Abstract
In women, ovary and adrenal gland produce androgens. Androgens are essential drivers of the primordial to antral follicle development, prior to serving as substrate for estrogen production in the later stages of folliculogenesis. Androgens play a crucial role in the follicular–stromal intertalk by fine tuning the extracellular matrix and vessel content of the ovarian stroma. Local auto-and paracrine factors regulate androgen synthesis in the pre-antral follicle. Androgen excess is a hallmark of polycystic ovary syndrome and is a key contributor in the exaggerated antral follicle formation, stromal hyperplasia and hypervascularity. Hyperandrogenaemia overrides the follicular–stromal dialog, resulting in follicular arrest and disturbed ovulation. On the other hand, androgen deficiency is likely to have a negative impact on fertility as well, and further research is needed to examine the benefits of androgen-replacement therapy in subfertility.
Keywords: androgen, folliculogenesis, stroma, angiogenesis, polycystic ovary syndrome
Introduction
The effect of androgens on female fertility is an emerging field in reproductive science at the interface of endocrinology and gynecology. In the following section, the authors aim to describe androgen action on the early growing follicle and its environment, and highlight new implications and developments in the field.
Follicle unit and ovarian structure
The single follicle is the fundamental unit of the ovary and is composed of an oocyte surrounded by specialized endocrine cells. It produces peptide hormones and sex steroids that modulate the maturation of the oocyte and regulate follicle cell growth and differentiation through local auto- and paracrine-signaling pathways. Secreted into the bloodstream, these hormones also exert endocrine effects and prepare the reproductive organs for fertilization and implantation. Primordial follicles are continuously recruited for growth, and this phenomenon, as well as the subsequent stages of follicular development, is considered to be locally regulated and independent of gonadotrophin action. While the majority of growing follicles are lost in atresia, a small cohort of antral follicles is recruited for further growth, dominance and ovulation under the cyclic stimulation of gonadotrophins.
The outer cortex of the ovary contains immature follicles and is a rigid, avascular environment. It is made up of tightly packed spindle-shaped fibroblasts, vasculature-related cells (smooth muscle cells and endothelial cells), inflammatory cells and precursor theca cells. The inner medulla is more elastic and composed of loose connective tissue and ovarian vasculature. The ovarian stroma consists of connective tissue and its extracellular matrix sustains the ovarian architecture and provides structural support to the growing follicles. The stroma also produces a variety of cytokines, chemokines and growth factors that tightly co-regulate—in an autocrine and paracrine fashion—the early growth phase of its enclosed follicles.
Androgens
In women, the main sources of circulating androgens are the adrenal glands and ovaries (Arlt, 2006). Dehydroepiandrosterone (DHEA), mainly from the adrenal glands, acts as a crucial precursor of sex steroids in the ovary and other target tissues (Labrie, 2010). Depending on the intracellular availability of steroidogenic enzymes in target tissues, DHEA is converted to androstenedione and testosterone, both of which can be aromatized to estrogens. Testosterone can also be converted to the much more potent 5α-dihydrotestosterone (DHT). Testosterone and DHT are the only two hormones that bind and activate the androgen receptor (AR). Serum levels of DHEA and androgens peak in the early reproductive years, followed by a steep decline with age (Davison et al., 2005).
Androgen action
Androgens exert their action mainly through the AR. The AR functions as a ligand-activated nuclear transcription factor (Gelmann, 2002). It has recently become clear that many effects of androgens in non-ovarian target tissues depend on other complex-signaling pathways, including rapid non-genomic pathways. The reported non-genomic effects of androgens at physiological concentrations appear to be mediated through the cytosolic AR, involved in the activation of mitogen-activated protein kinase-extracellular signal-related kinase (ERK) pathways (Kousteni et al., 2001). There is evidence that this non-genomic stimulation results in an enhancement of AR transcriptional activation, thereby creating an autocrine loop between AR and its ligand (Heinlein and Chang, 2002a, b). It is currently unclear whether androgens exert non-genomic actions in the ovary. Interestingly, the activation of the PI3-K/Akt pathway in the minutes following testosterone supplementation has been reported in neonatal mouse ovaries, an effect reverted by the AR-antagonist flutamide and suggestive of non-genomic androgen signaling (Yang et al., 2010).
AR expression in the ovary
ARs are expressed in all cell types of the ovarian follicle, including the oocyte, granulosa and theca cells (Sen and Hammes, 2010). Few studies have examined the distribution of AR in the connective tissue or stromal compartment. In bovine, non-human primates and humans, AR is expressed in the cortical stroma (Horie et al., 1992; Weil et al., 1998; Yang and Fortune, 2006). In rodents, cattle, primates and human, increasing concentrations of AR are detected in granulosa cells from the primary stage onward, peaking in the antral stage (Weil et al., 1998; Yang and Fortune, 2006; Rice et al., 2007; Sen and Hammes, 2010). The crucial role of the granulosa AR has been demonstrated by the phenotype of the granulosa cell-specific AR knockout mouse, which is subfertile, has reduced follicle progression, fewer ovulations and reduced litter size (Sen and Hammes, 2010).
Ovarian androgen supply
Primordial and primary follicles within the avascular ovarian cortex rely on passive diffusion of nutrients and growth factors from the surrounding stroma and the systemic circulation. Steroid hormones are lipophilic and can therefore readily enter cells (Oren et al., 2004); however, a central to periphery diffusion gradient is to be expected as steroids produced by growing follicles, and those delivered by the systemic circulation provide from the central medulla. The ovarian stroma seems to be steroidogenically silent in physiological premenopausal conditions (Young and McNeilly, 2010). Studies of fetal human ovaries have shown that the oocyte of the primordial follicle has the steroidogenic machinery in place to synthetize androgens, and that pre-granulosa cells express AR (Fowler et al., 2011). The relevance of this steroidogenic pathway remains currently unknown.
In the secondary stage, theca cells are recruited from the surrounding stroma and participate in providing a vascular network to the growing follicle (Young and McNeilly, 2010). Expression of the required steroidogenic enzymes (Star, P450c11a1, P450c17 and HSD3b), as well as luteinizing hormone (LH) receptor, begin at this stage and allow the theca cells to produce androgens (Logan et al., 2002).
Androgen effects on early stage follicles
Primordial to primary follicle transition
The primordial follicle is the smallest, and contains a meiotically arrested oocyte surrounded by squamous granulosa cells. The primordial follicle pool represents the reserve of follicles available and therefore determines the fertility potential of a female. This follicle reserve is established during fetal development in humans and in the immediate post-natal period in mice (Peters et al., 1975). The number of follicles within the reserve gradually declines via atresia with increasing age. Although this pool of follicles is considered to exist in a resting state, it is also dynamically and tightly regulated with continuous follicular fate decisions: many follicles will be lost to atresia, a large number remain dormant and only a select few are recruited into the growing pool (Tingen et al., 2009; Kim, 2012; Sanchez and Smitz, 2012). The first indication that a primordial follicle has been recruited to enter the growing pool is the change in granulosa cell shape, from squamous to cuboidal, followed by proliferation of the granulosa cells. On a molecular level, the phosphoinoside 3-kinase (PI3K) pathway seems to be central in regulating fate decisions in primordial follicles (Castrillon et al., 2003; Reddy et al., 2008). A basal degree of intra-oocyte PI3K activation is required for survival throughout the long dormancy of these follicles (Reddy et al., 2010). At the same time, PI3K signaling is inhibited by several molecules, such as PTEN-PDK1 (Reddy et al., 2008; Reddy et al., 2009) and FOXO3 (Castrillon et al., 2003; Liu et al., 2007; John et al., 2008), which ensures sustained dormancy of the primordial follicle pool. Inactivation of these PI3K repressors by KIT ligand and other growth factors plays a crucial role in the recruitment of the primordial follicle, at least in mice (Sanchez and Smitz, 2012).
Testosterone rapidly increases the intra-oocyte PI3K/Akt/FOXO3 pathway in mouse primordial follicles, increasing by >2-fold the ratio of primary to primordial follicles (Yang et al., 2010). Similar non-genomic activation of the PI3K/Akt pathway by androgens has been described in other target cells (Baron et al., 2004; Kang et al., 2004; Cinar et al., 2007). In rhesus monkeys, testosterone treatment appears to promote primordial follicle activation, with elevated intra-oocyte IGF1 signaling (Vendola et al., 1999a, b). It is unclear how androgens exert this effect, as AR is not detected, or is below the limit of detection, in this follicle class (Weil et al., 1998). Nevertheless, it is interesting that IGF1 receptor-mediated protection from apoptosis relies on PI3K activation (Shelton et al., 2004).
