Syntaxin 16 is a key regulator of cytokinesis, as it is required for the recruitment of both recycling endosome–associated Exocyst and ESCRT machinery during late telophase. Therefore these two distinct facets of cytokinesis are inextricably linked.
Abstract
Recently it was shown that both recycling endosome and endosomal sorting complex required for transport (ESCRT) components are required for cytokinesis, in which they are believed to act in a sequential manner to bring about secondary ingression and abscission, respectively. However, it is not clear how either of these complexes is targeted to the midbody and whether their delivery is coordinated. The trafficking of membrane vesicles between different intracellular organelles involves the formation of soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) complexes. Although membrane traffic is known to play an important role in cytokinesis, the contribution and identity of intracellular SNAREs to cytokinesis remain unclear. Here we demonstrate that syntaxin 16 is a key regulator of cytokinesis, as it is required for recruitment of both recycling endosome–associated Exocyst and ESCRT machinery during late telophase, and therefore that these two distinct facets of cytokinesis are inextricably linked.
INTRODUCTION
Cytokinesis is the terminal stage of the cell cycle that separates the newly formed daughter cells (Glotzer, 2005; Barr and Gruneberg, 2007). After separation of the genetic material and the formation of two nuclei, the mother cell divides by the formation of a furrow, which constricts the cytoplasm, leaving the two daughter cells connected by a thin intercellular bridge. Resolution of this bridge (abscission) then separates the daughter cells. Each of the steps in this process requires the cell to integrate changes in the cytoskeleton with membrane dynamics to accommodate changes in cell shape, surface area, and the production of specialized domains within the intercellular bridge that culminate in abscission (Glotzer, 2005; Barr and Gruneberg, 2007; Neto and Gould, 2011). Membrane trafficking to the furrow is believed to coordinate the delivery of various lipids and proteins essential for cytokinesis (Barr and Gruneberg, 2007; Prekeris and Gould, 2008; Fielding et al., 2012) and is also required for abscission. The latter involves both the secretory and endosomal systems (Goss and Toomre, 2008; Fielding et al., 2012; Schiel and Prekeris, 2013), with considerable evidence supporting an essential role for Rab11/recycling endosomes (Prekeris and Gould, 2008). Present models suggest that a complex network of interactions between Rab11 and its effector protein FIP3 regulates the accumulation of Rab11-positive vesicles in the midbody (Fielding et al., 2005; Wilson et al., 2005; Simon et al., 2008). These accumulated vesicles are believed to play a key role in thinning the intercellular bridge, forming so-called secondary ingression zones (for reviews, see Neto and Gould, 2011; Schiel and Prekeris, 2011, 2013; Fededa and Gerlich, 2012).
The Exocyst complex has been proposed as one mechanism by which both secretory and endosomal vesicles may be tethered within the midbody during cytokinesis. The Exocyst tethering complex comprises eight subunits (Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84) and is believed to mediate the interaction of post-Golgi vesicles with the plasma membrane before their fusion (TerBush et al., 1996; Munson and Novick, 2006). The Exocyst complex is required for cytokinesis, and all subunits have been identified in the furrow and/or midbody of mammalian cells during cytokinesis, where they often appear as ring-like structures adjacent to the midbody (Gromley et al., 2005; Neto and Gould, 2011). The Exocyst is believed to act to tether vesicles in the midbody, where vesicles often accumulate before abscission (discussed in Neto and Gould, 2011). However, the mechanism by which Exocyst components reach the midbody is not clear.
The terminal events in abscission are mediated by the action of the endosomal sorting complex required for transport (ESCRT) proteins (Carlton and Martin-Serrano, 2007, 2008; Lee et al., 2008; Bastos and Barr, 2010). Late in telophase, the centrosomal protein Cep55 localizes to the midbody of the intercellular bridge (Lee et al., 2008; Bastos and Barr, 2010) and serves to recruit tumor susceptibility gene-101 (ESCRT-I protein, Tsg101) and apoptosis-linked gene 2–interacting protein X (ALIX) to the midbody (Carlton and Martin-Serrano, 2007; Lee et al., 2008). ALIX and Tsg101 are then proposed to recruit ESCRT-III proteins to the midzone (Carlton and Martin-Serrano, 2007, 2008). Subsequently ESCRT-III proteins appear at the secondary ingression zones, where they are proposed to mediate the final abscission step (Elia et al., 2011; Guizetti et al., 2011). Of interest, the accumulation of the ESCRT machinery at the secondary ingression zones has been shown to depend on the Rab11 recycling endosomal system (Schiel et al., 2012). However, how ALIX, Tsg101, and other ESCRT components accumulate in the midbody remains largely unresolved, and whether their midbody accumulation is coordinated with Rab11-dependent membrane traffic is unclear.
Membrane traffic in eukaryotic cells is controlled by the formation of specific soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) complexes. Members of the target family of SNARE proteins (t-SNAREs) mark specific organelles (Hong, 2005). The formation of complexes between t-SNAREs and their cognate vesicle SNAREs (v-SNAREs) in donor membranes is sufficient to catalyze bilayer fusion, and controlling SNARE complex formation enables the cell to regulate membrane traffic (Carr and Rizo, 2010). Syntaxin 2 has been identified as a crucial t-SNARE for cytokinesis and is believed to mediate the fusion of secretory cargo with the plasma membrane in the furrow (Low et al., 2003). Little is known, however, regarding the identity and function of intracellular SNARE proteins involved in the trafficking steps required for cytokinesis. Here we examine the role of endosomal t-SNAREs in cytokinesis. We find that perturbation of syntaxin 16 (Sx16) function (either by overexpression of a “dominant-negative” mutant or knockdown using small interfering RNA [siRNA]) impairs cytokinesis. We show that Sx16 is required for the accumulation of recycling endosomes in the midbody and also for the accumulation of Exocyst components at the midbody in late telophase. We also observe that Sx16 is required for midbody accumulation of Cep55 and ALIX in late telophase, suggesting that Sx16-dependent trafficking is required for correct placement of both the Exocyst and ESCRT machinery during late telophase/abscission and that these two distinct facets of cytokinesis are tightly integrated.
