Abstract
Measuring iron content in the brain has important implications for a number of neurodegenerative diseases. Quantitative susceptibility mapping (QSM), derived from magnetic resonance images, has been used to measure total iron content in vivo and in post mortem brain. In this paper, we show how magnetic susceptibility from QSM correlates with total iron content measured by X-ray fluorescence (XRF) imaging and by inductively coupled plasma mass spectrometry (ICPMS). The relationship between susceptibility and ferritin iron was estimated at 1.10 ± 0.08 ppb susceptibility per μg iron/g wet tissue, similar to that of iron in fixed (frozen/thawed) cadaveric brain and previously published data from unfixed brains. We conclude that magnetic susceptibility can provide a direct and reliable quantitative measurement of iron content and that it can be used clinically at least in regions with high iron content.
Keywords: Brain iron, Ferritin, Quantitative susceptibility mapping (QSM), X-ray fluorescence imaging (XRF)
Introduction
Iron is an important endogenous biomarker for many neurological diseases and normal aging (Haacke et al., 2005; Schenck and Zimmerman, 2004). Previous histological work has shown that focally elevated iron deposition is associated with various neurological and psychiatric disorders, including multiple sclerosis (MS) (LeVine, 1997), Alzheimer’s disease (Bouras et al., 1997; Hallgren and Sourander, 1960; LeVine, 1997), Huntington’s disease (Chen et al., 1993; Dexter et al., 1991) and Parkinson’s disease (Chen et al., 1993; Dexter et al., 1991). Increased iron accumulation has been detected in chronic hemorrhage, MS lesions, cerebral infarction, anemia, thalassemia, hemochromatosis, and NBIA (neurodegeneration with brain iron accumulation) (Haacke et al., 2005). An in vivo non-invasive and quantitative estimation of non-heme iron deposition (predominantly ferritin) is essential to understand the cause of iron accumulation and its distribution patterns as well as its physiological role in any given disease (Bartzokis et al., 2007; Gerlach et al., 1994; Ke and Qian, 2003).
A variety of methods have been used in the past to quantify iron using magnetic resonance imaging (MRI) (Haacke et al., 2005). The standard workhorses in this area are T2 (House et al., 2007; Jensen et al., 2010; Mitsumori et al., 2012) and T2* (or R2* = 1/T2*) imaging methods that create T2* or R2* maps derived from multi-echo gradient (recalled) echo magnitude images. The latter are particularly useful since gradient echo sequences are very sensitive to the local susceptibility induced magnetic field inhomogeneity due to iron (Bartzokis et al., 1993; Haacke et al., 1989, 2005; Ordidge et al., 1994; Peters et al., 2007; Reichenbach et al., 1997). Further, T2* or R2* maps provide an important contrast mechanism to investigate brain tissue microstructure and to detect abnormal levels of brain iron (Bartzokis et al., 2007; Bouras et al., 1997; Chen et al., 1993; Dexter et al., 1991; Haacke et al., 2005, 2009; Hallgren and Sourander, 1960; LeVine, 1997; Wallis et al., 2008).
In this paper, we focus on susceptibility measurements from phase images. Phase has been used as a means to measure iron content (Haacke et al., 2007). However, phase is dependent on the geometry of the object and so it can be misinterpreted. The solution lies in using a susceptibility map reconstructed from the phase information. In theory, this approach is independent of field strength, echo time, the object’s relative orientation to the main field and the object’s shape (Cheng et al., 2009b; de Rochefort et al., 2010; Haacke et al., 2010; Kressler et al., 2010; Li et al., 2011; Liu et al., 2009; Marques and Bowtell, 2005; Schweser et al., 2011; Shmueli et al., 2009; Wharton and Bowtell, 2010; Yao et al., 2009). Recent work has suggested that susceptibility changes in the basal ganglia, thalamus and other deep gray matter nuclei have better correlation with iron concentration than phase information (Bilgic et al., 2012; Fukunaga et al., 2010; Langkammer et al., 2012b; Schweser et al., 2011, 2012; Shmueli et al., 2009; Wharton and Bowtell, 2010; Yao et al., 2009) and, therefore, quantitative susceptibility mapping (QSM) may provide a good means to study tissue iron content.
Currently, the neuroscience community relies upon the 50 year old data on iron in cadaveric brains published by Hallgren and Sourander (Hallgren and Sourander, 1958). Total iron in cadaveric brain has been measured using synchrotron X-ray fluorescence (XRF) iron mapping (Hopp et al., 2010; Zheng et al., 2012), proton-induced X-ray emission mapping (Butz et al., 2000), inductively coupled plasma mass spectrometry (ICPMS) measurements (Langkammer et al., 2010, 2012a) and atomic absorption spectrometry measurements (House et al., 2007). Among these, the first two techniques can provide a voxel by voxel quantification of iron content which can then be compared with MR iron quantification.
In this paper, our goal is to develop an absolute quantification scale by separating the iron induced susceptibility change from other potential sources by comparing ferritin-gelatin phantoms with quantified XRF iron maps of basal ganglia from cadaver brains and ICPMS iron values.
Materials and methods
Preparation of ferritin phantoms
Horse spleen ferritin (Ref. F4503, Sigma-Aldrich, USA) was used to prepare ferritin-gelatin phantoms. The iron concentration as determined by the supplier using ICPMS was 7.13 ± 0.15 mg/ml. The ferritin solution was first diluted by adding 4 ml of original solution with 16ml warm 7% gelatin resulting in a stock solution with iron concentration of about 1426 ± 30 μg/ml. This stock solution was serially diluted six times in warm gelatin by a factor of 2 each time. The ferritin-doped gelatin solutions as well as pure gelatin were loaded into straws and then embedded in a pure gelatin matrix. Total iron was measured in aliquots of the ferritin-doped gelatin by XRF and ICPMS. See the detailed scheme of the experiment in Table 1.
