Abstract
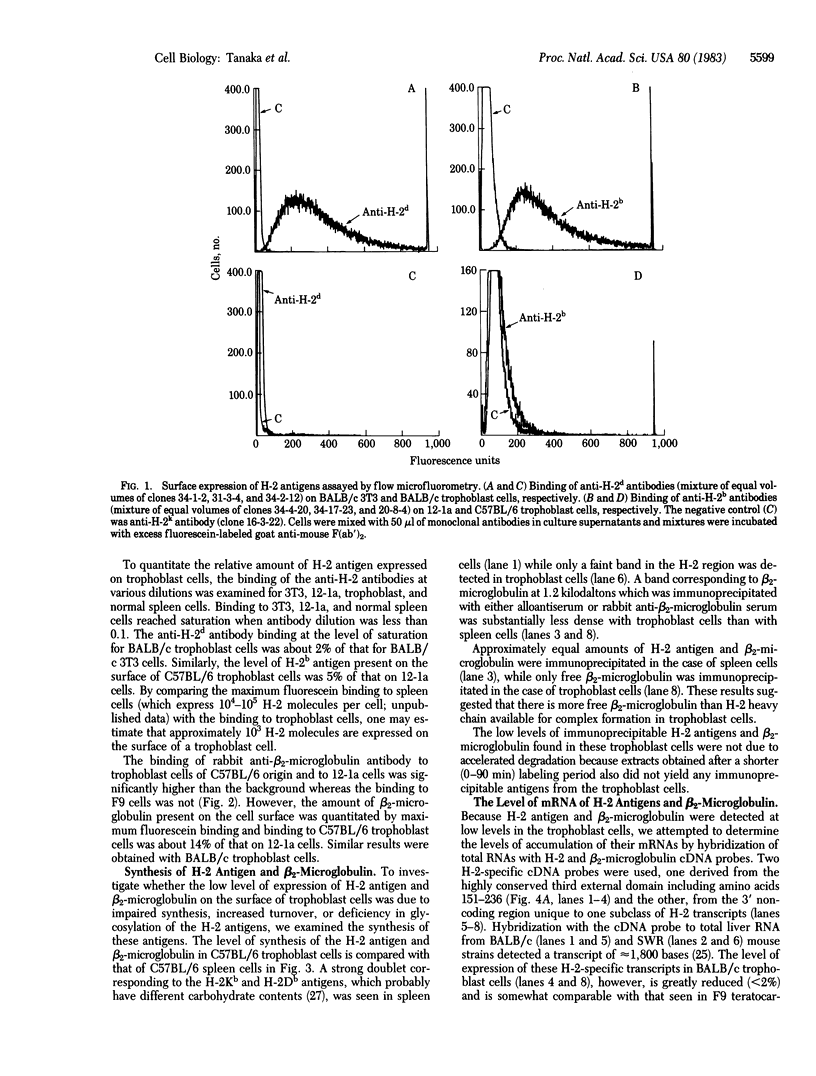
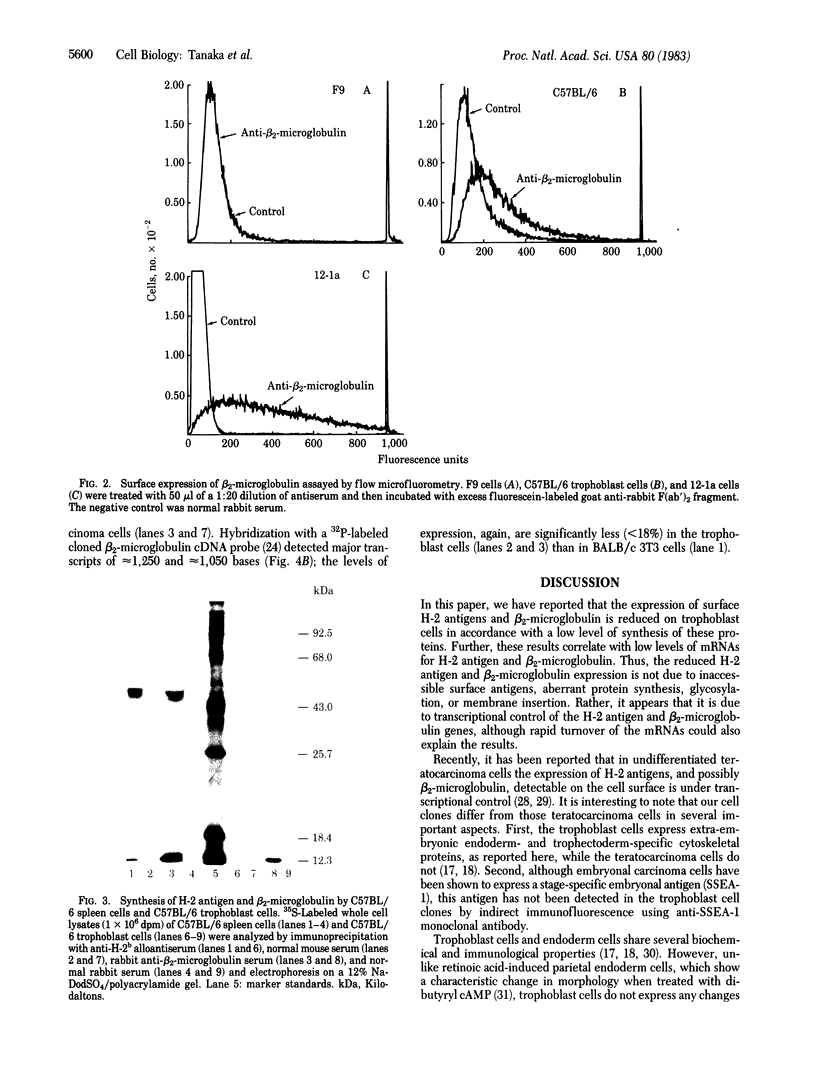
We have investigated the expression of H-2 antigen and beta 2-microglobulin in recently established mouse trophoblast cell clones. These clones, derived from C57BL/6 (H-2b) or BALB/c (H-2d) strains, synthesized extra-embryonic endoderm- and trophectoderm-specific cytoskeletal proteins, termed "Endo A" and "Endo B," and no detectable SSEA-1 embryonic antigen. Flow microfluorometry indicated that H-2 antigen expression on the cell surface of trophoblast cells was very low, corresponding to 2-5% of the amounts found on differentiated teratocarcinoma cells (12-1a) and BALB/c 3T3 cells, respectively. Expression of beta 2-microglobulin was reduced to approximately equal to 14% of the amounts found on 12-la cells. Immunoprecipitation and polyacrylamide gel electrophoretic analysis indicated that the synthesis of H-2 antigen and beta 2-microglobulin in the trophoblast cells was lower than that found in normal spleen cells. In addition low, but unequal, levels of mRNA specific for H-2 antigen and beta 2-microglobulin were found in trophoblast cells by blot hybridization with cDNA probes. The low mRNA levels may be due to transcriptional control of the genes encoding H-2 antigen and beta 2-microglobulin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arce-Gomez B., Jones E. A., Barnstable C. J., Solomon E., Bodmer W. F. The genetic control of HLA-A and B antigens in somatic cell hybrids: requirement for beta2 microglobulin. Tissue Antigens. 1978 Feb;11(2):96–112. doi: 10.1111/j.1399-0039.1978.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee-Hasrouni S., Lala P. K. Localization of paternal H-2K antigens on murine trophoblast cells in vivo. J Exp Med. 1982 Jun 1;155(6):1679–1689. doi: 10.1084/jem.155.6.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee-Hasrouni S., Lala P. K. MHC antigens on mouse trophoblast cells: paucity of Ia antigens despite the presence of H-2K and D. J Immunol. 1981 Nov;127(5):2070–2073. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cosman D., Kress M., Khoury G., Jay G. Tissue-specific expression of an unusual H-2 (class I)-related gene. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4947–4951. doi: 10.1073/pnas.79.16.