Background: Cysteine racemization occurs at heavy chain position 220 of an IgG1under high pH stress.
Results: d-Cysteine forms on both heavy and light chains of an IgGs under mild conditions.
Conclusion: The terminal cysteine impairs racemization on the κ light chain.
Significance: Because d-cysteine is naturally occurring on antibodies, its presence on therapeutic antibodies poses less of a safety concern.
Keywords: Antibodies, Disulfide, Mass Spectrometry (MS), Post Translational Modification, Protein Chemical Modification, Chirality, Light Chain, Thioether
Abstract
Under basic pH conditions, the heavy chain 220-light chain 214 (H220-L214) disulfide bond, found in the flexible hinge region of an IgG1, can convert to a thioether. Similar conditions also result in racemization of the H220 cysteine. Here, we report that racemization occurs on both H220 and L214 on an IgG1 with a λ light chain (IgG1λ) but almost entirely on H220 of an IgGl with a κ light chain (IgG1κ) under similar conditions. Likewise, racemization was detected at significant levels on H220 and L214 on endogenous human IgG1λ but only at the H220 position on IgG1κ. Low but measurable levels of d-cysteines were found on IgG2 cysteines in the hinge region, both with monoclonal antibodies incubated under basic pH conditions and on antibodies isolated from human serum. A simplified reaction mechanism involving reversible β-elimination on the cysteine is presented that accounts for both base-catalyzed racemization and thioether formation at the hinge disulfide.
Introduction
mAbs have been the principal biotherapeutic modality over the past decade (1, 2). Much of the product quality attribute monitoring for biotherapeutics, including mAbs, is for posttranslational modifications. The recent regulatory agency focus on “quality by design” places greater emphasis on how the product quality attributes affect the safety and efficacy of the drug. Those product quality attributes that must be controlled within certain ranges are considered critical quality attributes.
Many posttranslational modifications found in biotherapeutic proteins, including methionine oxidations, deamidations, glycations, and disulfide isoforms, form during production or storage (3). Some of the posttranslational modifications that increase in the IgG1 antibody subtype during storage are localized to the flexible hinge region between the Fab arms and the Fc region. Under stress conditions such as exposure to heat, chemicals, or UV light, fragmentation, thioether bond formation, and racemization of cysteines have been detected in this region (4–9). Although an IgG1 has 14 disulfide bonds, the only thioether bond that has been reported was at the position of the light chain-heavy chain (LC-HC)2 linkage (at H220-L214) (5). Cysteine racemization was localized to this specific disulfide bond as well on an IgG1 but only on the H220 and not the L214 position (8).
Recently, we explored the base-catalyzed thioether bond formation in human IgG1s (10). Formation rates were faster in antibodies containing λ light chains over those with the more common κ light chains. A reaction mechanism was proposed in which dehydrogenation occurred on either the H220 or L214 to form dehydroalanine as an intermediate in the thioether formation pathway. More dehydrogenation occurred on the cysteine L214 of the λ than the κ light chains, presumably due to its position, which on the λ light chain is one residue away from the C terminus. One prediction from the proposed mechanism, which was not explored in the previously reported study, was that reversibility of the dehydrogenation step would cause racemization of cysteine. Because dehydrogenation occurred on both the H220 and L214 in IgG1λ antibodies, racemization would also be expected to be detected on both cysteines in the H220-L214 disulfide bond. Here, we explore the racemization reaction in IgG1 and IgG2 antibodies and integrate these findings into a simplified reaction mechanism for both thioether formation and cysteine racemization.
EXPERIMENTAL PROCEDURES
Materials
Four recombinant human monoclonal antibodies, mAbA (IgG1κ), mAbB (IgG1λ), mAbC (IgG2κ), and mAbD (IgG2λ) were produced from CHO cells and purified at Amgen, Inc. Human IgGs (myIgG1κ, myIgG1λ, myIgG2κ, and myIgG2λ), purified from plasma of myeloma patients, were purchased from Sigma-Aldrich. Polyclonal endogenous IgGs were isolated from the serum of healthy human donors as described previously (11–13). Endoproteinase Lys-C was obtained from Wako Chemicals (Richmond, VA). Acetonitrile was from Sigma-Aldrich. TFA and dithiothreitol were purchased from Thermo Scientific Pierce (Rockford, IL). Synthetic peptides with L/D cysteine, TVAPTECS, TVAPTE(d-Cys)S, SFNRGEC, SFNRGE(d-Cys), SCDK, S(d-Cys)DK, were obtained from 21 Century Biochemicals (Marlboro, MA).
Forced Cysteine Racemization in Hydrogen/Deuterium Oxide
50 mm deuterated glycine-NaOH buffer (pH 9.3) was prepared by mixing 2.5 ml of 0.2 m glycine dissolved in D2O with 0.44 ml of 0.2 m NaOH (prepared in D2O). Monoclonal antibodies mAbA, mAbB, mAbC, and mAbD (at ∼30 mg/ml) were briefly dried with a centrifuge under vacuum and incubated in both H2O-based glycine-NaOH buffer (pH 9.1), and D2O-based glycine-NaOH buffer (pH 9.3) for 6 days at 56 °C.
Reduced Lys-C Enzymatic Digestion
To denature the antibodies, 50 μl of antibody solutions (∼3 mg/ml) were mixed with 10 μl of 1.0 m NaH2PO4, 10 μl of 0.4 m NH2OH, 6 μl of 36 mg/ml methionine, and 36 mg of urea. After vortexing, 3 μl of 20% TFA (v/v) was added and the mixture was incubated at 37 °C for 1 h. Prior to digestion, the denatured antibody mixture was neutralized with 14 μl of 1.0 m NaOH, followed by adding 26 μl of water and 1.2 μl of 100 mm EDTA. The digestion reaction was carried out by adding 10 μl of 1 μg/μl Lys-C and incubating the mixture at 37 °C overnight, followed by reduction with 30 mm dithiothreitol at 37 °C for 30 min.
pH Profile of Cys Racemization
To determine the pH effects on the formation of cysteine racemization, both mAbA and mAbB were diluted 18-fold to ∼3 mg/ml into different buffers to obtain specific pH values. To generate pH 7 and 8, 0.1 m phosphate buffer was used; for pH 9, 50 mm glycine-NaOH buffer was used. The final pH of each sample was measured to be 7.0, 8.0, and 9.1. Samples were then incubated at 37 °C up to 30 days in the dark.