Table 1.
Methodology and data processing.
| ICPMS | XRF | SWI Background phase removal | QSM | |
|---|---|---|---|---|
| Ferritin samples | √ | √ | Quadratic fitting | Forward fitting |
| Cadaveric brain | √ | SHARP | Truncated k-space division (Haacke et al., 2010) |
Rapid scanning X-ray fluorescence (RS-XRF)
All XRF measurements were conducted at the Stanford Synchrotron Radiation Lightsource (SSRL). RS-XRF images of ferritin phantoms and cadaveric brain were acquired at wiggler beam line 10–2 at SSRL. The samples were mounted onto a set of motorized stages oriented at 45° to the incident beam. The incident beam (12 keV) passing through a tantalum aperture produced a 100 μm × 100 μm spot on the sample which was raster-scanned in the beam using a dwell time of 15 ms/point. Fluorescent energy windows were centered for Fe (6.21–6.70 keV) as well as all other biologically interesting elements, scatter and total incoming counts. Elements were quantified in μg iron/g wet tissue by comparison of signal strength with XRF calibration standards (±5% uncertainty) (Micromatter, Vancouver, BC, Canada) according to Hopp and colleagues (Hopp et al., 2010) using Sam’s Microanalysis kit (Webb, 2010). An area of the ferritin-doped gelatin block was mapped and average counts were compared with XRF calibration standards.
Inductively coupled plasma mass spectrometry
To confirm the total iron content of the ferritin phantoms, 5 ml samples were taken from the straws after MR imaging and the iron content was determined by ICPMS using an ELAN 9000 system (PerkinElmer, Waltham, MA, USA) (American Environmental Testing Laboratory Inc., California). The samples were diluted to the range acceptable for ICPMS via serial dilutions.
Preparation of the cadaveric brain sample
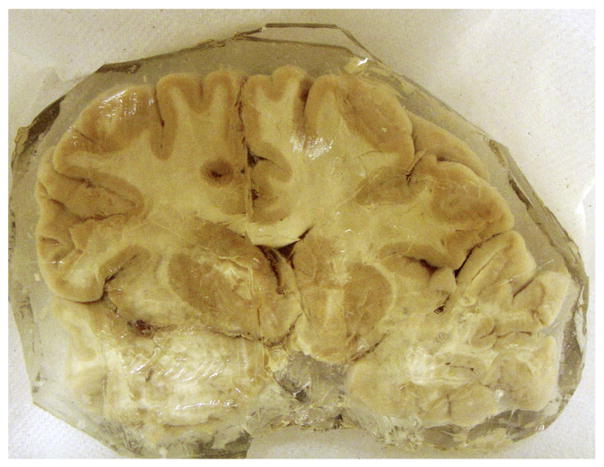
One frozen coronal section (96 mm long × 132 mm wide × 5 mm thick) of human cadaveric multiple sclerosis (MS) brain (MS 3852) (see Fig. 1) was obtained from the Human Brain and Spinal Fluid Resource Center, Los Angeles, CA, under the University of Saskatchewan ethics approval BioREB 06-250. Coronal sections showed extensive irregular demyelination throughout the brainstem. There were also a few small scattered demyelinating periventricular foci (bilateral). The surface of the sample (a 5 mm thick section) showed patchy areas of slight rarefaction without significant axonal loss or change in oligodendrocyte density. There were varying degrees of associated gliosis. The areas of rarefaction were associated with extensive demyelination. To reduce storage artifacts such as leaching of metals, fresh autopsy brain was flash frozen and the slices were shipped on dry ice and stored frozen until they were thawed by immersion in buffered formalin. After 6 h of fixation, the brain slice was drained and sealed in plastic prior to initial synchrotron imaging of the surface of the slice. To resolve regions of interest, the slices were embedded in gelatin for MR imaging. The brain hemispheres were sectioned to expose the region of interest and then the slice was sealed in metal-free thin polypropylene film. RS-XRF images were acquired and quantified at SSRL (see the detailed scheme of the experiment in Table 1).
Fig. 1.
Photograph of the cadaveric brain sample in gelatin.
MR imaging and image processing
Imaging and phase processing of ferritin samples
MR data of ferritin samples were collected on a 3 T Siemens Verio system using a multi-echo susceptibility weighted imaging (SWI) sequence with 11 echoes (TR = 40 ms, FA = 15°). The resolution was 1 mm × 1 mm × 1 mm with a matrix of 256 × 256 × 128. The shortest echo time was 5 ms with a 2.39 ms increment for the other 10 echoes. Magnitude and phase images were reconstructed from the raw data for each individual and combined channel. The geometry of the ferritin samples was segmented from multi-echo spin echo images (TR = 2000 ms, resolution 0.22 mm × 0.22 mm × 3 mm).
