Background: Prohibitin is essential in adipocyte differentiation and mitochondrial functions, but the regulative mechanisms of prohibitin by microRNA remain unclear.
Results: miR-27 negatively regulates adipogenesis by targeting prohibitin and impairing mitochondrial biogenesis, structure, and activity.
Conclusion: miR-27 targets prohibitin and suppresses adipocyte differentiation.
Significance: Manipulation of miR-27 may offer opportunities for the therapeutic modulation of adipogenesis in obesity.
Keywords: Adipogenesis, Cell Differentiation, MicroRNA, Mitochondria, Stem Cells, miR-27, Prohibitin
Abstract
Prohibitin (PHB) has been reported to play a crucial role in adipocyte differentiation and mitochondrial function. However, the regulative mechanism of PHB during adipogenesis remains unclear. In this study, we determined that the levels of both microRNA (miR)-27a and miR-27b were down-regulated following adipogenic induction of human adipose-derived stem cells, whereas the mRNA level of PHB was up-regulated. Overexpression of miR-27a or miR-27b inhibited PHB expression and adipocyte differentiation. Using PHB 3′-UTR luciferase reporter assay, we observed that miR-27a and miR-27b directly targeted PHB in human adipose-derived stem cells. A compensation of PHB partially restored the adipogenesis inhibited by miR-27. Moreover, we demonstrated the novel finding that ectopic expression of miR-27a or miR-27b impaired mitochondrial biogenesis, structure integrity, and complex I activity accompanied by excessive reactive oxygen species production. Our data suggest that miR-27 is an anti-adipogenic microRNA partly by targeting PHB and impairing mitochondrial function. Pharmacological modulation of miR-27 function may provide a new therapeutic strategy for the treatment of obesity.
Introduction
The recent discovery of microRNAs (miRNAs)2 has introduced a novel type of regulatory control over gene expression during plant and animal development (1, 2). The finding that some of these miRNAs are expressed in adipose tissue from obese mouse models and humans has rekindled an interest in post-transcriptional regulation during the development of obesity and has raised the question of the role of miRNAs during this process (3–6). miRNAs comprise a large family of ∼22-nucleotide single-stranded RNAs that decrease gene expression by binding to target mRNAs and leading to translational repression. miRNAs can therefore cause partial or full silencing of respective target genes. Furthermore, it seems that miRNAs can form extensive regulatory networks with a complexity comparable to that of transcription factors (7, 8). Hyperplasia and hypertrophy of adipocytes can cause the formation of and increase in adipose tissues and may then result in obesity (9). It is widely recognized that proliferation, differentiation, and apoptosis of preadipocytes are all related to the hyperplasia of adipose tissue (10). Elucidating the mechanisms involved in preadipocyte differentiation will be critical in the study of therapies for obesity. Our previous studies have demonstrated that miRNAs are involved in stem cell differentiation (11, 12). To profile the global changes in miRNA expression during adipogenesis, a miRNA expression array study was performed using total RNA extracted from human-derived preadipocytes and adipocytes (13). Several miRNAs, including miR-27, have been implicated in the process of accelerating or inhibiting preadipocyte differentiation (3, 14–18).
Prohibitin (PHB) is highly expressed in cells that rely heavily on mitochondrial function (19). Mitochondrial biogenesis and remodeling are considered to be necessary adjustments during adipogenesis as the cells become increasingly active in metabolism (20). A recent publication has shown that PHB deficiency in nematodes markedly reduces mitochondrial membrane potential and fat content early in adulthood (21). Our group has revealed that PHB silencing by synthetic siRNA induces down-regulation of peroxisome proliferator-activated receptor γ (PPARγ), reduction of adipogenesis, and dysfunction of mitochondria in the mouse 3T3-L1 cell line (22). PHB has been predicted to be a broadly conserved target of miR-27 by using the miRNA prediction database. Indeed, miR-27 has been observed to target PHB to promote proliferation in cancer cells (23, 24). In this study, we demonstrate that miR-27 suppresses adipogenesis by targeting PHB and impairing mitochondrial biogenesis and function in human ASC.
EXPERIMENTAL PROCEDURES
Human ASC Culture and Adipocyte Differentiation
Human ASC and their culture medium were purchased from Invitrogen. The cells were cultured in ASC growth medium containing basal medium, growth supplement, and 2 mmol/liter l-glutamine. The cultures were maintained in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. The medium was replaced every 2–3 days. Passages 5–6 were used for all experiments. To initiate adipocyte differentiation, overconfluent ASC (day 0) were treated with an adipogenic medium containing ASC basal medium, 10% FBS, 2 mmol/liter l-glutamine, 1 μmol/liter dexamethasone (Sigma), 10 μmol/liter insulin (Sigma), 0.5 mmol/liter isobutylmethylxanthine (Sigma), 200 μmol/liter indomethacin (Sigma), 100 units/ml penicillin, and 100 μg/ml streptomycin. The differentiation medium was changed every 3 days thereafter until the indicated times (22). For certain adipogenic experiments, 1 μm rosiglitazone (Thermo Fisher Scientific), a PPARγ agonist, was added to the adipogenic medium.
TaqMan miRNA Assay
Total RNA, including miRNA, was extracted from ASC by using a mirVana isolation kit (Invitrogen) according to the instructions of the manufacturer. Reverse transcription was performed using a TaqMan microRNA reverse transcription kit (Invitrogen) and reverse transcriptase primers from the specific TaqMan microRNA assays (Invitrogen). Equal amounts of total RNA (10 ng) were reverse-transcribed with 1 mm dNTPs, 50 units of reverse transcriptase, 4 units of RNase inhibitor, and specific miRNA reverse transcriptase primers in a total volume of 15 μl at 16 °C for 30 min, 42 °C for 30 min, and 85 °C for 5 min.
Real-time PCR for miRNA assay was then conducted on a LightCycler 480 system (Roche Applied Science) at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Each PCR contained 0.2 μl of the reverse transcription reaction product, 10 μl of TaqMan Universal PCR Master Mix (Invitrogen), 1 μl of a mixture of PCR primer/probe from the specific TaqMan MicroRNA assays, and 8.8 μl of nuclease-free water in a total volume of 20 μl. The relative miRNA levels were normalized to endogenous U6 snRNA expression for each sample (25).