LC-MS/MS Analysis of Lys-C Digests
The online LC-MS/MS analyses were carried out using an Agilent 1290 HPLC system, coupled with a Thermo Fisher Scientific LTQ-Orbitrap Elite mass spectrometer equipped with an electrospray ionization source. The Lys-C digest was injected onto a Waters BEH C18 Column (2.1 × 150 mm; pore size, 300 Å; particle size, 1.7 μm) with the column temperature maintained at 50 °C. The mobile phase A contained 0.2% (v/v) TFA and 0.8% (v/v) formic acid in water, and the mobile phase B was prepared in acetonitrile with 0.2% (v/v) TFA and 0.8% formic acid (v/v). A gradient (hold at 0% B for 2 min, 0% to 40% B for 120 min) was used to separate the digested peptides at a flow rate of 0.2 ml/min. The eluted peptides were monitored by both UV (214 and 280 nm wavelengths) and mass spectrometry. The mass spectrometer was set up to acquire one high-resolution full scan at 60,000 resolution (at m/z 400), followed by either a data-dependent scan mode or a preselected ion mode. The width for precursor ion isolation was set to 3.0 (m/z) with an activation Q of 0.25 and an activation time of 10 ms. The spray voltage was 3 kV, and the temperature for the heated capillary was 300 °C. The relative racemization level was quantified by calculating the ratio of the peak areas, extracted from the base peak ion chromatogram corresponding to the d-cysteine containing peptide, over the sum of the corresponding peak areas observed for both the d-cysteine and l-cysteine containing peptides.
RESULTS
Potential Racemization Discovered in Thioether-linked Peptides
Under high pH conditions, a human IgG1 antibody can form a thioether bond between a cysteine in the heavy chain hinge region (H220, Eu numbering) and a cysteine in the light chain (L214, κ LC, Kabat numbering). The thioether bond formed at this position can be detected as a non-reducible species possessing the combined mass of the LC and HC by reducing capillary electrophoresis or reducing RP-HPLC/MS, or can be detected by peptide mapping (5). Peptide mapping under non-reducing conditions has been used to characterize this species as well as quantify the relative levels (10). Digestions of a previously stressed IgG1 antibody containing a κ light chain (IgG1κ) with the protease Lys-C generates a thioether-linked peptide of the sequence (H)SC*DK/(L)SFNRGEC*, where the asterisks represent positions of the modified cystine linkage, also called a lanthionine, and the letters in parentheses represent the antibody polypeptide chains (H for heavy chain, L for light chain). The thioether-linked peptide cannot be cleaved with thiol reducing reagents such as dithiothreitol and has a mass 32 Da less than the parent disulfide-linked version. For IgG1κ, the thioether containing peptides can be resolved into two isobaric peaks by RP-HPLC, consistent with racemization on HC cysteine 220 (10). When the high pH incubations were performed in D2O, a mass increase of 1 Da was observed on both peaks. Tandem MS analysis indicated that the mass increase was associated with the HC cysteine on this peptide. These results indicate that dehydrogenation and rehydrogenation occurred on the HC cysteine during the reaction as had been proposed previously.
Thioethers also form at the same relative positions in IgG1λ antibodies (peptide (H)SC*DK/(L)TVAPTEC*S) incubated under similar conditions, which, similar to IgG1κ antibodies, resulted in peak splitting on the RP-HPLC peptide map analysis. However, multiple observations suggested that the dehydrogenation step occurred on both the HC and the LC cysteines for IgG1λ antibodies. First, although not completely resolved, further peak splitting of the thioether containing peptides occurred. Second, in high pH studies with D2O, two deuterium atoms could be incorporated per thioether-linked peptide. Third, tandem MS analyses showed that the deuterium was incorporated in both the HC 220 and the LC 214 cysteines. Taken together, the results suggested that dehydrogenation and rehydrogenation also occurred on the LC cysteine, which had not previously been observed. Thus, racemization might be expected to occur on the LC cysteine as well.
Racemization on Disulfide-linked Peptides
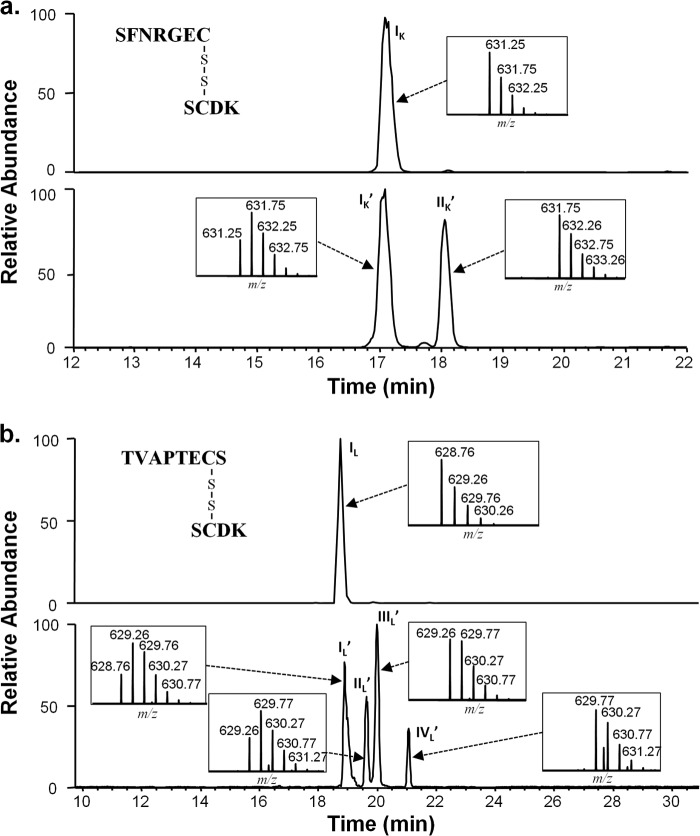
Peak splitting was also observed on the disulfide-linked parental LC-HC peptides (Fig. 1) involved in the thioether forming reaction. The disulfide-linked peptide SCDK/SFNRGEC obtained from an IgG1κ incubated at high pH, resolved into two major isobaric peaks (Fig. 1a, lower panel) when analyzed by non-reduced peptide mapping. High pH D2O incubations resulted in a 1-Da increase in mass in the peptide (Fig. 1a, lower panel, inset), localized on the HC cysteine (data not shown). Although nearly all of the peptide in the new peak (IIK′) had a 1-Da mass increase (631.75 versus 631.25 m/z, doubly charged) over a peptide from a non-deuterated sample, the peptides at the original position (IK′) contained a mixture of material with a +1 Da mass with material without mass shift. Peptide maps of IgG1λ after high pH treatments produced four peaks for the LC-HC disulfide-linked peptide in the same region (Fig. 1b, lower panel). Both a 1- and 2-Da increase in mass were detected on the IgG1λ HC-LC peptides when the incubations were performed in D2O. The isotopic distribution of the HC-LC peptide in the peak eluting at the position of the unincubated sample (IL′) contained a mixture of the original mass (m/z 628.76) and a +1 Da mass (629.26 m/z, doubly charged). A similar isotopic distribution was seen in peak IIL′. The distribution in peak IIIL′, was similar to peak IIK′ for IgG1κ, with, mainly a +1-Da shift (629.26 m/z) and little of the original mass. Finally, the fourth peak, IVL′ had mainly a mass shift of +2 Da (629.77 m/z), with very little of the +1 Da or original mass material. The combination of peak splitting, MS/MS characterization (data not shown), and the isotopic distribution results, suggested racemization on these LC-HC disulfide-linked peptides. The additional peaks and mass increases observed on the IgG1λ were suspected to due to the racemization on both HC 220 and LC cysteine 214 of these peptides. A +2-Da shift would indicate racemization on both cysteines in the same disulfide-linked peptide.
FIGURE 1.
