretion of hormone metabolites in premenopausal women. IARC Sci Publ 156: 375-377. PubMed: 12484210. [PubMed] [Google Scholar]
- 149. Brown BD, Thomas W, Hutchins A, Martini MC, Slavin JL (2002) Types of dietary fat and soy minimally affect hormones and biomarkers associated with breast cancer risk in premenopausal women. Nutr Cancer 43: 22-30. PubMed: 12467131. [DOI] [PubMed] [Google Scholar]
- 150. Kumar NB, Cantor A, Allen K, Riccardi D, Cox CE (2002) The specific role of isoflavones on estrogen metabolism in premenopausal women. Cancer 94: 1166-1174. doi: 10.1002/cncr.10320. PubMed: 11920488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lu LJ, Anderson KE, Grady JJ, Nagamani M (2001) Effects of an isoflavone-free soy diet on ovarian hormones in premenopausal women. J Clin Endocrinol Metab 86: 3045-3052. doi: 10.1210/jc.86.7.3045. PubMed: 11443166. [DOI] [PubMed] [Google Scholar]
- 152. Xu X, Duncan AM, Wangen KE, Kurzer MS (2000) Soy consumption alters endogenous estrogen metabolism in postmenopausal women. Cancer Epidemiol Biomarkers Prev 9: 781-786. PubMed: 10952094. [PubMed] [Google Scholar]
- 153. Duncan AM, Merz-Demlow BE, Xu X, Phipps WR, Kurzer MS (2000) Premenopausal equol excretors show plasma hormone profiles associated with lowered risk of breast cancer. Cancer Epidemiol Biomarkers Prev 9: 581-586. PubMed: 10868692. [PubMed] [Google Scholar]
- 154. Lu LJ, Cree M, Josyula S, Nagamani M, Grady JJ et al. (2000) Increased urinary excretion of 2-hydroxyestrone but not 16alpha-hydroxyestrone in premenopausal women during a soya diet containing isoflavones. Cancer Res 60: 1299-1305. PubMed: 10728690. [PubMed] [Google Scholar]
- 155. Duncan AM, Merz BE, Xu X, Nagel TC, Phipps WR et al. (1999) Soy isoflavones exert modest hormonal effects in premenopausal women. J Clin Endocrinol Metab 84: 192-197. doi: 10.1210/jc.84.1.192. PubMed: 9920082. [DOI] [PubMed] [Google Scholar]
- 156. Martini MC, Dancisak BB, Haggans CJ, Thomas W, Slavin JL (1999) Effects of soy intake on sex hormone metabolism in premenopausal women. Nutr Cancer 34: 133-139. PubMed: 10578479. [DOI] [PubMed] [Google Scholar]
- 157. McMichael-Phillips DF, Harding C, Morton M, Roberts SA, Howell A, et al. (1998) Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. American Journal of Clinical Nutrition 68: Suppl -1435S [DOI] [PubMed] [Google Scholar]
- 158. Nagata C, Takatsuka N, Inaba S, Kawakami N, Shimizu H (1998) Effect of soymilk consumption on serum estrogen concentrations in premenopausal Japanese women. J Natl Cancer Inst 90: 1830-1835. doi: 10.1093/jnci/90.23.1830. PubMed: 9839524. [DOI] [PubMed] [Google Scholar]
- 159. Cassidy A, Bingham S, Setchell KDR (1994) Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am J Clin Nutr 60: 333-340. PubMed: 8074062. [DOI] [PubMed] [Google Scholar]
- 160. Khan SA, Chatterton RT, Michel N, Bryk M, Lee O et al. (2012) Soy isoflavone supplementation for breast cancer risk reduction: a randomized phase II trial. Cancer. Prev Res (Phila Pa) 5: 309-319. doi: 10.1158/1940-6207.CAPR-11-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Maskarinec G, Morimoto Y, Heak S, Isaki M, Steinbrecher A et al. (2012) Urinary estrogen metabolites in two soy trials with premenopausal women. Eur J Clin Nutr 66: 1044-1049. doi: 10.1038/ejcn.2012.71. PubMed: 22713773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Maskarinec G, Ollberding NJ, Conroy SM, Morimoto Y, Pagano IS et al. (2011) Estrogen levels in nipple aspirate fluid and serum during a randomized soy trial. Cancer Epidemiol Biomarkers Prev 20: 1815-1821. doi: 10.1158/1055-9965.EPI-11-0363. PubMed: 21742946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Morimoto Y, Conroy SM, Pagano IS, Franke AA, Stanczyk FZ et al. (2011) Influence of diet on nipple aspirate fluid production and estrogen levels. Food Funct 2: 665-670. doi: 10.1039/c1fo10144g. PubMed: 21986640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Morimoto