RESULTS
Sx6, Sx16, and mVps45 are required for cytokinesis
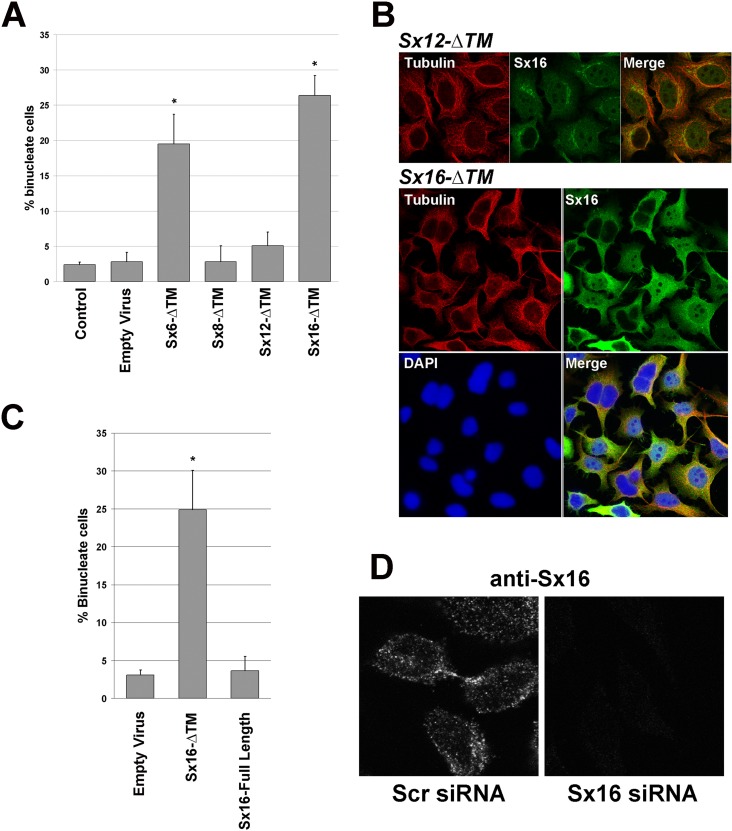
In an effort to determine which endosomal t-SNAREs may be involved in membrane trafficking in cytokinesis, we assayed the effect on cytokinesis of adenoviral-driven overexpression of t-SNAREs devoid of their transmembrane domain (hereafter referred to as -ΔTM mutants). Such mutants were previously shown to interfere with the endogenous t-SNAREs by acting as dominant interfering mutants (see, e.g., Scales et al., 2000; Perera et al., 2003; Choudhury et al., 2006; Proctor et al., 2006). HeLa cells were infected with recombinant adenovirus engineered to overexpress Sx6-ΔTM, Sx8-ΔTM, Sx12-ΔTM, or Sx16-ΔTM (see Supplemental Figure S1A) and the consequences for cytokinesis determined by quantifying the frequency of binuclear cells and comparing this with control (uninfected) cells or cells infected with empty adenovirus. Note that under the conditions used in all experiments reported here (multiplicity of infection of 30:1), >98% of cells express the mutant Sx-ΔTM in question (Supplemental Figure S1B). Figure 1, A and B, shows that overexpression of Sx6-ΔTM or Sx16-ΔTM, but not Sx8-ΔTM or Sx12-ΔTM, significantly increased the frequency of binucleate cells, suggestive of defective cytokinesis. The observed increase in binuclear cells was not observed upon overexpression of wild-type (full-length) Sx16 (Figure 1C). A potential role for Sx16 in cytokinesis is supported by our observation that endogenous Sx16 localized to the midbody in late telophase (Figure 1D); note that the specificity of antibody staining was verified by comparing signals in cells depleted of Sx16 by siRNA (Figure 1D). Finally, real-time image analysis revealed that overexpression of Sx16-ΔTM consistently increased the time taken for completion of abscission, such that 100% of cells examined (>25) took >2 h to proceed from the onset of furrowing to completion of abscission and ∼35% became binucleate; by contrast, >80% of control cells completed abscission in a time of <2 h, and none were binucleate (Figure 2). Knockdown of Sx6 or Sx16 using siRNA also resulted in a marked increase in the frequency of binuclear cells (Figure 3). Such data collectively argue that Sx6 and Sx16 play an important role in cytokinesis. Sx16 is a Qa-SNARE, known to interact with the Sec1/Munc18 family member mVps45 (Tellam et al., 1997; Simonsen et al., 1998; Dulubova et al., 2002; Struthers et al., 2009). Given that Sec1/Munc18 proteins are known to act as regulators of the SNARE machinery, we tested whether mVps45 is also required for cytokinesis. Knockdown of mVps45 by siRNA also resulted in a dramatic increase in the number of binucleate cells (Figure 2).
FIGURE 1:
Syntaxin 16 is required for cytokinesis. HeLa cells were incubated with adenovirus designed to express the indicated Sx-ΔTM construct at identical multiplicity of infection (in the experiment shown, 30:1), empty virus or not infected (control), as described in Materials and Methods. At 48 h later, cells were fixed and stained with anti-tubulin antibodies and DAPI and the frequency of binucleate cells counted. (A) Quantification of five independent experiments of this type. Sx6-ΔTM and Sx16-ΔTM significantly increased the frequency of binucleate cells (*p < 0.05), using at least three different batches of virus. (B) Typical fields of cells infected with Sx12-ΔTM or Sx16-ΔTM as indicated and stained for tubulin (red; tubulin), Sx16 (green; Sx16) or DNA (blue; DAPI). Note that for Sx16-ΔTM–infected cells, the high level of overexpression of Sx16-ΔTM required the use of reduced gain/laser power during collection of the image. (C) Comparison of the frequency of binucleate cells in cells infected with empty virus, Sx16-ΔTM, or Sx16-full length. (D) HeLa cells were transfected with an siRNA SmartPool designed to knock down Sx16 or a scrambled siRNA SmartPool control and then incubated for 48 h after transfection before fixation and staining with anti-Sx16. Data from a typical experiment. Left, typical cell in telophase, with endogenous Sx16 present near the midbody. See Figure 3 for Sx16 protein levels after knockdown. C and D are representative of three or more experiments of this type.
FIGURE 2:
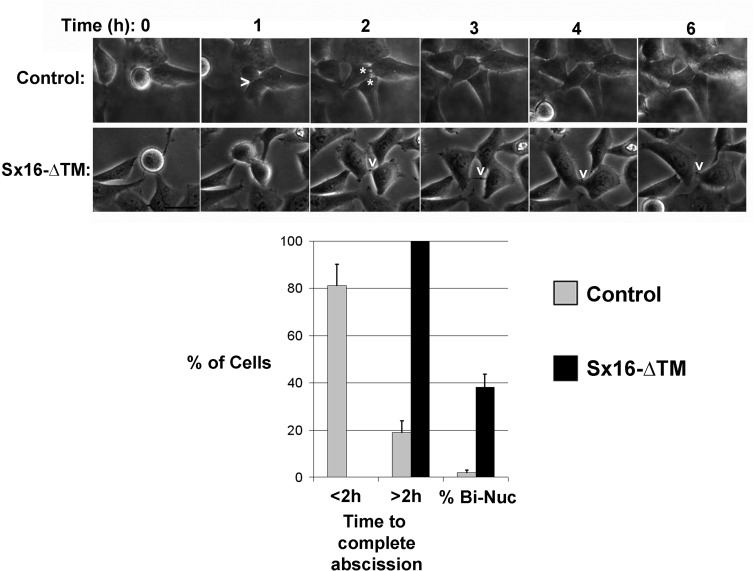
Sx16 is involved in abscission. Images from a typical time course of HeLa cell division, revealing delayed abscission in Sx16-ΔTM–expressing cells (bottom) compared with control cells (top). In control cells, a furrow is indicated by >, and cytokinesis is complete ∼1 h thereafter (two daughter cells indicated by asterisks in the next image). In Sx16-ΔTM–expressing cells, a long-lived midbody is indicated by the v; in the experiment shown, the cells remained connected for >4 h from the first clear appearance of the midbody (shown in the 2-h image). Data from >25 cells from three or more experiments in each group were binned according to the time taken to complete abscission after the start of furrowing into two groups, those taking <2 h, or those taking >2 h. The frequency of binucleate cells in this subgroup is also presented. Cells expressing Sx16-ΔTM consistently took longer to complete abscission, or failed abscission at a significantly greater rate, than their control counterparts.
FIGURE 3:
Sx16 and mVps45 knockdown perturbs cytokinesis. HeLa cells were transfected with siRNA SmartPools against either scrambled siRNA (control), GAPDH, Sx6, Sx16, or mVps45 and were incubated for 48 h after transfection before fixation and staining for tubulin/DAPI to allow quantification of the frequency of binucleate cells. (A) Quantification of three experiments of this type. Asterisk indicates significant increase compared with control (p < 0.05). (B) Typical immunoblot analysis to confirm the extent and specificity of knockdown. Lysates were prepared in parallel to the analysis shown in A and immunoblotted with the antibodies shown. The approximate positions of molecular weight markers are shown.
