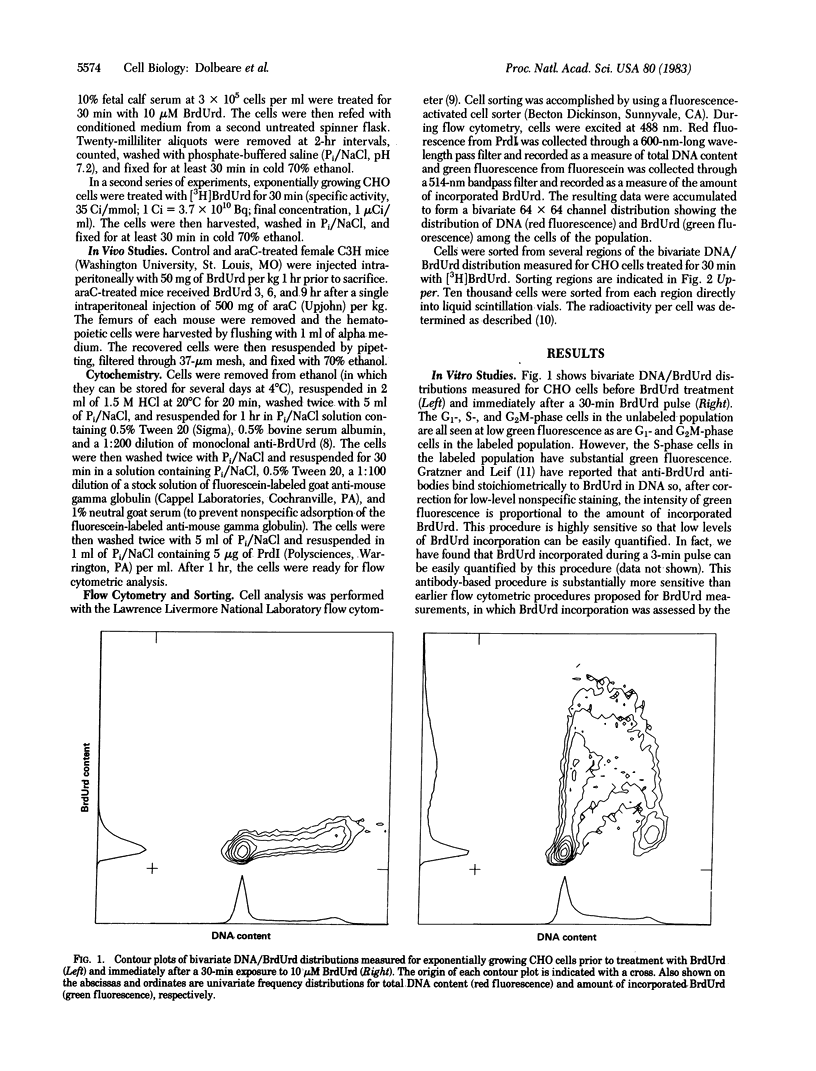
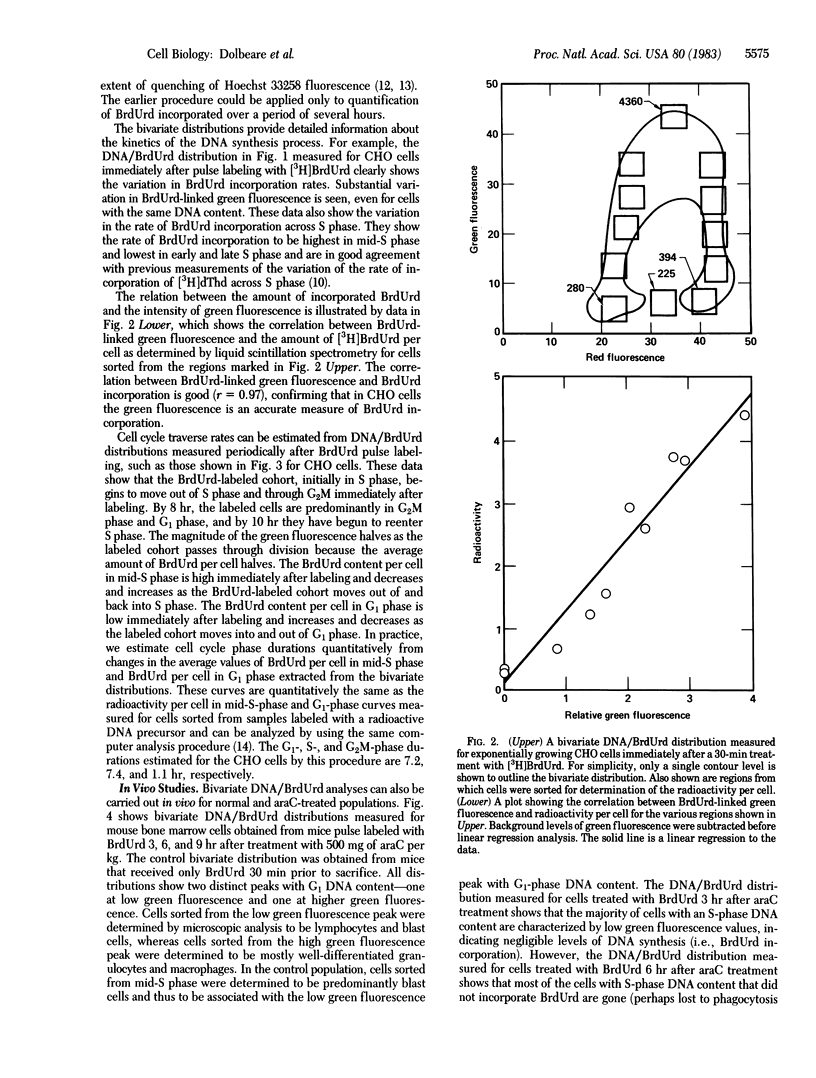
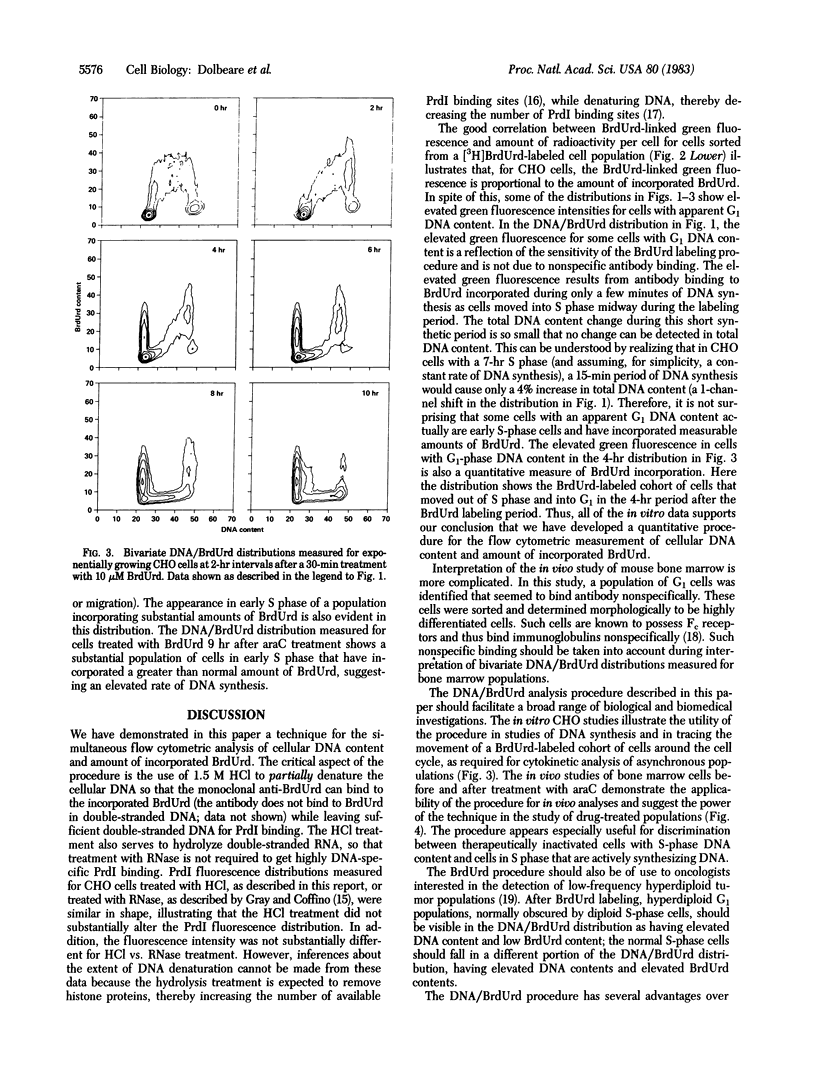
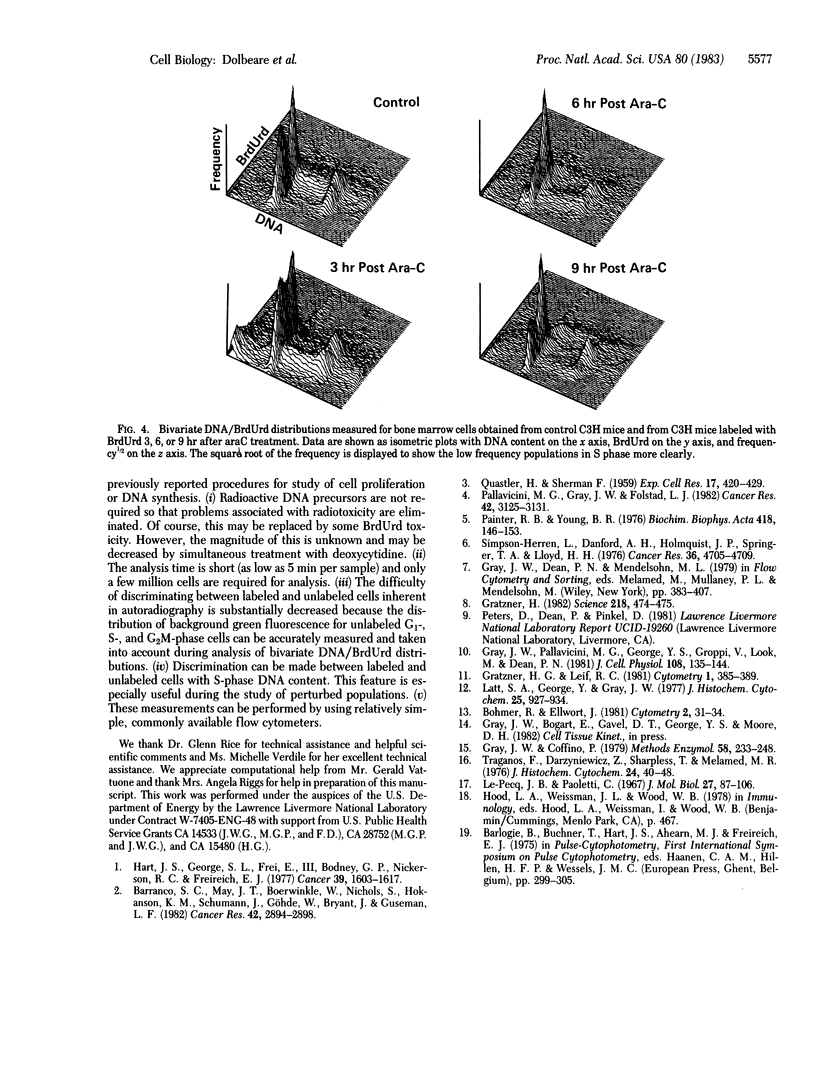
Abstract
We have developed a procedure for simultaneous flow cytometric measurement of cellular DNA content and amount of BrdUrd incorporated into cellular DNA. Propidium iodide was used as a fluorescent probe for total cellular DNA and a monoclonal antibody against BrdUrd was used as a probe for BrdUrd incorporated into DNA. Fluorescein-labeled goat anti-mouse antibody was used to fluorescently label the bound anti-BrdUrd probe. Bivariate DNA/BrdUrd distributions measured for Chinese hamster ovary cells labeled for 30 min with BrdUrd clearly show the G1-and G2M-phase cells to have low BrdUrd-linked fluorescence and the S-phase cells to have high BrdUrd-linked fluorescence. Cell cycle traverse rates were estimated for Chinese hamster ovary cells from bivariate distributions measured for samples taken periodically after pulse labeling with BrdUrd. Bivariate DNA/BrdUrd distributions were also applied in the analysis of the response of C3H murine bone