Primary to secondary follicle transition
Once activated, the oocyte begins to grow, forms the zona pellucida and establishes cell–cell contact with the surrounding granulosa cells. Intercellular communication occurs through gap junctions and is of paramount importance for the follicle, which must remain a coupled and coordinated unit throughout development. In fetal bovine ovaries, mid-gestational exposure to the anti-androgen flutamide decreases connexin 43 expression and the number of granulosa cell-oocyte gap junctions (Knapczyk-Stwora et al., 2013). In contrast, in a luteinized granulosa cell line, androgen excess decreases connexin 43 expression (Wu et al., 2010). Differences in granulosa differentiation could possibly explain this dual effect.
The secondary follicle is characterized by the acquisition of a second layer of granulosa cells. Testosterone stimulates, in a dose-dependent manner, the primary to secondary follicle transition in fetal bovine ovaries, and this effect is inhibited by the AR antagonist flutamide (Yang and Fortune, 2006). The mitogenic properties of androgens could be mediated partly through increased glucose metabolism via Glut4 signaling (Sato et al., 2008). In rhesus monkeys, testosterone administration up-regulates the expression of its own receptor in the granulosa cell (Weil et al., 1998); similar findings have been reported in ovaries from testosterone-treated transsexual women (Chadha et al., 1994).
Pre-antral to antral follicle transition
As the granulosa cell layers expand, the expression of follicle-stimulating hormone (FSH)- and LH receptors is detected, and although growth remains under control of intra-ovarian regulators at this stage, the follicle becomes gonadotrophin responsive. At this point, an antrum begins to form and granulosa cells differentiate toward mural and cumulus cell phenotypes. The most important development at this stage is theca cell recruitment and differentiation, with acquisition of steroidogenic function and neo-angiogenesis (Young and McNeilly, 2010), allowing the follicle to interact with systemic endocrine factors and enter the gonadotrophin-dependent phase.
Theca cell function is initially under auto/paracrine control, mainly by members of the tumor growth factor (TGF)-beta superfamily. One member of this family is GDF-9, an oocyte-derived growth differentiation factor. GDF-9 knockout mice fail to establish a theca cell layer (Dong et al., 1996). GDF-9 is also essential for inducing P450c17 expression and androgen synthesis in pre-antral follicles (Vitt et al., 2000b; Orisaka et al., 2009), while suppressing P450c19 (aromatase) activity (Vitt et al., 2000a), thereby ensuring an androgen-rich environment. Other members of the TGF-beta superfamily, the bone morphogenic proteins (BMP-4, BMP-6, BMP-7), act via insulin-like peptide 3 to down-regulate P450c17 and have shown to be potent suppressors of basal and LH-induced thecal androgen production (Glister et al., 2005; Glister et al., 2013). Several local-binding proteins, such as gremlin, chordin and follistatin, can reverse BMP proteins' function (Glister et al., 2005). In antral follicles, granulosa cells produce activin that suppresses androgen synthesis, and this effect is opposed by inhibin (Knight et al., 2012). Insulin, in synergy with LH, induces P450c17 and stimulates thecal androgen production (Franks et al., 1999), and KIT ligand promotes thecal function by Erk1/2-mediated up-regulation of steroidogenic factor 1 (Jin et al., 2005).
Anti-Müllerian hormone (AMH) is another member of the TGF-beta superfamily. In females, AMH is exclusively produced by granulosa cells from the primary stage onward, with peak levels in follicles that have reached the stage at which they may be selected for dominance (pre-antral stage in mice, antral stage in women), followed by a decline in granulosa cell AMH (but not in cumulus cells) as the follicle enters the gonadotrophin-dependent phase (Durlinger et al., 2002; Weenen et al., 2004; La Marca et al., 2009; Andersen et al., 2010; Dumesic and Richards, 2013). AMH levels reflect antral follicle count, and the serum AMH level has become an established clinical marker for ovarian reserve (Pigny et al., 2006; Kristensen et al., 2012; Visser et al., 2012). In mice (Durlinger et al., 2002) and in humans (Carlsson et al., 2006), AMH inhibits the primordial to primary follicle transition. Additionally, AMH has shown to decrease the FSH sensitivity of mouse antral follicles (Durlinger et al., 2001) and functions as a break for FSH recruitment of antral follicles. In vitro, AMH decreases the FSH-induced expression of aromatase in granulosa cells (Grossman et al., 2008), thereby increasing the intrafollicular androgen/estrogen ratio. In normo- and hyperandrogenic women, circulating levels of testosterone and AMH are positively correlated (Eldar-Geva et al., 2005; Piltonen et al., 2005; Nardo et al., 2009). In individual follicles, a similar positive association between the expression of AMH and AR is observed in polycystic ovary syndrome (PCOS) women and controls (Catteau-Jonard et al., 2008), suggesting an AR-driven stimulation of AMH synthesis.
In human small antral follicles, a positive correlation between follicular fluid androgen levels and FSH-receptor (FSH-R) expression in granulosa cells has recently been observed (Nielsen et al., 2011). Testosterone administration to rhesus monkeys up-regulates AR and FSH-R expression in granulosa cells, thereby robustly stimulating antral follicle growth (Vendola et al., 1998; Weil et al., 1998; Weil et al., 1999). Ligand-activated AR amplifies FSH action by increasing cAMP-mediated post-receptor signaling (Hillier and Tetsuka, 1997). This synergetic effect between androgens and FSH has also been demonstrated using in vitro murine follicular culture models (Murray et al., 1998; Wang et al., 2001; Lenie and Smitz, 2009).
Thus, at the antral stage, androgens, through the AR, not only enhance their own action but also prime the follicle for later FSH action, which in turn modulates LH responsiveness. We postulate that androgens stimulate AMH secretion, although this has not been demonstrated yet. This scenario, depicted in Fig. 1, would explain how the growing follicle is ‘protected’ from premature recruitment by FSH until it reaches the maturation stage for selection for dominance (Visser et al., 2006).
Figure 1.
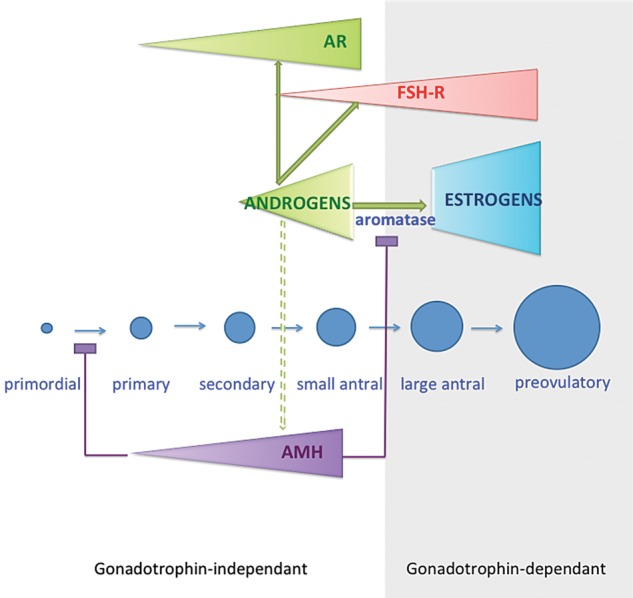
Working model for androgen action in the pre-antral follicle. Androgens are secreted by theca cells from the secondary stage on, and amplify their local effects by increasing their own receptor expression and activity. Androgens induce the expression of FSH-R and prime the follicle for further FSH-driven follicular growth and maturation. We postulate that androgens stimulate AMH secretion that in turn inhibits FSH-induced aromatase expression thereby maintaining a predominantly androgenic intrafollicular milieu. In this scenario the growing follicle is ‘protected’ from premature selection by FSH.