Real-time PCR Analysis
Real-time PCR for mRNA analysis was performed as described previously (22). Total RNA was isolated from ASC at the indicated times using an RNeasy mini kit (Qiagen). Isolated RNA was reverse-transcribed with an oligo(dT) primer using an Advantage RT-for-PCR kit (Clontech). Real-time PCR was performed using a LightCycler FastStart DNA Master SYBR Green I kit (Roche Applied Science) and a LightCycler real-time thermal cycler (Roche Applied Science). The primer pairs used were 5′-agtggtggctcgctttgatgct-3′ and 5′-gatgatggccgcctttttctgtt-3′ for human PHB, 5′-agcctcatgaagagccttcca-3′ and 5′-tccggaagaaacccttgca-3′ for human PPARγ, 5′-tgtgcagaaatgggatggaaa-3′ and 5′-caacgtcccttggcttatgct-3′ for human aP2, and 5′-ggaagggcaccaccaggagt-3′ and 5′-tgcagccccggacatctaag-3′ for human 18 S rRNA (used as an internal control). The amplified products were analyzed by electrophoresis on 2% E-Gel agarose (Invitrogen) to verify the primer specificity and PCR product size.
Creation and Transduction of Lentivirus
Lentiviral plasmids pLenti/miR-Control, pLenti/miR-27a, and pLenti/miR-27b were purchased from System Biosciences (Mountain View, CA); pLenti/Luc-UTR/Blank, pLenti/Luc-UTR/PHB, and pLenti/Luc-UTR/PHBmut were purchased from Applied Biological Materials (Richmond, British Columbia, Canada); pLenti/GFP was purchased from Invitrogen; and pLenti/PHB was created in our laboratory. The creation, concentration, titration, and ASC transduction of lentivirus were described previously (22). ASC transduction efficiency of lentivirus was evaluated by determining GFP expression of Lenti/miR-Control, Lenti/miR-27a, and Lenti/miR-27b by flow cytometry. Briefly, ASC were harvested after transduction of lentivirus for 3 days and analyzed on a Guava EasyCyte flow cytometer (EMD Millipore). The green fluorescence of the measured cell population (events = 5000) was gated using the Guava Express Plus program.
Immunoblotting
Cells were lysed in mammalian protein extraction reagent (Thermo Fisher Scientific) supplemented with protease inhibitor mixture (Sigma). The cell lysates were resolved by electrophoresis on 10% or 4–12% precast BisTris gel (Invitrogen). Proteins were transferred from the gel to a nitrocellulose membrane using an iBlot dry blotting system (Invitrogen). Specific proteins were detected using anti-PHB (BioLegend, San Diego, CA), anti-C/EBPβ (Cell Signaling, Danvers, MA), anti-PPARγ (Cell Signaling), anti-aP2 (Abcam, Cambridge, MA), and anti-HSP90 (Santa Cruz Biotechnology, Santa Cruz, CA) primary antibodies. HRP-conjugated anti-rabbit IgG or anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) was used as a secondary antibody. Blots were revealed with enhanced chemiluminescent reagents (Thermo Fisher Scientific).
Oil Red O Staining
Oil Red O (Sigma) staining was performed as described previously (22). Fourteen days after the induction of adipocyte differentiation, ASC in 35-mm dishes were washed with PBS and fixed with 10% formalin. The cells were washed once with 60% isopropyl alcohol and left to dry completely. The cells were then stained with 0.2% Oil Red O for 10 min, rinsed once with 60% isopropyl alcohol, and thoroughly washed four times with water. The dishes were scanned to obtain the pictures. The dye was then extracted with 100% isopropyl alcohol and quantified on a spectrophotometer (Molecular Devices, Sunnyvale, CA) by reading the absorbance at a wavelength of 510 nm.
Luciferase Reporter Assay
Luciferase activities in ASC were determined using a luciferase assay kit (Applied Biological Materials) and an Lmax microplate luminometer with SoftMax Pro software (Molecular Devices) according to the instructions of the manufacturers. In brief, ASC were transduced with Lenti/miR-Control, Lenti/miR-27a, or Lenti/miR-27b at a multiplicity of infection (m.o.i.) of 2. The following day, the cells were transduced with Lenti/Luc-UTR/Blank, Lenti/Luc-UTR/PHB, or Lenti/Luc-UTR/PHBmut at m.o.i. = 1. After 2 days of additional incubation, the cells were washed once with PBS. Cell lysis buffer, included in the kit, was added to the cells, followed by incubation at room temperature for 25 min. An equal volume of luciferase assay reagent was added to the cells. The luminescence was measured using a 10-s integration time between 30 min and 1 h after the addition of the assay reagent.
Detection of Mitochondrial DNA Content
Total DNA in ASC was isolated with a DNeasy DNA isolation kit (Qiagen). The DNA levels of the human mitochondrial ND1 (NADH dehydrogenase 1) gene and nuclear 18 S rRNA were determined by real-time PCR quantification. The relative mtDNA content was reflected by the ratio of DNA levels between mitochondrial ND1 and nuclear 18 S rRNA as described previously (26).
Mitochondrial Membrane Potential Assay
The mitochondrial membrane potential of ASC was measured by detecting the accumulation of tetramethylrhodamine ethyl ester (TMRE), a red fluorescent dye, in active mitochondria by flow cytometry. Briefly, ASC were incubated in 200 nmol/liter TMRE at 37 °C and 5% CO2 for 20 min. The cells were then washed with PBS once and trypsinized. The red fluorescence of the cell population (events = 10,000) was gated using the Guava Express Plus program in a Guava EasyCyte system.
MitoTracker Staining and Confocal Microscopy
ASC in Lab-Tek chamber slides (Thermo Fisher Scientific) were stained with 250 nmol/liter MitoTracker (Invitrogen) in serum-free DMEM for 15 min at 37 °C according to the manufacturer's instructions. Images were captured and analyzed using a Leica TCS SP5 confocal microscopy system (Leica Microsystems, Bannockburn, IL) as described previously (22).
Measurement of ATP Concentration
Three days post-transduction of ASC in a 96-well plate with Lenti/miR-Control, Lenti/miR-27a, or Lenti/miR-27b, the ATP concentration was measured using an ATP assay system bioluminescence detection kit (Promega, Madison, WI) and the Lmax microplate luminometer with SoftMax Pro software (22).
Reactive Oxygen Species (ROS) Detection
ROS were detected with the cell-permeable, peroxide-sensitive fluorophore CellROX Orange reagent (Invitrogen) according to the manufacturer's instructions. The dye is non-fluorescent while in a reduced state and exhibits bright orange fluorescence upon oxidation by ROS. ASC in a 96-well plate were transduced with Lenti/miR-Control, Lenti/miR-27a, or Lenti/miR-27b and cultured for 3 days. The cells were then incubated in 5 μmol/liter CellROX Orange reagent at 37 °C for 30 min, followed by washing twice with prewarmed PBS. Afterward, the plate was read on a GENios Plus microplate reader with universal reader control and data analysis software (Magellan V3.11, Tecan, San Jose, CA). To ensure that the CellROX Orange reagent was detecting hydrogen peroxide, cells were preincubated with 250 units/ml cell-permeable PEG-catalase (Sigma) at 37 °C for 2 h.
