Background: Lipid transport by the A-subfamily of ATP-binding cassette (ABC) transporters implicated in human disorders is poorly understood.
Results: ABCA1 and ABCA7 export phosphatidylcholine and phosphatidylserine across membranes, whereas ABCA4 imports phosphatidylethanolamine.
Conclusion: ABCA transporters actively flip phospholipids across membranes, a function severely reduced for disease-causing mutants.
Significance: ABCA-dependent phospholipid transport plays a crucial role in cellular lipid homeostasis.
Keywords: ABC Transporter, Cholesterol Metabolism, High Density Lipoprotein (HDL), Lipid Transport, Membrane Reconstitution, Phospholipid, ABCA1, ABCA4, Stargardt Disease, Tangier Disease
Abstract
ABCA1, ABCA7, and ABCA4 are members of the ABCA subfamily of ATP-binding cassette transporters that share extensive sequence and structural similarity. Mutations in ABCA1 cause Tangier disease characterized by defective cholesterol homeostasis and high density lipoprotein (HDL) deficiency. Mutations in ABCA4 are responsible for Stargardt disease, a degenerative disorder associated with severe loss in central vision. Although cell-based studies have implicated ABCA proteins in lipid transport, the substrates and direction of transport have not been firmly established. We have purified and reconstituted ABCA1, ABCA7, and ABCA4 into liposomes for fluorescent-lipid transport studies. ABCA1 actively exported or flipped phosphatidylcholine, phosphatidylserine, and sphingomyelin from the cytoplasmic to the exocytoplasmic leaflet of membranes, whereas ABCA7 preferentially exported phosphatidylserine. In contrast, ABCA4 transported phosphatidylethanolamine in the reverse direction. The same phospholipids stimulated the ATPase activity of these ABCA transporters. The transport and ATPase activities of ABCA1 and ABCA4 were reduced by 25% in the presence of 20% cholesterol. Nine ABCA1 Tangier mutants and the corresponding ABCA4 Stargardt mutants showed significantly reduced phospholipid transport activity and subcellular mislocalization. These studies provide the first direct evidence for ABCA1 and ABCA7 functioning as phospholipid transporters and suggest that this activity is an essential step in the loading of apoA-1 with phospholipids for HDL formation.
Introduction
ATP-binding cassette (ABC)3 transporters comprise a superfamily of membrane proteins that actively transport chemically diverse substrates across biological membranes. They typically consist of four core domains as follows: two transmembrane domains, which provide a pathway for the translocation of a substrate across the membrane, and two ATP-binding cassettes or nucleotide binding domains (NBD), which supply the energy for substrate transport through the binding and hydrolysis of ATP (1). In eukaryotes, ABC transporters exist as either full transporters in which all four domains are present within a single polypeptide chain or half-transporters in which an NBD and transmembrane domain reside within a polypeptide chain that assembles as homo- or heterodimers. In prokaryotes, the domains can exist as individual subunits or together in various combinations to generate an active transporter.
The human genome contains 48 human ABC transporters that have been classified into seven subfamilies (ABCA through ABCG) based on phylogenetic analysis (2). The ABCA subfamily is made up of 12 full transporters that share a high degree of similarity in sequence and structural organization (3, 4). They are organized as two nonidentical, tandem halves each containing a transmembrane domain followed by an NBD. A characteristic feature of ABCA transporters is the presence of large glycosylated exocytoplasmic domains (ECD) between the first membrane-spanning segment and a cluster of five membrane-spanning segments in both the N- and C-terminal halves (5). The importance of the ABCA subfamily is evident from genetic studies that have shown that mutations in four members (ABCA1, ABCA3, ABCA4, and ABCA12) are responsible for severe genetic disorders. Although the substrates of most ABCA members remain to be identified, disease-associated phenotypes, analysis of knock-out mice, and cell-based studies suggest that many ABCA proteins play a crucial role in cellular lipid homeostasis (6, 7).
ABCA1 consisting of 2261 amino acids is expressed in most tissues where it plays a central role in cellular cholesterol homeostasis and high density lipoprotein (HDL) formation (8). Mutations in ABCA1 are associated with Tangier disease, a disorder characterized by severe HDL deficiency in plasma, accumulation of cholesterol esters in macrophages, and an increased risk of atherosclerosis (9–12). Cell-based studies have implicated ABCA1 in the efflux of cholesterol and phospholipids from cells to lipid-poor apolipoprotein A1 (apoA-1) to generate nascent HDL, a pathway termed reverse cholesterol transport. Cross-linking and mutagenesis studies indicate that apoA-1 directly binds to ABCA1 through electrostatic interactions involving lysine residues within the ECDs of ABCA1 (13–16). MDCKII and HeLa cells expressing ABCA1-GFP show enhanced redistribution of endogenous PS and NBD-PS from the cytosolic side to the extracellular side of the plasma membrane (17). Many disease-associated ABCA1 mutants exhibit a decrease in phospholipid and cholesterol efflux compared with wild-type (WT) ABCA1 in heterologous cell expression studies (18–20). Abca1 knock-out mice show similar pathophysiological features observed in Tangier patients, including a significant decrease in HDL, reduced cholesterol and plasma phospholipids, and an accumulation of macrophage foam cells (21).
ABCA7, a 2146-amino acid protein sharing 54% sequence identity with ABCA1, is highly expressed in brain, lung, myelolymphatic tissues, kidneys, macrophages, and platelets (22). Given the high degree of homology of ABCA7 to ABCA1, it has been suggested that ABCA7 may also mediate the removal of phospholipids and cholesterol from cells to apolipoproteins (23). Cell-based studies have confirmed that ABCA7 expression promotes the efflux of PC and SM, but not cholesterol, to apoA-1 (24). However, cholesterol and phospholipid levels of bone marrow-derived macrophages from Abca7 knock-out mice were similar to that of WT mice leading to an uncertainty as to the function of ABCA7 as a lipid transporter (25).
ABCA4 is a 2273-amino acid transporter localized in light-sensitive outer segment disc membranes of rod and cone photoreceptor cells (26). Over 800 mutations in ABCA4 are known to cause Stargardt disease, a macular degeneration associated with severe loss in central vision (27, 28). ABCA4 is the only ABCA transporter that has been purified and reconstituted into liposomes for direct analysis of its substrate binding and transport activities (29, 30). ABCA4 binds and actively transports the Schiff base adduct of retinal and phosphatidylethanolamine (PE) known as N-retinylidene-PE from the lumen to the cytoplasmic leaflet of photoreceptor outer disc membranes. The transport of N-retinylidene-PE by ABCA4 facilitates the removal of retinal by the visual cycle thereby preventing the accumulation of potentially toxic retinoid compounds (31). This is supported by the finding that mice deficient in ABCA4 and individuals with Stargardt disease show an accumulation of retinoid compounds in photoreceptor and retinal pigment epithelial cells (32, 33). Recent single particle electron microscopic studies have provided a glimpse of the structural architecture of ABCA4 and revealed conformational changes that occur upon the binding of ATP (34).
Despite the importance of ABCA transporters in various physiological and pathological processes, relatively little is known about the substrates and direction of transport by the various members of this subfamily. This is largely due to the difficulty in isolating sufficient amounts of purified ABCA proteins for functional analysis and the lack of sensitive assays to measure substrate transport across membranes.
In this study, we have expressed, purified, and reconstituted ABCA1, ABCA4, and ABCA7. We show for the first time that ABCA1 and ABCA7 directly transport PC, PS, and SM from the cytoplasmic to the exocytoplasmic side of membranes, whereas ABCA4 transports PE in the opposite direction. Our studies further suggest that ABCA1 does not directly transport cholesterol, but instead it mediates the removal of cholesterol from cells most likely through the preloading of apoA-1 with phospholipids. As part of this study, we also describe the effect of Tangier mutations in ABCA1 and corresponding Stargardt mutations in ABCA4 on their ATPase and phospholipid transport activity.
EXPERIMENTAL PROCEDURES
Materials
Porcine brain polar lipid (BPL), cholesteryl hemisuccinate, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, 1,2-dioleoyl-sn-glycero-3-phosphocholine, 1,2-dioleoyl-sn-glycero-3-phosphoserine, and fluorescent-labeled 7-nitro-2-1,3-benzoxadiazol-4-yl or NBD-labeled phospholipids, C6 NBD-labeled PS (Fl-PS), C6-NBD-labeled PC (Fl-PC), C6-NBD-labeled PE (Fl-PE), C6-NBD-labeled PG (Fl-PG), and C6-NBD-labeled-SM (Fl-SM) were purchased from Avanti Polar Lipids (Alabaster, AL). Protease Inhibitor Mixture was obtained from Roche Applied Science. CHAPS was from Anatrace (Maumee, OH); [α-32P]ATP was from PerkinElmer Life Sciences. Anti-calnexin was from Abcam, and secondary anti-mouse and anti-rabbit antibodies conjugated to Alexa-488 and Alexa-594 was from Molecular Probes. Stock solutions of ATP, ADP, and AMP-PNP were adjusted to pH 7.5 with NaOH and filter-sterilized. The Rho1D4 antibody was purchased from University of British Columbia through Flintbox.
Generation of ABCA1, ABCA4, and ABCA7 Plasmids and Site-directed Mutagenesis
Human ABCA7 was a generous gift of Dr. Kazumitsu Ueda and was subcloned with a C-terminal 1D4 tag (Thr-Glu-Thr-Ser-Gln-Val-Ala-Pro-Ala) into a pCEP4 vector at NotI/HindIII sites (Invitrogen) (35). Human ABCA4 and human ABCA1 with a C-terminal 1D4 tag subcloned in pCEP4 vectors at its NotI/NheI and NotI/NotI sites, respectively, were used for site-directed mutagenesis as described previously (36, 37). Mutations introduced by overlap extension PCR using Pfu AD DNA polymerase in ABCA1 included S100C, W590S, F593L, N935S, T929I, C1477R, T1512M, R2081W, and P2150L. Corresponding ABCA4 mutations determined by amino acid alignment with ABCA1 included S100P, W605S, F608L, T959I, N965S, C1502R, T1537M, R2107P, and P2180L. ABCA1-MM was constructed to harbor the Walker A-motif lysine-to-methionine mutations K939M/K1952M by the nested PCR method; ABCA4-MM had the corresponding K969M/K1969M Walker A mutations (37), and ABCA7-MM had the K847M/K1833M Walker A mutations. The presence of these mutations was confirmed by DNA sequencing.