In order to reconstruct a susceptibility map, a pristine phase map was required. That is, the phase was unwrapped and all spurious phase information was removed. Phase images (TE = 21.73 ms) were unwrapped using Prelude in FSL (Jenkinson, 2003). To remove the low spatial frequency background field effects, phase from regions outside the straws were chosen, where there were minimal remnant dipole effects. First, a circular mask with a radius three times that of the straw was defined and centered on each straw and all the information inside this mask was removed from the images. The remaining signal was fit with a quadratic function and extrapolated back into the masked region. Then the estimated dipole phase was obtained by subtracting this modified background phase from the original phase. The susceptibility inside each of the ferritin straws was assumed to be uniform and was estimated using a least squares fitting of the forward simulated dipole phase with the estimated phase (Neelavalli et al., 2009). All the steps were performed in MATLAB R2009a. The results of each step are shown in Fig. 2.
Fig. 2.

Removing the background phase (TE = 21.73 ms). A) Geometry of the straws segmented from the spin echo images. B) Original phase. C) Background phase after extrapolation of magnetic fields into the straw regions. D) Subtraction of C from B to reveal pristine dipole effects due to the iron in the straws.
Imaging and image processing of cadaveric brain
MR images were collected on a 3 T Siemens Verio system using the same 11 echo SWI sequence but with different imaging parameters. The coronal images were acquired with a resolution 0.5 mm × 0.5 mm in phase encoding and readout direction and 0.7 mm in the slice select direction with a readout bandwidth of 465 Hz/pixel, a field-of-view of 256 mm × 192 mm with Nx = 512, Ny = 384 and Nz = 40. The shortest echo time was 5.68 ms with a 2.57 ms increment for the other 10 echoes. MR phase images (TE = 8.25 ms) were first unwrapped using Prelude in FSL (Jenkinson, 2003) and then the background phase was removed using TSVD-SHARP (Schweser et al., 2011) with a kernel size of 5 mm. An initial estimation of the susceptibility distribution was obtained using truncated k-space division, with a threshold value of 0.1. Due to the presence of some air bubbles near the brain tissue, the streaking artifacts would mask several important regions in the susceptibility map. Thus, the air bubbles were first extracted from the susceptiblity map by setting a threshold, since air has a much higher susceptibility relative to water than that of brain tissue. The extracted susceptibility maps of the air bubbles were used to predict their induced field variation through a forward field calculation. Finally, the predicted fields induced by the air bubbles were removed from the SHARP (Schweser et al., 2012) processed field map. The central region of these air bubbles in phase images was set to be zero, in order to reduce the streaking artifacts caused by the noise inside the bubble. This newly processed field map was used to generate the final susceptibility maps, using a truncated k-space division with a threshold of 0.1 (Haacke et al., 2010) via SPIN (Signal Processing in NMR, Detroit, MI, USA) software.
Results
Correlation between susceptiblity and ferritin iron content
The susceptibilities (TE = 21.73 ms) of the five empty straws embedded in gelatin were estimated at (9.46 ± 0.015; 9.64 ± 0.015; 9.46 ± 0.016; 9.65 ± 0.013; 9.46 ± 0.015) ppm. Assuming that the susceptibility difference between the air and gel is 9.4 ppm (Cheng et al., 2009a), the total susceptiblity measurement including the background removal, straw geometry segmentation error and least squares fitting had a bias of 1.42%.
The measured susceptibilities (TE = 21.73 ms) and iron concentrations of the six ferritin samples are listed in Table 2. The dipolar phase pattern outside the straw from the sample with the lowest iron concentration (39 ± 6 μg Fe/ml) had its sign reversed compared with other samples. This sample shows a negative susceptibility of −14ppb when using the forward fitting approach. One possible explanation for this could be a small baseline shift coming from an imperfect background removal. Since the iron concentration range that can be measured with XRF is broad, there was no need for dilution. In contrast, ICPMS requires dilution of samples to make iron concentration in the proper range for analysis. The results in Table 2 show that the iron content measured by two approaches (XRF and ICPMS) was essentially the same. The correlation slopes in Fig. 3 obtained from ICPMS (1.11 ± 0.06 ppb per μg iron/ml) and XRF imaging (1.10 ± 0.08 ppb per μg iron/ml) were close and both were less than the theoretical estimation of 1.27ppb per μg iron/ml from Schenck (1992).
Table 2.
Susceptibilities of ferritin phantoms as quantified from SWI phase data (TE = 21.73 ms) and iron concentrations measured by XRF and ICPMS. Data are shown as mean ± one standard deviation.
| Sample no. 1 | Sample no. 2 | Sample no. 3 | Sample no. 4 | Sample no. 5 | Sample no. 6 | Gelatin solution (7%) | |
|---|---|---|---|---|---|---|---|
| Susceptibility (ppb) (N = 19,205) | 840 ± 2.4 | 428 ± 1.3 | 271 ± 0.9 | 101 ± 0.4 | 39 ± 0.3 | −14 ± 0.2 | N.A. for forward fitting |
| XRF iron concentration (μg/ml) (N = 961) | 790 ± 94 | 395 ± 44 | 229 ± 32 | 110 ± 27 | 77 ± 16 | (Not available) | (Not available) |
| ICPMS iron concentration (μg/ml) | 772 ± 115 | 448 ± 67 | 240 ± 36 | 127 ± 19 | 66 ± 10 | 39 ± 6 | 0.23 ± 0.11 |
Standard deviation includes the spatial distribution variation in the straws.
Fig. 3.

Correlation between susceptibility measured by MRI and total iron measured by ICPMS and XRF for ferritin phantoms.
