Abstract
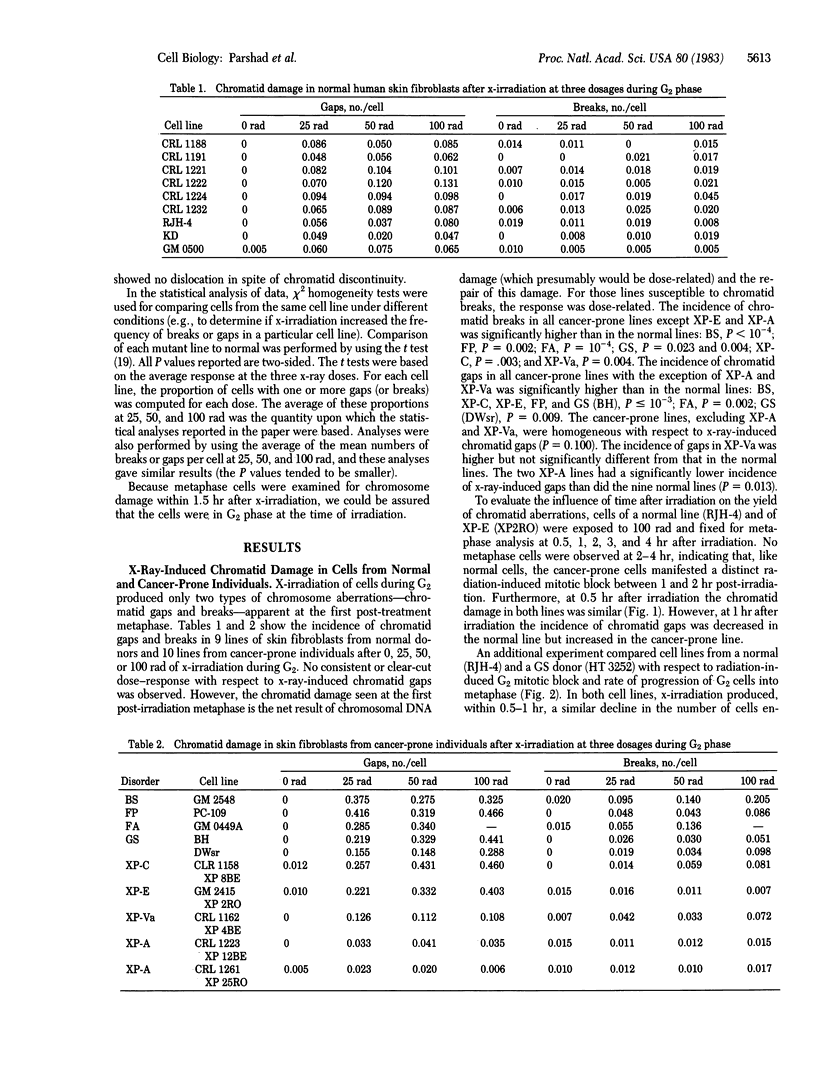
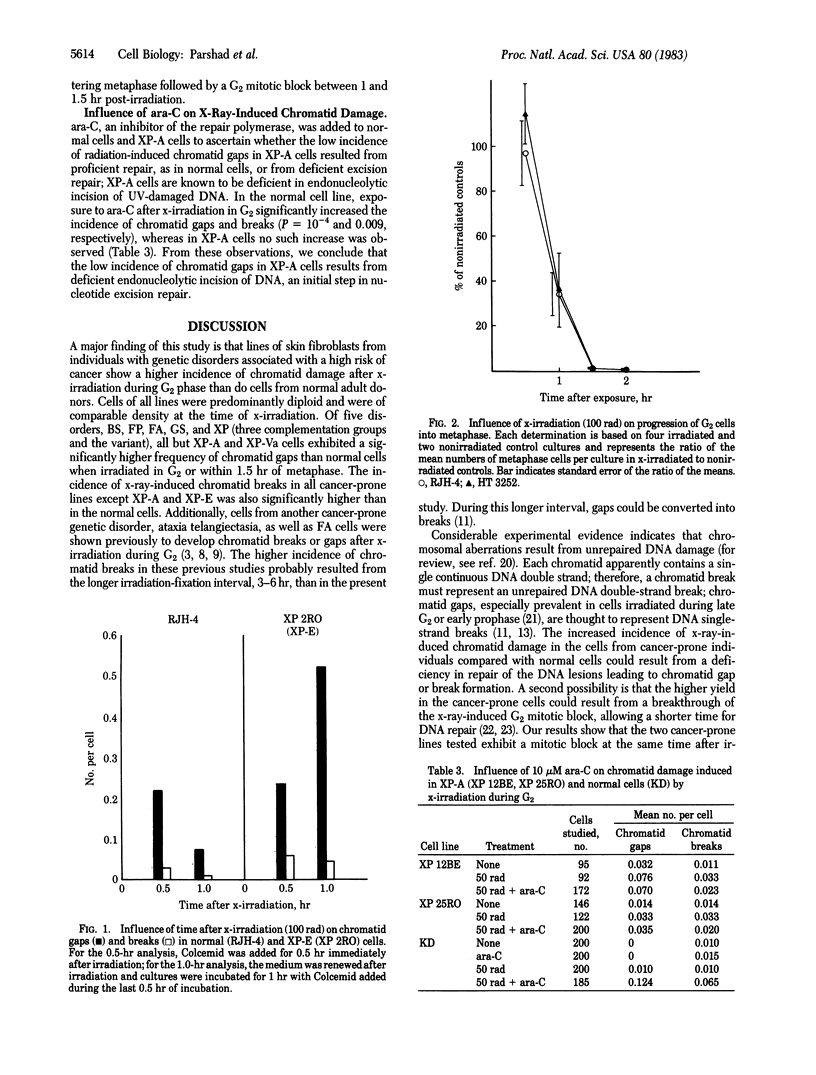
Ten lines of skin fibroblasts from individuals with genetic disorders predisposing to a high risk of cancer were compared with nine lines from normal adult donors with respect to chromatid damage after x-irradiation [25, 50, and 100 rad (0.25, 0.50, and 1 gray)] during G2 phase. The 10 cell lines represented five genetic disorders: Bloom syndrome, familial polyposis, Fanconi anemia, Gardner syndrome, and xeroderma pigmentosum, complementation groups A(XP-A), C(XP-C), E(XP-E), and variant (XP-Va). The incidence of chromatid breaks in all cancer-prone lines except XP-E and XP-A was significantly higher than in the normal lines. The incidence of chromatid gaps in all cancer-prone lines except XP-A and XP-Va was significantly higher than in the normal lines. Because each chromatid apparently contains a single continuous DNA double strand, chromatid breaks and gaps represent unrepaired DNA strand breaks arising directly or indirectly during excision repair of x-ray-induced DNA damage. These cytogenetic data together with results from use of the DNA repair inhibitor arabinofuranosyl cytosine (cytosine arabinoside) suggest that cells from all of these cancer-prone individuals are deficient in some step of DNA repair, predominantly excision repair operative during the G2-prophase period of the cell cycle. It appears that these DNA repair deficiencies are associated with a genetic predisposition to a high risk of cancer.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender M. A., Griggs H. G., Bedford J. S. Mechanisms of chromosomal aberration production. 3. Chemicals and ionizing radiation. Mutat Res. 1974 May;23(2):197–212. doi: 10.1016/0027-5107(74)90140-7. [DOI] [PubMed] [Google Scholar]
- Bigelow S. B., Rary J. M., Bender M. A. G2 chromosomal radiosensitivity in Fanconi's anemia. Mutat Res. 1979 Nov;63(1):189–199. doi: 10.1016/0027-5107(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968 May 18;218(5142):652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- Coquerelle T. M., Weibezahn K. F. Rejoining of DNA double-strand breaks in human fibroblasts and its impairment in one ataxia telangiectasia and two Fanconi strains. J Supramol Struct Cell Biochem. 1981;17(4):369–376. doi: 10.1002/jsscb.380170408. [DOI] [PubMed] [Google Scholar]
- Dubinin N. P., Soyfer V. N. Chromosome breakage and complete genic mutation production in molecular terms. Mutat Res. 1969 Sep-Oct;8(2):353–365. doi: 10.1016/0027-5107(69)90014-1. [DOI] [PubMed] [Google Scholar]