IgG1 Lys-C generated LC-HC disulfide-linked peptides after high pH (70 h, 50 °C, pH 9.3) incubations. The peptide map is performed and run under non-reducing conditions. a, peptides from IgG1κ. The top panel is the extracted ion chromatogram (XIC) of the peptide from the sample prior to incubation. The lower panel is the peptide after incubation. Shown is the D2O incubated sample. b, peptides from IgG1λ. The top panel is the XIC of the peptide from the sample prior to incubation. The lower panel is the peptide after incubation. Shown is the D2O incubated sample. Insets show the isotopic distribution for the doubly charged species in each of the labeled peaks. The y axis of the inset figure represents relative level. Because the chromatography for the top and bottom panels was performed on different days, reference peptides (of m/z 495.76 and 990.51, Δ retention time of 2.93) were used. The subscript K designates the κ light chain, and the L designates the λ light chain. The prime symbol indicates peptides from the D2O-incubated samples.
Cysteine Racemization using Reducing Peptide Mapping
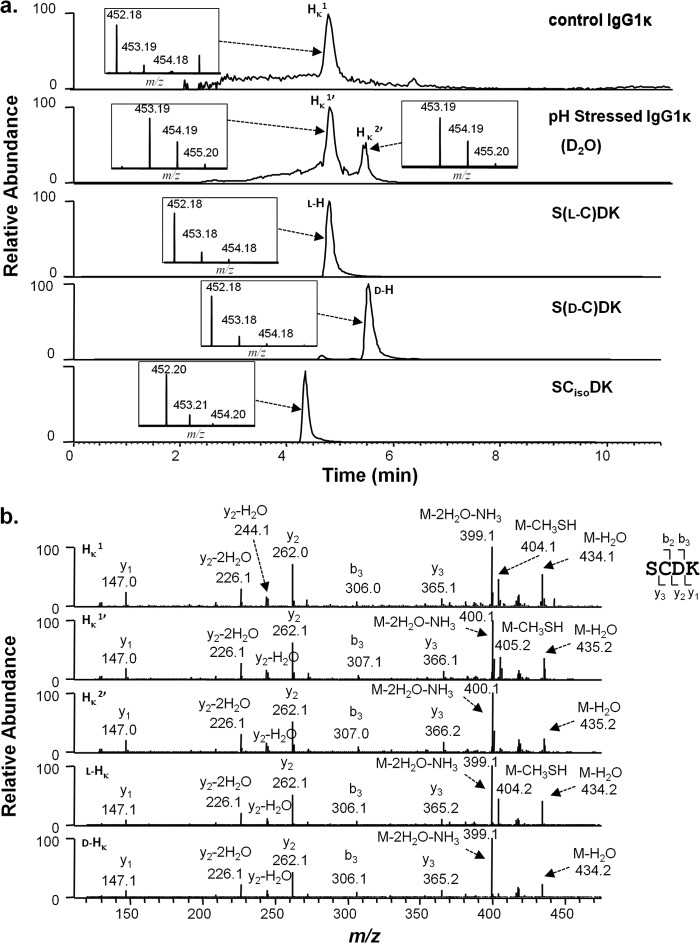
A series of experiments were performed to characterize the chemical changes occurring in the LC-HC linkage region upon high pH incubations. These incubations were also performed under the same conditions but in deuterated water (D2O). Peptides generated by the protease Lys-C were treated with dithiothreitol to reduce disulfide bonds and separated and analyzed by RP-HPLC/MS. Because the denaturation and protease digestion steps were performed in water, only non-exchangeable deuterium remained from those reactions. No quantitative or qualitative differences were observed in the UV chromatograms between the D2O- and the H2O-based reactions. The resultant reduced LC and HC peptides from the HC-LC linkage could be resolved on the same chromatographic run. Prior to incubation, the IgG1κ-reducing peptide map produced a single peak for the peptide SCDK (HK1; Fig. 2a, top panel, at 4.7 min), which is the HC portion of the disulfide-linked peptide SCDK/SFRGEC. Over time at a high pH (shown for 6 days, 56 °C, pH 9.3), IgG1κ generated a second peak at 5.5 min (HK2′, Fig. 2a, second panel from top). MS/MS analysis confirmed that both peaks are of the same sequence (Fig. 2b). Mass shifts in peptide SCDK were observed when the high pH incubations were performed in D2O. The peptide eluting at the position of HK1 (called HK1′ for D2O incubation experiments) showed mainly a +1-Da shift (453.19 m/z, singly charged species) over the unincubated sample. Peak HK2′ showed a similar mass shift. This +1 Da mass was localized to the cysteine by tandem MS performed on the HK1′ and HK2′ peptides (Fig. 2b).
FIGURE 2.
Cysteine racemization on IgG1κ HC from control and stressed samples by peptide mapping. a, XIC of peptides from control and stressed samples and models peptides with corresponding isotopic distribution (inset). The y axis of the inset represents relative level. The peptide map is performed and run under disulfide reducing conditions. Top panel, unstressed antibody. Second panel from top, antibody was incubated at 56 °C for 6 days at pH 9.3 in D2O. Third panel from top, synthetic peptide S(l-C)DK, called l-H. Fourth panel from top, synthetic peptide S(d-C)DK (called d-H). Bottom panel, synthetic peptide SCisoDK. b, MS2 spectra of selected peaks shown in a.
To identify the shifted peaks, synthetic peptides were purchased with l-cysteine or d-cysteine in position 220 of the IgG1 HC (sequence S(l-C)DK, called l-H, sequence S(d-C)DK, called d-H), or isoAsp at HC position 221 [S(l-C)isoDK, another known degradant on this peptide). Peptide l-H eluted at 4.7 min, the position of H1 (and H1′), whereas peptide d-H eluted at, 5.5 min the same position as H2 (and H2′). Some low level of l-H appears to exist in the d-H sample as indicated as a small isobaric peak eluting in Fig. 2a (second from bottom panel). The synthetic S(l-C)isoDK peptide eluted at 4.3 min (Fig. 2a, bottom panel). Little of the HC peptide containing isoD was observed in the original sample or in subsequent stressed samples. An isoaspartate containing peptide also would not be expected to result in a mass increase in D2O incubations. This deuterium incorporation approach was used by Amano et al. (8) to first identify racemization at the H220 position.