Correlation between susceptibility and iron in cadaveric brain
In order to correlate the susceptibility and XRF iron maps, images from both methods were co-registered (Fig. 4). ROIs marked in each image were used for a voxel by voxel comparison of susceptibility and iron measurements (Table 3). At TE = 8.25 ms, the correlation equations were found to be Y = 0.80(±0.01) (ppb susceptibility per μg iron/g wet tissue) * X (μg iron/g wet tissue) + 10.87(±2.9) (ppb susceptibility) and Y = 0.79(±0.02) * X − 3.66(±4.2) (ppb suscep rowsep=“1”tibility) for left and right hemisphere, respectively, as shown in Fig. 5 (A, B). The phase images at TE = 21.1 ms were also processed, the fitted equations were Y = 0.78(±0.02) * X − 4.36(±4) (ppb susceptibility) and Y = 0.79(±0.01) * X − 5.22(±2.8) (ppb susceptibility) for left and right hemispheres respectively. The slopes (0.80 and 0.79 ppb susceptibility per μg iron/g wet tissue) determined from the TE = 21.1 ms data were similar to those from TE = 8.25 ms (0.78 and 0.79). Although phase is clearly modified as a function of echo time, tissue susceptibility change is expected to be and here is shown to be independent of echo time (Haacke et al., 2010). The estimated susceptibility based on our simulation of the inverse process using the structures of a similar size showed an underestimate or bias of −14%.
Fig. 4.
Iron quantified from XRF Fe mapping (A, B) for left and right hemispheres; putative iron quantified as susceptibility (TE = 8.25 ms) (C, D). Images are co-registered and the ROIs used for a pixel by pixel correlation are outlined in both images. CN: caudate nucleus. PUT: putamen. GP: globus pallidus. ROIs were defined by excluding the edges in the map for each structure.
Table 3.
Average susceptibility of a cadaveric brain as quantified from SWI phase data (TE = 8.25 ms) and Fe measured using XRF imaging. ROIs are defined in Fig. 4. Data are shown as mean ± one standard deviation.
| CN (left) | PUT (left) | GP (left) | CN (right) | PUT (right) | GP (right) | |
|---|---|---|---|---|---|---|
| Susceptibility (ppb) | 111 ± 25 | 152 ± 18 | 273 ± 73 | 105 ± 31 | 121 ± 24 | 242 ± 40 |
| Iron estimated by XRF (μg/g wet tissue) | 153 ± 28 | 210 ± 35 | 338 ± 73 | 135 ± 28 | 179 ± 26 | 277 ± 40 |
Mean is estimated as the average within the ROI. Standard deviation includes the spatial distribution variation within the ROI.
Fig. 5.
Correlation between susceptibility and XRF iron measurements for all data points taken from each of the regions demarcated in Fig. 4. A: fitting for left hemisphere; B: fitting for right hemisphere.
Discussion
Using ferritin phantoms and a cadaveric brain sample, we have found that the susceptibility correlates reasonably well with the iron measured by XRF and/or ICPMS (Fig. 3). The cadaveric brain used in the study was from a person with multiple sclerosis. It is commonly assumed that the iron in normal and pathological MS brains is predominantly stored in the form of ferritin. As long as this assumption holds, the MS pathology will not affect the susceptibility/iron correlation slope. Our correlation of iron content with susceptibility for cadaveric brains (Fig. 5) was comparable with that obtained by Langkammer et al. (2012b). This is expected since we used SHARP with the same parameters to remove the background fields. The SWIM approach used in this paper underestimates the susceptibility by 14% for deep gray matter structures according to our simulations. The homogeneity-enabled incremental dipole inversion (HEIDI) method used by Langkammer et al. (2012b) underestimates the susceptibility by about 7% (Langkammer et al., 2012b; Schweser et al., 2012). Our slope (0.8 / (1–14%) ≅ 0.93) is close to that in Langkammer et al. (2012b) (0.89 / (1–7%) ≅ 0.957) for deep gray matter when these biases are accounted for. Since the cadaveric brain in our experiment was for-malin fixed and those in Langkammer et al. (2012b) were unfixed, this suggests that fixation may not change tissue susceptibility in deep gray matter.
However, the slope of 0.59 ppb susceptibility per μg iron/g wet tissue obtained from our in vivo data (Haacke, 2012) and other single orientation results that used Hallgren and Sourander’s equation as the iron baseline (Shmueli et al., 2009; Wharton and Bowtell, 2010) was smaller than the 0.8 ppb susceptibility per μg iron/g wet tissue obtained from our cadaveric brain data, even though they were processed with the same methods. Thus, there appears to be a difference between in vivo and ex vivo susceptibilities and their correlation with iron. The reason for this is unclear but could be due to the freezing and fixation process which could affect local susceptibilities of the tissue.
Formalin fixation might change MR signal but previous work on myelin susceptibility (Lee et al., 2012; Liu et al., 2011) demonstrated that the effect of formalin fixation on the susceptibility changes due to myelin was subtle. The similar iron/susceptibility slopes of fixed brain in our work and of the unfixed brains in the work of Langkammer and colleagues (Langkammer et al., 2012b) further supports the view that formalin fixation has negligible effect on susceptibility. The effects of fixation on R2 and thus R2* values, however, are known to be substantial (Dawe et al., 2009; Lee et al., 2012; Pfefferbaum et al., 2004; Schmierer et al., 2008) and are beyond the scope of this paper.