Transient Transfection and Purification of ABCA Proteins
HEK293T cells were transfected with plasmid DNA (10 μg per plate) using calcium phosphate and grown in a humidified incubator (5% CO2) at 37 °C in DMEM supplemented with 10% bovine growth serum. After 24 h post-transfection, a cell suspension from nine 10-cm dishes was added slowly to 1.5 ml of Buffer A (50 mm HEPES, pH 7.4, 0.2 mg/ml BPL, 0.002% cholesteryl hemisuccinate, 150 mm NaCl, 1 mm MgCl2, 10% glycerol, 1 mm DTT) containing 18 mm CHAPS and protease inhibitor and stirred for 45 min at 4 °C. The supernatant after a 10-min centrifugation at 100,000 × g (TLA110.4 rotor in a Beckman Optima TL centrifuge) was mixed with 50 μl of Rho1D4-Sepharose 2B for 1 h at 4 °C. The matrix was washed six times in Buffer A containing 10 mm CHAPS, 0.002% cholesteryl hemisuccinate. The protein was eluted three times with 150 μl each at 12 °C over 60 min in the same buffer with 0.2 mg/ml 1D4 peptide. A small fraction was analyzed by SDS-PAGE and Western blotting using Rho1D4 antibody. The concentrated eluted protein (20–70 ng of ABCA/μl) was promptly used for proteoliposome reconstitution.
Preparation of Liposomes and Reconstitution of ABCA1 in Proteoliposomes
For the preparation of unilamellar vesicles, the designated lipid compositions were prepared in chloroform and dried under N2. For fluorescent phospholipid (Fl-PL) transport assays, 1,2-dioleoyl-sn-glycero-3-phosphocholine was mixed at a weight ratio of 99.4:0.6 with Fl-PL lipid. The lipids were resuspended in buffer containing 20 mm HEPES, pH 7.4, 150 mm NaCl, 2 mm MgCl2, 10% glycerol, 3.5 mm CHAPS at a concentration of 2.5 mg/ml by bath sonication and incubated at room temperature for 3 h. In a typical reconstitution experiment, 250 μg of lipids were incubated with the ABCA protein (2–6 μg in Buffer A and 10 mm CHAPS) at 4 °C. Buffer B was added to dilute the CHAPS concentration to 4 mm CHAPS, and the mixture was incubated for 1 h at 4 °C. Detergent was removed by dialysis for at least 24 h at 4 °C with a minimum of 3 1-liter changes of Buffer C (10 mm HEPES, pH 7.4, 150 mm NaCl, 2 mm MgCl2, 10% sucrose, and 1 mm DTT).
Dynamic light scattering measurements with a Zetasizer Nanoseries S90 were used to determine the size of the lipid vesicles. Results are averaged over 10 consecutive measurements showing the mean diameter ± S.D.
Fl-PL Flipping Assay
Typically, 40 μl of liposomes containing Fl-PL reconstituted with the ABCA transporter were suspended in Buffer B (50 mm HEPES, pH 7.4, 0.2 mg/ml BPL, 0.002% cholesteryl hemisuccinate, 150 mm NaCl, 1 mm MgCl2, 10% glycerol, 1 mm DTT) to a total volume of 100 μl.
To initiate transport, 2 μl of a stock solution of ATP or AMP-PNP was added to the proteoliposomes to obtain a final concentration of 1 mm. The samples were incubated for 1 h at 37 °C prior to being transferred to a 100-μl quartz cuvette (path length of 1.0 cm). The fluorescence was recorded at 22 °C with a Varian spectrofluorometer using excitation and emission wavelengths of 468 nm (slit width, 2.5 nm) and 540 nm (slit width, 5.0 nm), respectively. After a base line was established (∼3 min), the fluorescence from Fl-PL in the outer leaflet of the proteoliposomes was quenched by the addition of 4 μl of sodium dithionite solution in 1 m Tris-HCl at a final concentration of 4 mm. When a base line was re-established (∼5 min), the proteoliposomes were permeabilized by adding 10 μl of 10% Triton X-100 (final concentration of 1%).
The percent Fl-PL accessible to dithionite (outer leaflet) in the proteoliposomes or protected from dithionite (inner leaflet) was calculated using Equations 1 and 2.
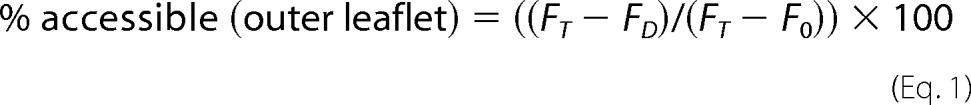 |
where FT is the total fluorescence of the sample before addition of dithionite, FC is the fluorescence of the sample following quenching with dithionite, and F0 is the fluorescence of the sample following permeabilization with Triton X-100. FT, FD, and F0 were determined by averaging the last 10 data points (covering 10 s) of the recorded traces. The net percent Fl-PL translocated from the inner to the outer leaflet of the proteoliposome in the 60-min period was calculated from the difference in transbilayer distribution between ABCA proteoliposomes with ATP and AMP-PNP.
Iodide Quenching of Fl-PL in ABCA Proteoliposomes
To assess the steady-state distribution of the Fl-PL across the membranes of proteoliposomes, collisional quenching of fluorescent lipid probes was performed with potassium iodide (KI) (38). ABCA protein was reconstituted using Buffer C but with 250 mm NaCl and devoid of sucrose. Excitation and emission wavelengths were set at 470 and 530 nm, respectively. Fluorescence intensity of Fl-PL-containing proteoliposomes was measured for samples consisting of 50 μl of vesicles diluted into 100 μl of buffer containing 10 mm HEPES, pH 7.4, 10 mm Na2S2O3, and the quencher KI (0 to 250 mm). NaCl was used to adjust the ionic strength to 250 mm and maintain the osmolarity of the solution, whereas Na2S2O3 prevented production of I2. Parallel samples were measured using NaCl instead of KI. Data were analyzed according to the modified Stern-Volmer Equation 2,
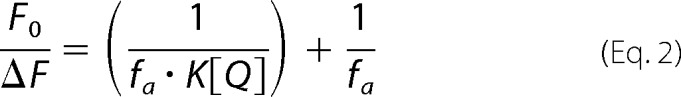 |
where F0 is the fluorescence intensity in the absence of the quencher; ΔF is the fluorescence intensity in the presence of the quencher at concentration [Q]; fa is the fraction of fluorescence that is accessible to the quencher, and K is the Stern-Volmer quenching constant.
ATPase Assays and Membrane Preparations
ATPase activity was measured using 100 μm [α-32P]ATP and thin layer chromatography as described previously (39). ATPase assays on BPL or synthetic proteoliposomes were performed as described earlier (29). Membranes from transfected cells were also prepared as described previously (37), except that the procedure was scaled down and the cell suspension originated from two 10-cm dishes of HEK293T cells. SDS-gel electrophoresis and Western blotting were carried out as described previously (29). Quantification of cell expression was measured using a LiCor imaging system with data obtained from three independent experiments and expressed as the mean ± S.D.
Immunofluorescence Microscopy
COS7 cells transfected with 1D4-tagged ABCA proteins were fixed in 4% paraformaldehyde, 100 mm phosphate buffer, pH 7.4, for 1 h and subsequently blocked and permeabilized with 10% normal goat serum and 0.2% Triton X-100 in phosphate buffer for 30 min. The cells were then labeled for 2 h at room temperature with Rho1D4 hybridoma culture fluid diluted 1:50 in phosphate buffer containing 2.5% normal goat serum and 0.1% Triton X-100 and an anti-calnexin polyclonal antibody (Abcam) diluted 1:200 in the same buffer. The cells were labeled with secondary 1:1000 diluted anti-mouse and anti-rabbit antibodies conjugated to Alexa-488 and Alexa-594 (Molecular Probes), respectively. Samples were visualized under a Zeiss LSM 700 confocal microscope. Relative quantification of co-labeling of ABCA1 and calnexin as an ER marker was determined using ImageJ software (rsb.info.nih.gov) as follows. In the micrographs, mean pixel background ± 3× SD intensity in a selected area without cells for both green and red channels was subtracted from the respective micrograph. Each cell was delimited with the region of interest tool, and a binary mask was formed to multiply both channels. Co-localization was analyzed using the JaCop plugin (Just Another Co-localization Plugin) for ImageJ, and Pearson's coefficient (r) was calculated with a JaCop plugin (40).
RESULTS
Purification and Reconstitution of ABCA Proteins into Proteoliposomes
ABCA1, ABCA7, and ABCA4 and corresponding mutant proteins in which lysine residues in the Walker A motifs (GXXGXK(S/T)) of NBD1 and NBD2 were replaced with methionine (ABCA-MM), all containing a 9-amino acid C-terminal 1D4 tag, were transiently expressed in HEK293 cells and purified from CHAPS-solubilized cell lysates on a Rho1D4 immunoaffinity matrix. A typical Coomassie Blue-stained SDS gel and Rho1D4 antibody-labeled Western blot of the cell lysates and immunoaffinity-purified ABCA1 and ABCA7 are shown in Fig. 1A. The level of expression and extent of purification were similar for the WT and mutant proteins. Similar results were obtained for ABCA4 as reported previously (29, 41). The 1D4 C-terminal tag did not affect the functional properties of ABCA4 (41).
FIGURE 1.