- Edwards M. J., Taylor A. M., Duckworth G. An enzyme activity in normal and ataxia telangiectasia cell lines which is involved in the repair of gamma-irradiation-induced DNA damage. Biochem J. 1980 Jun 15;188(3):677–682. doi: 10.1042/bj1880677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt R., Parshad R., Ewig R. A., Sanford K. K., Jones G. M., Tarone R. E., Kohn K. W. Fluorescent light-induced DNA crosslinkage and chromatid breaks in mouse cells in culture. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3809–3812. doi: 10.1073/pnas.75.8.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch-Kauffmann M., Schweiger M., Wagner E. F., Sperling K. Deficiency of DNA ligase activity in Fanconi's anemia. Hum Genet. 1978 Nov 24;45(1):25–32. doi: 10.1007/BF00277570. [DOI] [PubMed] [Google Scholar]
- Natarajan A. T., Meyers M. Chromosomal radiosensitivity of ataxia telangiectasia cells at different cell cycle stages. Hum Genet. 1979 Nov 1;52(1):127–132. doi: 10.1007/BF00284606. [DOI] [PubMed] [Google Scholar]
- Natarajan A. T., Obe G., van Zeeland A. A., Palitti F., Meijers M., Verdegaal-Immerzeel E. A. Molecular mechanisms involved in the production of chromosomal aberrations. II. Utilization of Neurospora endonuclease for the study of aberration production by X-rays in G1 and G2 stages of the cell cycle. Mutat Res. 1980 Feb;69(2):293–305. doi: 10.1016/0027-5107(80)90094-9. [DOI] [PubMed] [Google Scholar]
- Painter R. B., Young B. R. Radiosensitivity in ataxia-telangiectasia: a new explanation. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7315–7317. doi: 10.1073/pnas.77.12.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parshad R., Gantt R., Sanford K. K., Jones G. M., Tarone R. E. Repair of chromosome damage induced by X-irradiation during G2 phase in a line of normal human fibroblasts and its malignant derivative. J Natl Cancer Inst. 1982 Aug;69(2):409–414. [PubMed] [Google Scholar]
- Parshad R., Sanford K. K., Jones G. M., Tarone R. E., Hoffman H. A., Grier A. H. Susceptibility to fluorescent light-induced chromatid breaks associated with DNA repair deficiency and malignant transformation in culture. Cancer Res. 1980 Dec;40(12):4415–4419. [PubMed] [Google Scholar]
- Parshad R., Sanford K. K., Jones G. M., Tarone R. E. Neoplastic transformation of human cells in culture associated with deficient repair of light-induced chromosomal DNA damage. Int J Cancer. 1982 Aug 15;30(2):153–159. doi: 10.1002/ijc.2910300205. [DOI] [PubMed] [Google Scholar]