marrow cells to treatment in vivo with 1-beta-D-arabinofuranosylcytosine (araC). Bivariate distributions were measured for bone marrow cells taken from mice that were pulse labeled with BrdUrd at various times after treatment with araC. The resulting DNA/BrdUrd sequences show the kinetics of recovery from araC and allow discrimination of the araC sterilized cells.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barranco S. C., May J. T., Boerwinkle W., Nichols S., Hokanson K. M., Schumann J., Göhde W., Bryant J., Guseman L. F. Enhanced cell killing through the use of cell kinetics-directed treatment schedules for two-drug combinations in vitro. Cancer Res. 1982 Jul;42(7):2894–2898. [PubMed] [Google Scholar]
- Böhmer R. M., Ellwart J. Cell cycle analysis by combining the 5-bromodeoxyuridine/33258 Hoechst technique with DNA-specific ethidium bromide staining. Cytometry. 1981 Jul;2(1):31–34. doi: 10.1002/cyto.990020107. [DOI] [PubMed] [Google Scholar]
- Gratzner H. G., Leif R. C. An immunofluorescence method for monitoring DNA synthesis by flow cytometry. Cytometry. 1981 May;1(6):385–393. doi: 10.1002/cyto.990010606. [DOI] [PubMed] [Google Scholar]
