Abstract
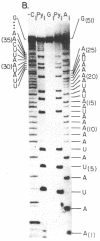
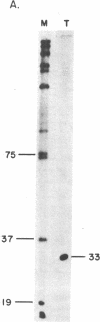
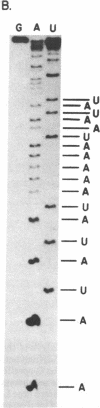
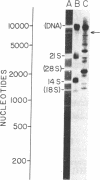
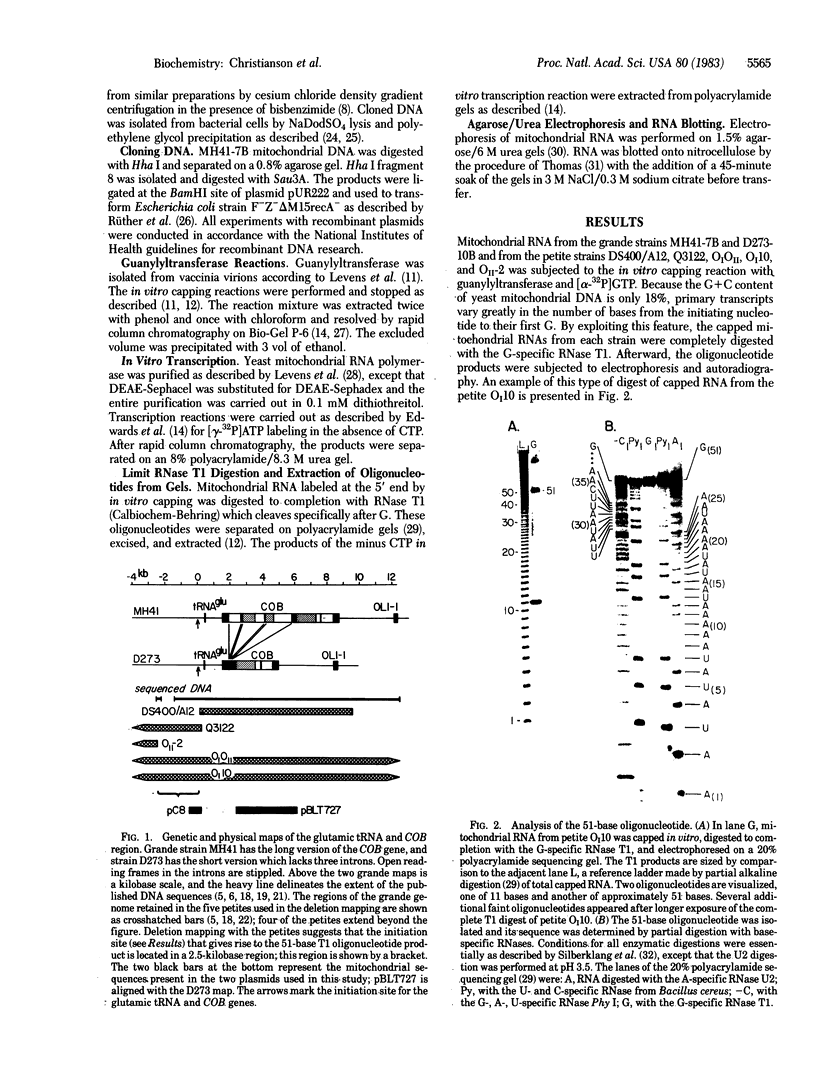
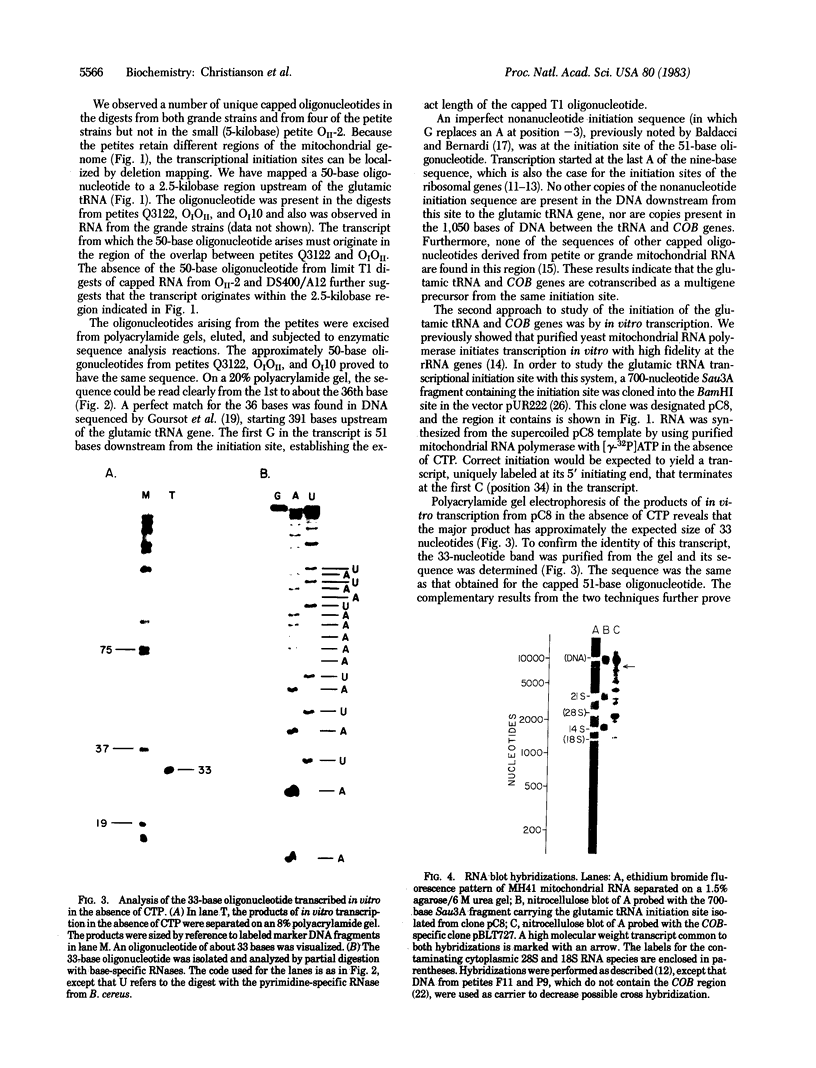
We have identified a single transcriptional initiation site for the glutamic tRNA and COB (cytochrome b) genes by using the complementary techniques of in vitro capping of RNA and in vitro transcription. In the capping reaction, mitochondrial RNA is labeled with [alpha-32P]GTP by vaccinia virus guanylyltransferase. This reaction is specific for the 5' ends of RNA retaining the terminal triphosphate of transcriptional initiation. Exploiting the extremely low G+C content (18%) of yeast mitochondrial DNA, we digested in vitro capped transcripts from various petite deletion mutants with the G-specific RNase T1. By petite deletion mapping, a capped transcript giving rise to a 51-base RNase T1-generated oligonucleotide was localized near the glutamic tRNA gene. When the sequence of this oligonucleotide was determined, it perfectly matched the DNA sequence 391 base upstream of the glutamic tRNA. Purified yeast mitochondrial RNA polymerase initiated transcription in vitro at the same site as shown by the sequence of the 33-base oligonucleotide product of the reaction performed in the absence of CTP. Initiation starts at a nonanucleotide sequence previously implicated in yeast mitochondrial transcriptional initiation. Because there is no evidence of an initiation site in the 1,050 bases between the glutamic tRNA and COB genes, the two genes are likely to be transcribed together. Further evidence of a long common transcript was provided by RNA blot hybridization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldacci G., Bernardi G. Replication origins are associated with transcription initiation sequences in the mitochondrial genome of yeast. EMBO J. 1982;1(8):987–994. doi: 10.1002/j.1460-2075.1982.tb01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg P. E., Lewin A., Christianson T., Rabinowitz M. Propagation of restriction fragments from the mitochondrial DNA of Saccharomyces cerevisiae in E. coli by means of plasmid vectors. Nucleic Acids Res. 1979;6(6):2133–2150. doi: 10.1093/nar/6.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonitz S. G., Homison G., Thalenfeld B. E., Tzagoloff A., Nobrega F. G. Assembly of the mitochondrial membrane system. Processing of the apocytochrome b precursor RNAs in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1982 Jun 10;257(11):6268–6274. [PubMed] [Google Scholar]
