Abstract
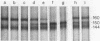
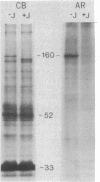
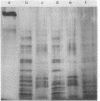
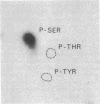
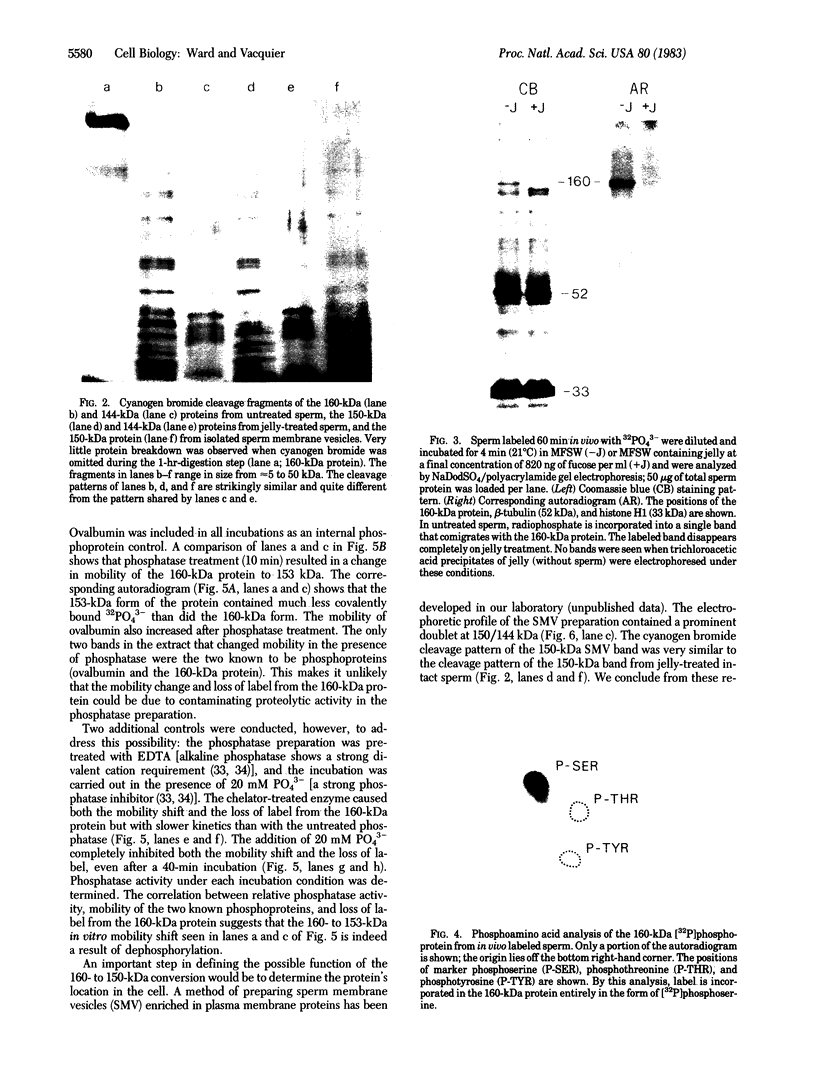
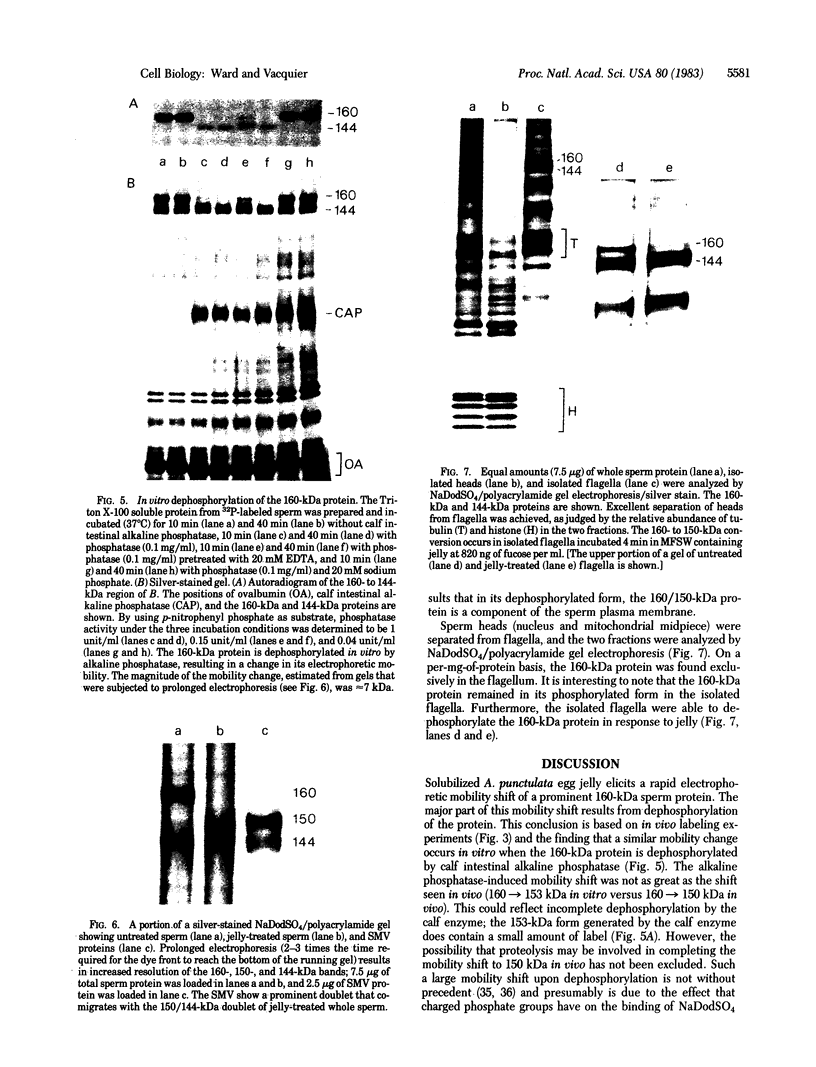
A 160-kilodalton (kDa) protein of Arbacia punctulata sperm is constitutively phosphorylated on serine residues, as shown by in vivo32PO43- labeling. Exposure of sperm to solubilized egg jelly results in the immediate dephosphorylation (within 5 sec) of this protein and a simultaneous increase in its electrophoretic mobility (to an apparent molecular mass of 150 kDa). In its dephosphorylated form (150 kDa), the protein is a major component of the flagellar plasma membrane. In fact, silver-stained polyacrylamide gels show the 160/150-kDa protein to be the third most abundant protein in the whole flagellum. The spermatozoan must pass through the egg jelly layer on its way to the egg surface. The jelly-induced dephosphorylation of such an abundant sperm membrane protein may be an important event in the successful interaction of sperm and egg during fertilization.
Keywords: Arbacia punctulata, sperm activation, sperm phosphoproteins, flagellar proteins
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Hunter T. Two structurally and functionally different forms of the transforming protein of PRC II avian sarcoma virus. Mol Cell Biol. 1982 Aug;2(8):890–896. doi: 10.1128/mcb.2.8.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier W., Singer S. J. On the mechanism of ATP-induced shape changes in human erythrocyte membranes. II. The role of ATP. J Cell Biol. 1977 Jun;73(3):647–659. doi: 10.1083/jcb.73.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collins F. A reevaluation of the fertilizin hypothesis of sperm agglutination and the description of a novel form of sperm adhesion. Dev Biol. 1976 Apr;49(2):381–394. doi: 10.1016/0012-1606(76)90182-2. [DOI] [PubMed] [Google Scholar]
- Collins F., Epel D. The role of calcium ions in the acrosome reaction of sea urchin sperm: regulation of exocytosis. Exp Cell Res. 1977 Apr;106(1):211–222. doi: 10.1016/0014-4827(77)90258-0. [DOI] [PubMed] [Google Scholar]
- Conway A. F., Metz C. B. Phospholipase activity of sea urchin sperm: its possible involvement in membrane fusion. J Exp Zool. 1976 Oct;198(1):39–47. doi: 10.1002/jez.1401980106. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross N. L. Isolation and electrophoretic characterization of the plasma membrane of sea-urchin sperm. J Cell Sci. 1983 Jan;59:13–25. doi: 10.1242/jcs.59.1.13. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Galani-Kranias E., Bick R., Schwartz A. Phosphorylation of a 100 000 dalton component and its relationship to calcium transport in sarcoplasmic reticulum from rabbit skeletal muscle. Biochim Biophys Acta. 1980 Apr 3;628(4):438–450. doi: 10.1016/0304-4165(80)90393-1. [DOI] [PubMed] [Google Scholar]