Detection of Mitochondrial Complex I/IV Activities
The activities of mitochondrial complexes I and IV were determined in whole cell lysates of ASC with complexes I and IV enzyme activity dipstick assay kits (Abcam), respectively, according to our previous description (22) and the manufacturer's instructions.
Statistics
All samples were prepared in a minimum of triplicates. Results from the quantitative studies are expressed as the mean ± S.D. of three independent experiments. Statistical analyses were performed by one-way analysis of variance, and comparisons between groups were performed using Student's t test. Differences were considered significant when p < 0.05.
RESULTS
miR-27a and miR-27b Are Predicted to Target Prohibitin and Are Down-regulated during Adipogenesis
Our previous studies have revealed that PHB is essential in adipocyte differentiation (22). To further investigate the regulation of PHB, computational prediction of miRNA families targeting PHB was performed using the TargetScan Database (version Human 6.0). Two miRNAs, hsa-miR-27 and hsa-miR-128, were predicted to be broadly conserved miRNA families among vertebrates targeting human PHB (Fig. 1A). The mRNA level of PHB and the levels of miR-27 and miR-128 were therefore examined in ASC during adipogenesis. The induced expression of adipogenic markers PPARγ and aP2 indicated that an adipogenic model was successfully established. An increase in the mRNA level of PHB was observed (Fig. 1B), which confirmed our previous observation at the protein level of PHB in ASC during adipogenesis (22). In addition, our data demonstrated that both miR-27a and miR-27b were down-regulated in ASC during adipogenesis (Fig. 1C), which was in accordance with the observation in mouse 3T3-L1 preadipocytes (15, 17, 27) and human ASC (16). miR-128 was not detected in ASC before or after adipogenic induction. Similar to our results, miR-128 has been previously reported to be expressed at very low levels or not at all in human ASC using miRNA profiling analysis (28, 29). These results suggest that miR-27 is associated with adipogenesis and may be a candidate miRNA family targeting PHB.
FIGURE 1.
Expression of prohibitin and miR-27 during adipogenesis. A, the mature sequences of miR-27a/b and miR-128 and the partial sequences of the 3′-UTR of PHB from various species are illustrated. The seed sites of miR-27a/b and its binding sites at the 3′-UTR of PHB are shown in green and by the green rectangle, respectively. The seed site of miR-128 and its binding sites at the 3′-UTR of PHB are shown in red and by the red rectangle, respectively. The minimum free energy (mfe) was calculated using the PicTar open-source software. hsa, Homo sapiens; tbe, Tupaia belangeri; mmu, Mus musculus; rno, Rattus norvegicus; ocu, Oryctolagus cuniculus; dno, Dasypus novemcinctus; oan, Ornithorhynchus anatinus. B and C, overconfluent ASC were treated with adipocyte differentiation medium for the indicated times (d, days). Total RNA, including miRNA, was then isolated. B, the relative mRNA expression levels of PHB, PPARγ, and aP2 were analyzed by RT-PCR. 18 S rRNA was used an internal control. The relative levels of each mRNA at 0 h were set to 1. *, p < 0.05; **, p < 0.01 compared with corresponding mRNA level at 0 h. C, the levels of miR-27a, miR-27b, and miR-128 were determined by RT-PCR. U6 snRNA was used as an internal control. The relative levels of miR-27a and miR-27b at 0 h were set to 1. miR-128 was not detected. *, p < 0.05; **, p < 0.01 compared with corresponding miR-27 level at 0 h. Data are means ± S.D.
miR-27a and miR-27b Repress Adipocyte Differentiation
To investigate the potential effects of miR-27 during adipogenesis, miR-27a and miR-27b were overexpressed using lentiviral constructs (m.o.i. = 2). The transduction efficiency of concentrated lentivirus in ASC was evaluated by detecting GFP expressed by the lentiviral construct using flow cytometry. Because of the high transduction efficiency (always in excess of 85%), no antibiotic selection was required (Fig. 2A). An increase of 3–4-fold in miR-27a/b in transduced ASC confirmed overexpression of miR-27a/b (Fig. 2B). In the adipogenic induction experiments, the protein levels of PHB and adipogenic markers C/EBPβ, PPARγ, and aP2 were decreased upon overexpression of miR-27a or miR-27b (Fig. 2C). Likewise, the mRNA of PHB in ASC was significantly reduced as well (Fig. 2D). The lipid accumulation in ASC was also remarkably attenuated at day 14 (Fig. 2E). These data indicate that miR-27 down-regulates PHB expression and suppresses adipocyte differentiation in ASC.
FIGURE 2.
miR-27a and miR-27b inhibit adipogenesis. ASC were transduced with Lenti/miR-Control (miR-Cont), Lenti/miR-27a (miR-27a), or Lenti/miR-27b (miR-27b) and cultured for 3 days to an overconfluent condition. A, the efficiency of lentivirus transduction was determined upon expression of GFP by lentivirus by flow cytometry. B, the levels of miR-27a and miR-27b in transduced ASC were examined by RT-PCR. U6 snRNA was used as an internal control. The relative levels of miR-27a and miR-27b in ASC transduced with Lenti/miR-Control were set to 1. **, p < 0.01 compared with the corresponding level of miR-27a or miR-27b treated with Lenti/miR-Control. C–E, ASC were treated with the adipocyte differentiation medium for the indicated days. C, the protein levels of PHB, C/EBPβ, PPARγ, and aP2 were analyzed with immunoblotting. HSP90 was used as a loading control. D, the mRNA content of PHB was analyzed by RT-PCR. 18 S rRNA was used as an internal control. **, p < 0.01 compared with the level of the Lenti/miR-Control group at day 0. #, p < 0.05; ##, p < 0.01 compared with the level of the Lenti/miR-Control group on the same day. E, ASC were stained with Oil Red O dye at day 14. The accumulated lipid was quantified using readings on a spectrophotometer at 510 nm for cell-released dye. **, p < 0.01 compared with the level of the Lenti/miR-Control group.
Prohibitin Targets miR-27a and miR-27b in ASC
Although hsa-miR-27a and hsa-miR-27b are located at chromosomes 19 and 9, respectively, their mature sequences exhibit a single-nucleotide difference, whereas the seed site sequence is identical. Both are predicted to target the same site of the human PHB 3′-UTR (Fig. 3A). To experimentally validate the targeting effect, a luciferase reporter construct expressing the 3′-UTR of human PHB (Lenti/Luc-UTR/PHB) was created. Another construct expressing the mutated 3′-UTR of PHB (Lenti/Luc-UTR/PHBmut), with a deletion of six nucleotides at the predicted miR-27 target site, was used as a control. Lenti/Luc-UTR/Blank was used as a negative control. Results from co-transduction experiments indicated that the relative luciferase activities in Lenti/Luc-UTR/PHB-treated ASC were significantly inhibited by either miR-27a or miR-27b, whereas the luciferase activities in Lenti/Luc-UTR/PHBmut-treated ASC were unaffected (Fig. 3B). These results suggest that PHB is a direct target of miR-27a and miR-27b in ASC.