- Parshad R., Taylor W. G., Sanford K. K., Camalier R. F., Gantt R., Tarone R. E. Fluorescent light-induced chromosome damage in human IMR-90 fibroblasts. Role of hydrogen peroxide and related free radicals. Mutat Res. 1980 Nov;73(1):115–124. doi: 10.1016/0027-5107(80)90140-2. [DOI] [PubMed] [Google Scholar]
- Paterson M. C., Smith P. J. Ataxia telangiectasia: an inherited human disorder involving hypersensitivity to ionizing radiation and related DNA-damaging chemicals. Annu Rev Genet. 1979;13:291–318. doi: 10.1146/annurev.ge.13.120179.001451. [DOI] [PubMed] [Google Scholar]
- Poon P. K., O'Brien R. L., Parker J. W. Defective DNA repair in Fanconi's anaemia. Nature. 1974 Jul 19;250(463):223–225. doi: 10.1038/250223a0. [DOI] [PubMed] [Google Scholar]
- Preston R. J. The effect of cytosine arabinoside on the frequency of X-ray-induced chromosome aberrations in normal human leukocytes. Mutat Res. 1980 Jan;69(1):71–79. doi: 10.1016/0027-5107(80)90177-3. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. Two forms of repair in the DNA of human cells damaged by chemical carcinogens and mutagens. Cancer Res. 1974 Dec;34(12):3318–3325. [PubMed] [Google Scholar]
- Remsen J. F., Cerutti P. A. Deficiency of gamma-ray excision repair in skin fibroblasts from patients with Fanconi's anemia. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2419–2423. doi: 10.1073/pnas.73.7.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M. S., Tonomura A. A high susceptibility of Fanconi's anemia to chromosome breakage by DNA cross-linking agents. Cancer Res. 1973 Aug;33(8):1829–1836. [PubMed] [Google Scholar]
- Savage J. R. Classification and relationships of induced chromosomal structual changes. J Med Genet. 1976 Apr;13(2):103–122. doi: 10.1136/jmg.13.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B. Repair deficient human disorders and cancer. Nature. 1978 Feb 23;271(5647):713–717. doi: 10.1038/271713a0. [DOI] [PubMed] [Google Scholar]
- Taylor A. M. Unrepaired DNA strand breaks in irradiated ataxia telangiectasia lymphocytes suggested from cytogenetic observations. Mutat Res. 1978 Jun;50(3):407–418. doi: 10.1016/0027-5107(78)90045-3. [DOI] [PubMed] [Google Scholar]
- Zampetti-Bosseler F., Scott D. Cell death, chromosome damage and mitotic delay in normal human, ataxia telangiectasia and retinoblastoma fibroblasts after x-irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1981 May;39(5):547–558. doi: 10.1080/09553008114550651. [DOI] [PubMed] [Google Scholar]