Y, Conroy SM, Pagano IS, Isaki M, Franke AA et al. (2012) Urinary estrogen metabolites during a randomized soy trial. Nutr Cancer 64: 307-314. doi: 10.1080/01635581.2012.648819. PubMed: 22293063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Atkinson C, Warren RM, Sala E, Dowsett M, Dunning AM et al. (2004) Red-clover-derived isoflavones and mammographic breast density: a double-blind, randomized, placebo-controlled trial [ISRCTN42940165. Breast Cancer Research 6: R170-R179. doi: 10.1186/bcr909. PubMed: 15084240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Powles TJ, Howell A, Evans DG, McCloskey EV, Ashley S et al. (2008) Red clover isoflavones are safe and well tolerated in women with a family history of breast cancer. Menopause Int 14: 6-12. doi: 10.1258/mi.2007.007033. PubMed: 18380954. [DOI] [PubMed] [Google Scholar]
- 167. Li N, Dong J, Hu Z, Shen H, Dai M (2010) Potentially functional polymorphisms in ESR1 and breast cancer risk: a meta-analysis. Breast Cancer Res Treat 121: 177-184. doi: 10.1007/s10549-009-0532-9. PubMed: 19760036. [DOI] [PubMed] [Google Scholar]
- 168. Messina M, Wu AH (2009) Perspectives on the soy-breast cancer relation. Am J Clin Nutr 89: 1673S-1679S. doi: 10.3945/ajcn.2009.26736V. PubMed: 19339397. [DOI] [PubMed] [Google Scholar]
- 169. Andres S, Abraham K, Appel KE, Lampen A (2011) Risks and benefits of dietary isoflavones for cancer. Crit Rev Toxicol 41: 463-506. doi: 10.3109/10408444.2010.541900. PubMed: 21438720. [DOI] [PubMed] [Google Scholar]
- 170. Anderson LN, Cotterchio M, Boucher B, Kreiger N (2011) Phytoestrogen intake from foods, during adolescence and adulthood, and the risk of breast cancer by estrogen and progesterone receptor (ERPR) tumor subgroup among Ontario women. American Journal of Epidemiology Conference: 3rd North American Congress of Epidemiology Montreal, QC Canada. Conference Start: 20110621. Conference End: 20110624. Conference Publication: (var.pagings) 173: S312. [Google Scholar]
- 171. Andres S, Lampen A (2011) Isolates isoflavones are not without risk for women in and after the menopause. Toxicology LettersConference: 47th Congress of the European Societies of Toxicology, EUROTOX 2011 Paris France. Conference Start: 20110828. Conference End: 20110831. Conference Publication: (varpagings) 205: S264. [Google Scholar]
- 172. Archer DF, Sturdee DW, Baber R, de Villiers TJ, Pines A et al. (2011) Menopausal hot flushes and night sweats: Where are we now? Climacteric 14: 515-528. doi: 10.3109/13697137.2011.608596. PubMed: 21848495. [DOI] [PubMed] [Google Scholar]
- 173. Asensi M, Ortega A, Mena S, Feddi F, Estrela JM (2011) Natural polyphenols in cancer therapy. Crit Rev Clin Lab Sci 48: 197-216. doi: 10.3109/10408363.2011.631268. PubMed: 22141580. [DOI] [PubMed] [Google Scholar]
- 174. Harris DM, Besselink E, Henning SM, Go VL, Heber D (2005) Phytoestrogens induce differential estrogen receptor alpha- or Beta-mediated responses in transfected breast cancer cells. Exp Biol Med (Maywood) 230: 558-568. PubMed: 16118406. [DOI] [PubMed] [Google Scholar]
- 175. Chen J, Liu L, Hou R, Shao Z, Wu Y et al. (2011) Calycosin promotes proliferation of estrogen receptor-positive cells via estrogen receptors and ERK1/2 activation in vitro and in vivo. Cancer Lett 308: 144-151. doi: 10.1016/j.canlet.2011.04.022. PubMed: 21612861. [DOI] [PubMed] [Google Scholar]
- 176. Koehler KF, Helguero LA, Haldosén LA, Warner M, Gustafsson JA (2005) Reflections on the discovery and significance of estrogen receptor beta. Endocr Rev 26: 465-478. doi: 10.1210/er.2004-0027. PubMed: 15857973. [DOI] [PubMed] [Google Scholar]
- 177. Chen JLY, Sperry J, Ip NY, Brimble MA (2011) Natural products targeting telomere maintenance. MedChemComm 2: 229-245. [Google Scholar]
- 178. Clemente A, Sonnante G, Domoney C (2011) Bowman-birk inhibitors from legumes and human gastrointestinal health: current status and perspectives. Curr Protein Pept Sci 12: 358-373. doi: 10.2174/138920311796391133. PubMed: 21418025. [DOI] [PubMed] [Google Scholar]