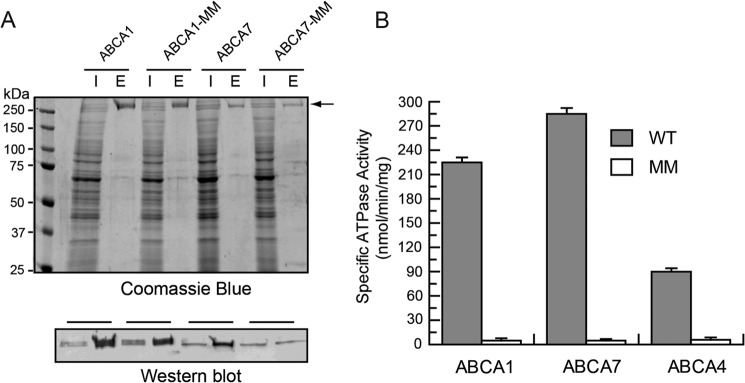
Purification and ATPase activity of ABCA1, ABCA7, and ABCA4. Wild type (WT) and mutant ABCA1, ABCA4, and ABCA7 in which lysine residues in the Walker A motifs of NBD1 and NBD2 were replaced with methionine (ABCA-MM) all containing a 9-amino acid C-terminal epitope were expressed in HEK293T cells and purified on a Rho1D4 immunoaffinity matrix. A, Coomassie Blue-stained gel and Rho1D4-labeled Western blot of the ABCA1 and ABCA7 detergent-solubilized cell lysate (lane labeled I) and purified protein eluted from the immunoaffinity column with the 1D4 peptide (lane labeled E). The arrow identifies the position of the ABCA1 and ABCA7 transporters. B, ATPase activity of WT and mutant ABCA proteins. Immunoaffinity purified WT ABCA1, ABCA7, and ABCA4 and their MM mutants were reconstituted into brain polar lipid for analysis of their ATPase activity. Results are the mean ± S.D. for three independent experiments.
The immunoaffinity-purified ABCA proteins were subsequently reconstituted into BPL at a lipid/protein ratio of ∼20:1 by weight for analysis of their ATPase activities. BPL was used in these initial studies because it contains a variety of known phospholipids (12.6% PC, 33.1% PE, 4.1% PI, 18.5% PS, and 0.8% phosphatidic acid) along with 30.9% unknown components (Avanti Polar Lipids). As shown in Fig. 1B, all WT ABCA proteins exhibited significant ATPase activity, whereas the ABCA-MM mutant proteins were devoid of activity.
Stimulation of ABCA1, ABCA4, and ABCA7 ATPase Activity by Specific Membrane Lipids
The phospholipid specificity of the ABCA transporters was studied by reconstituting the proteins in unilamellar liposomes consisting of 1,2-dioleoylphosphatidylglycerol (abbreviated here as PG) as the base phospholipid for ABCA1 and ABCA7, and 1,2-dioleoylphosphatidylcholine (abbreviated as PC) for ABCA4. The lipid composition was modified by replacing a portion of the base phospholipid with a defined amount of 1,2-dioleoylphospholipids (PC, PS, PE, PI, and PG), SM, or cholesterol. The mean diameter of the proteoliposomes as determined by dynamic light scattering was typically within the range of 50–100 nm (Table 1). The unilamellar nature of the vesicles was confirmed by cryoelectron microscopy and fluorescence lipid measurements in which greater than 50% of the Fl-PL was accessible to bleaching by sodium dithionite (see Fig. 3, A and B). The orientation of the reconstituted ABCA transporters in the liposomes was determined from the increase in ATPase activity following detergent permeabilization. In the case of the ABCA proteins reconstituted into PC vesicles, ∼60% of the protein was oriented in an inside-out configuration, i.e. nucleotide-binding domains exposed on the outside of the vesicles. ABCA proteins reconstituted in liposomes composed of PC/PS (7:3), PC/PE (7:3), or PG were oriented with 70–80% of the protein having their NBDs on the outer side of the proteoliposomes.
TABLE 1.
Size distribution of ABCA4-reconstituted unilamellar vesicles
The size of ABCA4-reconstituted vesicles for various lipid compositions was determined by dynamic light scattering. Mean values ± S.D. are given for 10 measurements. Values are representative of two independent vesicle preparations. Phospholipids had 1,2-dioleoyl (DO) fatty acyl chains. Chol is cholesterol.
| Lipid composition of vesicles (lipid ratio) | Size in nm (S.D.) |
|---|---|
| DOPG | 56 (±19) |
| DOPG/DOPC (7:3) | 66 (±26) |
| DOPG/DOPS (7:3) | 62 (±24) |
| DOPG/DOPE (7:3) | 58 (±28) |
| DOPG/Chol (7:3) | 79 (±24) |
| DOPG/SM (7:3) | 84 (±31) |
| DOPC | 76 (±22) |
| DOPC/DOPS (7:3) | 81 (±21) |
| DOPC/Chol (7:3) | 98 (±28) |
| DOPC/SM (7:3) | 106 (±34) |
| BPL | 91 (±29) |
FIGURE 3.
Differential direction of transport of fluorescent-labeled phospholipid across the lipid bilayer by ABCA1 and ABCA4. A, ABCA1 reconstituted into PG liposomes containing 0.6% (w/w) Fl-PC, and B, ABCA4 reconstituted into PG liposomes containing 0.6% (w/w) Fl-PE were incubated at 37 °C with 1 mm ATP or 1 mm AMP-PNP as a control. After 1 h, the fluorescence (λex = 465 nm, λem = 540 nm) was monitored at 22 °C. Dithionite (4 mm) was added to bleach fluorescent lipids on the outer leaflet of the liposomes. After the fluorescence stabilized, 1% (w/v) Triton X-100 (∼6 min) was added to bleach the remaining fluorescent lipids on the inner leaflet. ABCA1 showed reduced bleaching after ATP treatment relative to AMP-PNP treatment, whereas ABCA4 showed the reverse indicative of differential vectoral transport (flipping) of the fluorescent phospholipids by these transporters. C–E, rate of transport of Fl-PL across the lipid bilayer in the presence of 1 mm ATP (●) and AMP-PNP (○). C, Fl-PC transport by ABCA1 reconstituted into PG liposomes; D, Fl-PS transport by ABCA1 reconstituted into PG liposomes; E, Fl-PE transport by ABCA4 reconstituted into PC liposomes. F and G, determination of the fraction of Fl-PL accessible on the outer leaflet of proteoliposomes via collisional quenching of Fl-PL with iodide ions. PG liposomes reconstituted with ABCA1 and containing 0.6% (w/w) Fl-PC (F) and Fl-PS (G) and PG liposomes reconstituted with ABCA4 and containing 0.6% Fl-PE (H) were treated with 1 mm AMP-PNP or 1 mm ATP prior to iodide quenching. The data are presented as modified Stern-Volmer plots. F0 is the fluorescence intensity of the sample in the absence of quencher; ΔF is the fluorescence intensity at a given iodide ion concentration; KI is the concentration of potassium iodide. The connecting lines were obtained by linear regression. The inverse of the y intercept represents the fraction of Fl-PL that is accessible to the quencher. Errors bars represent S.D.
The effect of various phospholipids, sphingolipids, and cholesterol on the ATPase activity of reconstituted ABCA1, ABCA7, and ABCA4 is shown in Fig. 2, A–C. The ATPase activity of ABCA1 was stimulated 2- and 1.6-fold by the addition of 30 mol % PC and PS, respectively, with a smaller 1.4-fold increase for 30 mol % SM. In contrast, a decrease in activity was found when ABCA1 proteoliposomes were doped with 30 mol % PE, PI, cholesterol, or ceramide. These data indicate that PC, PS, and SM specifically stimulate the ATPase activity of ABCA1 over the ATPase activity in pure PG liposomes. The ATPase activity of ABCA7 was also stimulated by PC, PS, and SM, but PS was more effective than PC for this transporter. For comparison, the effect of these lipids on the ATPase activity of ABCA4 was also measured in PC liposomes containing 30 mol % added lipid. A maximal stimulation of 1.8-fold was observed for PE. In all cases, a decrease in ATPase activity was observed for PI, cholesterol, and ceramide.
FIGURE 2.
Effect of membrane lipids on the ATPase activities of ABCA1, ABCA7, and ABCA4. Immunoaffinity-purified ABCA1 and ABCA7 were reconstituted into PG liposomes, and ABCA4 was reconstituted into PC liposomes as the base lipids. The activity of the ABCA transporters in these liposomes was set at 100%. A–C, effect of various lipids was determined by replacing 30 mol % of the base lipid with the 1,2-dioleoylphospholipids, PC, PS, PE, or PI, cholesterol (Chol), sphingomyelin (SM), or ceramide (Cer). D–F, effect of increasing phospholipid (PL) concentration on the ATPase activity of ABCA1, ABCA7, and ABCA4. ATP hydrolysis was measured at 37 °C for 40 min. Results are the mean ± S.D. for three independent experiments.
The effect of varying the PC, PS, and PE contents of the liposomes on the ATPase activity of these ABCA transporters was also determined (Fig. 2, D and E). A maximum relative increase in ATPase activity of 75–85% was observed at 30% PC for ABCA1, 30% PS for ABCA7, and 20% PE for ABCA4.
Transport of Phospholipids by ABCA Transporters
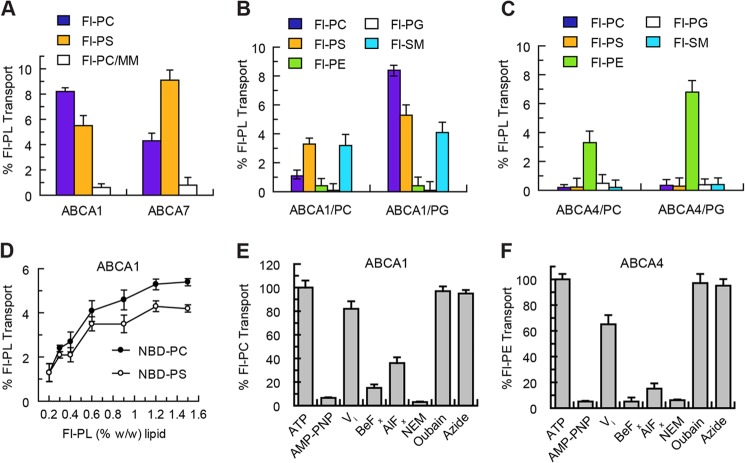
Stimulation of the ATPase activity of ABCA1, ABCA7, and ABCA4 by selected phospholipids suggests that these lipids may serve as substrates that are actively transported or flipped across the lipid bilayer by these transporters. To test this possibility and determine the direction of transport, we utilized the well established dithionite phospholipid fluorescence bleaching technique (42, 43). Proteoliposomes reconstituted with purified ABCA proteins were prepared with a low concentration (0.6%) of Fl-PL. ATP was added to the proteoliposomes to initiate active transport with the nonhydrolyzable derivative AMP-PNP added to the control sample. After 1 h, sodium dithionite, an impermeable reducing agent, was added to bleach the Fl-PL exposed on the outer leaflet of the proteoliposome. The amount of Fl-PL transported was determined from the difference in fluorescence between the ATP- and AMP-PNP-treated samples after dithionite addition. Fig. 3A shows typical fluorescence traces for the transport of Fl-PC across the lipid bilayer of PG liposomes reconstituted with ABCA1. ATP addition resulted in a decrease in the degree of dithionite bleaching (higher fluorescent signal) compared with the control AMP-PNP indicating that ABCA1 promoted an energy-dependent transport of Fl-PC from the outer to the inner leaflet of the proteoliposome, a direction that is consistent with PC transport from the cytoplasmic to the extracellular/lumenal side of biological membranes. Similar results were obtained for the transport of Fl-PC by ABCA7.