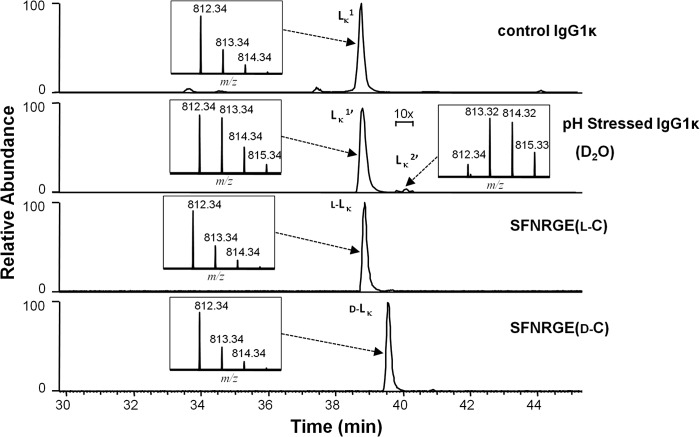
Very little racemization appeared to occur in the LC 214 cysteine of the IgG1κ when incubated under the high pH conditions described above. Fig. 3 shows analysis performed on the peptide SFNRGEC, the LC portion of the HC-LC disulfide peptide SCDK/SFNRGEC. The elution positions and isotopic distributions are given in Fig. 3. After high pH stress conditions, where significant racemization occurs on the HC 220 cysteine (Fig. 2a, second panel from top), the LC peptide elutes primarily as a single peak at 38.5 min in the chromatogram (Fig. 3, second panel from top). A very small peak, with the same mass as Lκ1, eluted at 39–40 min (called Lκ2′ in D2O incubations, with a 1-Da increase). Synthetic peptides of the sequence SFNRGEC with either l- or d-isomers of cysteine 214 eluted slightly differently from each other in the RP-HPLC chromatogram, with the l-cysteine containing peptide (l-LK) eluting at 38.5 min and the one with the d-cysteine (d-LK) eluting at 39.5 min (Fig. 3, third from top and bottom, respectively). MS/MS spectrum of LK2′ looked similar to the synthetic d-LK peptide, but the quality of the LK2′ spectrum was poor (spectrum not shown). For this reason, it was not possible to unambiguously assign the 1-Da increase in the D2O high pH stressed LK2′ peptide to the terminal cysteine. These results are consistent with those by Amano et al. (8) who observed racemization on HC cysteine 220 in an IgG1κ but not on the LC 214 cysteine.
FIGURE 3.
Cysteine racemization on IgG1κ LC. XIC of peptides and isotopic distribution (inset). The y axis of the inset represents relative level. Top panel, unstressed material. Second panel from top, antibody was incubated at 56 °C for 6 days at pH 9.3 in D2O. Third panel from top, synthetic peptide SFNRGE(l-C) (l-LK). Bottom panel, synthetic peptide SFNRGE(d-C) (d-LK).
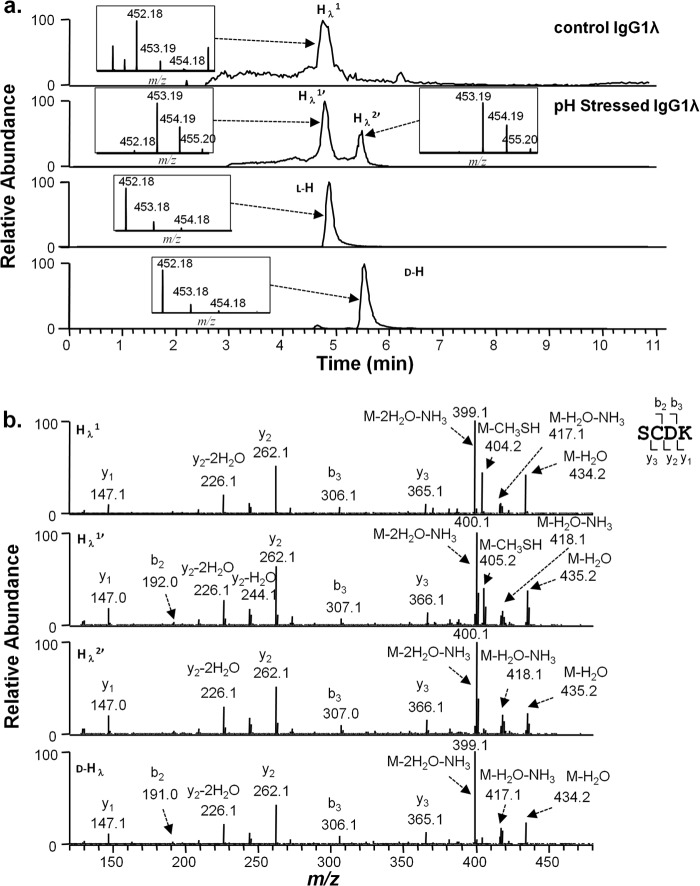
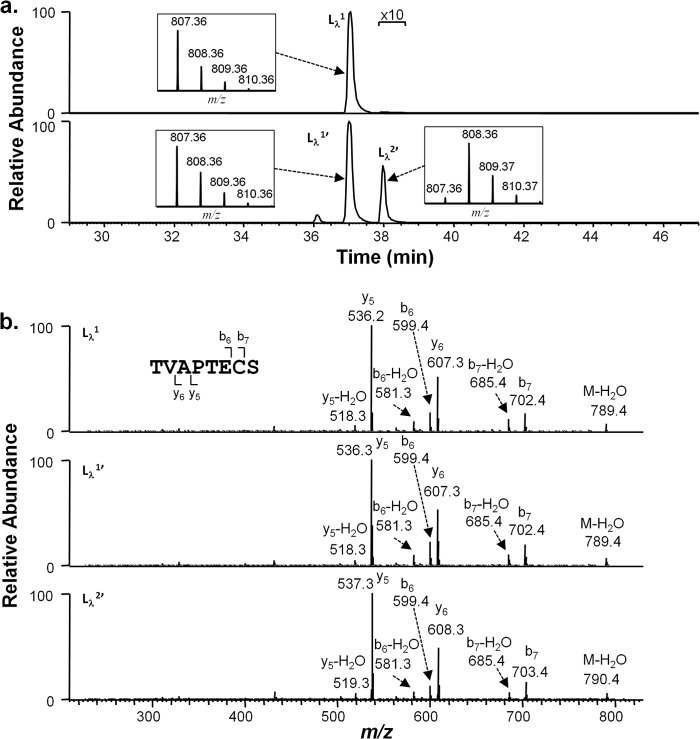
Identical high pH incubation experiments were performed on an IgG1λ antibody. Peptides generated by the protease Lys-C were separated by RP-HPLC under disulfide-reducing conditions and analyzed by MS. The HC and LC portions of the disulfide-linked peptide SCDK/TVAPTECS were analyzed as described above for the IgG1κ antibody. Results for the HC portion, SCDK, were similar to those observed for that portion of the IgG1κ HC-LC peptide. In an unstressed IgG1λ sample, the peptide eluted as a single peak at 4.7 min (Hλ1, Fig. 4a, top panel). After the antibody was stressed at high pH, a second peptide with the same mass was observed at position 5.5 min (Hλ2′, Fig. 4a, second panel from the top). High pH incubations in D2O resulted in a 1-Da mass increase (Fig. 4a, second panel from top) on both peaks (Hλ1′ and Hλ2′), which were localized to cysteine 220 (Fig. 4b, second and third panel from top).
FIGURE 4.
Cysteine racemization on IgG1λ HC. a, XIC of peptides and isotopic distribution (inset). The y axis of the inset represents relative level. Top panel, unstressed material. Second panel from top, antibody was incubated at 56 °C for 6 days at pH 9.3 in D2O. Third panel from top, synthetic peptide S(l-C)DK (l-H). Bottom panel, synthetic peptide S(d-C)DK (d-H). b, MS2 spectra of selected peaks shown in a.