The susceptibility/iron correlation slopes obtained from cadaveric and in vivo brains in Table 4 are generally smaller than the theoretical slope of 1.32 ppb susceptibility per μg iron/g wet tissue except for the slope obtained with myelin correction in Schweser et al. (2011). One possible reason for the smaller slope from the in vivo human brains is that there are still some forms of iron that are MR invisible although these may be in other species that are known to be present at low levels (Hopp et al., 2010). A second explanation for smaller slopes seen in our work (Haacke, 2012) and other work (Shmueli et al., 2009; Wharton and Bowtell, 2010) is that Hallgren and Sourander’s measurements of total iron (Hallgren and Sourander, 1958) may not be accurate. Third, susceptibility mapping is known to have a bias and leads to a smaller slope, but this bias can be potentially corrected (J. Liu et al., 2012; T. Liu et al., 2012; Schweser et al., 2012; Wharton and Bowtell, 2010). Other possible factors that have been explored include contributions of myelin (Duyn et al., 2007; Liu et al., 2011; Ogg et al., 1999), chemical exchange between water and macromolecular protons (Luo et al., 2010; Shmueli et al., 2011; Zhong et al., 2008) and microstructure orientation (He and Yablonskiy, 2009; Lee et al., 2010; Liu, 2010). Indeed, it could well be a combination of all these sources that lead to different measurements of iron in vivo and ex vivo. Despite these imperfections, the slopes for susceptibility versus iron content are generally consistent between both ex vivo studies (this paper and Langkammer et al., 2012b) and in vivo studies using similar susceptibility mapping methods (see Table 4) (Haacke, 2012; Shmueli et al., 2009; Wharton and Bowtell, 2010).
Table 4.
Correlation between susceptibility mapping and iron concentration.
| Authors | Correlation slopea | Structures | Background removal | QSM methodb | Myelin correction | Field (Tesla) | Sample | Iron |
|---|---|---|---|---|---|---|---|---|
| This paper | 1.11 | N.A. | Quadratic fitting | Forward fitting | N.A. | 3 T | Ferritin | ICPMS |
| This paper | 1.10 | N.A. | Quadratic fitting | Forward fitting | N.A. | 3 T | Ferritin | XRF |
| This paper | 0.80 | GP, PUT, CN | SHARP | TKD1 (SO) | No | 3 T | MS cadaveric brain (fixed) | XRF |
| Haacke (2012) | 0.59 | GP, PUT, CN | SHARP | TKD1 (SO) | No | 3 T | In vivo brains | H&Sc |
| Shmueli et al. (2009) | 0.56 | PUT, RN, SN | Polynomial fitting | TKD2 (SO) | No | 7 T | In vivo brain | H&S |
| Wharton and Bowtell (2010) | 0.75/0.6 | GP, SN, RN, PUT, CN, TH, GM | Simulated geometric effect + fitting | TKD3 (MO/SO) | No | 7 T | In vivo brains | H&S |
| Langkammer et al. (2012a) | 0.89 | GP, PUT, CN, TH | SHARP | HEIDI (SO) | No | 3 T | Unfixed cadaveric brains | ICPMS |
| Schweser et al. (2011) | 1.30 | GP, SN, DN, PUT, CN, TH, WM, GM | SHARP | MO regularization | Yes | 3 T | In vivo brains | H&S |
The unit of the slope for human brain is susceptibility/μg iron/g wet tissue (ρ = 1.04g/ml at 36.5 °C); the unit of the slope for the ferritin solution is ppb susceptibility/μg iron/ml and the corresponding theoretical value is 1.27.
SO: single orientation; MO: multiple orientation; TKD: thresholded k-space division. TKD1: Haacke et al., 2010; TKD2: Shmueli et al., 2009; TKD3: (Wharton and Bowtell, 2010).
H&S: (Hallgren and Sourander, 1958).
Conclusion
Our results suggest that susceptibility changes from iron measured in ex vivo studies reasonably reflect iron content even for in vivo studies, although the predicted values may be underestimated. Our study further demonstrates that the correlation of susceptibility with iron is consistent with other results in the literature and is independent of echo time and orientation. Thus, susceptibility would appear to be a direct and reliable quantitative indication of iron, especially for brain regions with high iron content. Susceptibility mapping provides a reliable tool for clinical investigations of iron that could be used to study changes in iron over time or within a given age-matched population.
Acknowledgments
This work is supported by the Canadian Institutes of Health Research (CIHR)/Heart and Stroke Foundation of Canada (HSFC) Synchrotron Medical Imaging Team Grant #CIF 99472.
This work was supported in part by the Telemedicine and Advanced Technology Research Center (TATRC) at the U.S. Army Medical Research and Materiel Command (USAMRMC) through award W81XWH-12-1-0522. The views, opinions and/or findings contained in this report are those of the author(s) and should not be construed as an official government position, policy or decision unless so designated by other documentation.
Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource, a Directorate of SLAC National Accelerator Laboratory and an Office of Science User Facility operated for the U.S. Department of Energy Office of Science by Stanford University. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program (P41RR001209).
The authors would like to thank Sam Webb at the Stanford Synchrotron Radiation Lightsource and Angela Auriat at the Dept. of Neurosurgery, Stanford University for their help in collecting XRF data. We thank Zahid Latif, Yang Xuan, Yimin Shen for their assistance with the MR protocols, and Dr. Wei Feng and Dr. Jaladhar Neelavalli for their help on susceptibility mapping. The authors would also like to thank Dr. Karin Shmueli at NINDS, National Institute of Health for her valuable discussions of the literature.
Footnotes
Conflict of interest
The authors have no Conflicts of interest.