Androgen effects on stroma and vasculature
Androgenized female-to-male transsexuals exhibit diffuse hyperplasia of the ovarian stroma with excessive collagen accumulation (Ikeda et al., 2013). In vitro, testosterone increases type I collagen production by fibroblasts (Jenkins et al., 2007). In the ovary and the endometrium, sex steroids regulate the expression of vascular endothelial growth factor (VEGF) (Shweiki et al., 1993). This endothelial-specific mitogen plays a crucial role in the natural neo-angiogenesis that occurs in the female reproductive tract (Perrot-Applanat et al., 2000). Clear spatiotemporal expression patterns of VEGF mRNA in steroid-producing cells and VEGF-R mRNA in adjacent endothelial cells are observed during various processes, such as neovascularization of the ovarian follicle, angiogenesis of the corpus luteum, tissue repair and uterine implantation in mice (Shweiki et al., 1993). VEGF and its receptor have been localized to human pre-antral follicles and the surrounding stroma (Abir et al., 2010). In vitro studies demonstrate that ligand-activated AR induces VEGF expression in human fetal prostatic fibroblasts (Levine et al., 1998) and in human aortic endothelial cells (Cai et al., 2011). Androgens activate hypoxia-inducible factor 1 (HIF1) (Mabjeesh et al., 2003; Shafighi et al., 2012), a known transcriptional activator of VEGF (Ferrara, 2004), and this AR-mediated effect is enhanced in hypoxic conditions (Mitani et al., 2011; Park et al., 2012). In human vascular endothelial and smooth muscle cells, androgen has a proliferative effect (Nheu et al., 2011). The androgen precursor DHEA is known to stimulate endothelial proliferation and angiogenesis through extracellular signal-regulated kinase 1/2-mediated mechanisms (Liu et al., 2008).
These in vitro studies throw new light on the mechanisms of androgen-promoted vascular proliferation in steroid target tissues. To date, the androgen effects on ovarian vasculogenesis remain unexplored. In the light of the ovarian cortical hypervascularity observed in androgen-excess conditions such as PCOS (see below), it would be worth investigating if a similar mechanism occurs in the ovary in vivo. A working model for androgen-promoted angiogenesis in the ovary is shown in Fig. 2.
Figure 2.
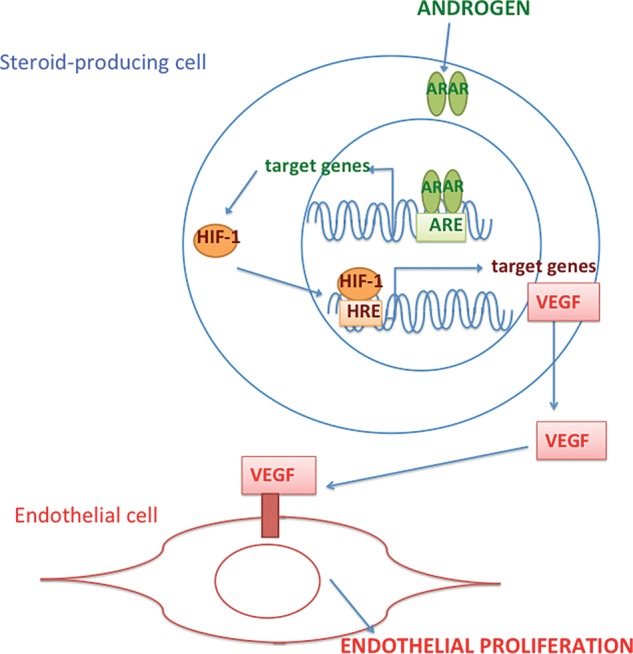
Working model for androgen action on endothelial cells. In steroid-producing cells, ligand-activated AR induces HIF-1 (Hypoxia-inducible factor 1) expression. HIF-1 is a transcription factor for VEGF. Secreted VEGF binds membrane receptors on the neighboring endothelial cells and potently stimulates proliferation.
New developments: implications for androgen excess and PCOS
PCOS is the most frequent cause of oligoanovulation and hyperandrogenaemia and affects 5–10% of women of reproductive age. PCOS is a heterogeneous condition, with a range of reproductive and long-term metabolic complications. A detailed description of this condition is beyond the scope of this review and is discussed elsewhere (Ehrmann et al., 1995; Fauser et al., 2012).
The etiology of PCOS is still unclear, but likely results from a genetic–environmental interaction. Animal studies are an important tool to study the effects of hyperandrogenaemia on the ovary and the metabolic system. Prenatally androgenized monkey (Abbott and Bacha, 2013) and sheep (Veiga-Lopez et al., 2011) exhibit adult reproductive and metabolic features that mimic PCOS in women. These important studies have focused the attention of the field on the role of prenatal/fetal androgen excess in the development of PCOS and metabolic syndrome later in life.
A similar reproductive–metabolic PCOS phenotype is obtained in rodents exposed to high doses of DHT before puberty (Manneras et al., 2007; van Houten et al., 2012). Findings through rodent PCOS models make extrapolation to the human situation more complex as, in contrast to women, rodents are poly-ovulators and lack adrenal androgen production. Also, the DHT doses employed are supraphysiologic compared with the PCOS situation.
With the physiological effects of androgens on early follicular development, stroma and ovarian vasculature in mind, we propose a ‘fresh’ look at this prevalent condition.
Intrinsic deregulation of early follicular development
The characteristic morphological feature of PCOS is the accumulation of small antral follicles in the ovarian cortex (Balen et al., 2003). There is growing consensus that follicular development in PCOS is deregulated from the very early, gonadotrophin-independent stage onward, resulting in follicular arrest and disruption of dominant follicle selection (Webber et al., 2003; Franks et al., 2008; Franks and Hardy, 2010). Hyperandrogenaemia is likely to be a key mediator in this process, but certainly not the only factor involved. Hyperinsulinaemia resulting from insulin resistance in PCOS is another prominent contributor (Nestler et al., 1998; Diamanti-Kandarakis and Dunaif, 2012). Intra-ovarian androgen excess reflects an intrinsic abnormality in theca cell function (Ehrmann et al., 1995), resulting from an increase in steroidogenic enzyme activity (Nelson et al., 2001; Franks and Hardy, 2010). LH- and insulin excess jointly promote thecal Cyp17 activity and subsequent androgen production (Bergh et al., 1993; Dumesic and Richards, 2013).
The elevated AMH levels typically observed in women with PCOS are probably due to a combination of an increased antral follicle pool (Pigny et al., 2006; Hart et al., 2010) coupled with an elevated production of AMH by individual granulosa cells (Dunson et al., 2002; Nardo et al., 2009; Arabzadeh et al., 2010). Androgen-lowering treatments typically decrease AMH levels in PCOS (Piltonen et al., 2005; Amer et al., 2009; Falbo et al., 2010). AMH-induced aromatase inhibition (Grossman et al., 2008) results in an even greater androgenic milieu, which negatively influences the delicate balance between androgens and FSH. In PCOS, circulating FSH levels are thought to be insufficient to reach the increased FSH threshold of the follicle required for selection (Hillier, 1994; Franks and Hardy, 2010). Therefore, restoring the androgen/FSH balance in PCOS by administration of low-dose exogenous FSH during controlled ovarian stimulation typically yields multiple oocytes.
Although there are concerns regarding the effect of androgens on oocyte quality and embryonic developmental competence for review, see Qiao and Feng, 2011), there is little evidence that impaired oocyte function is a significant contributor to PCOS subfertility, as normal cumulative conception rates can be achieved with appropriate ovulation-restoring treatment (Fauser et al., 2012).
Stromal hyperplasia, rigidity, hypervascularity and inflammation
Polycystic ovaries have an increased ovarian stromal volume, and ultrasound measurements correlate with the degree of hyperandrogenism (Kyei-Mensah et al., 1998; Fulghesu et al., 2007). Histological assessment of polycystic ovaries reveals increased thickness of the cortical and medullar stroma and hypervascularity (Hughesdon, 1982). Microarray data in PCOS show differential expression of genes involved in extracellular matrix formation (Jansen et al., 2004). Proteomic research in PCOS identifies differential expression of several proteins and filaments involved in fibrogenesis (Ma et al., 2007). PCOS women have higher levels of basic fibroblast growth factor in serum and follicular fluid (Artini et al., 2006). This exaggerated fibroblast proliferation, coupled with an increased extracellular matrix deposition greatly increases the rigidity of the ovarian cortical tissue. An important question is whether this stromal hyperrigidity in PCOS alters the steroidogenic behavior of the antral follicles and contributes to hyperandrogenaemia (Woodruff and Shea, 2011). Although non-permissive or ‘rigid’ culture conditions for mouse in vitro follicular culture are associated with decreased steroid production, this effect has only been studied in larger multi-layered follicles thus far (Xu et al., 2006).