- Christianson T., Edwards J., Levens D., Locker J., Rabinowitz M. Transcriptional initiation and processing of the small ribosomal RNA of yeast mitochondria. J Biol Chem. 1982 Jun 10;257(11):6494–6500. [PubMed] [Google Scholar]
- Church G. M., Slonimski P. P., Gilbert W. Pleiotropic mutations within two yeast mitochondrial cytochrome genes block mRNA processing. Cell. 1979 Dec;18(4):1209–1215. doi: 10.1016/0092-8674(79)90233-2. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Salle H., Jacq C., Slonimski P. P. Critical sequences within mitochondrial introns: pleiotropic mRNA maturase and cis-dominant signals of the box intron controlling reductase and oxidase. Cell. 1982 Apr;28(4):721–732. doi: 10.1016/0092-8674(82)90051-4. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. C., Levens D., Rabinowitz M. Analysis of transcriptional initiation of yeast mitochondrial DNA in a homologous in vitro transcription system. Cell. 1982 Dec;31(2 Pt 1):337–346. doi: 10.1016/0092-8674(82)90127-1. [DOI] [PubMed] [Google Scholar]
- Frontali L., Palleschi C., Francisci S. Transcripts of mitochondrial tRNA genes in Saccharomyces cerevisiae. Nucleic Acids Res. 1982 Nov 25;10(22):7283–7293. doi: 10.1093/nar/10.22.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goursot R., Mangin M., Bernardi G. Surrogate origins of replication in the mitochondrial genomes of ori-zero petite mutants of yeast. EMBO J. 1982;1(6):705–711. doi: 10.1002/j.1460-2075.1982.tb01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivell L. A., Borst P. Mitochondrial mosaics-maturases on the move. Nature. 1982 Aug 19;298(5876):703–704. doi: 10.1038/298703a0. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich A., Pajot P., Foucher M., Grandchamp C., Slonimski P. A pathway of cytochrome b mRNA processing in yeast mitochondria: specific splicing steps and an intron-derived circular DNA. Cell. 1980 Feb;19(2):321–329. doi: 10.1016/0092-8674(80)90506-1. [DOI] [PubMed] [Google Scholar]