References
- Bartzokis G, Aravagiri M, Oldendorf WH, Mintz J, Marder SR. Field dependent transverse relaxation rate increase may be a specific measure of tissue iron stores. Magn Reson Med. 1993;29:459–464. doi: 10.1002/mrm.1910290406. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA, Lu PH, Villablanca P, Altshuler LL, Carter M, Huang D, Edwards N, Mintz J. Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol Aging. 2007;28:414–423. doi: 10.1016/j.neurobiolaging.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Bilgic B, Pfefferbaum A, Rohlfing T, Sullivan EV, Adalsteinsson E. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. Neuroimage. 2012;59:2625–2635. doi: 10.1016/j.neuroimage.2011.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouras C, Giannakopoulos P, Good PF, Hsu A, Hof PR, Perl DP. A laser microprobe mass analysis of brain aluminum and iron in dementia pugilistica: comparison with Alzheimer’s disease. Eur Neurol. 1997;38:53–58. doi: 10.1159/000112903. [DOI] [PubMed] [Google Scholar]
- Butz T, Flagmeyer R-H, Heitmann J, Jamieson DN, Legge GJF, Lehmann D, Reibetanz U, Reinert T, Saint A, Spemann D, Szymanski R, Tröger W, Vogt J, Zhu J. The Leipzig high-energy ion nanoprobe: a report on first results. Nucl Instrum Methods Phys Res B. 2000;161–163:323–327. [Google Scholar]
- Chen JC, Hardy PA, Kucharczyk W, Clauberg M, Joshi JG, Vourlas A, Dhar M, Henkelman RM. MR of human postmortem brain tissue: correlative study between T2 and assays of iron and ferritin in Parkinson and Huntington disease. Am J Neuroradiol. 1993;14:275–281. [PMC free article] [PubMed] [Google Scholar]
- Cheng YCN, Hsieh CY, Neelavalli J, Haacke EM. Quantifying effective magnetic moments of narrow cylindrical objects in MRI. Phys Med Biol. 2009a;54:7025–7044. doi: 10.1088/0031-9155/54/22/018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YCN, Neelavalli J, Haacke EM. Limitations of calculating field distributions and magnetic susceptibilities in MRI using a Fourier based method. Phys Med Biol. 2009b;54:1169–1189. doi: 10.1088/0031-9155/54/5/005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe RJ, Bennett DA, Schneider JA, Vasireddi SK, Arfanakis K. Postmortem MRI of human brain hemispheres: T2 relaxation times during formaldehyde fixation. Magn Reson Med. 2009;61:810–818. doi: 10.1002/mrm.21909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rochefort L, Liu T, Kressler B, Liu J, Spincemaille P, Lebon V, Wu JL, Wang Y. Quantitative susceptibility map reconstruction from MR phase data using Bayesian regularization: validation and application to brain imaging. Magn Reson Med. 2010;63:194–206. doi: 10.1002/mrm.22187. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114:1953–1975. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- Duyn JH, van Gelderen P, Li TQ, de Zwart JA, Koretsky AP, Fukunaga M, Li TQ. High-field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci U S A. 2007;104:11796–11801. doi: 10.1073/pnas.0610821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga M, Li TQ, van Gelderen P, de Zwart JA, Shmueli K, Yao B, Lee J, Maric D, Aronova MA, Zhang G. Layer-specific variation of iron content in cerebral cortex as a source of MRI contrast. Proc Natl Acad Sci U S A. 2010;107 (8):3834–3839. doi: 10.1073/pnas.0911177107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach M, Ben-Shachar D, Riederer P, Youdim MB. Altered brain metabolism of iron as a cause of neurodegenerative diseases? J Neurochem. 1994;63:793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- Haacke EM. Clinical Intensive Course, Advanced Neuroimaging 1. : Intl Soc Mag Reson Med. Vol. 20. Melbourne, Australia: 2012. Quantitative measurements of iron. http://www.ismrm.org/12/WK_Neuro1.htm. [Google Scholar]
- Haacke E, Tkach J, Parrish T. Reducing T*2 dephasing in gradient field-echo imaging. Radiology. 1989;170:457–462. doi: 10.1148/radiology.170.2.2911669. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Cheng NY, House MJ, Liu Q, Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, Obenaus A. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging. 2005;23:1–25. doi: 10.1016/j.mri.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Ayaz M, Khan A, Manova ES, Krishnamurthy B, Gollapalli L, Ciulla C, Kim I, Petersen F, Kirsch W. Establishing a baseline phase behavior in magnetic resonance imaging to determine normal vs. abnormal iron content in the brain. J Magn Reson Imaging. 2007;26:256–264. doi: 10.1002/jmri.22987. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Makki M, Ge Y, Maheshwari M, Sehgal V, Hu J, Selvan M, Wu Z, Latif Z, Xuan Y, Khan O, Garbern J, Grossman RI. Characterizing iron deposition in multiple sclerosis lesions using susceptibility weighted imaging. J Magn Reson Imaging. 2009;29:537–544. doi: 10.1002/jmri.21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke EM, Tang J, Neelavalli J, Cheng YCN. Susceptibility mapping as a means to visualize veins and quantify oxygen saturation. J Magn Reson Imaging. 2010;32:663–676. doi: 10.1002/jmri.22276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The non-haemin iron in the cerebral cortex in Alzheimer’s disease. J Neurochem. 1960;5:307–310. doi: 10.1111/j.1471-4159.1960.tb13369.x. [DOI] [PubMed] [Google Scholar]