- Gratzner H. G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Gray J. W., Coffino P. Cell cycle analysis by flow cytometry. Methods Enzymol. 1979;58:233–248. doi: 10.1016/s0076-6879(79)58140-3. [DOI] [PubMed] [Google Scholar]
- Gray J. W., Pallavicini M. G., George Y. S., Groppi V., Look M., Dean P. N. Rates of incorporation of radioactive molecules during the cell cycle. J Cell Physiol. 1981 Aug;108(2):135–144. doi: 10.1002/jcp.1041080204. [DOI] [PubMed] [Google Scholar]
- Hart J. S., George S. L., Frei E., 3rd, Bodey G. P., Nickerson R. C., Freireich E. J. Prognotic significance of pretreatment proliferative activity in adult acute leukemia. Cancer. 1977 Apr;39(4):1603–1617. doi: 10.1002/1097-0142(197704)39:4<1603::aid-cncr2820390435>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Latt S. A., George Y. S., Gray J. W. Flow cytometric analysis of bromodeoxyuridine-substituted cells stained with 33258 Hoechst. J Histochem Cytochem. 1977 Jul;25(7):927–934. doi: 10.1177/25.7.70460. [DOI] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Painter R. B., Young B. R. Formation of nascent DNA molecules during inhibition of replicon initiation in mammalian cells. Biochim Biophys Acta. 1976 Jan 19;418(2):146–153. doi: 10.1016/0005-2787(76)90063-0. [DOI] [PubMed] [Google Scholar]
- Pallavicini M. G., Gray J. W., Folstad L. J. Quantitative analysis of the cytokinetic response of KHT tumors in vivo to 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1982 Aug;42(8):3125–3131. [PubMed] [Google Scholar]
- QUASTLER H., SHERMAN F. G. Cell population kinetics in the intestinal epithelium of the mouse. Exp Cell Res. 1959 Jun;17(3):420–438. doi: 10.1016/0014-4827(59)90063-1. [DOI] [PubMed] [Google Scholar]
- Simpson-Herren L., Sanford A. H., Holmquist J. P., Springer T. A., Lloyd H. H. Ambiguity of the thymidine index. Cancer Res. 1976 Dec;36(12):4705–4709. [PubMed] [Google Scholar]
- Traganos F., Darzynkiewicz Z., Sharpless T., Melamed M. R. Cytofluorometric studies on conformation of nucleic acids in situ. I. Restriction of acridine orange binding by chromatin proteins. J Histochem Cytochem. 1976 Jan;24(1):40–48. doi: 10.1177/24.1.1254934. [DOI] [PubMed] [Google Scholar]