- 179. Ciotta L, Leanza V, Pagano I, Ando A, Formuso C, et al. (2011) Clinical effects of phytoestrogens' treatment in women in post-menopause. Climacteric Conference: 13th World Congress on the Menopause Rome Italy. Conference Start: 20110608. Conference End: 20110611. Conference Publication: (varpagings) 14: 192. [Google Scholar]
- 180. Chu ZH, Liang XH, Zhou XL, Huang RF, Zhan Q, et al. (2011) Effects of deguelin on proliferation and apoptosis of MCF-7 breast cancer cells by phosphatidylinositol 3-kinase/Akt signaling pathway. Zhong Xi Yi Jie He Xue Bao 9: 533-538. [DOI] [PubMed] [Google Scholar]
- 181. Ju YH, Allred KF, Allred CD, Helferich WG (2006) Genistein stimulates growth of human breast cancer cells in a novel, postmenopausal animal model, with low plasma estradiol concentrations. Carcinogenesis 27: 1292-1299. doi: 10.1093/carcin/bgi370. PubMed: 16537557. [DOI] [PubMed] [Google Scholar]
- 182. Qin W, Zhu W, Shi H, Hewett JE, Ruhlen RL et al. (2009) Soy isoflavones have an antiestrogenic effect and alter mammary promoter hypermethylation in healthy premenopausal women. Nutr Cancer 61: 238-244. doi: 10.1080/01635580802404196. PubMed: 19235040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Amado NG, Fonseca BF, Cerqueira DM, Neto VM, Abreu JG (2011) Flavonoids: Potential Wnt/beta-catenin signaling modulators in cancer. Life Sci 89: 545-554. doi: 10.1016/j.lfs.2011.05.003. PubMed: 21635906. [DOI] [PubMed] [Google Scholar]
- 184. Choi EJ, Kim GH (2011) Anticancer mechanism of equol in 7,12-dimethylbenz(a) anthracene-treated animals. Int J Oncol 39: 747-754. PubMed: 21667019. [DOI] [PubMed] [Google Scholar]
- 185. Mai Z, Blackburn GL, Zhou JR (2007) Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells. Mol Carcinog 46: 534-542. doi: 10.1002/mc.20300. PubMed: 17295235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Mai Z, Blackburn GL, Zhou JR (2007) Soy phytochemicals synergistically enhance the preventive effect of tamoxifen on the growth of estrogen-dependent human breast carcinoma in mice. Carcinogenesis 28: 1217-1223. doi: 10.1093/carcin/bgm004. PubMed: 17234721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Boumendjel A, Macalou S, Valdameri G, Pozza A, Gauthier C et al. (2011) Targeting the multidrug ABCG2 transporter with flavonoidic inhibitors: In Vitro optimization and in vivo validation. Curr Med Chem 18: 3387-3401. doi: 10.2174/092986711796504736. PubMed: 21728961. [DOI] [PubMed] [Google Scholar]
- 188. Messina M, Nagata C, Wu AH (2006) Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer 55: 1-12. doi: 10.1207/s15327914nc5501_1. PubMed: 16965235. [DOI] [PubMed] [Google Scholar]
- 189. Nechuta SJ, Caan BJ, Chen WY, Lu W, Chen Z et al. (2012) Soy food intake after diagnosis of breast cancer and survival: an in-depth analysis of combined evidence from cohort studies of US and Chinese women. Am J Clin Nutr 96: 123-132. doi: 10.3945/ajcn.112.035972. PubMed: 22648714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Doyle C, Kushi LH, Byers T, Courneya KS, Demark-Wahnefried W et al. (2006) Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J Clin 56: 323-353. doi: 10.3322/canjclin.56.6.323. PubMed: 17135691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk of Breast Cancer By Study Quality (Isoflavones).
(TIF)
Risk of Breast Cancer by Menopausal Status (Isoflavones).
(TIF)
Risk of Breast Cancer by Dose of Soy Isoflavones.
(TIF)
Risk of Breast Cancer Recurrence with Intake of Soy Protein or Isoflavones.
(TIF)
Risk of Bias Across Studies.
(TIFF)
Medline Search Strategy.
(DOCX)
Case Control Studies of Soy and Risk of Breast Cancer.
(DOC)
Prospective Cohort Studies of Soy and Risk of Primary Breast Cancer.
(DOC)
Trials Assessing Effect of Soy on Hormonal Biomarkers and Estrogen Dependent Tissues.
(DOC)
(DOC)