Zaidi et al. (1995) used color Doppler ultrasound to demonstrate significantly increased blood flow velocity in polycystic ovaries, with blood vessels running in an almost linear configuration in the ovarian stroma. This vascular increase is mainly observed in the cortical area (Delgado-Rosas et al., 2009). Circulating VEGF levels are typically higher in PCOS and are a reliable marker for ovarian stromal blood flow (Agrawal et al., 1998). Laparoscopic ovarian drilling reduces Doppler indices of ovarian stromal blood flow (Parsanezhad et al., 2003), with concomitant reduction in circulating androgen (Kaaijk et al., 2000) and VEGF levels (El Behery et al., 2011). The increased blood supply to the PCOS cortex further fuels the connective tissue proliferation and, importantly, provides the enclosed follicles with inappropriate amounts of oxygen, nutrients, growth factors and hormones. The profoundly altered follicular microenvironment in PCOS is an important disruptor of the normal follicle dynamics.
PCOS is considered to be a low-grade inflammatory condition, as illustrated by elevated C-reactive protein (CRP) levels, inflammatory cytokines (such as IL-6) and hyperleucocytosis (Diamanti-Kandarakis et al., 2006). Further studies are required to determine the contribution of these inflammatory cells, and their secreted cytokines, chemokines and growth factors in the pathogenesis of PCOS. In physiological conditions, stromal cells differentiate as the follicle matures, and it has been postulated that ovarian macrophages are attracted toward activated follicles and stay associated with this follicle throughout its development (Tingen et al., 2011). In benign prostate hypertrophy, an androgen-dependent recruitment and infiltration of macrophages is well described and contributes to the stromal hyperplasia (Izumi et al., 2013). It is worth investigating if a similar epithelial–stromal interaction occurs in PCOS.
The dialog between the follicular and stromal compartment is an essential, and somewhat forgotten, concept that one has to keep in mind when unraveling the complex pathogenesis of PCOS. The described mutual interactions are visualized in Fig. 3.
Figure 3.
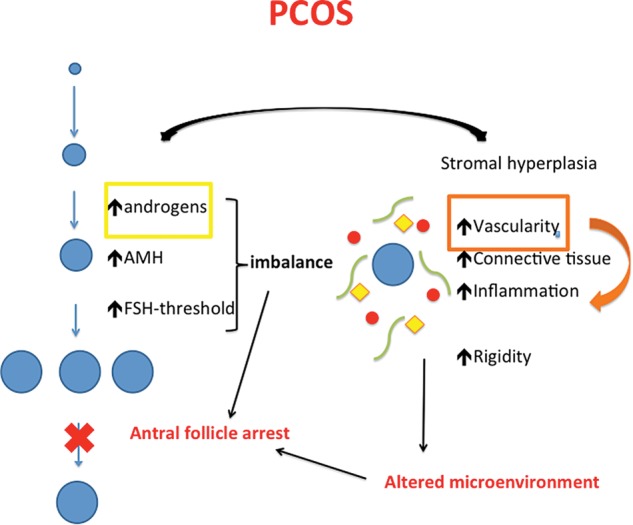
Working model for excess androgen action on follicle and stroma in PCOS. In PCOS, the disturbed balance between androgens, AMH and FSH leads to antral follicular arrest. Circulating FSH-levels are insufficient to reach the increased FSH-threshold of the follicles and selection for dominance does not occur. The hypervascular, rigid and inflammatory cortex negatively impacts the follicular dynamics. Exaggerated blood supply, partly mediated by local androgen overproduction, fuels the whole process. The blue ovals indicate follicles, red circles blood vessels, yellow squares inflammatory cells and green bowed lines stand for connective tissue.
Implications for clinical translation: androgen treatment in subfertility
Low ovarian reserve is characterized by an impaired quantity and quality of the ovarian follicles, resulting in a diminished fertility potential, with advancing age as an important determinant. In the last decade, the idea has emerged that androgen administration to the improperly growing follicles in this condition would result in a PCOS-like phenotype, thereby improving the oocyte yield during ovarian hyperstimulation. A number of controlled studies suggest that adjuvant DHEA and testosterone treatment can enhance fertility in women with low ovarian reserve (Casson et al., 2000; Barad and Gleicher, 2005; Barad and Gleicher, 2006; Wiser et al., 2010; Gleicher et al., 2010b; Gleicher and Barad, 2011; Sunkara and Coomarasamy, 2011). In a randomized, open-label trial in 33 women with low ovarian reserve, DHEA (75 mg/day) was associated with a higher oocyte yield and a significantly increased birth rate (Wiser et al., 2010). Decreased functional ovarian reserve not only results in longer time to conceive, but also comes with an increased risk for spontaneous miscarriage, aneuploidy and birth defects (Duncan et al., 2012). While mainstream thinking in the field incriminates degenerative changes that affect oocyte health (Navot et al., 1991), some researchers are opposed to the current dogma and speculate that primordial oocytes do not age, and that it is the follicular microenvironment that is prone to an age-related reduction in the quality (Gleicher and Barad, 2011; Gleicher et al., 2011). This hypothesis is based on limited clinical data suggesting that DHEA supplementation reduces age-related increases in aneuploidy (Gleicher et al., 2010a) and miscarriage (Gleicher et al., 2009). DHEA has the advantage of being a prohormone that is metabolized in target cells depending on the steroidogenic enzymes expressed, and therefore does not lead to supraphysiological androgen levels in the circulation. Despite the still weak clinical evidence and lack of rigorous randomized trials of sufficient size, it is estimated that approximately one-quarter of all IVF centers today use DHEA supplementation in women with low ovarian reserve (Sunkara et al., 2012).
Keeping in mind the crucial role of androgens in ovarian biology, we postulate that androgen deficiency has a negative impact on fertility. Female androgen excess is clearly recognized as detrimental for fertility, and the same causal relationship should be considered for androgen deficiency. Severe androgen deficiency is encountered in primary adrenal insufficiency (Addison's disease) due to the pathological loss in adrenal DHEA synthesis (Lebbe and Arlt, 2012). In a survey reporting on 269 Norwegian women with primary adrenal insufficiency, fertility was significantly reduced; the standardized incidence ratio for childbirth was 0.97 in the women before being diagnosed with adrenal failure, but dropped to 0.69 after the diagnosis had been established. This remained significantly reduced at 0.72 when excluding all women with premature ovarian failure (Erichsen et al., 2010). No studies thus far have looked at the effect of androgen-replacement therapy on fertility in adrenal insufficiency patients. The guidelines published by the Endocrine Society in 2006 recommend against making a diagnosis of androgen deficiency in women with normal adrenal function because of the lack of a clearly defined clinical syndrome, and of normative data on testosterone levels across women's lifespan that can be used to define the disorder (Wierman et al., 2006). Given recent publications and developments, it may be time to revisit this guidance, considering the development of mass-spectrometry-based highly sensitive testosterone assays, the availability of satisfactory forms of androgen administration and emerging data on short-term safety data on androgen replacement in women (for review, see Marie Lebbe, 2012).
Summary
During folliculogenesis, the maturing follicle undergoes dramatic shifts in its steroidogenic capacity and endocrine responsiveness. In the pre-antral stage, the follicle is an androgen-secreting and paracrine-signaling unit. Gonadotrophin receptor expression and responsiveness converts the follicle to a predominantly estradiol-secreting endocrine organ, with androgens as the essential substrate.
Androgens exert their effect via genomic and possibly non-genomic ways, and amplify their local effects via an autocrine stimulatory loop involving the AR. AR expression is highest in the small antral follicles, where the trophic effects of androgen are maximal and synergistic with FSH and AMH. Androgens stimulate extracellular matrix and possibly new blood vessel formation, especially in the low oxygen and low androgen milieu of the ovarian cortex.
Androgen excess in PCOS not only disturbs the delicate balance between androgens, AMH and FSH, but also crucially contributes to ovarian tissue remodeling: stromal hyperplasia and rigidity, hypervascularity and inflammation. This joint follicular–stromal deregulation is a key mechanism in the pathogenesis of PCOS. Future research is required to gain molecular insight into the central negative role of androgen excess in these processes, aiming to offer new therapeutic opportunities for restoring fertility in women with PCOS.