Sx16 is required for Rab11 accumulation in the intercellular bridge
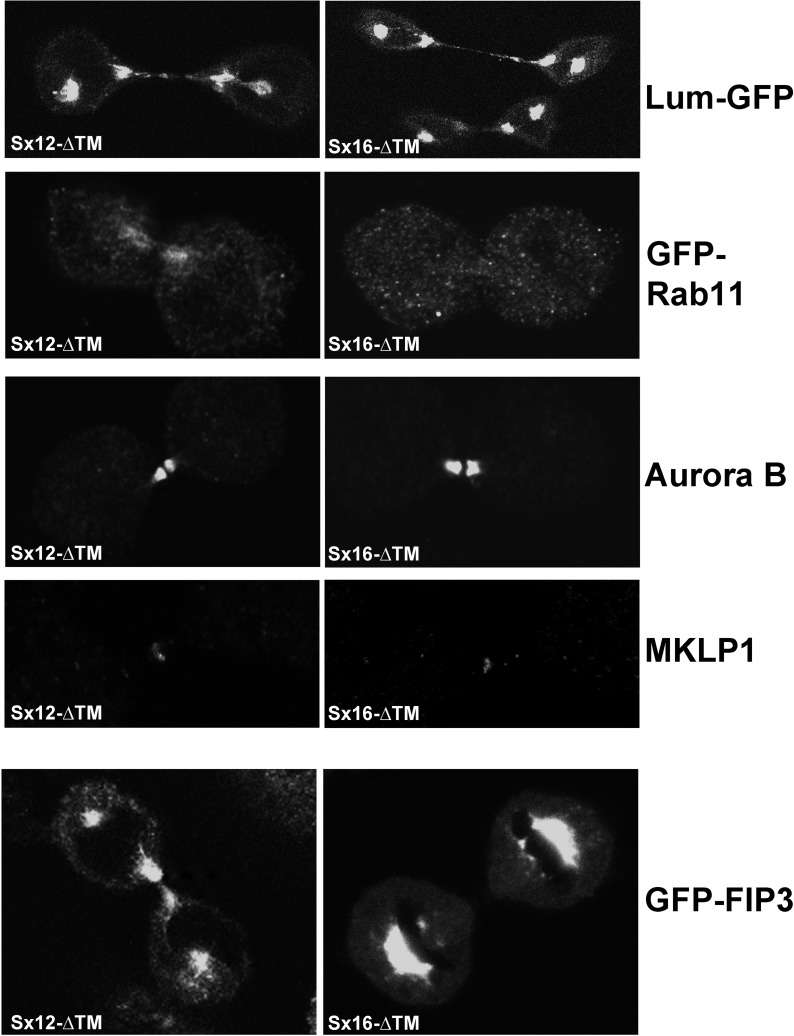
We next sought to ascertain which aspects of trafficking were perturbed in Sx16-defective cells. Recycling endosomal (Rab11-positive) vesicles and secretory vesicles traffic into the midbody during telophase. Both of these processes reflect traffic exiting the trans-Golgi network (TGN), where Sx16 has been proposed to control recycling between the TGN and endosomes (Simonsen et al., 1998; Dulubova et al., 2002). We therefore compared the distribution of Rab11-positive vesicles and vesicles containing lumenal–green fluorescent protein (GFP; Blum et al., 2000) as a marker for secretory cargo in cells infected with either Sx12-ΔTM or Sx16-ΔTM. We examined the distribution of GFP-Rab11 in cells in telophase and observed the characteristic accumulation of GFP-Rab11–positive vesicles in the furrow/midbody of 49 of 50 cells from three separate experiments using cells infected with Sx12-ΔTM (similar results were observed in cells infected with empty virus or Sx8-ΔTM; unpublished data). By comparison, GFP-Rab11 accumulation in the furrow/midbody of cells expressing Sx16-ΔTM was observed in only 3 of 52 cells (Figure 4). Similarly, GFP-FIP3 localization to the intercellular bridge was found to be significantly impaired in cells expressing Sx16-ΔTM (Figure 4). To identify secretory cargo, we expressed GFP fused to an amino-terminal signal peptide that targets the protein into the lumen of the endoplasmic reticulum (lum-GFP; Blum et al., 2000). This protein does not contain retention or retrieval motifs and thus does not traffic to endosomes, lysosomes, or multivesicular bodies. In marked contrast to the data for Rab11, expression of Sx16-ΔTM had little qualitative effect on the accumulation of lum-GFP–positive vesicles in the midbody at either early or late telophase (Figure 4). MKLP-1 and Aurora B staining of fixed cells likewise revealed no discernible difference in staining patterns of the intercellular bridge or midbody between these two populations of cells, suggesting that both the gross morphology of the midbody ring and the localization of key signaling molecules were not significantly impaired by Sx16-ΔTM (Figure 4).
FIGURE 4:
GFP-Rab11 traffic to the midbody/intercellular bridge is decreased in cells expressing Sx16-ΔTM. HeLa cells were transfected with plasmids driving overexpression of GFP-Rab11, lum-GFP, or GFP-FIP3 as indicted. At 6 h after transfection, cells were infected with adenovirus to overexpress Sx12-ΔTM or Sx16-ΔTM, and images were collected 48 h after infection. Representative images of cells in telophase, typical of three independent repeats. Parallel coverslips were fixed and stained using anti–Aurora B or anti-MKLP1, and representative images are shown.
Sx16 is required for correct Exocyst localization in telophase
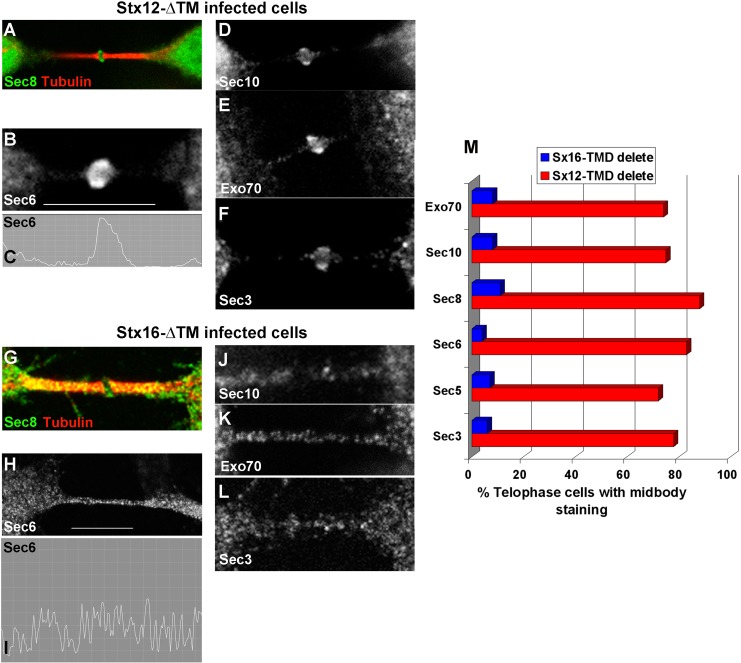
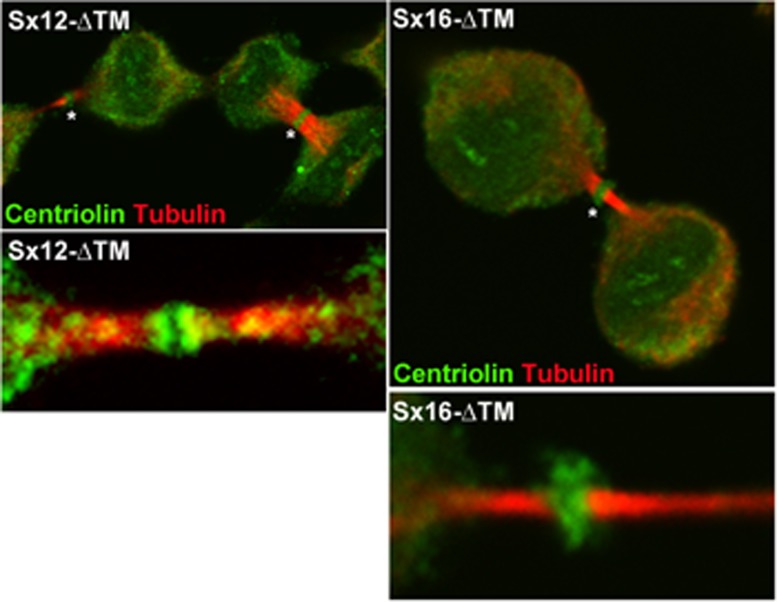
Rab11 interacts with the Exocyst complex, and depletion of the Exocyst both inhibits cytokinesis and prevents accumulation of vesicles in the midbody (Zhang et al., 2004; Fielding et al., 2005). Given the lack of localization of Rab11 to the midbody, we tested the hypothesis that Sx16-ΔTM may impede the delivery of Exocyst components to the midbody. We observed profound alterations in Exocyst distribution in cells expressing Sx16-ΔTM compared with controls. As previously reported (Fielding et al., 2005; Gromley et al., 2005; Martin-Cuadrado et al., 2005), we also observed that Sec3, Sec6, Sec8, Sec15, and Exo70 localize to a ring-like structure in the midbody in late telophase in control cells (unpublished data) and cells infected with Sx12-ΔTM (Figure 5). However, in cells expressing Sx16-ΔTM this characteristic ring structure was consistently absent. Instead these proteins appeared to populate multiple diffuse punctae throughout the intercellular bridge (Figure 5). Similar results were obtained upon Sx16-knockdown using siRNA (unpublished data). Arf6 accumulation in the intercellular bridge may also be involved in the delivery of recycling endosomes and interaction with the Exocyst (Fielding et al., 2005). We therefore examined the distribution of Arf6 in telophase in cells depleted of Sx16 or cells expressing Sx16-ΔTM (Supplemental Figure S2). Arf6 localization to the midbody was not affected by either manipulation. These data suggest that Sx16 is required for both the trafficking of Rab11 into the furrow and the delivery of Exocyst components into the midbody of dividing cells. Previous studies established that centriolin is essential for the anchoring of Exocyst components in the midbody of dividing cells (Gromley et al., 2005). Centriolin appears as a ring-like structure at the midbody in late telophase, and this structure was observed in cells overexpressing either Sx12-ΔTM or Sx16-ΔTM, suggesting that the mechanism by which centriolin accumulates at the midzone is not impaired by Sx16-ΔTM expression (Figure 6).