For comparison, this assay was used to measure the transport of Fl-PE across the membrane of liposomes reconstituted with ABCA4 (Fig. 3B). In this case, the addition of ATP resulted in an increase in dithionite-mediated fluorescence bleaching relative to the AMP-PNP indicating that ABCA4 transports Fl-PE in the opposite direction, i.e. from the extracellular/lumenal leaflet to the cytoplasmic leaflet of cells.
The time course for the flipping of phospholipids across the lipid bilayer was determined for ABCA1 and ABCA4 reconstituted into liposomes (Fig. 3, C–E). A linear increase in Fl-PC and Fl-PS flipped from the outer (cytoplasmic) to the inner (lumen) leaflet was observed over 60 min for ABCA1 reconstituted into PG liposomes. A linear decrease in the amount of Fl-PE in the inner leaflet was observed for ABCA4 reconstituted into PC liposomes.
ABCA1- and ABCA4-catalyzed phospholipid transport was also measured using the collisional fluorescence quenching method (Fig. 3, F–H) (38). In this technique, membrane-impermeable iodide ion was used to quench the fluorescence contribution of Fl-PL on the outer leaflet of the proteoliposomes after the addition of ATP or AMP-PNP. The results shown in a modified Stern-Volmer plot (44) confirm the results obtained using the dithionite fluorescence bleaching assay indicating that ABCA1 transports Fl-PC and Fl-PS from the outer to the inner leaflet of the proteoliposome (Fig. 3, F and G), whereas ABCA4 transports Fl-PE in the opposite direction (Fig. 3H).
Transport of Specific Lipids by ABCA1, ABCA7, and ABCA4
A number of fluorescent-labeled phospholipids, including Fl-PC, Fl-PS, Fl-PE, Fl-PG, and Fl-SM, were used to define the lipid specificity of the ABCA transporters. We first compared the specificity of ABCA1 and ABCA7 for Fl-PC and Fl-PS. As shown in Fig. 4A, ABCA1 showed a preference for PC over PS, whereas ABCA7 preferred PS over PC. In control experiments, the ATPase-deficient mutants (ABCA-MM) showed no significant transport activity. The differential specificity of ABCA1 and ABCA7 for PC and PS transport is in agreement with the differential stimulatory effect of these phospholipids on the ATPase activity of these transporters (Fig. 2).
FIGURE 4.
Transport of Fl-PL by ABCA1, ABCA7, and ABCA4. The dithionite Fl-PL bleaching assay was used to determine the phospholipid specificity and effect of nucleotides and inhibitors on phospholipid transport by ABCA proteins. A, phospholipid transport (flipping) by WT ABCA1 and ABCA7 and MM mutants reconstituted into PG liposomes containing 0.6% (w/w) Fl-PL. B, phospholipid transport by ABCA1 reconstituted into PC or PG liposomes. Unlabeled PC effectively competes with Fl-PC in PC liposomes reducing the transport of Fl-PS and Fl-SM (sphingomyelin) compared with liposomes composed of PG base lipid. C, phospholipid transport by ABCA4 reconstituted into PC and PG liposomes. D, effect of increasing Fl-PL concentration on transport by ABCA1 reconstituted into PG liposomes. E and F, effect of nucleotides, phosphate analogs, and inhibitors on the Fl-PC transport or flipping by ABCA1 (E) and Fl-PE by ABCA4 (F). Proteoliposomes were incubated at 37 °C for 60 min in the presence of AMP-PNP or ATP with or without the addition of 200 μm Vi, 200 μm AlFx, or 200 μm BeFx, 1 mm ouabain, and 1 mm azide. In the case of N-ethylmaleimide, the ABCA proteins were incubated with 10 mm N-ethylmaleimide for 25 min prior to Fl-PL transport. Results are the mean of three independent experiments. Error bars show S.E.
The transport of specific phospholipids by ABCA1 and ABCA4 was measured in PG and PC liposomes (Fig. 4, B and C). ABCA1 transported Fl-PC, Fl-PS, and Fl-SM but not Fl-PE or Fl-PG, whereas ABCA4 was highly specific for Fl-PE. The transport activity of ABCA1 was lower in PC vesicles compared with PG vesicles due in part to the competing effect of unlabeled PC for transport of Fl-PC.
The effect of Fl-PL concentration, various phosphate analogs, ATPase inhibitors, and protein-modifying reagents on phospholipid transport by ABCA1 was investigated. As shown in Fig. 4D, the transport of Fl-PC and Fl-PS was dependent on the concentration of fluorescent-labeled phospholipid reaching a limiting value above 1%. Addition of 200 μm beryllium fluoride (BeFx) and aluminum fluoride (AlFx) reduced the Fl-PC transport by 83 and 63%, respectively, whereas 1 mm vanadate had only a modest reduction in transport of 20% (Fig. 4E). Treatment of ABCA1 with N-ethylmaleimide prior to reconstitution resulted in the loss of phospholipid flippase activity, whereas ouabain and azide had no significant effect. Similar results were observed for the inhibition of Fl-PE transport by ABCA4 (Fig. 4F).
Effect of Cholesterol on Phospholipid Transport
Cell-based assays have implicated ABCA1 in the efflux of cholesterol as well as phospholipids from cells. To determine whether cholesterol affects phospholipid transport activity, ATP-dependent flipping of Fl-PC across the lipid bilayer was measured for proteoliposomes containing ABCA1 and increasing concentrations of cholesterol. As shown in Fig. 5A, a 30–35% decrease in Fl-PC flippase activity of ABCA1 was observed at 20–30% cholesterol. Cholesterol had a similar effect on the Fl-PE flippase activity of ABCA4 reconstituted into PC vesicles. A cholesterol-mediated decrease in phospholipid transport activity is in agreement with the decrease in ATPase activity of ABCA transporters by cholesterol (Fig. 2).
FIGURE 5.
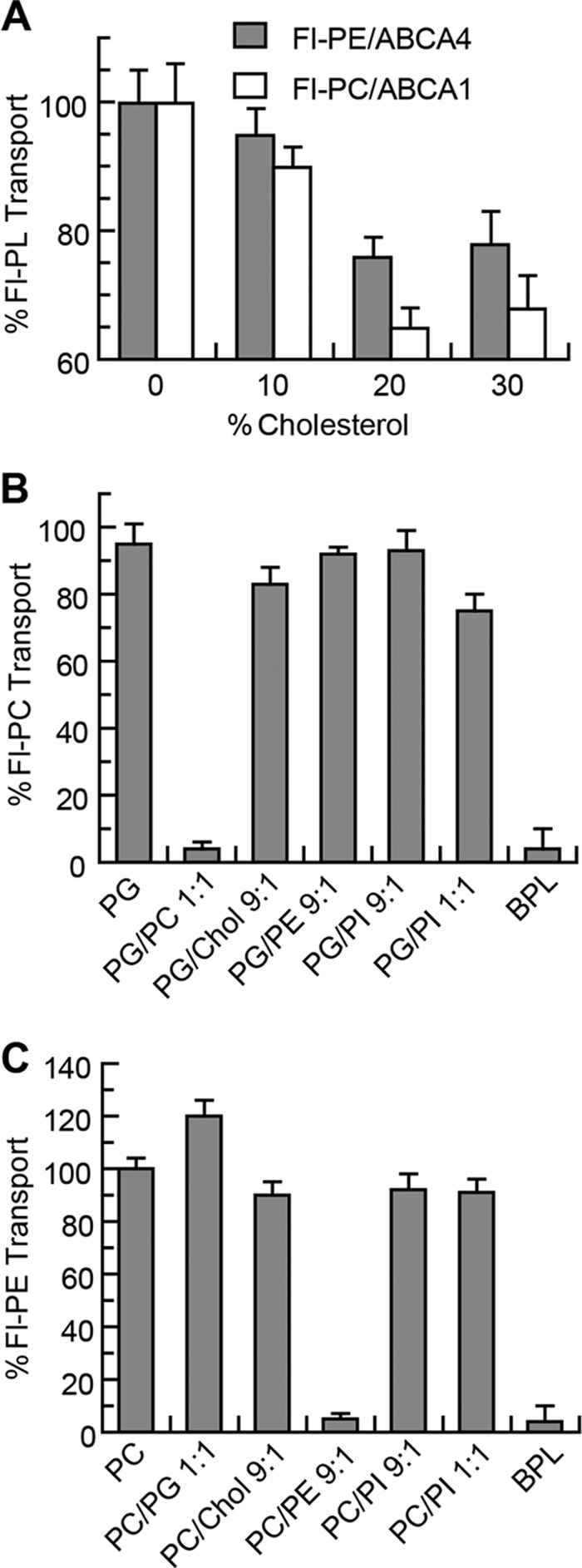
Effect of cholesterol and phospholipids on the transport activity of ABCA1 and ABCA4. A, Fl-PC transport activity of ABCA1 and Fl-PE transport activity of ABCA4 reconstituted in PG liposomes containing 0–30% (w/w) cholesterol (Chol). B, effect of PC, PE, and PI on the transport of Fl-PC by ABCA1 reconstituted into PG liposomes. Unlabeled PC and BPL effectively compete with Fl-PC for transport by ABCA1, whereas PI has little effect. C, effect of PC, PE, and PI on the transport of Fl-PE by ABCA4 reconstituted into PC liposomes. Unlabeled PE and BPL effectively compete with Fl-PE for transport by ABCA4, whereas PC and PI have little effect. Results are the mean ± S.D. for three independent experiments.