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Linnenbach A., Huebner K., Parnes J. R., Margulies D. H., Appella E., Seidman J. G. Control of expression of histocompatibility antigens (H-2) and beta 2-microglobulin in F9 teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5754–5758. doi: 10.1073/pnas.78.9.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk W. P., Temple A. Distribution of beta2 microglobulin and HLA in chorionic villi of human placentae. Nature. 1976 Aug 26;262(5571):799–802. doi: 10.1038/262799a0. [DOI] [PubMed] [Google Scholar]
- Fellous M., Colle A., Tonnelle C. The expression of human beta2-microglobulin on human spermatozoa. Eur J Immunol. 1976 Jan;6(1):21–24. doi: 10.1002/eji.1830060106. [DOI] [PubMed] [Google Scholar]
- Gachelin G., Fellous M., Guenet J. L., Jacob F. Developmental expression of an early embryonic antigen common to mouse spermatozoa and cleavage embryos, and to human spermatozoa: its expression during spermatogenesis. Dev Biol. 1976 Jun;50(2):310–320. doi: 10.1016/0012-1606(76)90154-8. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Barnstable C. J., Bodmer W. F., Snary D., Crumpton M. J. Expression of HLA system antigens on placenta. Transplantation. 1976 Dec;22(6):595–603. doi: 10.1097/00007890-197612000-00009. [DOI] [PubMed] [Google Scholar]
- Jay G., Ferrini U., Robinson E. A., Khoury G., Appella E. Cell-free synthesis of mouse H-2 histocompatibility antigens. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6562–6566. doi: 10.1073/pnas.76.12.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles B. B., Pan S., Solter D., Linnenbach A., Croce C., Huebner K. Expression of H-2, laminin and SV40 T and TASA on differentiation of transformed murine teratocarcinoma cells. Nature. 1980 Dec 11;288(5791):615–618. doi: 10.1038/288615a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Laskov R., Scharff M. D. Synthesis, assembly, and secretion of gamma globulin by mouse myeloma cells. I. Adaptation of the Merwin plasma cell tumor-11 to culture, cloning, and characterization of gamma globulin subunits. J Exp Med. 1970 Mar 1;131(3):515–541. doi: 10.1084/jem.131.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnenbach A., Huebner K., Croce C. M. DNA-transformed murine teratocarcinoma cells: regulation of expression of simian virus 40 tumor antigen in stem versus differentiated cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4875–4879. doi: 10.1073/pnas.77.8.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Log T., Chang K. S., Hsu Y. C. Carcinomas induced by cell lines cultivated from normal mouse placentas. Int J Cancer. 1981 Mar 15;27(3):365–372. doi: 10.1002/ijc.2910270316. [DOI] [PubMed] [Google Scholar]