FIGURE 5:
Sx16-ΔTM prevents the midbody accumulation of the Exocyst. HeLa cells were infected with Sx12-ΔTM (A–F) virus as described and immunostained with antibodies against different Exocyst components, as labeled. Sec8 (A), Sec6 (B), Sec10 (D), Exo70 (E), or Sec3 (F) localized to a ring-like structure in the midbody of the majority of Sx12-ΔTM cells in telophase (∼80%; see bar graph in M). By contrast, in cells expressing Sx16-ΔTM, Sec8 (G), Sec6 (H), Sec10 (J), Exo70 (K), or Sec3 (L) exhibited no such ring-like structure, but each Exocyst component was present within punctate structures throughout the midbody. The line graphs (C and I) indicate the intensity of staining for Sec6 in the region demarcated by the white bar in the two cases. (M) Quantification of data from three experiments of this type, encompassing >100 cells for each Exocyst component. Microtubule staining was omitted except for A and G in order to clearly show the distribution of Exocyst staining. Antibodies used in this figure were as described in Materials and Methods.
FIGURE 6:
Sx16-ΔTM does not prevent the midbody accumulation of centriolin. HeLa cells were infected with Sx16-ΔTM or Sx12-ΔTM virus as described and immunostained for centriolin (green) or tubulin (red). Representative images from five independent experiments of this type. Asterisk indicates the midbody ring-like structure. Bottom, typical higher-magnification images of the midbody areas.
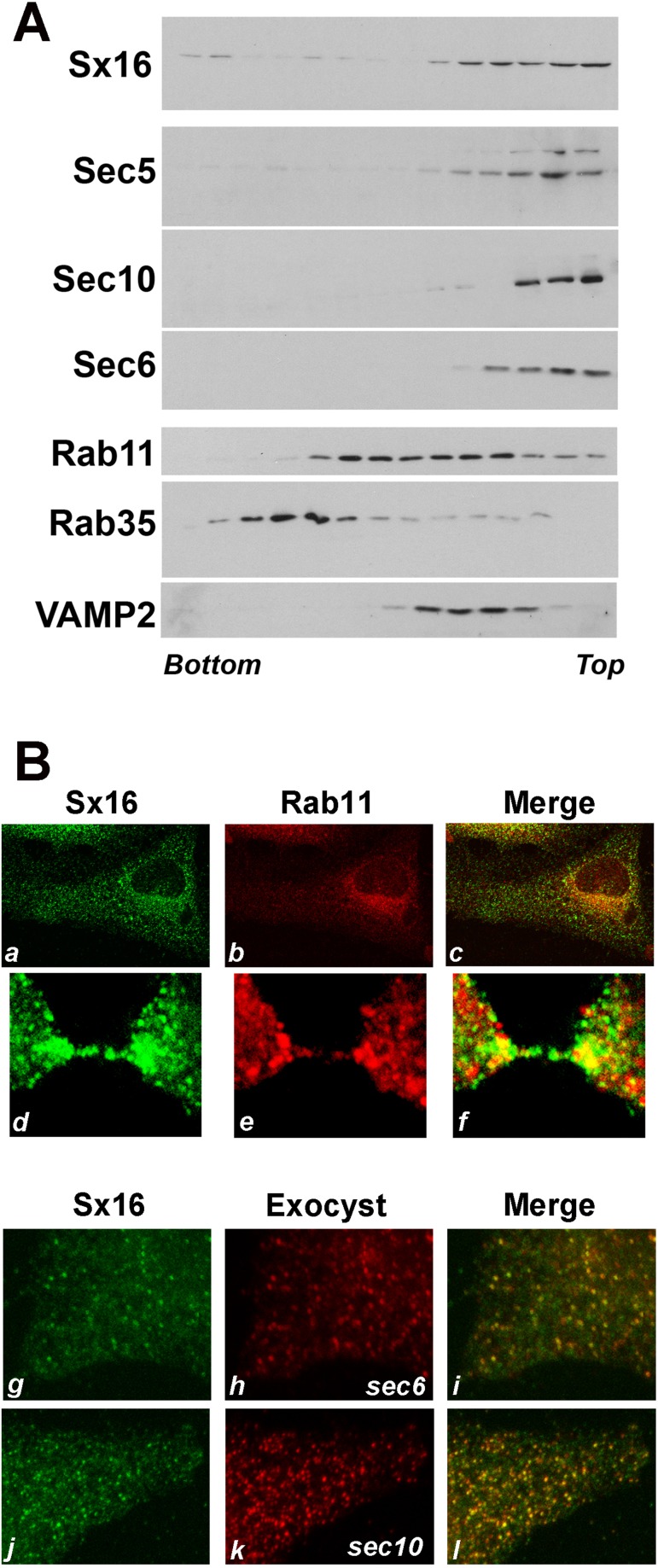
These observations prompt the question of whether Sx16-ΔTM has a direct or indirect effect on the trafficking of Exocyst proteins. To address this, we first isolated membranes from cells and analyzed the distribution of these proteins after iodixanol gradient centrifugation. This technique revealed extensive overlap between Sx16-positive and Exocyst-positive membranes. More limited overlap was observed between Sx16/Exocyst- and Rab11-containing fractions (Figure 7A). Similar results were reported by Chen et al. (2006). In support of these observations, immunofluorescence analysis revealed some overlap between Rab11-positive and Sx16-positive structures, particularly in the perinuclear region, but only limited colocalization in the periphery (Figure 7B, a–c). This is consistent with other studies, which reported up to 80% colocalization between these proteins (albeit in a different cell type; Manickam et al., 2011; Jung et al., 2013). Analysis of cells in late telophase also revealed colocalization of these proteins in the furrow adjacent to the intercellular bridge and partial overlap between Rab11- and Sx16-positive structures in the intercellular bridge (Figure 7B, d–f). Similar analysis of Sx16 and Exocyst proteins revealed extensive overlap in the perinuclear region (unpublished data), which, unlike Rab11, was also clearly evident in more peripheral structures (Figure 7B, g–l).