To further assess the effect of lipid environment on the phospholipid flippase activity of these transporters, ABCA1 and ABCA4 were reconstituted into PG- and PC-based liposomes containing various phospholipids. The addition of 10% PE or 10 or 50% PI had little effect on the transport activity of ABCA1 relative to its activity in pure PG vesicles. However, addition of 10% unlabeled PC or BPL, which contains ∼10% PC, suppressed Fl-PC transport. Similarly, Fl-PE transport by ABCA4 was unaffected by the addition of PI but was reduced by the addition of unlabeled 10% PE and BPL. These results indicate that cholesterol modestly reduces the phospholipid flippase activity of ABCA1 and ABCA4 presumably by altering the lipid environment of the protein. Unlabeled PC decreases the Fl-PC flippase activity of ABCA1, and unlabeled PE decreases the Fl-PE flippase activity of ABCA4 through the competing effect of these unlabeled PLs for transport of the Fl-PL.
Expression and Purification of Disease-causing ABCA1 and ABCA4 Mutants
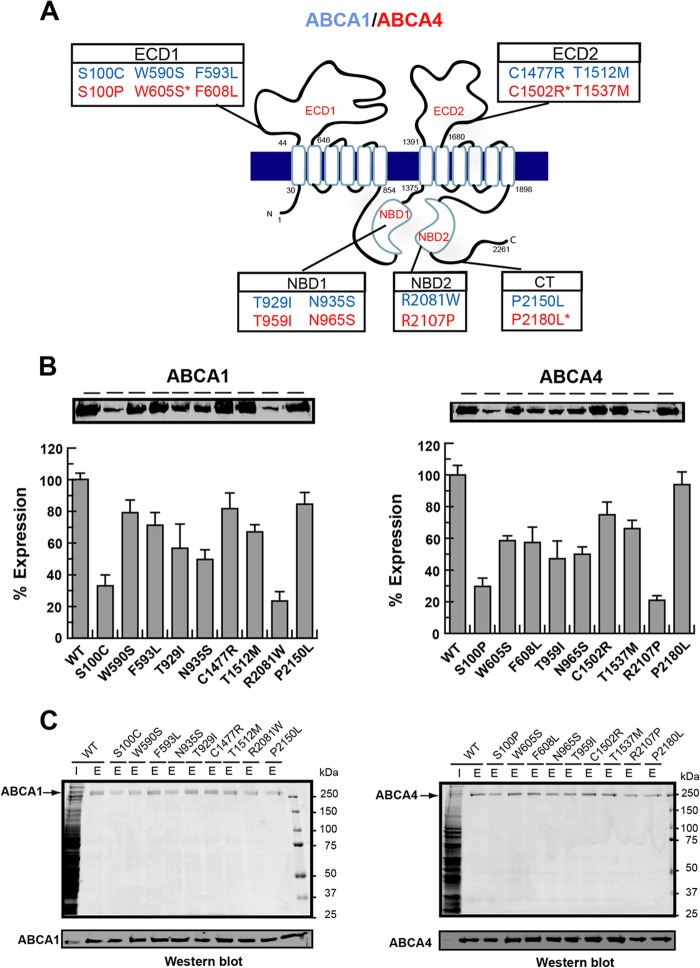
As part of this study, we have generated a number of disease-causing mutations in ABCA1 and ABCA4 to determine their effect on the expression and functional properties of these transporters. We focused our studies on nine missense mutations in ABCA1 known to cause Tangier disease, including three (S100C, W590S, and F593L) in ECD1, two (T929I and N935S) in the NBD1, two (C1477R and T1512M) in ECD2, one (R2081W) in NBD2, and one (P2150L) in the C-terminal segment as shown in Fig. 6A (blue). Alignment of the ABCA1 and ABCA4 sequences indicated that six mutations in corresponding positions in ABCA4 are associated with Stargardt disease (S100P, F608L, N965S, T959I, T1537M, and R2107P) (Fig. 6A, red). Mutations in residues Trp-605 and Pro-2180 of ABCA4 have yet to be linked to Stargardt disorder. These two residues were replaced with serine and leucine, respectively, (W605S and P2180L) corresponding to the disease-causing substitutions in ABCA1. Finally, the null mutant C1502X associated with Stargardt disease was modified to C1502R to reflect the primary sequence change of the corresponding C1477R mutant in ABCA1 linked to Tangier disease.
FIGURE 6.
Expression and purification of ABCA1 and ABCA4 disease-associated mutants. A, topological diagram of human ABCA1 and ABCA4 showing the position of the nine ABCA1 mutants associated with Tangier disease mutants (blue) and related ABCA4 mutants, many of which are associated with Stargardt disease (red) in relation to the various domains (exocytoplasmic domains ECD1 and ECD2, nucleotide binding domains NBD1 and NBD2, and C-terminal domain (CT)). Some disease alleles such as W590S, C1477R, and P2150L (ABCA1) have conserved residues in ABCA4, but mutations in these positions in ABCA4 have yet to be linked to Stargardt disease. B, expression profile of ABCA1 and ABCA4 disease-associated mutants relative to the WT protein. Proteins were expressed in HEK293T cells, and extracts were resolved by SDS-gel electrophoresis. An example of a Western blot labeled with the Rho1D4 antibody is shown along with quantitation from three independent experiments. C, purification of mutants on a Rho1D4 immunoaffinity matrix. Upper panel is a Coomassie Blue-stained gel of the WT extract (I) and 1D4 peptide-eluted protein (E). Lower panel is a Western blot labeled with the Rho1D4 antibody.
These mutants were expressed in HEK293T cells and purified by immunoaffinity chromatography. Fig. 6B shows the expression profile of the ABCA1 and ABCA4 mutants. The levels of expression of the ABCA1 and ABCA4 mutants were generally lower than the corresponding WT proteins with the ABCA1 mutants S100C and R2081W and the corresponding ABCA4 mutants S100P and R2107P expressing at levels less than 25% of WT and the remaining mutants expressing in the range of 35–90% of the WT proteins. Despite the differences in expression levels, all mutants could be purified by immunoaffinity chromatography on the Rho1D4-Sepharose matrix in sufficient quantities for functional studies (Fig. 6C).
ATPase Activities of Disease Variants
The purified ABCA1 and ABCA4 mutants were reconstituted into liposomes containing BPL to determine the effect of the mutations on the ATPase activity of these proteins. Variants in the ECD1 (S100C, W590S, and F593L), NBD1 (T929I and N935S), and NBD2 (R2081W) of ABCA1 showed significantly reduced ATPase activities in the range of 20–35% of WT activity (Fig. 7A). By contrast, the T1512M mutation in ECD2 and P2150L in the C-terminal segment exhibited ATPase activity similar to the WT protein. The C1477R mutant in ECD2 showed an intermediate reduction in activity. ABCA4 variants showed a similar ATPase activity profile as the ABCA1 mutants with the exception of the T1537M mutation of ABCA4, which was significantly lower than the corresponding T1512M mutant in ABCA1.
FIGURE 7.
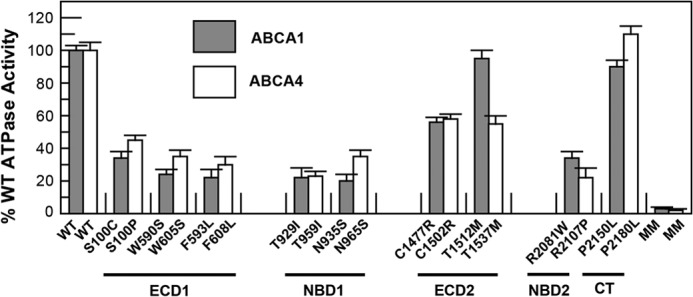
Relative ATPase activity of ABCA1 and ABCA4 disease-associated mutants. WT and mutant ABCA1 and ABCA4 containing the 1D4 epitope were expressed and purified from HEK293T cells by immunoaffinity chromatography and reconstituted into liposomes composed of BPL for analysis of ATPase activity. The ATPase activity of the disease-associated mutants was lower than that of the WT protein but significantly higher than the ATPase-deficient MM mutant. Results are the mean ± S.D. for three independent experiments.
Phospholipid Flippase Activity of Disease Variants
The ATP-dependent phospholipid transport properties of the ABCA1 and ABCA4 mutants were also investigated by the fluorescence dithionite bleaching protocol. As shown in Fig. 8, A and B, the Fl-PC flippase activity of the ABCA1 mutants and the Fl-PE flippase activity of ABCA4 mutants have a similar profile with the ABCA1 variants C1477R, T1512M, and P2150L and corresponding ABCA4 variants C1502R, T1537M, and P2180L showing transport activities ranging from 60 to 80% of the WT protein and the other mutants showing reduced activity in the range of 20–40% of the WT protein. Interestingly, the PS transport activity of most mutants showed a larger decrease in activity than the PC transport activity relative to the respective transport activity of the WT proteins.
FIGURE 8.