FIGURE 3.

Prohibitin is a direct target of miR-27a and miR-27b. A, schematic representation of hsa-miR-27a and hsa-miR-27b binding to the human PHB 3′-UTR. The seed sites of mature miR-27a and miR-27b are highlighted. The dashes indicate the six nucleotides (CTGTGA) that deleted in the mutant construct (Lenti/Luc-UTR/PHBmut). B, ASC were transduced with Lenti/miR-Control (miR-Cont), Lenti/miR-27a (miR-27a), or Lenti/miR-27b (miR-27b) at m.o.i. = 2 and incubated overnight. ASC were then transduced with Lenti/Luc-UTR/Blank, Lenti/Luc-UTR/PHB, or Lenti/Luc-UTR/PHBmut at m.o.i. = 1 as indicated. Following an additional 2 days of incubation, the luciferase activities in ASC were determined by luminometry. **, p < 0.01.
Replenishment of PHB Restores the Adipogenesis Attenuated by miR-27
It has been reported that miR-27 directly targets PPARγ and represses adipogenesis (16, 17). Our previous study revealed that PHB silencing inhibits PPARγ expression and adipocyte differentiation; additionally, an increase in PHB occurs earlier than that in PPARγ during adipogenesis, implying that PHB is located upstream of PPARγ in the signaling transduction pathway of adipogenesis (22). In an analysis using a miRNA target prediction website (PicTar), the PicTar scores for miR-27a and miR-27b binding to PHB (5.3307 and 5.2602, respectively) were higher than those for binding to PPARγ (4.2331 and 4.2280, respectively). We therefore tested the effect of replenishment of PHB by co-transducing Lenti/PHB and the effect of supplementation of rosiglitazone, a PPARγ agonist, on the adipogenesis inhibited by overexpression of miR-27. Our results indicated that PHB levels were increased by the transduction of Lenti/PHB, but not by the administration of rosiglitazone (Fig. 4, A and C). The levels of the adipogenic marker aP2 and lipid accumulation were slightly decreased by a gain of function of PHB, which was in agreement with our previous observations (22) and a recent report in 3T3-L1 cells with uncertain mechanisms (30). Of particular note was that the reduction of the levels of aP2 and lipid by miR-27a/b was partially restored by overexpression of PHB (Fig. 4, A and B). However, the reduction in the levels of aP2 and lipid by miR-27a/b was not restored upon administration of rosiglitazone compared with vehicle (Fig. 4, C and D). These data suggest that the anti-adipogenic activity of miR-27 is more tendentious via targeting PHB. The decrease in the PPARγ level may be partially due to an indirect effect of PHB silencing by miR-27.
FIGURE 4.
PHB, but not rosiglitazone, restores adipogenesis inhibited by miR-27. ASC were transduced with Lenti/miR-Control (miR-Cont), Lenti/miR-27a (miR-27a), or Lenti/miR-27b (miR-27b). A and B, the cells were then transduced with Lenti/PHB on the following day. Lenti/GFP was used as a control for Lenti/PHB. After an additional 2 days of incubation, ASC were induced to adipocyte differentiation. At day 14 of adipogenic induction, the cells were subjected to immunoblot analysis (A) and Oil Red O staining (B). The accumulated lipid was quantified using readings on a spectrophotometer at 510 nm for cell-released dye. **, p < 0.01 compared with co-transduction of Lenti/miR-Control and Lenti/GFP; ##, p < 0.01 compared with co-transduction of miR-27a and Lenti/GFP; #, p < 0.05 compared with co-transduction of miR-27b and Lenti/GFP. C and D, 3 days after lentiviral transduction and expression, ASC were treated with adipogenic medium plus 1 μm rosiglitazone (Rosi) or vehicle (Veh). ASC were subject to immunoblot analysis (C) and Oil Red O staining (D) after 14 days of adipogenic induction. *, p < 0.05; **, p < 0.01 compared with Lenti/miR-Control treated with vehicle.
Effects of miR-27 on Mitochondria in ASC
Our previous studies revealed the essential roles of PHB in mitochondrial biogenesis and morphology during adipogenesis (22), as well as in stabilizing the mitochondrial membrane potential in granulosa cells (31). Because PHB is a direct target of miR-27, as shown above, we therefore examined the mitochondrial content, mitochondrial membrane potential, and mitochondrial morphology during adipogenesis upon overexpression of miR-27a or miR-27b. Our results demonstrated that the content of relative mtDNA was doubled in ASC subject to adipogenic induction. The amount of mtDNA was partially suppressed upon overexpression of miR-27a or miR-27b whether or not the cells were subject to adipogenic induction (Fig. 5A). The mitochondrial membrane potential was examined using TMRE to label the active mitochondria. TMRE is a cell-permeant, positively charged dye that readily accumulates in active mitochondria due to their relative negative charge. Our data revealed that the mitochondrial membrane potential of ASC, with or without adipocyte differentiation, was significantly impaired by administration of miR-27a or miR-27b (Fig. 5, B and C). MitoTracker analysis revealed that instead of normal tubular mitochondria, nearly 20% of the miR-27a- or miR-27b-overexpressing ASC consisted of fragmented mitochondria before or after cellular adipogenesis (Fig. 5D).
FIGURE 5.
Effects of miR-27 on mitochondrial content, membrane potential, and morphology. ASC were transduced with Lenti/miR-Control (miR-Cont), Lenti/miR-27a (miR-27a), or Lenti/miR-27b (miR-27b). Three days later, the cells were induced to adipocyte differentiation with adipogenic inducers for a period from days 0 to 14. A, the relative mtDNA content was evaluated by a ratio of the DNA level of mitochondrial ND1 to that of nuclear 18 S rRNA. The relative mtDNA content in ASC overexpressing Lenti/miR-Control at day 0 was set to 1. B and C, the mitochondrial membrane potential of ASC was measured by detecting the accumulation of TMRE, a red fluorophore, in active mitochondria by flow cytometry. The cells without the addition of TMRE were used to facilitate the gating of red events. A statistical analysis of the percentage of TMRE-positive cells was shown in C. D, ASC at days 0 and 14 were stained with MitoTracker Red. The mitochondrial morphology was analyzed a confocal microscopy system. Scale bar = 10 μm. The bar graph represents the percentage of cell populations with fragmented mitochondria. *, p < 0.05; **, p < 0.01 compared with Lenti/miR-Control at day 0. *, p < 0.05; ##, p < 0.01 compared with Lenti/miR-Control at day 14.