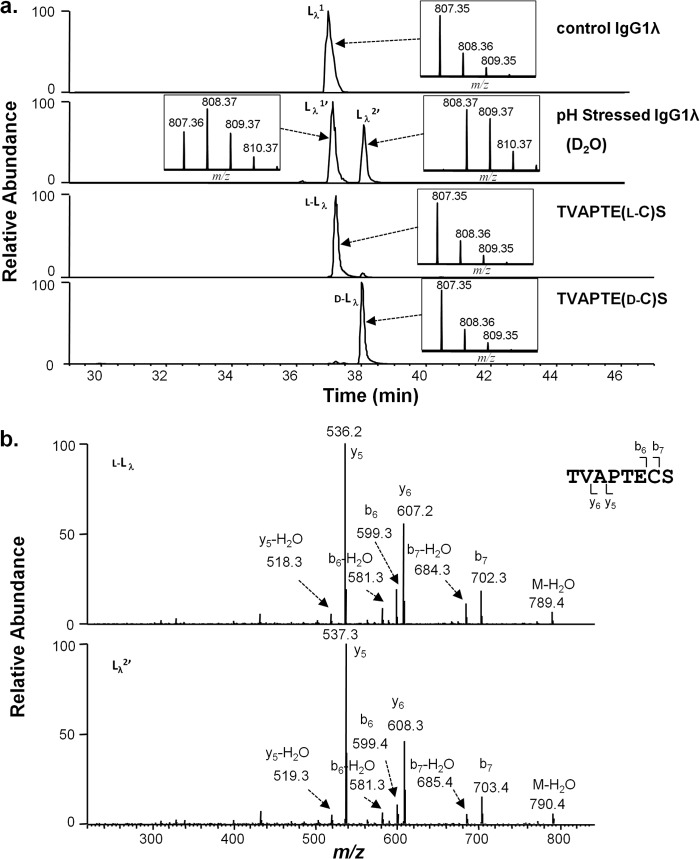
In contrast to the IgG1κ high pH incubations where only a single peak was observed for the resultant LC peptide from the HC-LC linkage (Lκ1), a second later eluting peak (38.2 min versus 37 min) was observed for the LC peptide TVAPTECS from IgG1λ, which grew in intensity over time (Fig. 5a, second panel from top). When the IgG1λ was incubated in D2O at high pH, a 1-Da increase in mass was observed on both the peak eluting at the original position (Lλ1′) and at the new position (Lλ2′, see Fig. 5a, second panel from top). MS/MS analysis of this peptide (Lλ2′, Fig. 5b, lower panel) was similar to the peptide Lλ1 in the original sample, with deuterium incorporated in the LC cysteine residue. These results suggest that racemization occurred on both the LC and HC cysteines of the disulfide-linked peptide SCDK/TVAPTECS from IgG1λ. To confirm this racemization, synthetic versions the C-terminal lambda LC peptide TVAPTECS were obtained with either d- or l-cysteine at position 214. The peptide with l-cysteine (l-Lλ, Fig. 5a, third panel from top) eluted at the position of the peptide from the untreated sample (Lλ1, Fig. 5a, top panel), whereas the d-cysteine containing peptide (d-Lλ, Fig. 5a, bottom panel) eluted at the same position as Lλ2′ (Fig. 5a, second panel from top). These results confirm that racemization occurs to a similar degree on both the LC cysteine and HC cysteine involved in the HC-LC linkage of an IgG1λ, when the protein is incubated at high pH.
FIGURE 5.
Cysteine racemization on IgG1λ LC. a, XIC of peptides and isotopic distribution (inset). The y axis of the inset represents relative level. Top panel, unstressed material. Second panel from top, antibody was incubated at 56 °C for 6 days at pH 9.3 in D2O. Third panel from top, synthetic peptide TVAPTE(l-C)S (l-Lλ). Bottom panel, synthetic peptide TVAPTE(d-C)S (d-Lλ). b, MS2 spectra of selected peaks shown in a.
Effect of pH on Racemization Reaction Rate
Racemization rates were measured in the IgG1κ and IgG1λ antibodies by monitoring the generation of Lys-C peptides containing the d-cysteine by MS relative to the l-cysteine peptide signal. HC cysteine 220 racemization rates increased with increasing pH, for both the IgG1κ and IgG1λ antibodies (Fig. 6). No significant racemization was observed in the IgG1κ LC cysteine 214 over this pH range (7–9) as expected. However, racemization rates in the IgG1λ LC cysteine were similar to those observed for the HC cysteine, in both magnitude and pH profile.
FIGURE 6.
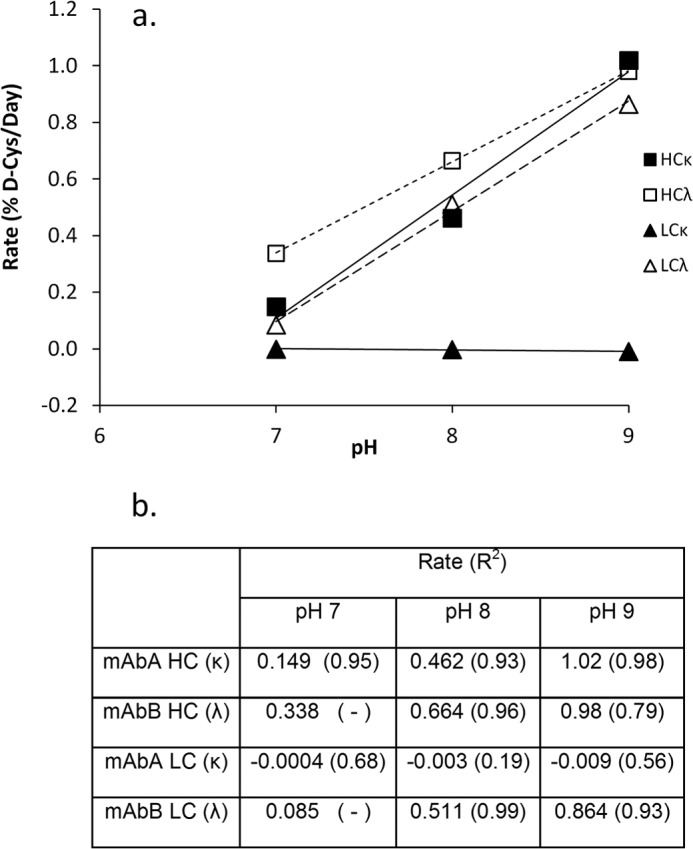
d-Cysteine formation rates at different pH values. a, plot showing the d-cysteine formation rates at the pH values shown. Each point was calculated from a time course slope using four points for up to 30 days. For certain samples, where the correlation coefficient (R2) is not listed, only two time points were used. b, table of the calculated d-cysteine formation rates and their correlation coefficients (R2). Values are in units of % d-cysteine/day. For conditions, see “Experimental Procedures.”