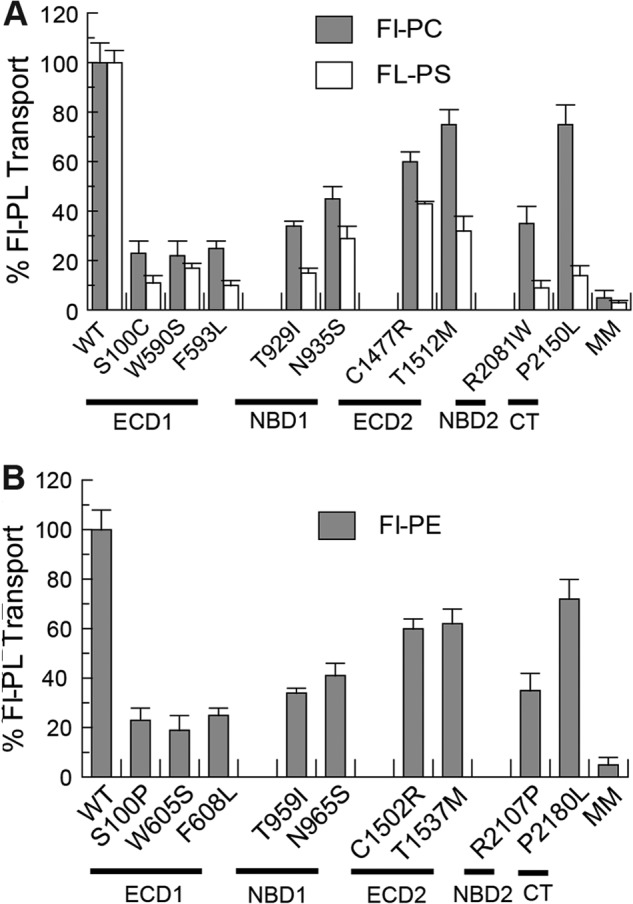
Relative Fl-PL transport activity of ABCA1 and ABCA4 disease-associated mutants. A, purified WT ABCA1 and disease-causing mutants were reconstituted into PG liposomes containing 0.6% Fl-PC or Fl-PS for analysis of transport activity by the dithionite fluorescence bleaching assay. B, purified WT ABCA4 and mutants were reconstituted into PC liposomes containing 0.6% Fl-PE for transport activity measurements. Results are the means ± S.D. for three independent experiments.
Immunofluorescence Localization of WT and Mutant ABCA1 and ABCA4 Proteins in Transfected COS7 Cells
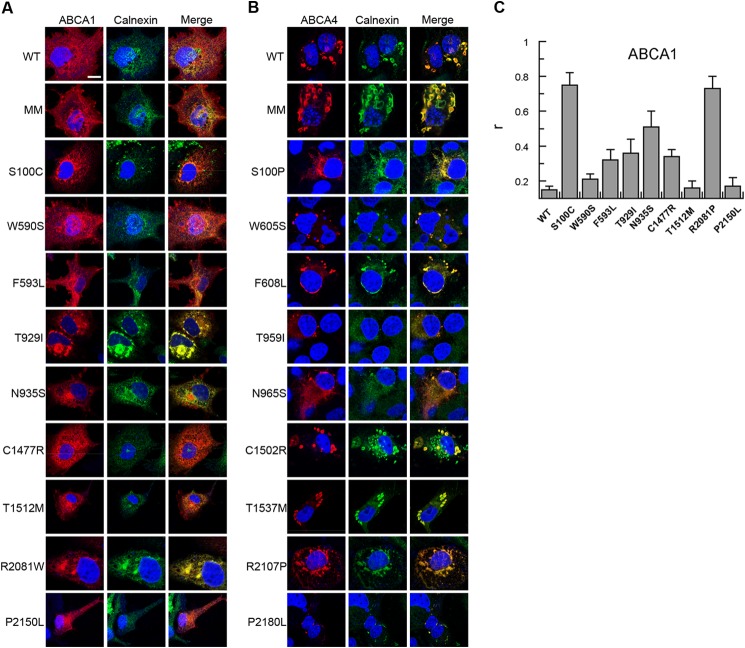
The effect of disease-causing mutations on the localization of ABCA1 and ABCA4 in transfected COS7 cells was investigated by immunofluorescence microscopy. In agreement with earlier studies, WT ABCA1 localized primarily to the plasma membrane and intracellular endosomes, whereas WT ABCA4 was present in large intracellular vesicles that also contained calnexin (Fig. 9, A and B) (20, 37, 41). The ABCA1-MM and ABCA4-MM showed a similar distribution as their WT counterparts indicating that these functionally inactive mutants fold into a native-like conformation allowing their exit from the ER. In contrast, some of the mutants, including ABCA1 mutants T929I and R2081W and related ABCA4 mutants T959I and R2107P, showed partial or complete co-localization with calnexin in a reticular pattern characteristic of the ER. This indicates that some of the mutants are highly misfolded and retained in the ER by the cellular quality control system. The extent of co-localization of ABCA1 mutants with calnexin is quantified from double labeling experiments in Fig. 9C.
FIGURE 9.
Localization of ABCA1 and ABCA4 disease mutants by immunofluorescence microscopy. COS7 cells expressing WT or disease-associated mutants of ABCA1 (A) or ABCA4 (B) were double labeled with the Rho1D4 monoclonal antibody (red) to localize ABCA1 or ABCA4 and a calnexin polyclonal antibody (green) as an ER marker for analysis by confocal scanning microscopy. WT and MM mutant ABCA1 localize to plasma membrane and endosomes, whereas most Tangier-causing mutants showed variable co-localization with the calnexin in a characteristic ER reticular pattern. WT and MM mutant ABCA4 are localized to large intracellular vesicles that also contain calnexin. Many ABCA4 mutants showed a reticular pattern of labeling within the cells characteristic of ER localization. Scale bar represents 10 μm. C, extent of co-localization of ABCA1 mutants and calnexin in transfected COS7 cells quantified using ImageJ analysis software. Pearson's coefficient (r) provided a relative indicator of co-localization with r = 0 indicating no co-localization and r = 1 indicating complete co-localization.
DISCUSSION
Although previous cell-based studies have implicated ABCA1 and ABCA7 in the efflux of phospholipids and cholesterol from cells as a critical step in the reverse cholesterol transport pathway, it has been unclear if these proteins directly transport these lipids across the membrane or simply mediate lipid transfer to apo-A1 through an indirect “regulatory” mechanism (45). In this study, we provide the first direct biochemical evidence that ABCA1 and ABCA7 transport or flip specific phospholipids from the cytoplasmic leaflet to the exocytoplasmic leaflet of the lipid bilayer, and we determine the phospholipid specificity for these ABCA transporters. This was achieved through analysis of ATP-dependent transport of Fl-PL by these transporters and supported by the stimulation of their ATPase activity by the same phospholipids.
Immunoaffinity-purified ABCA1, ABCA7, and ABCA4 exhibited significant ATPase activity upon reconstitution into liposomes consisting of BPL, in contrast to the ABCA-MM Walker A mutants that were devoid of activity. The ATPase activity of ABCA1 and ABCA7 (∼220–280 nmol/min/mg) was within the range of values observed in previous studies (37, 46) but was significantly lower than values observed for other ABC proteins (1.7–1.9 μmol/min/mg) such as P-glycoprotein and the TAP complex (47, 48). The ATPase activity of ABCA1 in PG liposomes was activated ∼2-fold by PC and to a lesser degree by PS and SM, whereas the activity of ABCA7 showed a preference for PS over PC. Little if any activation was observed for PE in contrast to ABCA4, which was specific for PE. In agreement with an earlier report (46), cholesterol did not activate the ATPase activity of ABCA1, but instead it decreased the activity of ABCA1, ABCA7, and ABCA4 by 20–30%. This general inhibitory effect most likely occurs through the effect of cholesterol on overall lipid environment of the reconstituted transporters.
Analysis of the phospholipid transport properties of ABCA1, ABCA7, and ABCA4 was a central focus of this study. Previously, we had shown that ABCA4 functions as an importer flipping N-retinylidene-PE from the lumen to the cytosolic leaflet of photoreceptor disc membranes and proteoliposomes (29). In this study, we have used both the Fl-PL dithionite bleaching assay and the Fl-PL collisional quenching method to further examine phospholipid transport by reconstituted ABCA1, ABCA7, and ABCA4. Although these ABCA transporters share a high degree of sequence identity (∼50%), ABCA1 and ABCA7 functioned as exporters flipping phospholipids from the cytoplasmic to the exocytoplasmic leaflet of membranes, whereas ABCA4 transported PE in the opposite direction. ABCA1 actively transported PC, PS, and SM with a preference for PC, whereas ABCA7 preferentially transported PS in general agreement with the phospholipid ATPase activation studies. Cholesterol inhibited the phospholipid transport activity of ABCA1, ABCA7, and ABCA4 to the same degree that it inhibited their ATPase activity suggesting that cholesterol is not co-transported with phospholipids. Our results are consistent with a previous study reporting the inability of the photoactivatable cholesterol derivative to bind ABCA1(49) and suggest that cholesterol is not directly transported by ABCA1. A direct cholesterol transport assay, however, is needed to further support this contention.
Several models have been proposed to explain ABCA1-mediated cholesterol and phospholipid efflux from cells to apoA-1. In the concurrent model, PC and cholesterol are effluxed together to apoA-1 by ABCA1 (50). In the two-step model, ABCA1 first mediates PC efflux to apoA-1, and this apoA-1 PC complex subsequently accepts cholesterol from the outer leaflet of the plasma membrane independent of ABCA1 (49, 51). In a third model, ABCA1 mediates the translocation of phospholipids to the outer leaflet to generate a phospholipid imbalance between the inner and outer leaflets resulting in membrane bending. The resulting protrusions promote transient binding of apoA-1 to the membrane and a solubilization of the exovesiculated lipid domain leading to the loading of apoA-1 with phospholipids and cholesterol for the generation of nascent HDL particles (52–54). Other models stress the importance of the interconversion of ABCA1 monomers to dimers and membrane meso-domain organization in apoA-1 binding and HDL formation (55, 56).
Our studies support the active export of the phospholipids by ABCA1 as an important initial step in reverse cholesterol transport and argue against the direct transport of cholesterol by ABCA1 as postulated in the concurrent model. ABCA1 may simply flip PC, PS, and SM from the cytoplasmic to the extracellular leaflet of cells thereby altering the surface area of the opposing leaflets resulting in cell protrusions that facilitate the loading of apoA-1 with cholesterol and phospholipids. Alternatively, ABCA1 may directly efflux phospholipids to apoA-1 directly bound to ABCA1 as a preloading step prior to the loading of cholesterol as suggested in the two-step model. Because we were unable to reconstitute the ABCA1·apoA-1 complex into liposomes, we could not determine whether ABCA1 can directly translocate phospholipids onto apoA-1 or if ABCA1 primarily functions as a phospholipid flippase. Further studies are needed to delineate between these mechanisms.