The lack of PHB reduces mitochondrial membrane integrity, disrupts oxidative phosphorylation, and results in an augmentation of ROS levels (32–35). Our recent study illustrated that ROS formation and mitochondrial complex I activity are increased upon PHB silencing in mouse 3T3-L1 preadipocytes (22). In light of this, we investigated the role of miR-27 in mitochondrial function in ASC. Our results showed that ROS levels increased by nearly 3-fold in either miR-27a- or miR-27b-overexpressing ASC, whereas the content of ATP was unaffected. The increase in ROS could be ablated when the cells were preincubated with PEG-catalase, a hydrogen peroxide scavenger, indicating the specificity of the oxidation by ROS (Fig. 6, A and B). These results suggest that the function of the mitochondrial oxidative phosphorylation system is affected upon miR-27 forced expression in ASC. To further investigate the underlying mechanisms of the extra ROS generation, the activities of mitochondrial complex I were examined. Our data demonstrated a reduction in complex I activity in miR-27a- or miR-27b-overexpressing ASC before or after adipogenesis (Fig. 6C). Taken together, our findings highlight that the impairment of mitochondrial electron transport in the oxidative phosphorylation system may be a mediator for the anti-adipogenic effect of miR-27.
FIGURE 6.
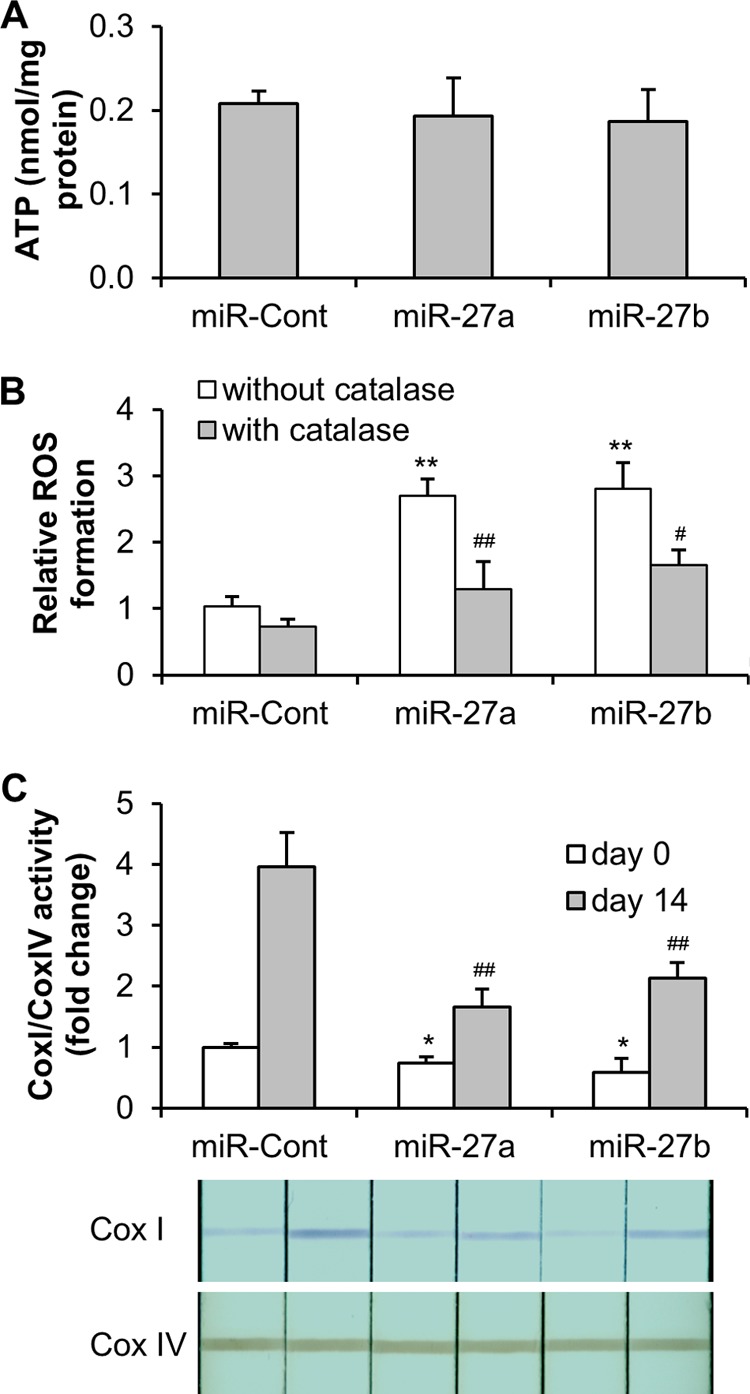
Effects of miR-27 on mitochondrial function. ASC were transduced with Lenti/miR-Control (miR-Cont), Lenti/miR-27a (miR-27a), or Lenti/miR-27b (miR-27b) and incubated for 3 days. A, the ATP concentration in the cells was detected by luminescence assay and was normalized to the protein concentration. B, ROS formation in ASC was determined using CellROX Orange reagent staining and flow cytometry, followed by preincubation with or without 250 units/ml PEG-catalase for 2 h. **, p < 0.01 compared with Lenti/miR-Control without catalase preincubation; ##, p < 0.01 compared with miR-27a without catalase preincubation; #, p < 0.05 compared with miR-27b without catalase preincubation. C, ASC were induced to adipocyte differentiation from days 0 to 14. The activities of complexes I (Cox I) and IV (Cox IV) were determined in whole cell lysates of ASC with mitochondrial dipstick assay kits. The activity of complex I was normalized to the activity of complex IV. The complex I/complex IV activity in the cells transduced with Lenti/miR-Control at day 0 was set to 1. *, p < 0.05 compared with Lenti/miR-Control at day 0; ##, p < 0.01 compared with Lenti/miR-Control at day 14.
DISCUSSION
Identifying functional miRNA-gene regulatory modules is a challenging task because one gene can be regulated by multiple miRNAs, and one miRNA can regulate a large number of genes (36, 37). miR-27 has been experimentally validated to target PHB in cancer cells (23, 24). Our group and others have recently reported that PHB deficiency reduces adipocyte differentiation and adipose accumulation and impairs mitochondrial structure and function (21, 22). Here, we have further demonstrated that miR-27 inhibits adipogenesis and impairs mitochondria by targeting PHB in human ASC.