- He X, Yablonskiy DA. Biophysical mechanisms of phase contrast in gradient echo MRI. Proc Natl Acad Sci U S A. 2009;106:13558–13563. doi: 10.1073/pnas.0904899106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp K, Gh Popescu BF, McCrea RPE, Harder SL, Robinson CA, Haacke EM, Rajput AH, Rajput A, Nichol H. Brain iron detected by SWI high pass filtered phase calibrated with synchrotron X-ray fluorescence. J Magn Reson Imaging. 2010;31:1346–1354. doi: 10.1002/jmri.22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House MJ, Pierre TGS, Kowdley KV, Montine T, Connor J, Beard J, Berger J, Siddaiah N, Shankland E, Jin LW. Correlation of proton transverse relaxation rates (R2) with iron concentrations in postmortem brain tissue from Alzheimer’s disease patients. Magn Reson Med. 2007;57:172–180. doi: 10.1002/mrm.21118. [DOI] [PubMed] [Google Scholar]
- Jenkinson M. A fast, automated, n-dimensional phase unwrapping algorithm. Magn Reson Med. 2003;49:193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jensen JH, Tang H, Tosti CL, Swaminathan SV, Nunez A, Hultman K, Szulc KU, Wu EX, Kim D, Sheth S, Brown TR, Brittenham GM. Separate MRI quantification of dispersed (ferritin-like) and aggregated (hemosiderin-like) storage iron. Magn Reson Med. 2010;63:1201–1209. doi: 10.1002/mrm.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Qian ZM. Iron misregulation in the brain: a primary cause of neurodegenerative disorders. Lancet Neurol. 2003;2:246–253. doi: 10.1016/s1474-4422(03)00353-3. [DOI] [PubMed] [Google Scholar]
- Kressler B, de Rochefort L, Liu T, Spincemaille P, Jiang Q, Wang Y. Nonlinear regularization for per voxel estimation of magnetic susceptibility distributions from MRI field maps. IEEE Trans Med Imaging. 2010;29:273–281. doi: 10.1109/TMI.2009.2023787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langkammer C, Krebs N, Goessler W, Scheurer E, Ebner F, Yen K, Fazekas F, Ropele S. Quantitative MR imaging of brain iron: a postmortem validation study. Radiology. 2010;257:455–462. doi: 10.1148/radiol.10100495. [DOI] [PubMed] [Google Scholar]
- Langkammer C, Krebs N, Goessler W, Scheurer E, Yen K, Fazekas F, Ropele S. Susceptibility induced gray–white matter MRI contrast in the human brain. Neuroimage. 2012a;59:1413–1419. doi: 10.1016/j.neuroimage.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langkammer C, Schweser F, Krebs N, Deistung A, Goessler W, Scheurer E, Sommer K, Reishofer G, Yen K, Fazekas F, Ropele S, Reichenbach JR. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage. 2012b;62:1593–1599. doi: 10.1016/j.neuroimage.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Shmueli K, Fukunaga M, van Gelderen P, Merkle H, Silva AC, Duyn JH. Sensitivity of MRI resonance frequency to the orientation of brain tissue microstructure. Proc Natl Acad Sci U S A. 2010;107:5130–5135. doi: 10.1073/pnas.0910222107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Shmueli K, Kang BT, Yao B, Fukunaga M, Gelderen Pv, Palumbo S, Bosetti F, Silva AC, Duyn JH. The contribution of myelin to magnetic susceptibility-weighted contrasts in high-field MRI of the brain. Neuroimage. 2012;59:3967–3975. doi: 10.1016/j.neuroimage.2011.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine SM. Iron deposits in multiple sclerosis and Alzheimer’s disease brains. Brain Res. 1997;760:298–303. doi: 10.1016/s0006-8993(97)00470-8. [DOI] [PubMed] [Google Scholar]
- Li W, Wu B, Liu C. Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. Neuroimage. 2011;55:1645–1656. doi: 10.1016/j.neuroimage.2010.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. Susceptibility tensor imaging. Magn Reson Med. 2010;63:1471–1477. doi: 10.1002/mrm.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Spincemaille P, de Rochefort L, Kressler B, Wang Y. Calculation of susceptibility through multiple orientation sampling (COSMOS): a method for conditioning the inverse problem from measured magnetic field map to susceptibility source image in MRI. Magn Reson Med. 2009;61:196–204. doi: 10.1002/mrm.21828. [DOI] [PubMed] [Google Scholar]
- Liu C, Li W, Johnson GA, Wu B. High-field (9.4 T) MRI of brain dysmyelination by quantitative mapping of magnetic susceptibility. Neuroimage. 2011;56:930–938. doi: 10.1016/j.neuroimage.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Xu W, Spincemaille P, Avestimehr AS, Wang Y. Accuracy of the morphology enabled dipole inversion (MEDI) algorithm for quantitative susceptibility mapping in MRI. IEEE Trans Med Imaging. 2012a;31:816–824. doi: 10.1109/TMI.2011.2182523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu T, Rochefort Ld, Ledoux J, Khalidov I, Chen W, Tsiouris AJ, Wisnieff C, Spincemaille P, Prince MR, Wang Y. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage. 2012b;59:2560–2568. doi: 10.1016/j.neuroimage.2011.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, He X, d’Avignon DA, Ackerman JJH, Yablonskiy DA. Protein induced water 1H MR frequency shifts: contributions from magnetic susceptibility and exchange effects. J Magn Reson. 2010;202:102–108. doi: 10.1016/j.jmr.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques JP, Bowtell R. Application of Fourier-based method for rapid calculation of field inhomogeneity due to spatial variation of magnetic susceptibility. Concepts Magn Reson B Magn Reson Eng. 2005;25:65–78. [Google Scholar]