As part of this study, we determined the effect of disease-causing missense mutations in ECD1, NBD1, ECD2, and NBD2, the C terminus of ABCA1, and corresponding mutations in ABCA4 on protein expression, phospholipid-specific ATPase activity, ATP-dependent phospholipid flippase activity, and subcellular localization. The mutants were expressed at levels ranging from 20 to 100% WT and were readily purified by immunoaffinity chromatography after solubilization in CHAPS buffer. The ATPase and phospholipid transport activities of the various mutants were in general lower than WT ranging from 20 to 80% that of WT activity. Interestingly, the overall profiles of the ABCA1 and ABCA4 mutants were strikingly similar. The ABCA1/ABCA4 mutants in the ECD1 (S100C/S100P, W590S/W605S, and F593L/F608L) displayed the lowest activities (20–30% WT), whereas those in the ECD2 (C1477R/C1502R and T1512M/T1537M) and the P2150L/P2180L mutants in the C terminus showed the highest activities (60–100% WT). This suggests that the structure-function relationships of ABCA1 and ABCA4 are broadly similar despite the fact that they transport different phospholipids in different directions. A low resolution structure of ABCA4 has recently been determined by single particle EM (34). One may surmise that ABCA1 will have a similar overall structure and undergo similar conformational changes as ABCA4 during the transport cycle, although the high affinity access phospholipid-binding site most likely resides on different sides of the proteins with the initial PL-binding site for ABCA1 on the cytoplasmic side and the PE binding for ABCA4 on the exocytoplasmic (lumen/extracellular) side.
Several reports have described the effect of some of the Tangier-associated mutations on apoA-1 binding, phospholipid and cholesterol efflux, and subcellular localization of ABCA1 (18–20). In these studies, the disease-associated mutants all showed reduced activity particularly with regard to phospholipid and cholesterol efflux. Our data on the effect of disease-associated mutations on phospholipid transport broadly agrees with their effect on phospholipid efflux as reported in the literature. For example, the P2150L showed only mild loss in phospholipid transport and efflux, whereas R2081W showed a more pronounced effect. However, the extent of reduction in these activities differs for some mutants. For example, the C1477R mutant shows intermediate (∼60% WT) phospholipid transport activity, whereas it shows minimum (<20% WT) phospholipid efflux activity. It is possible that this mutant is relatively active in flipping PC/PS across the bilayer, but due to its more limited ability to bind apoA-1, it shows a more marked decrease in phospholipid efflux activity. The Cys-1477 residue has been implicated in intramolecular disulfide bonding between the ECDs of ABCA1 (57), which may be required for efficient binding of apoA-1. Finally, our results showing that some mutants with limited functional activity are able to exit the ER is in agreement with previous studies showing that some, but not all, ABCA1 and ABCA4 mutants show subcellular localization similar to the WT proteins.
To date, relatively few eukaryotic ABC transporters have been purified for identification of their substrates and analysis of their transport mechanism. The immunoaffinity purification and reconstitution methodology used here to characterize ABCA1, ABCA4, and ABCA7 have broad application for analysis of other ABC transporters as well as other membrane proteins (58, 59). It will be of particular interest to identify the putative lipid substrates and direction of transport for ABCA3 associated with neonatal surfactant deficiency and pediatric interstitial lung disease and ABCA12 linked to harlequin ichthyosis as well as other members of the ABCA subfamily, including ABCA2.
Acknowledgment
We thank Professor Kazumitsu Ueda for the gift of the ABCA7 plasmid.
This work was supported, in whole or in part, by National Institutes of Health Grant EY002422.
This article was selected as a Paper of the Week.
- ABC
- ATP-binding cassette
- NBD
- nucleotide binding domain
- ECD
- exocytoplasmic domain
- ABCA-MM
- ABCA-double Walker A mutant Lys-to-Met
- BPL
- brain polar lipid
- apoA-1
- apolipoprotein A1
- PC
- phosphatidylcholine
- PS
- phosphatidylserine
- PE
- phosphatidylethanolamine
- PG
- phosphatidylglycerol
- SM
- sphingomyelin
- Fl-PL
- fluorescent phospholipid
- AMP-PNP
- adenosine 5′-(β,γ-imino)triphosphate
- ER
- endoplasmic reticulum
- PI
- phosphatidylinositol
- BeFx
- beryllium fluoride.
REFERENCES
- 1. Higgins C. F. (1992) ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8, 67–113 [DOI] [PubMed] [Google Scholar]
- 2. Dean M., Annilo T. (2005) Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genomics Hum. Genet. 6, 123–142 [DOI] [PubMed] [Google Scholar]
- 3. Piehler A. P., Ozcürümez M., Kaminski W. E. (2012) A-subclass ATP-binding cassette proteins in brain lipid homeostasis and neurodegeneration. Front. Psychiatry 3, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coleman J. A., Quazi F., Molday R. S. (2013) Mammalian P4-ATPases and ABC transporters and their role in phospholipid transport. Biochim. Biophys. Acta 1831, 555–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bungert S., Molday L. L., Molday R. S. (2001) Membrane topology of the ATP binding cassette transporter ABCR and its relationship to ABC1 and related ABCA transporters: identification of N-linked glycosylation sites. J. Biol. Chem. 276, 23539–23546 [DOI] [PubMed] [Google Scholar]
- 6. Quazi F., Molday R. S. (2011) Lipid transport by mammalian ABC proteins. Essays Biochem. 50, 265–290 [DOI] [PubMed] [Google Scholar]
- 7. Wenzel J. J., Piehler A., Kaminski W. E. (2007) ABC A-subclass proteins: gatekeepers of cellular phospho- and sphingolipid transport. Front. Biosci. 12, 3177–3193 [DOI] [PubMed] [Google Scholar]
- 8. Oram J. F., Vaughan A. M. (2006) ATP-binding cassette cholesterol transporters and cardiovascular disease. Circ. Res. 99, 1031–1043 [DOI] [PubMed] [Google Scholar]
- 9. Brooks-Wilson A., Marcil M., Clee S. M., Zhang L. H., Roomp K., van Dam M., Yu L., Brewer C., Collins J. A., Molhuizen H. O., Loubser O., Ouelette B. F., Fichter K., Ashbourne-Excoffon K. J., Sensen C. W., Scherer S., Mott S., Denis M., Martindale D., Frohlich J., Morgan K., Koop B., Pimstone S., Kastelein J. J., Genest J., Jr., Hayden M. R. (1999) Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22, 336–345 [DOI] [PubMed] [Google Scholar]
- 10. Bodzioch M., Orsó E., Klucken J., Langmann T., Böttcher A., Diederich W., Drobnik W., Barlage S., Büchler C., Porsch-Ozcürümez M., Kaminski W. E., Hahmann H. W., Oette K., Rothe G., Aslanidis C., Lackner K. J., Schmitz G. (1999) The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 22, 347–351 [DOI] [PubMed] [Google Scholar]
- 11. Rust S., Rosier M., Funke H., Real J., Amoura Z., Piette J. C., Deleuze J. F., Brewer H. B., Duverger N., Denèfle P., Assmann G. (1999) Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 22, 352–355 [DOI] [PubMed] [Google Scholar]
- 12. Singaraja R. R., Brunham L. R., Visscher H., Kastelein J. J., Hayden M. R. (2003) Efflux and atherosclerosis: the clinical and biochemical impact of variations in the ABCA1 gene. Arterioscler. Thromb. Vasc. Biol. 23, 1322–1332 [DOI] [PubMed] [Google Scholar]
- 13. Chroni A., Liu T., Fitzgerald M. L., Freeman M. W., Zannis V. I. (2004) Cross-linking and lipid efflux properties of apoA-I mutants suggest direct association between apoA-I helices and ABCA1. Biochemistry 43, 2126–2139 [DOI] [PubMed] [Google Scholar]
- 14. Wang N., Silver D. L., Costet P., Tall A. R. (2000) Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 275, 33053–33058 [DOI] [PubMed] [Google Scholar]
- 15. Fitzgerald M. L., Morris A. L., Chroni A., Mendez A. J., Zannis V. I., Freeman M. W. (2004) ABCA1 and amphipathic apolipoproteins form high-affinity molecular complexes required for cholesterol efflux. J. Lipid Res. 45, 287–294 [DOI] [PubMed] [Google Scholar]
- 16. Nagao K., Kimura Y., Ueda K. (2012) Lysine residues of ABCA1 are required for the interaction with apoA-I. Biochim. Biophys. Acta 1821, 530–535 [DOI] [PubMed] [Google Scholar]
- 17. Alder-Baerens N., Müller P., Pohl A., Korte T., Hamon Y., Chimini G., Pomorski T., Herrmann A. (2005) Headgroup-specific exposure of phospholipids in ABCA1-expressing cells. J. Biol. Chem. 280, 26321–26329 [DOI] [PubMed] [Google Scholar]
- 18. Singaraja R. R., Visscher H., James E. R., Chroni A., Coutinho J. M., Brunham L. R., Kang M. H., Zannis V. I., Chimini G., Hayden M. R. (2006) Specific mutations in ABCA1 have discrete effects on ABCA1 function and lipid phenotypes both in vivo and in vitro. Circ. Res. 99, 389–397 [DOI] [PubMed] [Google Scholar]
- 19. Fitzgerald M. L., Morris A. L., Rhee J. S., Andersson L. P., Mendez A. J., Freeman M. W. (2002) Naturally occurring mutations in the largest extracellular loops of ABCA1 can disrupt its direct interaction with apolipoprotein A-I. J. Biol. Chem. 277, 33178–33187 [DOI] [PubMed] [Google Scholar]
- 20. Tanaka A. R., Abe-Dohmae S., Ohnishi T., Aoki R., Morinaga G., Okuhira K., Ikeda Y., Kano F., Matsuo M., Kioka N., Amachi T., Murata M., Yokoyama S., Ueda K. (2003) Effects of mutations of ABCA1 in the first extracellular domain on subcellular trafficking and ATP binding/hydrolysis. J. Biol. Chem. 278, 8815–8819 [DOI] [PubMed] [Google Scholar]