Further studies are required to determine which subgroup of subfertile women could benefit from the positive effects of androgens on pre-antral follicle development.
Authors' roles
M.L. conceived the article and wrote the first draft of this review. T.K.W. then commented on the manuscript and both authors approved the final version for publication.
Funding
This work was supported by Medical Research Council UK (Research Fellowship RRAK 16242 to M.L.) and by Project 1 of the U54HD076188 Center for Reproductive Health after Disease from the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Specialized Cooperative Centers Program in Reproduction and Infertility Research (SCCPIR) to T.K.W.
Conflict of interest
None declared.
Acknowledgements
The authors wish to thank Dr Stacey C. Tobin for editorial support.
References
- Abbott DH, Bacha F. Ontogeny of polycystic ovary syndrome and insulin resistance in utero and early childhood. Fertil Steril. 2013;100:2–11. doi: 10.1016/j.fertnstert.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abir R, Ao A, Zhang XY, Garor R, Nitke S, Fisch B. Vascular endothelial growth factor A and its two receptors in human preantral follicles from fetuses, girls, and women. Fertil Steril. 2010;93:2337–2347. doi: 10.1016/j.fertnstert.2009.01.111. [DOI] [PubMed] [Google Scholar]
- Agrawal R, Sladkevicius P, Engmann L, Conway GS, Payne NN, Bekis J, Tan SL, Campbell S, Jacobs HS. Serum vascular endothelial growth factor concentrations and ovarian stromal blood flow are increased in women with polycystic ovaries. Hum Reprod. 1998;13:651–655. doi: 10.1093/humrep/13.3.651. [DOI] [PubMed] [Google Scholar]
- Amer SA, Li TC, Ledger WL. The value of measuring anti-Mullerian hormone in women with anovulatory polycystic ovary syndrome undergoing laparoscopic ovarian diathermy. Hum Reprod. 2009;24:2760–2766. doi: 10.1093/humrep/dep271. [DOI] [PubMed] [Google Scholar]
- Andersen CY, Schmidt KT, Kristensen SG, Rosendahl M, Byskov AG, Ernst E. Concentrations of AMH and inhibin-B in relation to follicular diameter in normal human small antral follicles. Hum Reprod. 2010;25:1282–1287. doi: 10.1093/humrep/deq019. [DOI] [PubMed] [Google Scholar]
- Arabzadeh S, Hossein G, Rashidi BH, Hosseini MA, Zeraati H. Comparing serum basal and follicular fluid levels of anti-Mullerian hormone as a predictor of in vitro fertilization outcomes in patients with and without polycystic ovary syndrome. Ann Saudi med. 2010;30:442–447. doi: 10.4103/0256-4947.71063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt W. Androgen therapy in women. Eur J Endocrinol. 2006;154:1–11. doi: 10.1530/eje.1.02062. [DOI] [PubMed] [Google Scholar]
- Artini PG, Monti M, Matteucci C, Valentino V, Cristello F, Genazzani AR. Vascular endothelial growth factor and basic fibroblast growth factor in polycystic ovary syndrome during controlled ovarian hyperstimulation. Gynecol Endocrinol. 2006;22:465–470. doi: 10.1080/09513590600906607. [DOI] [PubMed] [Google Scholar]
- Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003;9:505–514. doi: 10.1093/humupd/dmg044. [DOI] [PubMed] [Google Scholar]
- Barad DH, Gleicher N. Increased oocyte production after treatment with dehydroepiandrosterone. Fertil Steril. 2005;84:756. doi: 10.1016/j.fertnstert.2005.02.049. [DOI] [PubMed] [Google Scholar]
- Barad D, Gleicher N. Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Hum Reprod. 2006;21:2845–2849. doi: 10.1093/humrep/del254. [DOI] [PubMed] [Google Scholar]
- Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, Morel L. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem. 2004;279:14579–14586. doi: 10.1074/jbc.M306143200. [DOI] [PubMed] [Google Scholar]
- Bergh C, Carlsson B, Olsson JH, Selleskog U, Hillensjo T. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil Steril. 1993;59:323–331. doi: 10.1016/s0015-0282(16)55675-1. [DOI] [PubMed] [Google Scholar]
- Cai J, Hong Y, Weng C, Tan C, Imperato-McGinley J, Zhu YS. Androgen stimulates endothelial cell proliferation via an androgen receptor/VEGF/cyclin A-mediated mechanism. Am J Physiol Heart Circ Physiol. 2011;300:H1210–H1221. doi: 10.1152/ajpheart.01210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21:2223–2227. doi: 10.1093/humrep/del165. [DOI] [PubMed] [Google Scholar]
- Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000;15:2129–2132. doi: 10.1093/humrep/15.10.2129. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Catteau-Jonard S, Jamin SP, Leclerc A, Gonzales J, Dewailly D, di Clemente N. Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J C Endocrinol Metab. 2008;93:4456–4461. doi: 10.1210/jc.2008-1231. [DOI] [PubMed] [Google Scholar]
- Chadha S, Pache TD, Huikeshoven JM, Brinkmann AO, van der Kwast TH. Androgen receptor expression in human ovarian and uterine tissue of long-term androgen-treated transsexual women. Hum Pathol. 1994;25:1198–1204. doi: 10.1016/0046-8177(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Cinar B, Mukhopadhyay NK, Meng G, Freeman MR. Phosphoinositide 3-kinase-independent non-genomic signals transit from the androgen receptor to Akt1 in membrane raft microdomains. J Biol Chem. 2007;282:29584–29593. doi: 10.1074/jbc.M703310200. [DOI] [PubMed] [Google Scholar]
- Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- Delgado-Rosas F, Gaytan M, Morales C, Gomez R, Gaytan F. Superficial ovarian cortex vascularization is inversely related to the follicle reserve in normal cycling ovaries and is increased in polycystic ovary syndrome. Hum Reprod. 2009;24:1142–1151. doi: 10.1093/humrep/dep008. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Paterakis T, Kandarakis HA. Indices of low-grade inflammation in polycystic ovary syndrome. Ann NY Acad Sci. 2006;1092:175–186. doi: 10.1196/annals.1365.015. [DOI] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Richards JS. Ontogeny of the ovary in polycystic ovary syndrome. Fertil Steril. 2013;100:23–38. doi: 10.1016/j.fertnstert.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan FE, Hornick JE, Lampson MA, Schultz RM, Shea LD, Woodruff TK. Chromosome cohesion decreases in human eggs with advanced maternal age. Aging Cell. 2012;11:1121–1124. doi: 10.1111/j.1474-9726.2012.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17:1399–1403. doi: 10.1093/humrep/17.5.1399. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, Rose UM, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–4899. doi: 10.1210/endo.142.11.8486. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002;124:601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Barnes RB, Rosenfield RL. Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev. 1995;16:322–353. doi: 10.1210/edrv-16-3-322. [DOI] [PubMed] [Google Scholar]
- El Behery MM, Diab AE, Mowafy H, Ebrahiem MA, Shehata AE. Effect of laparoscopic ovarian drilling on vascular endothelial growth factor and ovarian stromal blood flow using 3-dimensional power Doppler. Int J Gynaecol Obstet. 2011;112:119–121. doi: 10.1016/j.ijgo.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Eldar-Geva T, Margalioth EJ, Gal M, Ben-Chetrit A, Algur N, Zylber-Haran E, Brooks B, Huerta M, Spitz IM. Serum anti-Mullerian hormone levels during controlled ovarian hyperstimulation in women with polycystic ovaries with and without hyperandrogenism. Hum Reprod. 2005;20:1814–1819. doi: 10.1093/humrep/deh873. [DOI] [PubMed] [Google Scholar]
- Erichsen MM, Husebye ES, Michelsen TM, Dahl AA, Lovas K. Sexuality and fertility in women with Addison's disease. J Clin Endocrinol Metabol. 2010;95:4354–4360. doi: 10.1210/jc.2010-0445. [DOI] [PubMed] [Google Scholar]