- Lamb M. R., Anziano P. Q., Glaus K. R., Hanson D. K., Klapper H. J., Perlman P. S., Mahler H. R. Functional domains in introns. RNA processing intermediates in cis- and trans-acting mutants in the penultimate intron of the mitochondrial gene for cytochrome b. J Biol Chem. 1983 Feb 10;258(3):1991–1999. [PubMed] [Google Scholar]
- Lazowska J., Jacq C., Slonimski P. P. Sequence of introns and flanking exons in wild-type and box3 mutants of cytochrome b reveals an interlaced splicing protein coded by an intron. Cell. 1980 Nov;22(2 Pt 2):333–348. doi: 10.1016/0092-8674(80)90344-x. [DOI] [PubMed] [Google Scholar]
- Levens D., Lustig A., Rabinowitz M. Purification of mitochondrial RNA polymerase from Saccharomyces cerevisiae. J Biol Chem. 1981 Feb 10;256(3):1474–1481. [PubMed] [Google Scholar]
- Levens D., Ticho B., Ackerman E., Rabinowitz M. Transcriptional initiation and 5' termini of yeast mitochondrial RNA. J Biol Chem. 1981 May 25;256(10):5226–5232. [PubMed] [Google Scholar]
- Lewin A., Morimoto R., Rabinowitz M. Restriction enzyme analysis of mitochondrial DNAs of petite mutants of yeast: classification of petites, and deletion mapping of mitochondrial genes. Mol Gen Genet. 1978 Jul 25;163(3):257–275. doi: 10.1007/BF00271955. [DOI] [PubMed] [Google Scholar]
- Locker J. Analytical and preparative electrophoresis of RNA in agarose-urea. Anal Biochem. 1979 Oct 1;98(2):358–367. doi: 10.1016/0003-2697(79)90154-4. [DOI] [PubMed] [Google Scholar]
- Locker J., Rabinowitz M. Transcription in yeast mitochondria: analysis of the 21 S rRNA region and its transcripts. Plasmid. 1981 Nov;6(3):302–314. doi: 10.1016/0147-619x(81)90038-x. [DOI] [PubMed] [Google Scholar]
- Locker J., Synenki R. M., Merten S., Rabinowitz M. Eukaryotic features of mitochondiral transcription and gene structure in yeast. Ann N Y Acad Sci. 1981;361:105–118. [PubMed] [Google Scholar]
- Morimoto R., Rabinowitz M. Physical mapping of the yeast mitochondrial genome: derivation of the fine structure and gene map of strain D273-10B and comparison with a strain (MH41-7B) differing in genome size. Mol Gen Genet. 1979 Feb 16;170(1):25–48. [PubMed] [Google Scholar]
- Nobrega F. G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1980 Oct 25;255(20):9828–9837. [PubMed] [Google Scholar]