As PHB silencing by artificial siRNA inhibits adipogenesis in the mouse 3T3-L1 preadipocyte cell line (22), we therefore hypothesized that PHB silencing by natural miRNA can inhibit adipogenesis in human ASC. miRNAs are short (21–23 nucleotides) RNAs that bind to the 3′-UTR of target genes. Computational prediction of miRNA targeting human PHB with the TargetScan Database results in two broadly conserved miRNA families, hsa-miR-27 and hsa-miR-128. Although it is more likely for miRNAs in animals to have only a partially complementary sequence of nucleotides to bond with the target mRNA, nucleotides 2–7 of miRNA (its “seed region”) nevertheless have to be perfectly complementary (38). Both miR-27 and miR-128 have an exact match at positions 2–8 of the mature miRNA (the seed region + position 8), followed by an adenosine to the 3′-UTR of PHB. miR-128 has been implicated to be an anti-onco-miRNA that represses cell growth, motility, and invasiveness and induces chemotherapeutic sensitivity and apoptosis (39–42). Our data show that miR-128 is undetected in human ASC before and after adipogenic induction, which is in accordance with the miRNA profiling analysis (28, 29). There are two isoforms in the miR-27 family, miR-27a and miR-27b. Interestingly, both miR-27a and miR-27b demonstrate down-regulation patterns following adipogenic induction of ASC, whereas PHB exhibits an up-regulation pattern. miR-27a is an intergenic miRNA, and miR-27b is an intronic miRNA. Both are highly conserved during evolution. In contrast to miR-128, miR-27a is reported to be an onco-miRNA that promotes cell growth (23, 24). In addition, miR-27a and miR-27b have been identified to be anti-adipogenic and to target PPARγ (16, 17). Notably, our results address the chronological changes in the decrease in miR-27a/b, the increase in PHB, and then the increase in PPARγ during adipogenesis, suggesting that PHB is located upstream of PPARγ in the signaling transduction pathway of adipocyte differentiation.
Mature miRNA is part of an active RNA-induced silencing complex that contains Argonaute proteins and many other associated proteins. Gene silencing may occur either through mRNA degradation or by preventing mRNA from being translated. It has been demonstrated that if there is a complete complementation between miRNA and the target mRNA sequence, Argonaute-2 (Ago2) can cleave the mRNA and lead to direct mRNA degradation. Otherwise, silencing is achieved only through preventing translation (37). Indeed, our data reveal that both the mRNA and protein levels of PHB are decreased by miR-27a/b before and after adipogenesis because miR-27a/b has an exact match with the target sequence at the 3′-UTR of PHB. miRNAs have now been implicated in the control of a wide range of biological functions, including development, differentiation, metabolism, growth, and apoptosis. In this study, we showed that both miR-27a and miR-27b attenuate adipocyte differentiation in human ASC, which is similar to the previous observations in the mouse 3T3-L1 cell line and human ASC (16, 17). Although miR-27 has been implicated to target PPARγ in HeLa cells and HEK293 cells (16, 17) and to target PHB in human cancer cell lines (23, 24), it is still important to experimentally validate here that both miR-27a and miR-27b directly target PHB in human ASC because the miRNA-mRNA target relationships differ among tissues, cells, and conditions (36). Partial restoration of adipogenesis resulting from the compensation of PHB further confirms the function of miR-27a and miR-27b as negative modulators of adipogenesis via targeting PHB in ASC. In contrast, administration of rosiglitazone, an agonist of PPARγ, has not been observed to significantly restore the anti-adipogenic effect of miR-27a or miR-27b. Our data imply that the decrease in PPARγ levels may be due partially to the direct effect of miR-27 and/or the indirect effect of PHB silencing by miR-27.
Numerous studies have consistently demonstrated that mitochondrial biogenesis is markedly enhanced during adipogenesis as cellular metabolism becomes more active. The enhancement is possibly the result of activation or promoted expression of nuclear encoded mitochondrial genes that are under the control of adipogenic transcription factors (20, 22, 43, 44). PHB is an evolutionarily conserved protein that is located mainly at the inner mitochondrial membrane and has been implicated in diverse cellular processes (45, 46). Studies from our group and others have demonstrated that a loss of PHB results in reduced adipocyte differentiation in vitro and fat accumulation in vivo; meanwhile, fragmented mitochondrial morphology, accompanied by decreases in mitochondrial biogenesis, membrane potential, and complex I activity and an increase in ROS production, has been observed (21, 22, 33). In this study, we observed repression of adipogenesis and impairment of mitochondrial biogenesis, structure integrity, and functional performance upon ectopic expression of miR-27a or miR-27b in ASC, which are similar to the effects of PHB deficiency. This novel finding of the role of miR-27 in mitochondria is most likely to be observed in other areas of study due to the multifunctional character of the mitochondrion. Stable ATP production in this situation may be the consequence of compensatory mechanisms at play in mitochondria, involving an increase in electron flow through complex II and/or III (33). Although down-regulation of miR-27a, accompanied by a reduction in the mitochondrial membrane potential and induction of ROS, has been observed in cancer cells treated with certain anticancer drugs, there is no evidence showing the direct effect of miR-27 on mitochondria thus far (47, 48). Interestingly, miR-128, a brain-enriched miRNA that targets PHB at the same position as miR-27, has been recently described to cause the loss of mitochondrial membrane potential and enhancement of ROS production (49). To further investigate the role of PHB as a mediator in mitochondrial impairment induced by miR-27 in ASC, the possible restoration of mitochondrial function may be examined upon a compensation of PHB.
Our findings provide evidence that miR-27 is an anti-adipogenic miRNA partly by targeting PHB and impairing mitochondrial functions and therefore may be a therapeutic candidate for the treatment of obesity. Interestingly, miR-27 has been recently reported to be pro-angiogenic as well. Thus, administration of miR-27 may also be beneficial to patients suffering from ischemic cardio-cerebrovascular diseases, which are often associated with obesity (50). However, a reduction in adipocyte differentiation and fat storage may lead to lipids being stored in the liver with detrimental side effects such as insulin resistance or steatosis (51). In addition, miR-27 has been implicated to facilitate the growth and survival of human cancer cells (24, 52). Therefore, the therapeutic effectiveness of miR-27 needs to be stringently evaluated in in vivo studies.
Acknowledgment
We thank Clayton Naddell (Morehouse School of Medicine) for editing and proofreading the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants G12RR003034 and SC2GM099629 (to D. L.).
- miRNA/miR
- microRNA
- PHB
- prohibitin
- PPARγ
- peroxisome proliferator-activated receptor γ
- ASC
- adipose-derived stem cell(s)
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- C/EBPβ
- CCAAT/enhancer-binding protein β
- TMRE
- tetramethylrhodamine ethyl ester
- ROS
- reactive oxygen species
- m.o.i.
- multiplicity of infection.