- Falbo A, Rocca M, Russo T, D'Ettore A, Tolino A, Zullo F, Orio F, Palomba S. Serum and follicular anti-Mullerian hormone levels in women with polycystic ovary syndrome (PCOS) under metformin. J Ovarian Res. 2010;3:16. doi: 10.1186/1757-2215-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38. doi: 10.1016/j.fertnstert.2011.09.024. e25. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Fowler PA, Anderson RA, Saunders PT, Kinnell H, Mason JI, Evans DB, Bhattacharya S, Flannigan S, Franks S, Monteiro A, et al. Development of steroid signaling pathways during primordial follicle formation in the human fetal ovary. J Clin Endocrinol Metab. 2011;96:1754–1762. doi: 10.1210/jc.2010-2618. [DOI] [PubMed] [Google Scholar]
- Franks S, Hardy K. Aberrant follicle development and anovulation in polycystic ovary syndrome. Ann Endocrinol. 2010;71:228–230. doi: 10.1016/j.ando.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Franks S, Gilling-Smith C, Watson H, Willis D. Insulin action in the normal and polycystic ovary. Endocrinol Metab Clin N Am. 1999;28:361–378. doi: 10.1016/s0889-8529(05)70074-8. [DOI] [PubMed] [Google Scholar]
- Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14:367–378. doi: 10.1093/humupd/dmn015. [DOI] [PubMed] [Google Scholar]
- Fulghesu AM, Angioni S, Frau E, Belosi C, Apa R, Mioni R, Xamin N, Capobianco GP, Dessole S, Fruzzetti F, et al. Ultrasound in polycystic ovary syndrome—the measuring of ovarian stroma and relationship with circulating androgens: results of a multicentric study. Hum Reprod. 2007;22:2501–2508. doi: 10.1093/humrep/dem202. [DOI] [PubMed] [Google Scholar]
- Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Barad DH. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR) Reprod Biol Endocrinol. 2011;9:67. doi: 10.1186/1477-7827-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleicher N, Ryan E, Weghofer A, Blanco-Mejia S, Barad DH. Miscarriage rates after dehydroepiandrosterone (DHEA) supplementation in women with diminished ovarian reserve: a case control study. Reprod Biol Endocrinol. 2009;7:108. doi: 10.1186/1477-7827-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Barad DH. Dehydroepiandrosterone (DHEA) reduces embryo aneuploidy: direct evidence from preimplantation genetic screening (PGS) Reprod Biol Endocrinol. 2010a;8:140. doi: 10.1186/1477-7827-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Barad DH. Improvement in diminished ovarian reserve after dehydroepiandrosterone supplementation. Reprod Biomed Online. 2010b;21:360–365. doi: 10.1016/j.rbmo.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Barad DH. Defining ovarian reserve to better understand ovarian aging. Reprod Biol Endocrinol. 2011;9:23. doi: 10.1186/1477-7827-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glister C, Richards SL, Knight PG. Bone morphogenetic proteins (BMP) -4, -6, and -7 potently suppress basal and luteinizing hormone-induced androgen production by bovine theca interna cells in primary culture: could ovarian hyperandrogenic dysfunction be caused by a defect in thecal BMP signaling? Endocrinology. 2005;146:1883–1892. doi: 10.1210/en.2004-1303. [DOI] [PubMed] [Google Scholar]
- Glister C, Satchell L, Bathgate RA, Wade JD, Dai Y, Ivell R, Anand-Ivell R, Rodgers RJ, Knight PG. Functional link between bone morphogenetic proteins and insulin-like peptide 3 signaling in modulating ovarian androgen production. Proc Natl Acad Sci USA. 2013;110:E1426–E1435. doi: 10.1073/pnas.1222216110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman MP, Nakajima ST, Fallat ME, Siow Y. Mullerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil Steril. 2008;89:1364–1370. doi: 10.1016/j.fertnstert.2007.03.066. [DOI] [PubMed] [Google Scholar]
- Hart R, Doherty DA, Norman RJ, Franks S, Dickinson JE, Hickey M, Sloboda DM. Serum antimullerian hormone (AMH) levels are elevated in adolescent girls with polycystic ovaries and the polycystic ovarian syndrome (PCOS) Fertil Steril. 2010;94:1118–1121. doi: 10.1016/j.fertnstert.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev. 2002a;23:175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002b;16:2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- Hillier SG. Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod. 1994;9:188–191. doi: 10.1093/oxfordjournals.humrep.a138480. [DOI] [PubMed] [Google Scholar]
- Hillier SG, Tetsuka M. Role of androgens in follicle maturation and atresia. Bailliere's Clin Obstet Gynaecol. 1997;11:249–260. doi: 10.1016/s0950-3552(97)80036-3. [DOI] [PubMed] [Google Scholar]
- Horie K, Takakura K, Fujiwara H, Suginami H, Liao S, Mori T. Immunohistochemical localization of androgen receptor in the human ovary throughout the menstrual cycle in relation to oestrogen and progesterone receptor expression. Hum Reprod. 1992;7:184–190. doi: 10.1093/oxfordjournals.humrep.a137614. [DOI] [PubMed] [Google Scholar]
- Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called ‘hyperthecosis. Obstet Gynecol Survey. 1982;37:59–77. doi: 10.1097/00006254-198202000-00001. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Baba T, Noguchi H, Nagasawa K, Endo T, Kiya T, Saito T. Excessive androgen exposure in female-to-male transsexual persons of reproductive age induces hyperplasia of the ovarian cortex and stroma but not polycystic ovary morphology. Hum Reprod. 2013;28:453–461. doi: 10.1093/humrep/des385. [DOI] [PubMed] [Google Scholar]
- Izumi K, Mizokami A, Lin WJ, Lai KP, Chang C. Androgen receptor roles in the development of benign prostate hyperplasia (BPH) Am J Pathol. 2013;182:1942–1949. doi: 10.1016/j.ajpath.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen E, Laven JS, Dommerholt HB, Polman J, van Rijt C, van den Hurk C, Westland J, Mosselman S, Fauser BC. Abnormal gene expression profiles in human ovaries from polycystic ovary syndrome patients. Mol Endocrinol. 2004;18:3050–3063. doi: 10.1210/me.2004-0074. [DOI] [PubMed] [Google Scholar]
- Jenkins C, Milsted A, Doane K, Meszaros G, Toot J, Ely D. A cell culture model using rat coronary artery adventitial fibroblasts to measure collagen production. BMC Cardiovasc Disord. 2007;7:13. doi: 10.1186/1471-2261-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Han CS, Zhang XS, Yuan JX, Hu ZY, Liu YX. Signal transduction of stem cell factor in promoting early follicle development. Mol Cell Endocrinol. 2005;229:3–10. doi: 10.1016/j.mce.2004.10.006. [DOI] [PubMed] [Google Scholar]
- John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321:197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaijk EM, Sasano H, Suzuki T, Beek JF, van Der Veen F. Distribution of steroidogenic enzymes involved in androgen synthesis in polycystic ovaries: an immunohistochemical study. Mol Hum Reprod. 2000;6:443–447. doi: 10.1093/molehr/6.5.443. [DOI] [PubMed] [Google Scholar]
- Kang HY, Cho CL, Huang KL, Wang JC, Hu YC, Lin HK, Chang C, Huang KE. Nongenomic androgen activation of phosphatidylinositol 3-kinase/Akt signaling pathway in MC3T3-E1 osteoblasts. J Bone Miner Res. 2004;19:1181–1190. doi: 10.1359/JBMR.040306. [DOI] [PubMed] [Google Scholar]
- Kim JY. Control of ovarian primordial follicle activation. Clin Exp Reprod Med. 2012;39:10–14. doi: 10.5653/cerm.2012.39.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapczyk-Stwora K, Grzesiak M, Slomczynska M. In utero exposure to the anti-androgen flutamide influences connexin 43 and beta-catenin expression in porcine fetal gonads. Domest Anim Endocrinol. 2013;44:185–194. doi: 10.1016/j.domaniend.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Knight PG, Satchell L, Glister C. Intra-ovarian roles of activins and inhibins. Mol Cell Endocrinol. 2012;359:53–65. doi: 10.1016/j.mce.2011.04.024. [DOI] [PubMed] [Google Scholar]
- Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- Kristensen SL, Ramlau-Hansen CH, Andersen CY, Ernst E, Olsen SF, Bonde JP, Vested A, Toft G. The association between circulating levels of antimullerian hormone and follicle number, androgens, and menstrual cycle characteristics in young women. Fertil Steril. 2012;97:779–785. doi: 10.1016/j.fertnstert.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Kyei-Mensah AA, LinTan S, Zaidi J, Jacobs HS. Relationship of ovarian stromal volume to serum androgen concentrations in patients with polycystic ovary syndrome. Hum Reprod. 1998;13:1437–1441. doi: 10.1093/humrep/13.6.1437. [DOI] [PubMed] [Google Scholar]
- Labrie F. DHEA, important source of sex steroids in men and even more in women. Progr Brain Res. 2010;182:97–148. doi: 10.1016/S0079-6123(10)82004-7. [DOI] [PubMed] [Google Scholar]
- La Marca A, Broekmans FJ, Volpe A, Fauser BC, Macklon NS. Anti-Mullerian hormone (AMH): what do we still need to know? Hum Reprod. 2009;24:2264–2275. doi: 10.1093/humrep/dep210. [DOI] [PubMed] [Google Scholar]
- Lebbe M, Arlt W. What is the best diagnostic and therapeutic management strategy for an Addison patient during pregnancy? Clin Endocrinol. 2013;78:497–502. doi: 10.1111/cen.12097. [DOI] [PubMed] [Google Scholar]
- Lenie S, Smitz J. Functional AR signaling is evident in an in vitro mouse follicle culture bioassay that encompasses most stages of folliculogenesis. Biol Reprod. 2009;80:685–695. doi: 10.1095/biolreprod.107.067280. [DOI] [PubMed] [Google Scholar]
- Levine AC, Liu XH, Greenberg PD, Eliashvili M, Schiff JD, Aaronson SA, Holland JF, Kirschenbaum A. Androgens induce the expression of vascular endothelial growth factor in human fetal prostatic fibroblasts. Endocrinology. 1998;139:4672–4678. doi: 10.1210/endo.139.11.6303. [DOI] [PubMed] [Google Scholar]
- Liu L, Rajareddy S, Reddy P, Du C, Jagarlamudi K, Shen Y, Gunnarsson D, Selstam G, Boman K, Liu K. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development. 2007;134:199–209. doi: 10.1242/dev.02667. [DOI] [PubMed] [Google Scholar]
- Liu D, Iruthayanathan M, Homan LL, Wang Y, Yang L, Wang Y, Dillon JS. Dehydroepiandrosterone stimulates endothelial proliferation and angiogenesis through extracellular signal-regulated kinase 1/2-mediated mechanisms. Endocrinology. 2008;149:889–898. doi: 10.1210/en.2007-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan KA, Juengel JL, McNatty KP. Onset of steroidogenic enzyme gene expression during ovarian follicular development in sheep. Biol Reprod. 2002;66:906–916. doi: 10.1095/biolreprod66.4.906. [DOI] [PubMed] [Google Scholar]
- Ma X, Fan L, Meng Y, Hou Z, Mao YD, Wang W, Ding W, Liu JY. Proteomic analysis of human ovaries from normal and polycystic ovarian syndrome. Mol Hum Reprod. 2007;13:527–535. doi: 10.1093/molehr/gam036. [DOI] [PubMed] [Google Scholar]
- Mabjeesh NJ, Willard MT, Frederickson CE, Zhong H, Simons JW. Androgens stimulate hypoxia-inducible factor 1 activation via autocrine loop of tyrosine kinase receptor/phosphatidylinositol 3′-kinase/protein kinase B in prostate cancer cells. Clin Cancer Res. 2003;9:2416–2425. [PubMed] [Google Scholar]
- Manneras L, Cajander S, Holmang A, Seleskovic Z, Lystig T, Lonn M, Stener-Victorin E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148:3781–3791. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- Marie Lebbe DH, Nicole R, Wiebke A. Androgen replacement therapy in women. Expert Rev Endocrinol Metab. 2012;7:515–529. doi: 10.1586/eem.12.45. 2012;7:14. [DOI] [PubMed] [Google Scholar]
- Mitani T, Yamaji R, Higashimura Y, Harada N, Nakano Y, Inui H. Hypoxia enhances transcriptional activity of androgen receptor through hypoxia-inducible factor-1alpha in a low androgen environment. J Steroid Biochem Mol Biol. 2011;123:58–64. doi: 10.1016/j.jsbmb.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Murray AA, Gosden RG, Allison V, Spears N. Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fertil. 1998;113:27–33. doi: 10.1530/jrf.0.1130027. [DOI] [PubMed] [Google Scholar]
- Nardo LG, Yates AP, Roberts SA, Pemberton P, Laing I. The relationships between AMH, androgens, insulin resistance and basal ovarian follicular status in non-obese subfertile women with and without polycystic ovary syndrome. Hum Reprod. 2009;24:2917–2923. doi: 10.1093/humrep/dep225. [DOI] [PubMed] [Google Scholar]
- Navot D, Bergh PA, Williams MA, Garrisi GJ, Guzman I, Sandler B, Grunfeld L. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337:1375–1377. doi: 10.1016/0140-6736(91)93060-m. [DOI] [PubMed] [Google Scholar]
- Nelson VL, Qin KN, Rosenfield RL, Wood JR, Penning TM, Legro RS, Strauss JF, 3rd, McAllister JM. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:5925–5933. doi: 10.1210/jcem.86.12.8088. [DOI] [PubMed] [Google Scholar]
- Nestler JE, Jakubowicz DJ, de Vargas AF, Brik C, Quintero N, Medina F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab. 1998;83:2001–2005. doi: 10.1210/jcem.83.6.4886. [DOI] [PubMed] [Google Scholar]
- Nheu L, Nazareth L, Xu GY, Xiao FY, Luo RZ, Komesaroff P, Ling S. Physiological effects of androgens on human vascular endothelial and smooth muscle cells in culture. Steroids. 2011;76:1590–1596. doi: 10.1016/j.steroids.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Nielsen ME, Rasmussen IA, Kristensen SG, Christensen ST, Mollgard K, Wreford Andersen E, Byskov AG, Yding Andersen C. In human granulosa cells from small antral follicles, androgen receptor mRNA and androgen levels in follicular fluid correlate with FSH receptor mRNA. Mol Hum Reprod. 2011;17:63–70. doi: 10.1093/molehr/gaq073. [DOI] [PubMed] [Google Scholar]
- Oren I, Fleishman SJ, Kessel A, Ben-Tal N. Free diffusion of steroid hormones across biomembranes: a simplex search with implicit solvent model calculations. Biophys J. 2004;87:768–779. doi: 10.1529/biophysj.103.035527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orisaka M, Jiang JY, Orisaka S, Kotsuji F, Tsang BK. Growth differentiation factor 9 promotes rat preantral follicle growth by up-regulating follicular androgen biosynthesis. Endocrinology. 2009;150:2740–2748. doi: 10.1210/en.2008-1536. [DOI] [PubMed] [Google Scholar]
- Park C, Kim Y, Shim M, Lee Y. Hypoxia enhances ligand-occupied androgen receptor activity. Biochem Biophys Res Commun. 2012;418:319–323. doi: 10.1016/j.bbrc.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Parsanezhad ME, Bagheri MH, Alborzi S, Schmidt EH. Ovarian stromal blood flow changes after laparoscopic ovarian cauterization in women with polycystic ovary syndrome. Hum Reprod. 2003;18:1432–1437. doi: 10.1093/humrep/deg244. [DOI] [PubMed] [Google Scholar]
- Perrot-Applanat M, Ancelin M, Buteau-Lozano H, Meduri G, Bausero P. Ovarian steroids in endometrial angiogenesis. Steroids. 2000;65:599–603. doi: 10.1016/s0039-128x(00)00117-3. [DOI] [PubMed] [Google Scholar]
- Peters H, Byskov AG, Himelstein-Braw R, Faber M. Follicular growth: the basic event in the mouse and human ovary. J Reprod Fertil. 1975;45:559–566. doi: 10.1530/jrf.0.0450559. [DOI] [PubMed] [Google Scholar]
- Pigny P, Jonard S, Robert Y, Dewailly D. Serum anti-Mullerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941–945. doi: 10.1210/jc.2005-2076. [DOI] [PubMed] [Google Scholar]
- Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Mullerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820–1826. doi: 10.1093/humrep/deh850. [DOI] [PubMed] [Google Scholar]
- Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2011;17:17–33. doi: 10.1093/humupd/dmq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Reddy P, Adhikari D, Zheng W, Liang S, Hamalainen T, Tohonen V, Ogawa W, Noda T, Volarevic S, Huhtaniemi I, et al. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet. 2009;18:2813–2824. doi: 10.1093/hmg/ddp217. [DOI] [PubMed] [Google Scholar]