- Osinga K. A., De Haan M., Christianson T., Tabak H. F. A nonanucleotide sequence involved in promotion of ribosomal RNA synthesis and RNA priming of DNA replication in yeast mitochondria. Nucleic Acids Res. 1982 Dec 20;10(24):7993–8006. doi: 10.1093/nar/10.24.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinga K. A., Tabak H. F. Initiation of transcription of genes for mitochondrial ribosomal RNA in yeast: comparison of the nucleotide sequence around the 5'-ends of both genes reveals a homologous stretch of 17 nucleotides. Nucleic Acids Res. 1982 Jun 25;10(12):3617–3626. doi: 10.1093/nar/10.12.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Rüther U., Koenen M., Otto K., Müller-Hill B. pUR222, a vector for cloning and rapid chemical sequencing of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4087–4098. doi: 10.1093/nar/9.16.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer C., Haid A., Grosch G., Schweyen R. J., Kaudewitz F. Pathways of transcript splicing in yeast mitochondria. Mutations in intervening sequences of the split gene COB reveal a requirement for intervening sequence-encoded products. J Biol Chem. 1981 Jul 25;256(14):7610–7619. [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Thalenfeld B. E., Hill J., Tzagoloff A. Assembly of the mitochondrial membrane system. Characterization of the oxi2 transcript and localization of its promoter in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1983 Jan 10;258(1):610–615. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ommen G. J., Boer P. H., Groot G. S., De Haan M., Roosendaal E., Grivell L. A., Haid A., Schweyen R. J. Mutations affecting RNA splicing and the interaction of gene expression of the yeast mitochondrial loci cob and oxi-3. Cell. 1980 May;20(1):173–183. doi: 10.1016/0092-8674(80)90245-7. [DOI] [PubMed] [Google Scholar]
- Weiss-Brummer B., Rödel G., Schweyen R. J., Kaudewitz F. Expression of the split gene cob in yeast: evidence for a precursor of a "maturase" protein translated from intron 4 and preceding exons. Cell. 1982 Jun;29(2):527–536. doi: 10.1016/0092-8674(82)90169-6. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Marotta R., Faugeron-Fonty G., Goursot R., Mangin M., Baldacci G., Bernardi G. The origins of replication of the yeast mitochondrial genome and the phenomenon of suppressivity. Nature. 1981 Jul 2;292(5818):75–78. doi: 10.1038/292075a0. [DOI] [PubMed] [Google Scholar]