Racemization on IgG2
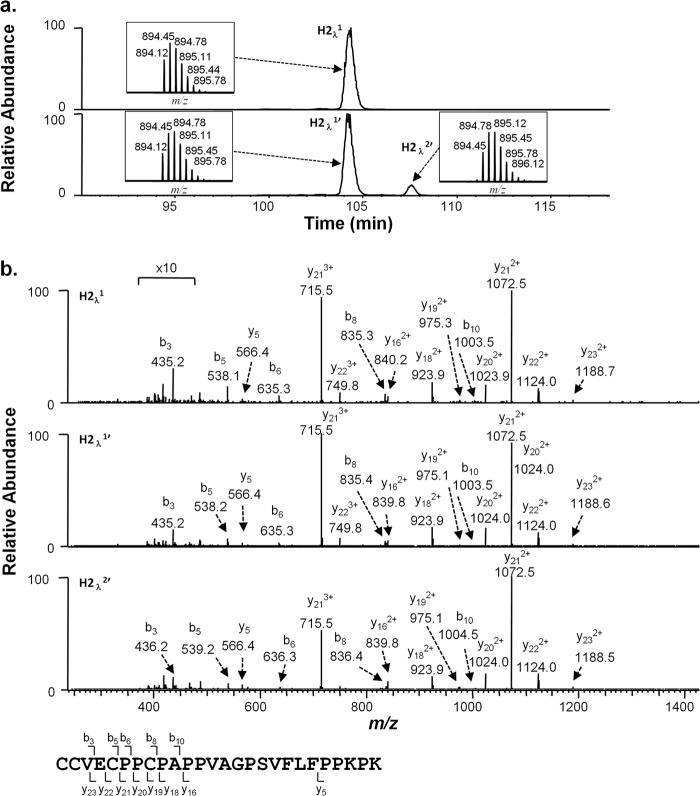
Because racemization has previously only been observed at HC 220 of an IgG1 antibody, it has been proposed that the CDK in the primary sequence possesses unique properties facilitating this reaction (8). To test whether this specific sequence is required, we probed for racemization in IgG2 antibodies under high pH conditions both with and without D2O. Incubations of a monoclonal IgG2λ at pH 9.3 at 56 °C generated d-cysteine in the LC peptide TVAPTECS (Fig. 7). A 1-Da increase in mass was observed in the peak containing d-cysteine (Lλ2′, Fig. 7a, bottom panel). Even though this peak was very small and is shown 10× the normal scale in the figure for clearer viewing, MS/MS spectrum (Fig. 7b, bottom panel) clearly indicates that deuterium was incorporated in this LC cysteine. The small peak eluting at 36 min (Fig. 7a, bottom panel) was not identified but does not involve cysteine racemization (data not shown). Racemization was also observed on the HC portion of the HC-LC linkage. IgG2 antibodies exist as a collection of disulfide isoforms with alternate linkages between the LC C-terminal cysteine and HC cysteines (14, 15). Each light chain can link to either the Fab HC cysteine 131 (Eu numbering) in the Fab arm or to cysteines 219/220 (Eu numbering) in the HC hinge region. The Lys-C hinge peptide, sequence CCVECPPCPAPPVAGPSVFLFPPKPK (N-terminal cysteine numbered 219), which links to the LC in two of the IgG2 disulfide isoforms (14), was probed for evidence of racemization. As shown in Fig. 8a (second panel from top), a second HC hinge peptide RP-HPLC peak (H2λ2′) forms over time in IgG2λ antibody samples incubated at high pH. When the incubations were performed in D2O, a +1-Da shift was observed for this peak (triply charged state shown). Tandem MS analysis localized the mass shift to the N-terminal three amino acids of the peptide (CCV), but limited fragmentation prevented more specific assignments (Fig. 8b). High pH incubations of a monoclonal IgG2κ antibody yielded identical changes in its HC hinge peptide (data not shown). Small amounts of racemization were also found in the κ LC cysteine 214 in this sample (data not shown).
FIGURE 7.
Cysteine racemization on IgG2λ LC. a, XIC of peptides and isotopic distribution (inset). The y axis of the inset represents relative level. Top panel, unstressed material. Bottom panel, antibody was incubated at 56 °C for 6 days at pH 9.3 in D2O. The portion of the chromatogram marked with a bracket was expanded 10 times on both panels for easier visualization. b, MS2 spectra of selected peaks shown in a.
FIGURE 8.
Cysteine racemization on IgG2λ HC hinge region. a, XIC of peptides and isotopic distribution (inset). The y axis of the inset represents relative level. Top panel, unstressed material. Bottom panel, antibody was incubated at 56 °C for 6 days at pH 9.3 in D2O. The portion of the chromatogram marked with a bracket was expanded 10 times on both panels for easier visualization. b, MS2 spectra of material selected peaks shown in a.
Racemization in Vivo
Resolution of the d- and l-cysteine containing peptides by RP-HPLC under reducing conditions was used as the basis for measuring the relative levels of racemization naturally occurring on endogenous human antibodies. Lys-C peptide mapping with MS detection was performed on myeloma IgG1 and IgG2 proteins with either κ or λ light chains and on antibodies obtained from the serum of healthy subjects. Results from these analyses are shown in Table 1. Significant amounts of d-cysteine in HC 220 were detected in IgG1, both for IgG1κ and IgG1λ (4.1 and 5.8%, respectively). LC d-cysteine levels on the IgG1 myeloma proteins are in line with what would be expected by incubating antibodies in vitro under physiological conditions. The light chain on the myeloma IgG1λ protein contained 2.7% d-cysteine at position 214, whereas the light chain on myeloma IgG1κ had only 0.09% at the analogous position. Small amounts of d-cysteine were also detected in myeloma IgG2 proteins. For a myeloma IgG2λ protein, similar amounts were seen in the LC and HC cysteines. In contrast, on a myeloma IgG2κ protein, lower amounts of d-cysteine were found in the HC hinge, and only very low amounts were detected in the LC 214.
TABLE 1.
d-Cysteine on HC Hinge (H220) and LC C-terminal (L214) in naturally occurring antibodies
All values are given in percentages of the total cysteine for that position. For endogenous IgG1 and IgG2 values in parentheses are the range seen between the two values. For the endogenous (Endog) IgG sample, the values in parentheses are S.D. from different samples and include both sample variation and analysis variation. Single analyses are provided for the myeloma samples. The general relative S.D. from independent analyses is ∼6% for replicate samples containing 30% d-cysteine. NA, not applicable.
| HC | λ LC | κ LC | |
|---|---|---|---|
| Myeloma IgG1κ | 4.1 | NA | 0.09 |
| Myeloma IgG1λ | 5.8 | 2.7 | NA |
| Myeloma IgG2κ | 0.28 | NA | 0.07 |
| Myeloma IgG2λ | 0.62 | 0.76 | NA |
| Endog IgG1 (n = 2) | 6.8 (1.9) | 3.2 (1.4) | 0.02 (0.05) |
| Endog IgG2 (n = 2) | 0.58 (0.04) | 0.86 (0.24) | 0.04 (0.02) |
| Endog IgG (n = 4) | 7.4 (1.3) | 3.2 (0.46) | 0.07 (0.09) |
d-Cysteine levels at these two positions were also measured on IgGs isolated from healthy volunteers. Polyclonal IgG1 and IgG2 fractions were obtained by separation through protein A chromatography. Each sample will therefore be a mixture of antibodies containing λ or κ light chains. The d-cysteine amounts measured were similar to those seen with the myeloma proteins. An unfractionated endogenous IgG sample will have on average about two times as much IgG1 as IgG2, so the d-cysteine levels in this sample more closely matched the purified IgG1 sample as expected.