- Reddy P, Zheng W, Liu K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol Metab. 2010;21:96–103. doi: 10.1016/j.tem.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Rice S, Ojha K, Whitehead S, Mason H. Stage-specific expression of androgen receptor, follicle-stimulating hormone receptor, and anti-Mullerian hormone type II receptor in single, isolated, human preantral follicles: relevance to polycystic ovaries. J Clin Endocrinol Metab. 2007;92:1034–1040. doi: 10.1210/jc.2006-1697. [DOI] [PubMed] [Google Scholar]
- Sanchez F, Smitz J. Molecular control of oogenesis. Biochim Biophys Acta. 2012;1822:1896–1912. doi: 10.1016/j.bbadis.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Sato K, Iemitsu M, Aizawa K, Ajisaka R. Testosterone and DHEA activate the glucose metabolism-related signaling pathway in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E961–E968. doi: 10.1152/ajpendo.00678.2007. [DOI] [PubMed] [Google Scholar]
- Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24:1393–1403. doi: 10.1210/me.2010-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafighi M, Olariu R, Brun C, Fathi AR, Djafarzadeh S, Jakob SM, Hunger RE, Banic A, Constantinescu MA. The role of androgens on hypoxia-inducible factor (HIF)-1alpha-induced angiogenesis and on the survival of ischemically challenged skin flaps in a rat model. Microsurgery. 2012;32:475–481. doi: 10.1002/micr.21996. [DOI] [PubMed] [Google Scholar]
- Shelton JG, Steelman LS, White ER, McCubrey JA. Synergy between PI3K/Akt and Raf/MEK/ERK pathways in IGF-1R mediated cell cycle progression and prevention of apoptosis in hematopoietic cells. Cell Cycle. 2004;3:372–379. [PubMed] [Google Scholar]
- Shweiki D, Itin A, Neufeld G, Gitay-Goren H, Keshet E. Patterns of expression of vascular endothelial growth factor (VEGF) and VEGF receptors in mice suggest a role in hormonally regulated angiogenesis. J Clin Invest. 1993;91:2235–2243. doi: 10.1172/JCI116450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara SK, Coomarasamy A. Androgen pretreatment in poor responders undergoing controlled ovarian stimulation and in vitro fertilization treatment. Fertil Steril. 2011;95:e73–e74. doi: 10.1016/j.fertnstert.2011.04.083. author reply e5. [DOI] [PubMed] [Google Scholar]
- Sunkara SK, Coomarasamy A, Arlt W, Bhattacharya S. Should androgen supplementation be used for poor ovarian response in IVF? Hum Reprod. 2012;27:637–640. doi: 10.1093/humrep/der464. [DOI] [PubMed] [Google Scholar]
- Tingen C, Kim A, Woodruff TK. The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol Hum Reprod. 2009;15:795–803. doi: 10.1093/molehr/gap073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingen CM, Kiesewetter SE, Jozefik J, Thomas C, Tagler D, Shea L, Woodruff TK. A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction. 2011;141:809–820. doi: 10.1530/REP-10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houten EL, Kramer P, McLuskey A, Karels B, Themmen AP, Visser JA. Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology. 2012;153:2861–2869. doi: 10.1210/en.2011-1754. [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Steckler TL, Abbott DH, Welch KB, MohanKumar PS, Phillips DJ, Refsal K, Padmanabhan V. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biol Reprod. 2011;84:87–96. doi: 10.1095/biolreprod.110.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendola K, Zhou J, Wang J, Bondy CA. Androgens promote insulin-like growth factor-I and insulin-like growth factor-I receptor gene expression in the primate ovary. Hum Reprod. 1999a;14:2328–2332. doi: 10.1093/humrep/14.9.2328. [DOI] [PubMed] [Google Scholar]
- Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod. 1999b;61:353–357. doi: 10.1095/biolreprod61.2.353. [DOI] [PubMed] [Google Scholar]
- Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-Mullerian hormone: a new marker for ovarian function. Reproduction. 2006;131:1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- Visser JA, Schipper I, Laven JS, Themmen AP. Anti-Mullerian hormone: an ovarian reserve marker in primary ovarian insufficiency. Nat Rev Endocrinol. 2012;8:331–341. doi: 10.1038/nrendo.2011.224. [DOI] [PubMed] [Google Scholar]
- Vitt UA, Hayashi M, Klein C, Hsueh AJ. Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol Reprod. 2000a;62:370–377. doi: 10.1095/biolreprod62.2.370. [DOI] [PubMed] [Google Scholar]
- Vitt UA, McGee EA, Hayashi M, Hsueh AJ. In vivo treatment with GDF-9 stimulates primordial and primary follicle progression and theca cell marker CYP17 in ovaries of immature rats. Endocrinology. 2000b;141:3814–3820. doi: 10.1210/endo.141.10.7732. [DOI] [PubMed] [Google Scholar]
- Wang H, Andoh K, Hagiwara H, Xiaowei L, Kikuchi N, Abe Y, Yamada K, Fatima R, Mizunuma H. Effect of adrenal and ovarian androgens on type 4 follicles unresponsive to FSH in immature mice. Endocrinology. 2001;142:4930–4936. doi: 10.1210/endo.142.11.8482. [DOI] [PubMed] [Google Scholar]
- Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, Franks S. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017–1021. doi: 10.1016/s0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- Weil SJ, Vendola K, Zhou J, Adesanya OO, Wang J, Okafor J, Bondy CA. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab. 1998;83:2479–2485. doi: 10.1210/jcem.83.7.4917. [DOI] [PubMed] [Google Scholar]
- Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84:2951–2956. doi: 10.1210/jcem.84.8.5929. [DOI] [PubMed] [Google Scholar]
- Wierman ME, Basson R, Davis SR, Khosla S, Miller KK, Rosner W, Santoro N. Androgen therapy in women: an Endocrine Society Clinical Practice guideline. J Clin Endocrinol Metab. 2006;91:3697–3710. doi: 10.1210/jc.2006-1121. [DOI] [PubMed] [Google Scholar]
- Wiser A, Gonen O, Ghetler Y, Shavit T, Berkovitz A, Shulman A. Addition of dehydroepiandrosterone (DHEA) for poor-responder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study. Hum Reprod. 2010;25:2496–2500. doi: 10.1093/humrep/deq220. [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet. 2011;28:3–6. doi: 10.1007/s10815-010-9478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Yang JG, Yang JJ, Lin YM, Tsai HD, Lin CY, Kuo PL. Androgen excess down-regulates connexin43 in a human granulosa cell line. Fertil Steril. 2010;94:2938–2941. doi: 10.1016/j.fertnstert.2010.06.077. [DOI] [PubMed] [Google Scholar]
- Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75:916–923. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- Yang MY, Fortune JE. Testosterone stimulates the primary to secondary follicle transition in bovine follicles in vitro. Biol Reprod. 2006;75:924–932. doi: 10.1095/biolreprod.106.051813. [DOI] [PubMed] [Google Scholar]
- Yang JL, Zhang CP, Li L, Huang L, Ji SY, Lu CL, Fan CH, Cai H, Ren Y, Hu ZY, et al. Testosterone induces redistribution of forkhead box-3a and down-regulation of growth and differentiation factor 9 messenger ribonucleic acid expression at early stage of mouse folliculogenesis. Endocrinology. 2010;151:774–782. doi: 10.1210/en.2009-0751. [DOI] [PubMed] [Google Scholar]
- Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction. 2010;140:489–504. doi: 10.1530/REP-10-0094. [DOI] [PubMed] [Google Scholar]
- Zaidi J, Campbell S, Pittrof R, Kyei-Mensah A, Shaker A, Jacobs HS, Tan SL. Ovarian stromal blood flow in women with polycystic ovaries—a possible new marker for diagnosis? Hum Reprod. 1995;10:1992–1996. doi: 10.1093/oxfordjournals.humrep.a136222. [DOI] [PubMed] [Google Scholar]