- Garbers D. L., Kopf G. S. The regulation of spermatozoa by calcium cyclic nucleotides. Adv Cyclic Nucleotide Res. 1980;13:251–306. [PubMed] [Google Scholar]
- Garbers D. L., Tubb D. J., Kopf G. S. Regulation of sea urchin sperm cyclic AMP-dependent protein kinases by an egg associated factor. Biol Reprod. 1980 Apr;22(3):526–532. doi: 10.1093/biolreprod/22.3.526. [DOI] [PubMed] [Google Scholar]
- Garbers D. L., Watkins H. D., Hansbrough J. R., Smith A., Misono K. S. The amino acid sequence and chemical synthesis of speract and of speract analogues. J Biol Chem. 1982 Mar 25;257(6):2734–2737. [PubMed] [Google Scholar]
- Hansbrough J. R., Garbers D. L. Sodium-dependent activation of sea urchin spermatozoa by speract and monensin. J Biol Chem. 1981 Mar 10;256(5):2235–2241. [PubMed] [Google Scholar]
- Hansbrough J. R., Garbers D. L. Speract. Purification and characterization of a peptide associated with eggs that activates spermatozoa. J Biol Chem. 1981 Feb 10;256(3):1447–1452. [PubMed] [Google Scholar]
- Hunter T., Hutchinson M. A., Eckhart W., Friedmann T., Esty A., La Porte P., Deininger P. Organization of T antigens in the polyoma virus genome. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):131–139. doi: 10.1101/sqb.1980.044.01.015. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee H. C., Johnson C., Epel D. Changes in internal pH associated with initiation of motility and acrosome reaction of sea urchin sperm. Dev Biol. 1983 Jan;95(1):31–45. doi: 10.1016/0012-1606(83)90004-0. [DOI] [PubMed] [Google Scholar]
- Levine A. E., Walsh K. A., Fodor E. J. Evidence of an acrosin-like enzyme in sea urchin sperm. Dev Biol. 1978 Apr;63(2):299–306. doi: 10.1016/0012-1606(78)90135-5. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nikodem V., Fresco J. R. Protein fingerprinting by SDS-gel electrophoresis after partial fragmentation with CNBr. Anal Biochem. 1979 Sep 1;97(2):382–386. doi: 10.1016/0003-2697(79)90089-7. [DOI] [PubMed] [Google Scholar]
- Sano M. Subcellular localizations of guanylate cyclase and 3',5'-cyclic nucleotide phosphodiesterase in sea urchin sperm. Biochim Biophys Acta. 1976 Apr 23;428(2):525–531. doi: 10.1016/0304-4165(76)90061-1. [DOI] [PubMed] [Google Scholar]