DISCUSSION
In recent work, we proposed a base-catalyzed reaction mechanism for thioether formation in IgG1, whose features were similar to those proposed by Cohen et al. (4) but with additional elements to highlight the influence of LC type on the reaction. Certain portions of this model are shown in Fig. 9 for a general disulfide bond. In a first step, a disulfide bond undergoes reversible β elimination, transforming one of the cysteines into dehydroalanine. This species is relatively unstable and can go on to form a thioether bond (4, 10) or can convert to its original disulfide bond. Supporting this mechanism, dehydroalanine was detected in the pH stressed λ LC samples samples (peak at 34 min; m/z = 773.4) at levels of <1% (data not shown). Dehydroalanine was not found on the corresponding HC peptide, most likely because of its early chromatographic elution. Reformation of the disulfide bond would involve rehydrogenation on the cysteine α carbon. Because chirality on that carbon is lost during dehydrogenation, subsequent non-enzymatic hydrogenation produces a mixture of d- and l-cysteine. d-cysteine would be an expected by product of a thioether reaction involving β elimination; thus, it should be detected in other thioether containing proteins such as long lived eye lens proteins (16, 17).
FIGURE 9.
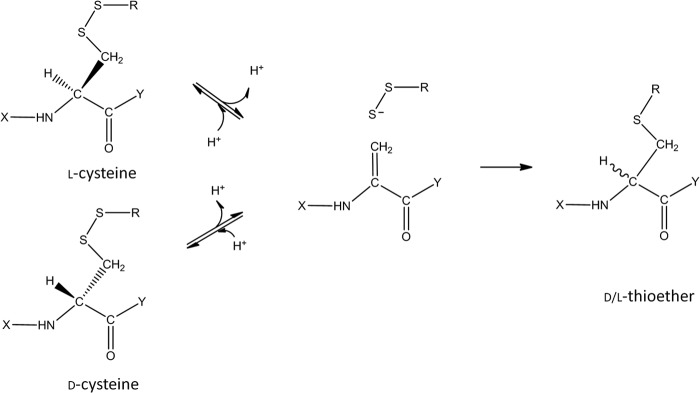
Simplified reaction scheme for base-catalyzed cysteine racemization and thioether formation.
Dehydrogenation/rehydrogenation was confirmed in the thioether formation mechanism by use of D2O. All thioether products formed in D2O contained at least a +1-Da mass shift (associated with the cysteine), but some of the IgGλ thioether product appeared to also contain a 2-Da mass increase. MS analysis suggested that the 2-Da increase was the result of dehydrogenation and rehydrogenation occurring on both the LC and the HC cysteines involved in the thioether linkage. Reversibility of the β elimination partial reaction is one possible explanation for the additional mass increase. If a fraction of the dehydroalanine that formed proceeds to thioether, whereas the rest reverts to its original disulfide bond, then some of the thioether product would bear the signs of multiple dehydrogenations. Proof of this could be found through deuterium incorporation on the HC and λ LC cysteines and by racemization at these positions.
In the simplified reaction scheme shown in Fig. 9, one cysteine linked in a disulfide bond undergoes dehydrogenation producing a dehydroalanine. Although either the LC of HC cysteine could potentially be dehydrogenated, the efficiency of this step would be greatly affected by the “Y” functional group. Similar dehydrogenation efficiencies were observed when one or more amino acids are attached to the C terminus of the cysteine, as found for the IgG1 HC 220 or λ LC 214 cysteines. Dehydrogenation reactions rates were much lower, however, when the functional group is a hydroxyl group, as found on the κ LC 214 cysteine. The resultant acidic carboxyl group in this case disfavors this reaction, possibly through charge repulsion.
Amano et al. (8) had presented an alternative reaction mechanism for racemization in the IgG1 hinge region involving a cyclic imidazoline intermediate. Their mechanism was developed to explain racemization that was found uniquely on HC cysteine 220 of an IgG1κ antibody. Only a single deuterium was incorporated in high pH incubations and only at this one HC cysteine. Our observations are consistent with theirs, but we also find that significant racemization can occur on cysteine 214 of the λ light chain. Therefore, a more likely explanation for our collection of observations is not that the HC sequence is unique but that the proximity of the carboxylic charge group at the C terminus inhibits the racemization on the κ LC cysteine. In fact, levels of racemization on λ LC cysteine 214 were similar to the amounts found on the HC 220 on the same antibody. Dehydrogenation on either the LC or HC cysteines also result in thioether formation of the HC-LC bond, which also accounts for the higher thioether levels on IgG1 antibodies with λ LCs over those with κ LCs.
Base catalyzed racemization and thioether formation is much more favorable at the HC220-LC214 disulfide bond of an IgG1 than at other disulfide positions of IgG1 or any positions in an IgG2. Moreover, greater levels of d-cysteine were formed on the λ LC cysteine 214 in an IgG1 antibody than on an IgG2 antibody stressed at high pH. This result is consistent with the observation that about four times as much λ LC 214 d-cysteine was found on endogenous IgG1 than an endogenous IgG2, yet very little racemization is detected at other positions. What is unique about this position? One factor that can influence the reaction rate is solvent accessibility. The ranking of HC-LC bond sensitivity to chemical reduction is IgG1λ > IgG1κ > IgG2λ > IgG2κ (18), similar to the racemization rankings. Other disulfide bonds such as the HC-HC interchain links also are solvent accessible but are less prone to racemization or thioether formation. It is not known whether the multiple hinge disulfides or other features in the hinge retard these reactions.
Racemization on the HC-LC-linked cysteines was found on endogenous human antibodies. The levels observed were consistent with a base-catalyzed formation mechanism. For example, the d-cysteine formation rate of the LC214 in an IgG1λ antibody under physiological conditions (pH 7.4, 37 °C) is expected to be between the measured rates at pH 7 (0.08%/day) and pH 8 (0.51%/day). Assuming a 14 to 21 day half-life for endogenous antibodies (19–21) and a linear interpolation of the rates between pH 7 and 8, we would estimate 3.6 to 5.4% d-cysteine in LC 214 of endogenous IgG1λ. This is similar to the measured value of 3.2 (S.D. = 1.4, see Table 1). Thus, cysteine racemization is a posttranslational modification occurring on both therapeutic and endogenous antibodies at significant levels, though it is not typically mentioned as a cysteine-related attribute (22, 23). Upon injection, a therapeutic antibody would be expected to form both thioethers (10) and d-cysteines at the HC-LC position at similar rates.
Racemization levels measured here can be use to estimate the relative efficiencies of the dehydroalanine intermediate partial reactions shown in Fig. 9. Either the reaction proceeds to form a thioether or dehydrogenation occurs, generating d- and l-cysteine. If the d- and l-cysteine were to form in equal amounts, the rehydrogenation reaction could be estimated by doubling the measured levels of d-cysteine produced. Because thioether formation rates were determined under the same conditions used in this racemization study (10), these two rates could be compared. Between 40 and 55% of the dehydrogenated intermediate in the pH 8 and 9 reactions (at 37 °C) formed thioether products, with the remaining reversing to the racemized cysteine. These values are similar to the efficiencies calculated from the levels of d-cysteine and thioether found in endogenous IgG1 antibodies.