- Mitsumori F, Watanabe H, Takay N, Garwood M, Auerbac EJ, Michaeli S, Mangia S. Toward understanding transverse relaxation in human brain through its field dependence. Magn Reson Med. 2012;68:947–953. doi: 10.1002/mrm.23301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelavalli J, Cheng YCN, Jiang J, Haacke EM. Removing background phase variations in susceptibility weighted imaging using a fast, forward-field calculation. J Magn Reson Imaging. 2009;29:937–948. doi: 10.1002/jmri.21693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg RJ, Langston JW, Haacke EM, Steen RG, Taylor JS. The correlation between phase shifts in gradient-echo MR images and regional brain iron concentration. Magn Reson Imaging. 1999;17:1141–1148. doi: 10.1016/s0730-725x(99)00017-x. [DOI] [PubMed] [Google Scholar]
- Ordidge R, Gorell J, Deniau J, Knight R, Helpern J. Assessment of relative brain iron concentrations using T2-weighted and T*2-weighted MRI at 3 Tesla. Magn Reson Med. 1994;32:335–341. doi: 10.1002/mrm.1910320309. [DOI] [PubMed] [Google Scholar]
- Peters AM, Brookes MJ, Hoogenraad FG, Gowland PA, Francis ST, Morris PG, Bowtell R. T2* measurements in human brain at 1.5, 3 and 7 T. Magn Reson Imaging. 2007;25:748–753. doi: 10.1016/j.mri.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Adalsteinsson E, Garrick T, Harper C. Postmortem MR imaging of formalin-fixed human brain. Neuroimage. 2004;21:1585–1595. doi: 10.1016/j.neuroimage.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Reichenbach J, Venkatesan R, Yablonskiy D, Thompson M, Lai S, Haacke E. Theory and application of static field inhomogeneity effects in gradient-echo imaging. Magn Reson Imaging. 1997;7:266–279. doi: 10.1002/jmri.1880070203. [DOI] [PubMed] [Google Scholar]
- Schenck JF. Health and physiological effects of human exposure to whole-body four-Tesla magnetic fields during MRI. Ann N Y Acad Sci. 1992;649:285–301. doi: 10.1111/j.1749-6632.1992.tb49617.x. [DOI] [PubMed] [Google Scholar]
- Schenck JF, Zimmerman EA. High-field magnetic resonance imaging of brain iron: birth of a biomarker? NMR Biomed. 2004;17:433–445. doi: 10.1002/nbm.922. [DOI] [PubMed] [Google Scholar]
- Schmierer K, Wheeler-Kingshott CAM, Tozer DJ, Boulby PA, Parkes HG, Yousry TA, Scaravilli F, Barker GJ, Tofts PS, Miller DH. Quantitative magnetic resonance of postmortem multiple sclerosis brain before and after fixation. Magn Reson Med. 2008;59:268–277. doi: 10.1002/mrm.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweser F, Deistung A, Lehr BW, Reichenbach JR. Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? Neuroimage. 2011;54:2789–2807. doi: 10.1016/j.neuroimage.2010.10.070. [DOI] [PubMed] [Google Scholar]
- Schweser F, Sommer K, Deistung A, Reichenbach JR. Quantitative susceptibility mapping for investigating subtle susceptibility variations in the human brain. Neuroimage. 2012;62:2083–2100. doi: 10.1016/j.neuroimage.2012.05.067. [DOI] [PubMed] [Google Scholar]
- Shmueli K, de Zwart JA, van Gelderen P, Li TQ, Dodd SJ, Duyn JH. Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data. Magn Reson Med. 2009;62:1510–1522. doi: 10.1002/mrm.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmueli K, Dodd SJ, Li TQ, Duyn JH. The contribution of chemical exchange to MRI frequency shifts in brain tissue. Magn Reson Med. 2011;65:35–43. doi: 10.1002/mrm.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis IW, Paley MNJ, Graham JM, Grunewald RA, Wignall EL, Joy HM, Griffiths PD. MRI assessment of basal ganglia iron deposition in Parkinson’s disease. J Magn Reson Imaging. 2008;28:1061–1067. doi: 10.1002/jmri.21563. [DOI] [PubMed] [Google Scholar]
- Web SM. AIP Conf Proc. 2010;1365:196–199. http://dx.doi.org/10.1063/1.3625338. [Google Scholar]
- Wharton S, Bowtell R. Whole-brain susceptibility mapping at high field: a comparison of multiple- and single-orientation methods. Neuroimage. 2010;53:515–525. doi: 10.1016/j.neuroimage.2010.06.070. [DOI] [PubMed] [Google Scholar]
- Yao B, Li TQ, Gelderen PV, Shmueli K, de Zwart JA, Duyn JH. Susceptibility contrast in high field MRI of human brain as a function of tissue iron content. Neuroimage. 2009;44:1259–1266. doi: 10.1016/j.neuroimage.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Haacke EM, Webb S, Nichol H. Imaging of stroke: a comparison between X-ray fluorescence and magnetic resonance imaging methods. Magn Reson Imaging. 2012;30:1416–1423. doi: 10.1016/j.mri.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong K, Leupold J, von Elverfeldt D, Speck O. The molecular basis for gray and white matter contrast in phase imaging. Neuroimage. 2008;40:1561–1566. doi: 10.1016/j.neuroimage.2008.01.061. [DOI] [PubMed] [Google Scholar]