- 21. McNeish J., Aiello R. J., Guyot D., Turi T., Gabel C., Aldinger C., Hoppe K. L., Roach M. L., Royer L. J., de Wet J., Broccardo C., Chimini G., Francone O. L. (2000) High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc. Natl. Acad. Sci. U.S.A. 97, 4245–4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim W. S., Guillemin G. J., Glaros E. N., Lim C. K., Garner B. (2006) Quantitation of ATP-binding cassette subfamily-A transporter gene expression in primary human brain cells. Neuroreport 17, 891–896 [DOI] [PubMed] [Google Scholar]
- 23. Abe-Dohmae S., Ueda K., Yokoyama S. (2006) ABCA7, a molecule with unknown function. FEBS Lett. 580, 1178–1182 [DOI] [PubMed] [Google Scholar]
- 24. Wang N., Lan D., Gerbod-Giannone M., Linsel-Nitschke P., Jehle A. W., Chen W., Martinez L. O., Tall A. R. (2003) ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. J. Biol. Chem. 278, 42906–42912 [DOI] [PubMed] [Google Scholar]
- 25. Linsel-Nitschke P., Jehle A. W., Shan J., Cao G., Bacic D., Lan D., Wang N., Tall A. R. (2005) Potential role of ABCA7 in cellular lipid efflux to apoA-I. J. Lipid Res. 46, 86–92 [DOI] [PubMed] [Google Scholar]
- 26. Illing M., Molday L. L., Molday R. S. (1997) The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J. Biol. Chem. 272, 10303–10310 [DOI] [PubMed] [Google Scholar]
- 27. Allikmets R., Singh N., Sun H., Shroyer N. F., Hutchinson A., Chidambaram A., Gerrard B., Baird L., Stauffer D., Peiffer A., Rattner A., Smallwood P., Li Y., Anderson K. L., Lewis R. A., Nathans J., Leppert M., Dean M., Lupski J. R. (1997) A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat. Genet. 15, 236–246 [DOI] [PubMed] [Google Scholar]
- 28. Nasonkin I., Illing M., Koehler M. R., Schmid M., Molday R. S., Weber B. H. (1998) Mapping of the rod photoreceptor ABC transporter (ABCR) to 1p21-p22.1 and identification of novel mutations in Stargardt's disease. Hum. Genet. 102, 21–26 [DOI] [PubMed] [Google Scholar]
- 29. Quazi F., Lenevich S., Molday R. S. (2012) ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nat. Commun. 3, 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beharry S., Zhong M., Molday R. S. (2004) N-Retinylidene-phosphatidylethanolamine is the preferred retinoid substrate for the photoreceptor-specific ABC transporter ABCA4 (ABCR). J. Biol. Chem. 279, 53972–53979 [DOI] [PubMed] [Google Scholar]
- 31. Molday R. S., Zhong M., Quazi F. (2009) The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim. Biophys. Acta 1791, 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weng J., Mata N. L., Azarian S. M., Tzekov R. T., Birch D. G., Travis G. H. (1999) Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell 98, 13–23 [DOI] [PubMed] [Google Scholar]
- 33. Mata N. L., Tzekov R. T., Liu X., Weng J., Birch D. G., Travis G. H. (2001) Delayed dark-adaptation and lipofuscin accumulation in abcr+/− mice: implications for involvement of ABCR in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 42, 1685–1690 [PubMed] [Google Scholar]
- 34. Tsybovsky Y., Orban T., Molday R. S., Taylor D., Palczewski K. (2013) Molecular organization and ATP-induced conformational changes of ABCA4, the photoreceptor-specific ABC transporter. Structure 21, 854–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abe-Dohmae S., Ikeda Y., Matsuo M., Hayashi M., Okuhira K., Ueda K., Yokoyama S. (2004) Human ABCA7 supports apolipoprotein-mediated release of cellular cholesterol and phospholipid to generate high density lipoprotein. J. Biol. Chem. 279, 604–611 [DOI] [PubMed] [Google Scholar]
- 36. Tsybovsky Y., Wang B., Quazi F., Molday R. S., Palczewski K. (2011) Posttranslational modifications of the photoreceptor-specific ABC transporter ABCA4. Biochemistry 50, 6855–6866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahn J., Beharry S., Molday L. L., Molday R. S. (2003) Functional interaction between the two halves of the photoreceptor-specific ATP binding cassette protein ABCR (ABCA4). Evidence for a non-exchangeable ADP in the first nucleotide binding domain. J. Biol. Chem. 278, 39600–39608 [DOI] [PubMed] [Google Scholar]
- 38. Rajasekharan A., Gummadi S. N. (2011) Flip-flop of phospholipids in proteoliposomes reconstituted from detergent extract of chloroplast membranes: kinetics and phospholipid specificity. PLoS One 6, e28401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ahn J., Wong J. T., Molday R. S. (2000) The effect of lipid environment and retinoids on the ATPase activity of ABCR, the photoreceptor ABC transporter responsible for Stargardt macular dystrophy. J. Biol. Chem. 275, 20399–20405 [DOI] [PubMed] [Google Scholar]
- 40. Bolte S., Cordelières F. P. (2006) A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 [DOI] [PubMed] [Google Scholar]
- 41. Zhong M., Molday L. L., Molday R. S. (2009) Role of the C terminus of the photoreceptor ABCA4 transporter in protein folding, function, and retinal degenerative diseases. J. Biol. Chem. 284, 3640–3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Romsicki Y., Sharom F. J. (2001) Phospholipid flippase activity of the reconstituted P-glycoprotein multidrug transporter. Biochemistry 40, 6937–6947 [DOI] [PubMed] [Google Scholar]
- 43. Coleman J. A., Kwok M. C., Molday R. S. (2009) Localization, purification, and functional reconstitution of the P4-ATPase Atp8a2, a phosphatidylserine flippase in photoreceptor disc membranes. J. Biol. Chem. 284, 32670–32679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lakowicz J. R. (1980) Fluorescence spectroscopic investigations of the dynamic properties of proteins, membranes and nucleic acids. J. Biochem. Biophys. Methods 2, 91–119 [DOI] [PubMed] [Google Scholar]
- 45. Szakács G., Langmann T., Ozvegy C., Orsó E., Schmitz G., Váradi A., Sarkadi B. (2001) Characterization of the ATPase cycle of human ABCA1: implications for its function as a regulator rather than an active transporter. Biochem. Biophys. Res. Commun. 288, 1258–1264 [DOI] [PubMed] [Google Scholar]
- 46. Takahashi K., Kimura Y., Kioka N., Matsuo M., Ueda K. (2006) Purification and ATPase activity of human ABCA1. J. Biol. Chem. 281, 10760–10768 [DOI] [PubMed] [Google Scholar]
- 47. Urbatsch I. L., Wilke-Mounts S., Gimi K., Senior A. E. (2001) Purification and characterization of N-glycosylation mutant mouse and human P-glycoproteins expressed in Pichia pastoris cells. Arch. Biochem. Biophys. 388, 171–177 [DOI] [PubMed] [Google Scholar]
- 48. Herget M., Kreissig N., Kolbe C., Schölz C., Tampé R., Abele R. (2009) Purification and reconstitution of the antigen transport complex TAP: a prerequisite for determination of peptide stoichiometry and ATP hydrolysis. J. Biol. Chem. 284, 33740–33749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang N., Silver D. L., Thiele C., Tall A. R. (2001) ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J. Biol. Chem. 276, 23742–23747 [DOI] [PubMed] [Google Scholar]
- 50. Smith J. D., Le Goff W., Settle M., Brubaker G., Waelde C., Horwitz A., Oda M. N. (2004) ABCA1 mediates concurrent cholesterol and phospholipid efflux to apolipoprotein A-I. J. Lipid Res. 45, 635–644 [DOI] [PubMed] [Google Scholar]
- 51. Fielding P. E., Nagao K., Hakamata H., Chimini G., Fielding C. J. (2000) A two-step mechanism for free cholesterol and phospholipid efflux from human vascular cells to apolipoprotein A-1. Biochemistry 39, 14113–14120 [DOI] [PubMed] [Google Scholar]
- 52. Hassan H. H., Denis M., Lee D. Y., Iatan I., Nyholt D., Ruel I., Krimbou L., Genest J. (2007) Identification of an ABCA1-dependent phospholipid-rich plasma membrane apolipoprotein A-I binding site for nascent HDL formation: implications for current models of HDL biogenesis. J. Lipid Res. 48, 2428–2442 [DOI] [PubMed] [Google Scholar]
- 53. Iatan I., Bailey D., Ruel I., Hafiane A., Campbell S., Krimbou L., Genest J. (2011) Membrane microdomains modulate oligomeric ABCA1 function: impact on apoAI-mediated lipid removal and phosphatidylcholine biosynthesis. J. Lipid Res. 52, 2043–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vedhachalam C., Duong P. T., Nickel M., Nguyen D., Dhanasekaran P., Saito H., Rothblat G. H., Lund-Katz S., Phillips M. C. (2007) Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J. Biol. Chem. 282, 25123–25130 [DOI] [PubMed] [Google Scholar]
- 55. Nagao K., Tomioka M., Ueda K. (2011) Function and regulation of ABCA1-membrane meso-domain organization and reorganization. FEBS J. 278, 3190–3203 [DOI] [PubMed] [Google Scholar]
- 56. Nagata K. O., Nakada C., Kasai R. S., Kusumi A., Ueda K. (2013) ABCA1 dimer-monomer interconversion during HDL generation revealed by single-molecule imaging. Proc. Natl. Acad. Sci. U.S.A. 110, 5034–5039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hozoji M., Kimura Y., Kioka N., Ueda K. (2009) Formation of two intramolecular disulfide bonds is necessary for ApoA-I-dependent cholesterol efflux mediated by ABCA1. J. Biol. Chem. 284, 11293–11300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wong J. P., Reboul E., Molday R. S., Kast J. (2009) A carboxyl-terminal affinity tag for the purification and mass spectrometric characterization of integral membrane proteins. J. Proteome Res. 8, 2388–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reboul E., Dyka F. M., Quazi F., Molday R. S. (2013) Cholesterol transport via ABCA1: new insights from solid-phase binding assay. Biochimie 95, 957–961 [DOI] [PubMed] [Google Scholar]