- Morello D., Daniel F., Baldacci P., Cayre Y., Gachelin G., Kourilsky P. Absence of significant H-2 and beta 2-microglobulin mRNA expression by mouse embryonal carcinoma cells. Nature. 1982 Mar 18;296(5854):260–262. doi: 10.1038/296260a0. [DOI] [PubMed] [Google Scholar]
- Oshima R. G. Identification and immunoprecipitation of cytoskeletal proteins from murine extra-embryonic endodermal cells. J Biol Chem. 1981 Aug 10;256(15):8124–8133. [PubMed] [Google Scholar]
- Ozato K., Mayer N. M., Sachs D. H. Monoclonal antibodies to mouse major histocompatibility complex antigens. Transplantation. 1982 Sep;34(3):113–120. doi: 10.1097/00007890-198209000-00001. [DOI] [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Parnes J. R., Seidman J. G. Structure of wild-type and mutant mouse beta 2-microglobulin genes. Cell. 1982 Jun;29(2):661–669. doi: 10.1016/0092-8674(82)90182-9. [DOI] [PubMed] [Google Scholar]
- Ramanathan L., Dubois G. C., Robinson E. A., Appella E. Purification and characterization of mouse beta-2 microglobulin: allelic variants from two different strains. Mol Immunol. 1982 Mar;19(3):435–446. doi: 10.1016/0161-5890(82)90209-7. [DOI] [PubMed] [Google Scholar]
- Sawicki J. A., Magnuson T., Epstein C. J. Evidence for expression of the paternal genome in the two-cell mouse embryo. Nature. 1981 Dec 3;294(5840):450–451. doi: 10.1038/294450a0. [DOI] [PubMed] [Google Scholar]
- Sellens M. H. Antigen expression on early mouse trophoblast. Nature. 1977 Sep 1;269(5623):60–61. doi: 10.1038/269060a0. [DOI] [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A. 1978 Nov;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Swartzendruber D. E., Lehman J. M. Neoplastic differentiation: interaction of simian virus 40 and polyoma virus with murine teratocarcinoma cells in vitro. J Cell Physiol. 1975 Apr;85(2 Pt 1):179–187. doi: 10.1002/jcp.1040850204. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Chowdhury K., Chang K. S., Israel M., Ito Y. Isolation and characterization of polyoma virus mutants which grow in murine embryonal carcinoma and trophoblast cells. EMBO J. 1982;1(12):1521–1527. doi: 10.1002/j.1460-2075.1982.tb01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]