FIGURE 7:
Sx16 and Exocyst components localize to vesicles of similar density. (A) HeLa cells in telophase were harvested, and membranes in a postnuclear supernatant were fractionated on the basis of density using iodixanol gradient analysis. Representative immunoblots of the indicated proteins. Denser membranes are at the bottom of the tube. The experiment was repeated twice more, with qualitatively similar results. Note that both Rab11-positive and Rab35-positive membranes were differentially localized to those containing Sx16. (B) HeLa cells were infected with Sx16-ΔTM or Sx12-ΔTM virus as described and immunostained with anti-Sx16 (a, d, g, and j; green) or anti-Rab11 (b, e; red) or anti-sec6 or anti-sec10 (h, k respectively; red). Merged images are shown in c, f, i, and j. Data from a typical experiment.
Sx16 modulates Cep55 and ALIX localization
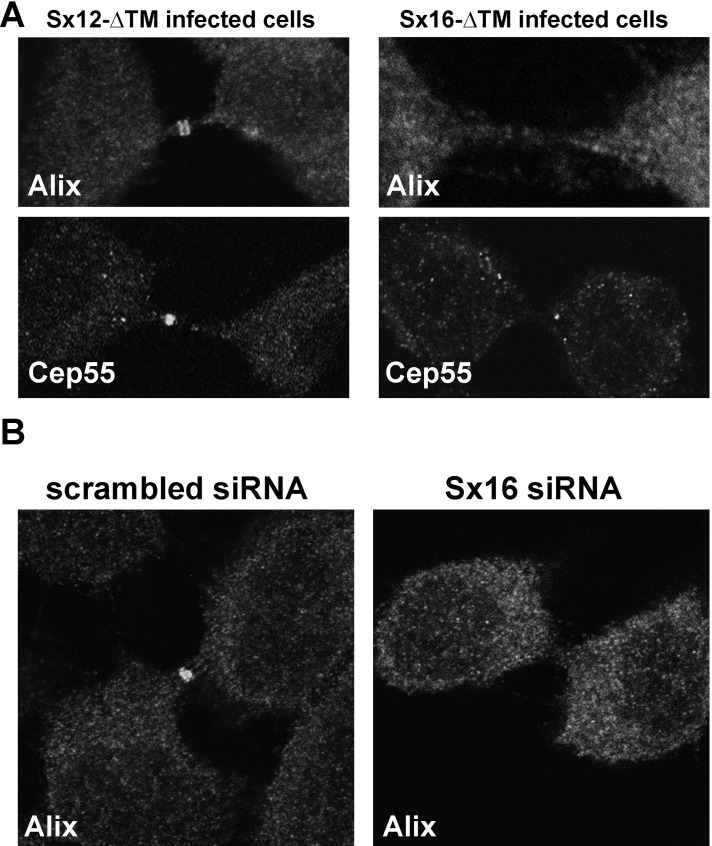
We next tested the hypothesis that the Sx16-dependent delivery of Exocyst components (Figure 5) and recycling endosomes (Figure 4) to the midbody was a prerequisite for accumulation of ESCRT components at this site. Present models suggest that ESCRT recruitment to the midbody initially involves anchoring of ALIX and/or Tsg101 to the midbody-resident protein Cep55 (Lee et al., 2008). Cep55 localization to the midbody was altered in 55% of cells expressing Sx16-ΔTM compared with controls (Figure 8A). These alterations were manifest as either absent or reduced levels of accumulation compared with control (Sx12-ΔTM–expressing cells). Similar changes were observed when RFP-Cep55 was studied (unpublished data). This was paralleled by marked diminution of ALIX accumulation in the midbody, a phenotype that was more prevalent and was observed in 92% of cells examined (Figure 8A). Reduced ALIX staining in the midbody was also evident after knockdown of Sx16 using siRNA (Figure 8B). We were unable to immunostain endogenous Tsg101 using reagents available to us. These data suggest that Sx16-dependent trafficking is required for the delivery of Cep55 and ALIX to the midbody. Of interest, we found that ALIX, Tsg101, and Cep55 all localize to the same fractions of an iodixanol gradient as Sx16 and largely distinct from those marked by TfR and Rab11 (Supplemental Figure S3). Attempts to localize ESCRT proteins using immunofluorescence are complicated, as most of the ESCRTs exhibit cytosolic diffuse staining, which masks the staining on, for example, endosomes (Bissig et al., 2013). Given that we could detect ALIX and Cep55 in the midbody, we examined the distribution of these proteins in the intercellular bridge but could not observe any significant overlap between Sx16 and these proteins (Supplemental Figure S3).
FIGURE 8:
Sx16-ΔTM or Sx16 knockdown prevents the midbody accumulation of ALIX and causes reduced levels of Cep55. HeLa cells were infected with Sx16-ΔTM or Sx12-ΔTM virus as described. Cells were immunostained with antibodies against ALIX or Cep55. (A) Typical immunofluorescence staining of ALIX at the midbody of control (Sx12-ΔTM–infected) cells, revealed as a characteristic double ring–like structure. Cep55 also localized to the midbody of these cells. By contrast, ALIX did not localize to the midbody of Sx16-ΔTMxinfected cells, and Cep55 localization was impaired in more than half of the cells analyzed (see the text for explanation). The data shown are representative of three experiments of this type. (B) HeLa cells were transfected with an siRNA SmartPool designed to knock down Sx16 or a scrambled siRNA SmartPool control, then incubated for 48 h after transfection before fixation and staining with anti-ALIX as indicated. Data from a representative experiment, repeated three times with similar results. In all experiments, the extent of Sx16 knock down was >85% as determined by immunoblotting (unpublished data).
ALIX knockdown does not prevent Exocyst accumulation in the midbody
We next sought to determine whether the accumulation of Exocyst components in the midbody ring required the assembly of ALIX at the midbody. To address this, we depleted HeLa cells of ALIX using siRNA and examined the distribution of Exocyst components by immunofluorescence microscopy (Supplemental Figure S4). As shown, ALIX knockdown did not impair the accumulation of Exocyst components as rings adjacent to the midbody but did increase the frequency of binucleate cells.
DISCUSSION
Recent years have seen rapid growth in our understanding of the molecular events in cytokinesis, with good evidence for secretory and endosomal traffic playing a central role in abscission (Gromley et al., 2005; Wilson et al., 2005; Goss and Toomre, 2008). A model has been proposed in which the accumulation of recycling endosomes in the intercellular bridge leads to the formation of so-called secondary ingression sites, which act as a foci for the action of the ESCRT complex to drive abscission (Elia et al., 2011; Guizetti et al., 2011; Neto and Gould, 2011; Schiel and Prekeris, 2011). Consistent with this, Rab11 and its effector FIP3 are required for abscission, as is the ESCRT machinery, the formation of secondary ingression sites is Rab11-dependent (reviewed in Neto and Gould, 2011; Schiel and Prekeris, 2011; Fededa and Gerlich, 2012), and Rab11 is required for localization of ESCRT-III components to these secondary ingression sites, where they are believed to mediate abscission (Schiel et al., 2012). The accumulation of Rab11-positive vesicles in the intercellular bridge involves interaction with the Exocyst protein-targeting complex. Such data suggest that these two distinct facets of cytokinesis—Rab11-vesicle accumulation and subsequent ESCRT localization—may be coordinated. In this study we identified a role for the endosomal t-SNARE Sx16 in cytokinesis and used perturbation of this t-SNARE as a means to address several unresolved questions: specifically, the role of membrane trafficking in the delivery of Exocyst components to the midbody and whether the accumulation of Rab11-vesicles is a prerequisite for the early stages of ESCRT accumulation in the midbody, in particular the arrival of Cep55 or ALIX.