REFERENCES
- 1. Lagos-Quintana M., Rauhut R., Lendeckel W., Tuschl T. (2001) Identification of novel genes coding for small expressed RNAs. Science 294, 853–858 [DOI] [PubMed] [Google Scholar]
- 2. Lagos-Quintana M., Rauhut R., Yalcin A., Meyer J., Lendeckel W., Tuschl T. (2002) Identification of tissue-specific microRNAs from mouse. Curr. Biol. 12, 735–739 [DOI] [PubMed] [Google Scholar]
- 3. Xie H., Lim B., Lodish H. F. (2009) MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes 58, 1050–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martinelli R., Nardelli C., Pilone V., Buonomo T., Liguori R., Castanò I., Buono P., Masone S., Persico G., Forestieri P., Pastore L., Sacchetti L. (2010) miR-519d overexpression is associated with human obesity. Obesity 18, 2170–2176 [DOI] [PubMed] [Google Scholar]
- 5. Alexander R., Lodish H., Sun L. (2011) MicroRNAs in adipogenesis and as therapeutic targets for obesity. Expert Opin. Ther. Targets 15, 623–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGregor R. A., Choi M. S. (2011) microRNAs in the regulation of adipogenesis and obesity. Curr. Mol. Med. 11, 304–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ke X. S., Liu C. M., Liu D. P., Liang C. C. (2003) MicroRNAs: key participants in gene regulatory networks. Curr. Opin. Chem. Biol. 7, 516–523 [DOI] [PubMed] [Google Scholar]
- 8. Hobert O. (2004) Common logic of transcription factor and microRNA action. Trends Biochem. Sci. 29, 462–468 [DOI] [PubMed] [Google Scholar]
- 9. Spiegelman B. M., Flier J. S. (1996) Adipogenesis and obesity: rounding out the big picture. Cell 87, 377–389 [DOI] [PubMed] [Google Scholar]
- 10. Naaz A., Holsberger D. R., Iwamoto G. A., Nelson A., Kiyokawa H., Cooke P. S. (2004) Loss of cyclin-dependent kinase inhibitors produces adipocyte hyperplasia and obesity. FASEB J. 18, 1925–1927 [DOI] [PubMed] [Google Scholar]
- 11. Huang H., Xie C., Sun X., Ritchie R. P., Zhang J., Chen Y. E. (2010) miR-10a contributes to retinoid acid-induced smooth muscle cell differentiation. J. Biol. Chem. 285, 9383–9389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xie C., Huang H., Sun X., Guo Y., Hamblin M., Ritchie R. P., Garcia-Barrio M. T., Zhang J., Chen Y. E. (2011) MicroRNA-1 regulates smooth muscle cell differentiation by repressing Kruppel-like factor 4. Stem Cells Dev. 20, 205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ortega F. J., Moreno-Navarrete J. M., Pardo G., Sabater M., Hummel M., Ferrer A., Rodriguez-Hermosa J. I., Ruiz B., Ricart W., Peral B., Fernández-Real J. M. (2010) MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation. PLoS ONE 5, e9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Q., Li Y. C., Wang J., Kong J., Qi Y., Quigg R. J., Li X. (2008) miR-17–92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc. Natl. Acad. Sci. U.S.A. 105, 2889–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin Q., Gao Z., Alarcon R. M., Ye J., Yun Z. (2009) A role of miR-27 in the regulation of adipogenesis. FEBS J. 276, 2348–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karbiener M., Fischer C., Nowitsch S., Opriessnig P., Papak C., Ailhaud G., Dani C., Amri E. Z., Scheideler M. (2009) microRNA miR-27b impairs human adipocyte differentiation and targets PPARγ. Biochem. Biophys. Res. Commun. 390, 247–251 [DOI] [PubMed] [Google Scholar]
- 17. Kim S. Y., Kim A. Y., Lee H. W., Son Y. H., Lee G. Y., Lee J. W., Lee Y. S., Kim J. B. (2010) miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARγ expression. Biochem. Biophys. Res. Commun. 392, 323–328 [DOI] [PubMed] [Google Scholar]
- 18. Kim Y. J., Hwang S. J., Bae Y. C., Jung J. S. (2009) MiR-21 regulates adipogenic differentiation through the modulation of TGF-β signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells 27, 3093–3102 [DOI] [PubMed] [Google Scholar]
- 19. Coates P. J., Nenutil R., McGregor A., Picksley S. M., Crouch D. H., Hall P. A., Wright E. G. (2001) Mammalian prohibitin proteins respond to mitochondrial stress and decrease during cellular senescence. Exp. Cell Res. 265, 262–273 [DOI] [PubMed] [Google Scholar]
- 20. Wilson-Fritch L., Burkart A., Bell G., Mendelson K., Leszyk J., Nicoloro S., Czech M., Corvera S. (2003) Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol. Cell. Biol. 23, 1085–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Artal-Sanz M., Tavernarakis N. (2009) Prohibitin couples diapause signalling to mitochondrial metabolism during ageing in C. elegans. Nature 461, 793–797 [DOI] [PubMed] [Google Scholar]
- 22. Liu D., Lin Y., Kang T., Huang B., Xu W., Garcia-Barrio M., Olatinwo M., Matthews R., Chen Y. E., Thompson W. E. (2012) Mitochondrial dysfunction and adipogenic reduction by prohibitin silencing in 3T3-L1 cells. PLoS ONE 7, e34315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu T., Tang H., Lang Y., Liu M., Li X. (2009) MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer Lett. 273, 233–242 [DOI] [PubMed] [Google Scholar]
- 24. Fletcher C. E., Dart D. A., Sita-Lumsden A., Cheng H., Rennie P. S., Bevan C. L. (2012) Androgen-regulated processing of the oncomir miR-27a, which targets prohibitin in prostate cancer. Hum. Mol. Genet. 21, 3112–3127 [DOI] [PubMed] [Google Scholar]
- 25. Yin K. J., Deng Z., Hamblin M., Xiang Y., Huang H., Zhang J., Jiang X., Wang Y., Chen Y. E. (2010) Peroxisome proliferator-activated receptor δ regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. J. Neurosci. 30, 6398–6408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liou C. W., Lin T. K., Chen J. B., Tiao M. M., Weng S. W., Chen S. D., Chuang Y. C., Chuang J. H., Wang P. W. (2010) Association between a common mitochondrial DNA D-loop polycytosine variant and alteration of mitochondrial copy number in human peripheral blood cells. J. Med. Genet. 47, 723–728 [DOI] [PubMed] [Google Scholar]