Product quality attribute monitoring during manufacturing and storage ensures consistent safety and efficacy of therapeutic proteins. Lot-to-lot variations in the levels of some attribute are minimized due to concerns that they may pose a safety risk, such as a greater immunogenic potential. Although efficacy would be another potential concern, it was not examined here. For the d-cysteine, knowledge that it is naturally occurring and in a conserved region of the molecule is taken into account when assessing its safety risk.
Acknowledgments
We gratefully acknowledge Dr. Andy Goetze for providing endogenous IgG samples and Diana Liu for kind support and discussions.
Footnotes
- LC-HC
- light chain-heavy chain
- XIC
- extracted ion chromatogram.
REFERENCES
- 1. Maggon K. (2007) Monoclonal antibody “gold rush.” Curr. Med. Chem. 14, 1978–1987 [DOI] [PubMed] [Google Scholar]
- 2. Weiner L. M., Surana R., Wang S. (2010) Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 10, 317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu H., Gaza-Bulseco G., Faldu D., Chumsae C., Sun J. (2008) Heterogeneity of monoclonal antibodies. J. Pharm. Sci. 97, 2426–2447 [DOI] [PubMed] [Google Scholar]
- 4. Cohen S. L., Price C., Vlasak J. (2007) β-elimination and peptide bond hydrolysis: two distinct mechanisms of human IgG1 hinge fragmentation upon storage. J. Am. Chem. Soc. 129, 6976–6977 [DOI] [PubMed] [Google Scholar]
- 5. Tous G. I., Wei Z., Feng J., Bilbulian S., Bowen S., Smith J., Strouse R., McGeehan P., Casas-Finet J., Schenerman M. A. (2005) Characterization of a novel modification to monoclonal antibodies: thioether cross-link of heavy and light chains. Anal. Chem. 77, 2675–2682 [DOI] [PubMed] [Google Scholar]
- 6. Mozziconacci O., Kerwin B. A., Schöneich C. (2010) Exposure of a monoclonal antibody, IgG1, to UV-light leads to protein dithiohemiacetal and thioether cross-links: a role for thiyl radicals? Chem. Res. Toxicol. 23, 1310–1312 [DOI] [PubMed] [Google Scholar]
- 7. Yan B., Yates Z., Balland A., Kleemann G. R. (2009) Human IgG1 Hinge Fragmentation as the Results of H2O2-mediated Radical Cleavage. J. Biol. Chem. 284, 35390–35402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amano M., Hasegawa J., Kobayashi N., Kishi N., Nakazawa T., Uchiyama S., Fukui K. (2011) Specific racemization of heavy-chain cysteine-220 in the hinge region of immunoglobulin γ1 as a possible cause of degradation during storage. Anal. Chem. 83, 3857–3864 [DOI] [PubMed] [Google Scholar]
- 9. Cordoba A. J., Shyong B. J., Breen D., Harris R. J. (2005) Non-enzymatic hinge region fragmentation of antibodies in solution. J. Chromatog. B 818, 115–121 [DOI] [PubMed] [Google Scholar]
- 10. Zhang Q., Schenauer M. R., McCarter J. D., Flynn G. C. (2013) IgG1 thioether bond formation in vivo. J. Biol. Chem. 288, 16371–16382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Y. D., van Enk J. Z., Flynn G. C. (2009) Human antibody Fc deamidation in vivo. Biologicals 37, 313–322 [DOI] [PubMed] [Google Scholar]
- 12. Flynn G. C., Chen X., Liu Y. D., Shah B., Zhang Z. (2010) Naturally occurring glycan forms of human immunoglobulins G1 and G2. Mol. Immunol. 47, 2074–2082 [DOI] [PubMed] [Google Scholar]
- 13. Pan H., Chen K., Pulisic M., Apostol I., Huang G. (2009) Quantitation of soluble aggregates in recombinant monoclonal antibody cell culture by pH-gradient protein A chromatography. Anal. Biochem. 388, 273–278 [DOI] [PubMed] [Google Scholar]
- 14. Wypych J., Li M., Guo A., Zhang Z., Martinez T., Allen M. J., Fodor S., Kelner D. N., Flynn G. C., Liu Y. D., Bondarenko P. V., Ricci M. S., Dillon T. M., Balland A. (2008) Human IgG2 antibodies display disulfide-mediated structural isoforms. J. Biol. Chem. 283, 16194–16205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dillon T. M., Ricci M. S., Vezina C., Flynn G. C., Liu Y. D., Rehder D. S., Plant M., Henkle B., Li Y., Deechongkit S., Varnum B., Wypych J., Balland A., Bondarenko P. V. (2008) Structural and functional characterization of disulfide isoforms of the human IgG2 subclass. J. Biol. Chem. 283, 16206–16215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bessems G. J., Rennen H. J., Hoenders H. J. (1987) Lanthionine, a protein cross-link in cataractous human lenses. Exp. eye res. 44, 691–695 [DOI] [PubMed] [Google Scholar]
- 17. Linetsky M., Hill J. M., LeGrand R. D., Hu F. (2004) Dehydroalanine crosslinks in human lens. Exp. Eye Res. 79, 499–512 [DOI] [PubMed] [Google Scholar]
- 18. Hutterer K. M., Hong R. W., Lull J., Zhao X., Wang T., Pei R., Le M. E., Borisov O., Piper R., Liu Y. D., Petty K., Apostol I., Flynn G. C. (2013) Monoclonal antibody disulfide reduction during manufacturing: Untangling process effects from product effects. MAbs. 5, 608–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vlug A., Nieuwenhuys E. J., van Eijk R. V., Geertzen H. G., van Houte A. J. (1994) Nephelometric measurements of human IgG subclasses and their reference ranges. Annales de Biologie Clinique 52, 561–567 [PubMed] [Google Scholar]
- 20. Alyanakian M. A., Bernatowska E., Scherrmann J. M., Aucouturier P., Poplavsky J. L. (2003) Pharmacokinetics of total immunoglobulin G and immunoglobulin G subclasses in patients undergoing replacement therapy for primary immunodeficiency syndromes. Vox Sang. 84, 188–192 [DOI] [PubMed] [Google Scholar]
- 21. Out T. A., McDonald J. R., Woldhuis-Kant J., Nieuwenhuys E. J. (1984) The effect of reduction of IgM on the quantitative determination of IgM by radial immunodiffusion and turbidimetry. Clin. Chim. Acta 144, 115–126 [DOI] [PubMed] [Google Scholar]
- 22. Eon-Duval A., Broly H., Gleixner R. (2012) Quality Attributes of Therapeutic Proteins: An Assessment of Impact on Safety and Efficacy as Part of a Quality by Design Development Approach. Biotechnol. Prog. 28, 608–622 [DOI] [PubMed] [Google Scholar]
- 23. Liu H., May K. (2012) Disulfide bond structures of IgG molecules. Mabs 4, 17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]