t-SNARE proteins have been implicated in cytokinesis. These include the Golgi-localized Sx5 (Xu et al., 2002), a syntaxin shown to be required for the maintenance of normal Golgi function during mitosis, and the plasma membrane-localized t-SNARE Sx2, which was shown to modulate abscission (Low et al., 2003). Perturbation of Sx2 function specifically prevented abscission, with little effect on furrowing, consistent with a role for the docking and fusion of vesicles with the plasma membrane in late telophase (Low et al., 2003). This observation is important, as it suggests that the specific trafficking of vesicles into the intercellular bridge is a prerequisite of this fusion event, prompting us to examine the role of intracellular syntaxin in cytokinesis. We identified Sx16 and its cognate SNARE partner Sx6 as important SNAREs in cytokinesis. Overexpression of Sx16-ΔTM (or Sx16 depletion by siRNA) consistently increased the frequency of binuclear cells and prolonged the abscission stage of cytokinesis (Figures 1–3). Consistent with an important role for Sx16 in cytokinesis, levels of Sx16 transcript are elevated in a wide array of human cancers (Supplemental Figure S5), notably colon/colorectal cancers, for which Sx16 overexpression was in the top 3% of four different cohorts in the Oncomine database. Similar elevations in Sx6 in human cancers have been noted by other groups (Riggs et al., 2012). Overexpression of Sx8-ΔTM or Sx12-ΔTM was without effect on cytokinesis, suggesting that the endosomal trafficking pathways regulated by these t-SNAREs do not play significant roles in cytokinesis. Expression of Sx16-ΔTM prevented accumulation of Rab11-GFP and the Rab11 effector FIP3 in the intercellular bridge or midbody of dividing cells but was without effect on the localization of lum-GFP, suggesting that the predominant effect of expression of Sx16-ΔTM was on endosomal traffic, not secretory traffic, into the intercellular bridge (Figure 4). Given that Rab11 and Rab11-FIP3 accumulation in the midbody was previously shown to be mediated, at least in part, via interactions with the Exocyst complex (Fielding et al., 2005), we examined the localization of this complex in cells expressing Sx16-ΔTM. Exocyst localization to the characteristic midbody ring-like structure (Gromley et al., 2005) in late telophase was absent in cells expressing Sx16-ΔTM (Gromley et al., 2005). Instead, the Exocyst components were present in multiple punctae throughout the midbody (Figure 5). These data suggest that the correct localization of Exocyst components into the midbody ring requires functional Sx16. One possible explanation of this is provided by our observation that Sx16 and the Exocyst components reside on populations of vesicles with similar density (Figure 7A). This analysis is supported by immunofluorescence studies, which reveal that Sx16 and Exocyst components exhibit considerable overlap, particularly in the periphery (Figure 7B) and in the perinuclear region (unpublished data). These data are consistent with the notion that Sx16 exerts a direct effect on the delivery of Exocyst components to the midbody. Studies from the Doxsey lab show that localization of the Exocyst to the midbody requires the centrosomal protein centriolin (Gromley et al., 2005). Overexpression of Sx16-ΔTM had no discernible effect on centriolin distribution, suggesting that the mechanism of centriolin localization is independent of Sx16. Consistent with these observations, it has been reported that inhibition of MNK1 kinase abrogates both accumulation of luminal GFP in the midbody and localization of centriolin to the stem body, suggesting that distinct pathways regulate these processes (Rannou et al., 2012). Arf6, another protein proposed to mediate interaction of Rab11-FIP3 vesicles in the midbody, was also unaffected by Sx16-ΔTM expression (Supplemental Figure S2). These data suggest that distinct trafficking pathway(s) may be involved in the delivery of these proteins to the midbody. Such observations are consistent with an important role for multiple different endosomal trafficking routes into the intercellular bridge. In addition to Rab11/FIP3 endosomes, others have shown important roles for Rab35-containing endosomes, which also deliver oculocerebrorenal syndrome of Lowe protein and septin 2 (Dambournet et al., 2011), an Arf6/Rab35-dependent trafficking event in abscission (Chesneau et al., 2012), and also an important role for endosomes enriched in phosphatidylinositol-3-phosphate, which accumulate at the abscission site (Schiel et al., 2013).
The inability of cells expressing Sx16-ΔTM to accumulate Rab11 or Exocyst components in the midbody prompted us to examine the localization of other midbody proteins involved in abscission, but at temporally earlier stages. Cep55 localization to the midbody is required for abscission, where it recruits the ESCRT-I protein Tsg101 and the ESCRT-associated protein ALIX (Carlton and Martin-Serrano, 2007; Lee et al., 2008). Cep55, ALIX, and Tsg101 accumulate in the midbody before the arrival of Rab11, suggesting that delivery of these proteins is an earlier event in cytokinesis (Schiel et al., 2012). Strikingly, we find that overexpression of Sx16-ΔTM or depletion of Sx16 using siRNA consistently prevented the accumulation of endogenous ALIX, suggesting that Sx16-dependent trafficking pathways are required for this process (Figure 8). The effect on Cep55 localization was more variable, however, with reduced or absent Cep55 localization to the midbody observed in ∼50% of cells. Of interest, others have shown that in BRCA−/− cells, correct Cep55 localization to the midbody is insufficient for ALIX or Tsg101 accumulation, suggesting that multiple pathways of membrane trafficking may affect the delivery of these key abscission proteins (Mondal et al., 2012). Nevertheless, our data clearly reveal that Sx16 is required for the correct placement of ALIX in the midbody. How Sx16 might function in this regard is not clear. We identified some overlap between Sx16 and ALIX/Cep55 on density gradient analysis, but this is not manifest by overlap of these proteins in the intercellular bridge (Supplemental Figure S3). It is important to note, however, that the overlap between Sx16 and ALIX or other ESCRTs is difficult to assess by conventional immunofluorescence, as most of the ESCRTs exhibit cytosolic diffuse staining (Bissig et al., 2013). Hence whether the effect of perturbation of Sx16 on ESCRT delivery is direct or indirect cannot presently be determined.
The data presented here extend our understanding of the membrane trafficking events that underpin abscission. We show that Sx16 is required for abscission, that this t-SNARE controls the delivery of the Exocyst to the midbody ring of mammalian cells in telophase, and that in the absence of the Exocyst, recycling endosomes do not accumulate in the intercellular bridge. Colocalization of Sx16 and ESCRT proteins offers the tentative hypothesis that Sx16 is involved directly in trafficking ESCRT components to the intercellular bridge. We extend this model to show that Sx16 is required for accumulation of ALIX in the midbody, suggesting that the important role of recycling endosome accumulation in telophase is coupled to the delivery of Cep55 and ALIX, thereby revealing that recycling endosome traffic and ESCRT accumulation are linked stages in abscission.
MATERIALS AND METHODS
Antibodies, DNA, viruses, and reagents
Mouse anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and rabbit anti-GFP antibodies were purchased from Abcam (Cambridge, United Kingdom). Mouse anti–syntaxin 6 antibody was obtained from BD Biosciences (San Jose, CA). Rat anti-tubulin (clone YL1/2) was from Millipore (Watford, United Kingdom). Alexa Fluor 568 goat anti-rat immunoglobulin G was purchased from Invitrogen (Paisley, United Kingdom). Anti-FIP3, Rab11, and other antibodies were as described in Fielding et al. (2005). Antibodies against the Exocyst components were as follows: anti-Sec5 and anti-Exo84 were from Lifespan Biosciences (Seattle, WA), anti-Sec3 (11690-1-AP) and anti-Rab35 were from Proteintech (Taipei, Taiwan), anti-Sec10 was from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-Rab11 and anti–transferrin receptor were from Zymed (San Francisco, CA); anti-Sec3, Sec5, Sec8, Exo70, and Exo84 were provided by S. A. Baldwin (University of Leeds, Leeds, United Kingdom; Fielding et al., 2005). Sec6 (56979) and Sec8 (13254) monclonal antibodies were from Abcam. Abcam anti-EXO70 was used in some experiments (product 57402). AntiSec10 was from Abnova (product number H00056915-M0). Anti-ALIX was from Cell Signaling (Beverly, MA), and anti-Tsg101, anti-MKLP1, and Anti-Cep55 were from Abcam. Polyclonal anti–syntaxin 6, 8, and 12/13 were as described in Perera et al. (2003). Anti-centriolin was generated in rabbits by Eurogentec (Seraing, Belgium) using a double-peptide procedure with the following peptides: residues 2–16 and 2311–2325 of human centriolin (KKGSQQKIFSKAKIP-Cys and Cys-DSQLGQNQEKNASAR, respectively) coupled to a carrier via the indicated Cys. Lipofectamine 2000 and 4,6-diamidino-2-phenyindole dilactate (DAPI) were from Invitrogen. Red fluorescent protein–Cep55 was generously provided by J. Martin-Serrano (Kings College, London, United Kingdom) and lum-GFP by R. Blum (Universitätsklinikum Würzburg, Germany).