- Schackmann R. W., Christen R., Shapiro B. M. Membrane potential depolarization and increased intracellular pH accompany the acrosome reaction of sea urchin sperm. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6066–6070. doi: 10.1073/pnas.78.10.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schackmann R. W., Shapiro B. M. A partial sequence of ionic changes associated with the acrosome reaction of Strongylocentrotus purpuratus. Dev Biol. 1981 Jan 15;81(1):145–154. doi: 10.1016/0012-1606(81)90357-2. [DOI] [PubMed] [Google Scholar]
- Schroeder T. E., Christen R. Polymerization of actin without acrosomal exocytosis in starfish sperm. Visualization with NBD-phallacidin. Exp Cell Res. 1982 Aug;140(2):363–371. doi: 10.1016/0014-4827(82)90125-2. [DOI] [PubMed] [Google Scholar]
- SeGall G. K., Lennarz W. J. Chemical characterization of the component of the jelly coat from sea urchin eggs responsible for induction of the acrosome reaction. Dev Biol. 1979 Jul;71(1):33–48. doi: 10.1016/0012-1606(79)90080-0. [DOI] [PubMed] [Google Scholar]
- SeGall G. K., Lennarz W. J. Jelly coat and induction of the acrosome reaction in echinoid sperm. Dev Biol. 1981 Aug;86(1):87–93. doi: 10.1016/0012-1606(81)90318-3. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Nomura K., Ohtake H., Isaka S. Purification and the primary structure of sperm-activity peptides from the jelly coat of sea urchin eggs. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1238–1244. doi: 10.1016/0006-291x(81)90752-x. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Swarup G., Garbers D. L. Phosphoprotein phosphatase activity of sea urchin spermatozoa. Biol Reprod. 1982 Jun;26(5):953–960. doi: 10.1095/biolreprod26.5.953. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Kiehart D. P., Sardet C., Tilney M. Polymerization of actin. IV. Role of Ca++ and H+ in the assembly of actin and in membrane fusion in the acrosomal reaction of echinoderm sperm. J Cell Biol. 1978 May;77(2):536–550. doi: 10.1083/jcb.77.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsanyi M., Heilmeyer L. M., Jr Phosphorylation of the 100 000 Mr Ca2+-transport ATPase by Ca2+ or cyclic AMP-dependent and -independent protein kinases. FEBS Lett. 1981 Aug 31;131(2):223–228. doi: 10.1016/0014-5793(81)80372-9. [DOI] [PubMed] [Google Scholar]
- Ward G. E., Vacquier V. D., Michel S. The increased phosphorylation of ribosomal protein S6 in Arbacia punctulata is not a universal event in the activation of sea urchin eggs. Dev Biol. 1983 Feb;95(2):360–371. doi: 10.1016/0012-1606(83)90037-4. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Matsui T., Aketa K. Purification and characterization of a chymotrypsin-like enzyme from sperm of the sea urchin, Hemicentrotus pulcherrimus. Eur J Biochem. 1982 Feb;122(1):57–62. doi: 10.1111/j.1432-1033.1982.tb05847.x. [DOI] [PubMed] [Google Scholar]