- 27. Qin L., Chen Y., Niu Y., Chen W., Wang Q., Xiao S., Li A., Xie Y., Li J., Zhao X., He Z., Mo D. (2010) A deep investigation into the adipogenesis mechanism: profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/β-catenin signaling pathway. BMC Genomics 11, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang K. H., Kao A. P., Singh S., Yu S. L., Kao L. P., Tsai Z. Y., Lin S. D., Li S. S. (2010) Comparative expression profiles of mRNAs and microRNAs among human mesenchymal stem cells derived from breast, face, and abdominal adipose tissues. Kaohsiung J. Med. Sci. 26, 113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cho J. A., Park H., Lim E. H., Lee K. W. (2011) MicroRNA expression profiling in neurogenesis of adipose tissue-derived stem cells. J. Genet. 90, 81–93 [DOI] [PubMed] [Google Scholar]
- 30. Ande S. R., Xu Z., Gu Y., Mishra S. (2012) Prohibitin has an important role in adipocyte differentiation. Int. J. Obes. 36, 1236–1244 [DOI] [PubMed] [Google Scholar]
- 31. Chowdhury I., Xu W., Stiles J. K., Zeleznik A., Yao X., Matthews R., Thomas K., Thompson W. E. (2007) Apoptosis of rat granulosa cells after staurosporine and serum withdrawal is suppressed by adenovirus-directed overexpression of prohibitin. Endocrinology 148, 206–217 [DOI] [PubMed] [Google Scholar]
- 32. Kasashima K., Sumitani M., Satoh M., Endo H. (2008) Human prohibitin 1 maintains the organization and stability of the mitochondrial nucleoids. Exp. Cell Res. 314, 988–996 [DOI] [PubMed] [Google Scholar]
- 33. Schleicher M., Shepherd B. R., Suarez Y., Fernandez-Hernando C., Yu J., Pan Y., Acevedo L. M., Shadel G. S., Sessa W. C. (2008) Prohibitin-1 maintains the angiogenic capacity of endothelial cells by regulating mitochondrial function and senescence. J. Cell Biol. 180, 101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bogenhagen D. F., Rousseau D., Burke S. (2008) The layered structure of human mitochondrial DNA nucleoids. J. Biol. Chem. 283, 3665–3675 [DOI] [PubMed] [Google Scholar]
- 35. Wang Y., Bogenhagen D. F. (2006) Human mitochondrial DNA nucleoids are linked to protein folding machinery and metabolic enzymes at the mitochondrial inner membrane. J. Biol. Chem. 281, 25791–25802 [DOI] [PubMed] [Google Scholar]
- 36. Krek A., Grün D., Poy M. N., Wolf R., Rosenberg L., Epstein E. J., MacMenamin P., da Piedade I., Gunsalus K. C., Stoffel M., Rajewsky N. (2005) Combinatorial microRNA target predictions. Nat. Genet. 37, 495–500 [DOI] [PubMed] [Google Scholar]
- 37. Lim L. P., Lau N. C., Garrett-Engele P., Grimson A., Schelter J. M., Castle J., Bartel D. P., Linsley P. S., Johnson J. M. (2005) Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433, 769–773 [DOI] [PubMed] [Google Scholar]
- 38. Mazière P., Enright A. J. (2007) Prediction of microRNA targets. Drug Discov. Today 12, 452–458 [DOI] [PubMed] [Google Scholar]
- 39. Evangelisti C., Florian M. C., Massimi I., Dominici C., Giannini G., Galardi S., Buè M. C., Massalini S., McDowell H. P., Messi E., Gulino A., Farace M. G., Ciafrè S. A. (2009) MiR-128 up-regulation inhibits Reelin and DCX expression and reduces neuroblastoma cell motility and invasiveness. FASEB J. 23, 4276–4287 [DOI] [PubMed] [Google Scholar]
- 40. Papagiannakopoulos T., Friedmann-Morvinski D., Neveu P., Dugas J. C., Gill R. M., Huillard E., Liu C., Zong H., Rowitch D. H., Barres B. A., Verma I. M., Kosik K. S. (2012) Pro-neural miR-128 is a glioma tumor suppressor that targets mitogenic kinases. Oncogene 31, 1884–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhu Y., Yu F., Jiao Y., Feng J., Tang W., Yao H., Gong C., Chen J., Su F., Zhang Y., Song E. (2011) Reduced miR-128 in breast tumor-initiating cells induces chemotherapeutic resistance via Bmi-1 and ABCC5. Clin. Cancer Res. 17, 7105–7115 [DOI] [PubMed] [Google Scholar]
- 42. Adlakha Y. K., Saini N. (2013) miR-128 exerts pro-apoptotic effect in a p53 transcription-dependent and -independent manner via PUMA-Bak axis. Cell Death Dis. 4, e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wilson-Fritch L., Nicoloro S., Chouinard M., Lazar M. A., Chui P. C., Leszyk J., Straubhaar J., Czech M. P., Corvera S. (2004) Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J. Clin. Invest. 114, 1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gesta S., Bezy O., Mori M. A., Macotela Y., Lee K. Y., Kahn C. R. (2011) Mesodermal developmental gene Tbx15 impairs adipocyte differentiation and mitochondrial respiration. Proc. Natl. Acad. Sci. U.S.A. 108, 2771–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nijtmans L. G., de Jong L., Artal Sanz M., Coates P. J., Berden J. A., Back J. W., Muijsers A. O., van der Spek H., Grivell L. A. (2000) Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J. 19, 2444–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Artal-Sanz M., Tavernarakis N. (2009) Prohibitin and mitochondrial biology. Trends Endocrinol. Metab. 20, 394–401 [DOI] [PubMed] [Google Scholar]
- 47. Pathi S. S., Jutooru I., Chadalapaka G., Sreevalsan S., Anand S., Thatcher G. R., Safe S. (2011) GT-094, a NO-NSAID, inhibits colon cancer cell growth by activation of a reactive oxygen species-microRNA-27a: ZBTB10-specificity protein pathway. Mol. Cancer Res. 9, 195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jutooru I., Chadalapaka G., Abdelrahim M., Basha M. R., Samudio I., Konopleva M., Andreeff M., Safe S. (2010) Methyl 2-cyano-3,12-dioxooleana-1,9-dien-28-oate decreases specificity protein transcription factors and inhibits pancreatic tumor growth: role of microRNA-27a. Mol. Pharmacol. 78, 226–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adlakha Y. K., Saini N. (2011) MicroRNA-128 downregulates Bax and induces apoptosis in human embryonic kidney cells. Cell. Mol. Life Sci. 68, 1415–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Urbich C., Kaluza D., Frömel T., Knau A., Bennewitz K., Boon R. A., Bonauer A., Doebele C., Boeckel J. N., Hergenreider E., Zeiher A. M., Kroll J., Fleming I., Dimmeler S. (2012) MicroRNA-27a/b controls endothelial cell repulsion and angiogenesis by targeting semaphorin 6A. Blood 119, 1607–1616 [DOI] [PubMed] [Google Scholar]
- 51. Rosen E. D., MacDougald O. A. (2006) Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7, 885–896 [DOI] [PubMed] [Google Scholar]
- 52. Zhu H., Wu H., Liu X., Evans B. R., Medina D. J., Liu C. G., Yang J. M. (2008) Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem. Pharmacol. 76, 582–588 [DOI] [PMC free article] [PubMed] [Google Scholar]