Adenovirus engineered to express wild-type Sx16, Sx16-ΔTM, Sx12-ΔTM, Sx8-ΔTM, and Sx6-ΔTM was previously described (Perera et al., 2003; Proctor et al., 2006). Large-scale preparations of virus were made by ViraQuest (North Liberty, IA). siRNA SmartPools targeting human Sx16 or human vps45 were purchased from Thermo Fisher Scientific (Loughborough, United Kingdom.
Cell culture, transfection, and imaging
HeLa cells and HeLa cells stably expressing GFP-FIP3 were cultured as described in Wilson et al. (2005). Cells were transfected with plasmid DNA or siRNAs using Lipofectamine 2000 following the manufacturer's instructions. Cells were processed for transgene expression/knockdown analysis 24–72 h posttransfection. For adenovirus infection, cells were incubated in serum-free medium for 1 h, and then virus was added for 6 h. Thereafter, serum was added to 10% (vol/vol) and the incubation continued overnight. The medium was replaced the following day and the cells used 24–48 h later, depending on the experiment.
For time-lapse microscopy, cells were grown on collagen-coated coverslips and viewed 24 h later using a 20× lens in an environmental chamber at 5% CO2, 37°C. Images were captured using an AxioCam Mrm camera (Carl Zeiss, Jena, Germany).
Immunofluorescence
Cells were grown on 12-well plates containing ethanol-sterilized 13-mm-diameter glass coverslips for 24 h before processing. Cells were fixed with 4% (wt/vol) p-formaldehyde and permeabilized with 0.1% (vol/vol) Triton-X100 (in phosphate-buffered saline [PBS]) for 4 min. Cells were blocked in immunofluorescence (IF) buffer (PBS containing 0.2% [wt/vol] fishskin gelatin and 0.1% [vol/vol] goat serum) and then incubated with primary antibody for 1 h at room temperature. After washing, cells were further incubated with secondary antibodies diluted in IF buffer for 1 h in the dark. For nuclear staining, DAPI was added to wash buffers. Coverslips were mounted using Shandon Immuno-Mount (Thermo Fisher Scientific) and analyzed using a 63× Zeiss oil immersion objective on a Zeiss confocal microscope equipped with a Zeiss LSM5 Pascal instrument.
Membrane fractionation analysis
HeLa cells were washed in ice-cold HES (25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 250 mM sucrose, 1 mM EDTA, pH 7.4) and scraped into the same buffer containing protease inhibitor tablets. The cell scrapings were then passed 12 times through a ball-bearing homogenizer using a clearance of 14 μm (Isobiotec, Heidelberg, Germany) and centrifuged at 2000 × g to produce a postnuclear fraction. The latter was mixed with an equal volume of 60% iodixanol and loaded into a centrifuge tube. Equal volumes of 29 and 10% iodixanol in HES were overlaid, and the tubes were centrifuged for 3 h at 60,000 × g. Fractions were collected and resuspended directly into Laemmli buffer (or first precipitated using trichloroacetic acid) before SDS–PAGE and immunoblot analysis as described in Fielding et al. (2005).
Supplementary Material
Acknowledgments
This work was supported by grants from Cancer Research UK (C25017/A9006 and A13082) and a grant from the Association for International Cancer Research to G.W.G. We thank J. Martin-Serrano and R. Blum for plasmids, S. Baldwin for antibodies, and Musab Bhutta, Nia Bryant, Andy Fielding, and Rytis Prekeris for discussion of results and/or helpful comments on the manuscript. This work is dedicated to Helia Neto, who was badly injured during this study and has been unable to return to laboratory work.
Abbreviations used:
- ALIX
apoptosis-linked gene-2 interacting protein X
- DAPI
4’,6-diamidino-2-phenylindole
- ESCRT
endosomal sorting complex required for transport
- GFP
green fluorescent protein
- SNARE
soluble N-ethylmaleimide–sensitive factor attachment protein receptor
- Sx
syntaxin
- TGN
trans-Golgi network
- Tsg101
tumor susceptibility gene 101
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E13-06-0302) on October 9, 2013.
H.N., A.K., and L.C.C. performed and designed experiments; A.K. and L.C.C. assisted with manuscript editing. G.W.G. conceived the study, secured funding, designed and performed experiments, and wrote the manuscript.
All authors declare no competing financial interests.
REFERENCES
- Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131:847–860. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Bastos RN, Barr FA. Plk1 negatively regulates Cep55 recruitment to the midbody to ensure orderly abscission. J Cell Biol. 2010;191:751–760. doi: 10.1083/jcb.201008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissig C, Lenoir M, Velluz MC, Kufareva I, Abagyan R, Overduin M, Gruenberg J. Viral infection controlled by a calcium-dependent lipid-binding module in ALIX. Dev Cell. 2013;25:364–373. doi: 10.1016/j.devcel.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum R, Stephens DJ, Schulz I. Lumenal targeted GFP, used as a marker of soluble cargo, visualises rapid ERGIC to Golgi traffic by a tubulo-vesicular network. J Cell Sci. 2000;113(Pt 18):3151–3159. doi: 10.1242/jcs.113.18.3151. [DOI] [PubMed] [Google Scholar]
- Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316:1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- Carlton J, Martin-Serrano J. Differential requirements for Alix and ESCRT-III in cytokinesis and HIV release. Proc Natl Acad Sci USA. 2008;105:10541–10546. doi: 10.1073/pnas.0802008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CM, Rizo J. At the junction of SNARE and SM protein function. Curr Opin Cell Biol. 2010;22:488–495. doi: 10.1016/j.ceb.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.-W., Inoue M, Hsu SC, Saltiel AR. RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis. J Biol Chem. 2006;281:38609–38616. doi: 10.1074/jbc.M512847200. [DOI] [PubMed] [Google Scholar]
- Chesneau L, Dambournet D, Machicoane M, Kouranti I, Fukuda M, Goud B, Echard A. An ARF6/Rab35 GTPase cascade for endocytic recycling and successful cytokinesis. Curr Biol. 2012;22:147–153. doi: 10.1016/j.cub.2011.11.058. [DOI] [PubMed] [Google Scholar]
- Choudhury A, Marks DL, Proctor KM, Gould GW, Pagano RE. Regulation of caveolar endocytosis by syntaxin 6-dependent delivery of membrane components to the cell surface. Nat Cell Biol. 2006;8:317–328. doi: 10.1038/ncb1380. [DOI] [PubMed] [Google Scholar]
- Dambournet D, Machicoane M, Chesneau L, Sachse M, Rocancourt M, El Marjou A, Formstecher E, Salomon R, Goud B, Echard A. Rab35 GTPase and OCRL phosphatase remodel lipids and F-actin for successful cytokinesis. Nat Cell Biol. 2011;13:981–988. doi: 10.1038/ncb2279. [DOI] [PubMed] [Google Scholar]
- Dulubova I, Yamaguchi T, Gao Y, Min S-W, Huryeva I, Sudhof TC, Rizo J. How Tlg2p/syntaxin 16 “snares” Vps45. EMBO J. 2002;21:3620–3631. doi: 10.1093/emboj/cdf381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia N, Sougrat R, Spurlin TA, Hurley JH, Lippincott-Schwartz J. Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proc Natl Acad Sci USA. 2011;108:4846–4851. doi: 10.1073/pnas.1102714108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fededa JP, Gerlich DW. Molecular control of animal cell cytokinesis. Nat Cell Biol. 2012;14:440–447. doi: 10.1038/ncb2482. [DOI] [PubMed] [Google Scholar]
- Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R, Gould GW. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 2005;24:3389–3399. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding AB, Willox AK, Okeke E, Royle SJ. Clathrin-mediated endocytosis is inhibited during mitosis. Proc Natl Acad Sci USA. 2012;109:6572–6577. doi: 10.1073/pnas.1117401109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- Goss JW, Toomre DK. Both daughter cells traffic and exocytose membrane at the cleavage furrow during mammalian cytokinesis. J Cell Biol. 2008;181:1047–1054. doi: 10.1083/jcb.200712137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromley A, Yeaman C, Rosa J, Redick S, Chen C-T, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. Centriolin anchoring of Exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Guizetti J, Schermelleh L, Mantler J, Maar S, Poser I, Leonhardt H, Muller-Reichert T, Gerlich DW. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science. 2011;331:1616–1620. doi: 10.1126/science.1201847. [DOI] [PubMed] [Google Scholar]
- Hong W. SNAREs and traffic. Biochim Biophys Acta. 2005;1744:493–517. [PubMed] [Google Scholar]
- Jung JJ, Inamdar SM, Tiwari A, Ye D, Lin F, Choudhury A. Syntaxin 16 regulates lumen formation during epithelial morphogenesis. PLoS One. 2013;8:e61857. doi: 10.1371/journal.pone.0061857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Elia N, Ghirlando R, Lippincott-Schwartz J, Hurley JH. Midbody targeting of the ESCRT machinery by a non-canonical coiled coil in CEP55. Science. 2008;322:576–580. doi: 10.1126/science.1162042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low SH, Li X, Miura M, Kudo N, Quinones B, Weimbs T. Syntaxin 2 and endobrevin are required for the terminal step of cytokinesis in mammalian cells. Dev Cell. 2003;4:753–759. doi: 10.1016/s1534-5807(03)00122-9. [DOI] [PubMed] [Google Scholar]
- Manickam V, Tiwari A, Jung JJ, Bhattacharya R, Goel A, Mukhopadhyay D, Choudhury A. Regulation of vascular endothelial growth factor receptor 2 trafficking and angiogenesis by Golgi localized t-SNARE syntaxin 6. Blood. 2011;117:1425–1435. doi: 10.1182/blood-2010-06-291690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cuadrado AB, Morrell JL, Konomi M, An H, Petit C, Osumi M, Balasubramanian M, Gould KL, Del Rey F, de Aldana CR. Role of septins and the exocyst complex in the function of hydrolytic enzymes responsible for fission yeast cell separation. Mol Biol Cell. 2005;16:4867–4881. doi: 10.1091/mbc.E04-12-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal G, Rowley M, Guidugli L, Wu J, Pankratz VS, Couch FJ. BRCA2 localization to the midbody by filamin A regulates cep55 signaling and completion of cytokinesis. Dev Cell. 2012;23:137–152. doi: 10.1016/j.devcel.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nat Struct Biol. 2006;13:577–581. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- Neto H, Gould GW. The regulation of abscission by multi-protein complexes. J Cell Sci. 2011;124:3199–3207. doi: 10.1242/jcs.083949. [DOI] [PubMed] [Google Scholar]
- Perera HK, Clarke M, Morris NJ, Hong W, Chamberlain LH, Gould GW. Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes. Mol Biol Cell. 2003;14:2946–2958. doi: 10.1091/mbc.E02-11-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris R, Gould GW. Breaking up is hard to do—membrane traffic in cytokinesis. J Cell Sci. 2008;121:1569–1576. doi: 10.1242/jcs.018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor KM, Miller SC, Bryant NJ, Gould GW. Syntaxin 16 controls the intracellular sequestration of GLUT4 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2006;347:433–438. doi: 10.1016/j.bbrc.2006.06.135. [DOI] [PubMed] [Google Scholar]
- Rannou Y, Salaun P, Benaud C, Khan J, Dutertre S, Giet R, Prigent C. MNK1 kinase activity is required for abscission. J Cell Sci. 2012;125:2844–2852. doi: 10.1242/jcs.058081. [DOI] [PubMed] [Google Scholar]
- Riggs KA, Hasan N, Humphrey D, Raleigh C, Nevitt C, Corbin D, Hu C. Regulation of integrin endocytic recycling and chemotactic cell migration by syntaxin 6 and VAMP3 interaction. J Cell Sci. 2012;125:3827–3839. doi: 10.1242/jcs.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales SJ, Chen YA, Yoo BY, Patel SM, Doung Y-C, Scheller RH. SNAREs contribute to the specificity of membrane fusion. Neuron. 2000;26:457–464. doi: 10.1016/s0896-6273(00)81177-0. [DOI] [PubMed] [Google Scholar]
- Schiel JA, Childs C, Prekeris R. Endocytic transport and cytokinesis: from regulation of the cytoskeleton to midbody inheritance. Trends Cell Biol. 2013;23:319–327. doi: 10.1016/j.tcb.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiel JA, Prekeris R. ESCRT or endosomes? Tales of the separation of two daughter cells. Commun Integr Biol. 2011;4:606–608. doi: 10.4161/cib.4.5.16789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiel JA, Prekeris R. Membrane dynamics during cytokinesis. Curr Opin Cell Biol. 2013;25:92–98. doi: 10.1016/j.ceb.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiel JA, Simon GC, Zaharris C, Weisz J, Castle D, Wu CC, Prekeris R. FIP3-endosome-dependent formation of the secondary ingression mediates ESCRT-III recruitment during cytokinesis. Nat Cell Biol. 2012;14:1068–1078. doi: 10.1038/ncb2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GC, Schonteich E, Wu CC, Piekny A, Ekiert D, Yu X, Gould GW, Glotzer M, Prekeris R. Sequential Cyk-4 binding to ECT2 and FIP3 regulates cleavage furrow ingression and abscission during cytokinesis. EMBO J. 2008;27:1791–1803. doi: 10.1038/emboj.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Bremnes B, Ronning E, Aasland R, Stenmark H. Syntaxin-16, a putative Golgi t-SNARE. Eur J Cell Biol. 1998;75:223–231. doi: 10.1016/S0171-9335(98)80116-7. [DOI] [PubMed] [Google Scholar]
- Struthers MS, Shanks SG, MacDonald C, Carpp LN, Drozdowska AM, Kioumourtzoglou D, Furgason MLM, Munson M, Bryant NJ. Functional homology of mammalian syntaxin 16 and yeast Tlg2p reveals a conserved regulatory mechanism. J Cell Sci. 2009;122:2292–2299. doi: 10.1242/jcs.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellam JT, James DE, Stevens TH, Piper RC. Identification of a mammalian Golgi Sec1p-like protein, mVps45. J Biol Chem. 1997;272:6187–6193. doi: 10.1074/jbc.272.10.6187. [DOI] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Wilson GM, Fielding AB, Simon GC, Yu X, Andrews PD, Hames RS, Frey AM, Peden AA, Gould GW, Prekeris R. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell. 2005;16:849–860. doi: 10.1091/mbc.E04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Brill JA, Hsien J, McBride R, Boulianne GL, Trimble WS. Syntaxin 5 is required for cytokinesis and spermatid differentiation in Drosophila. Dev Biol. 2002;251:294–306. doi: 10.1006/dbio.2002.0830. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem. 2004;279:43027–43034. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.