Background: Cells require mechanisms to regulate the potentially destructive action of RNases.
Results: RNase R is largely bound to ribosomes in growing cells dependent on tmRNA-SmpB and nonstop mRNA.
Conclusion: Ribosome binding stabilizes RNase R and sequesters it away from action on many functional RNAs.
Significance: This work defines a previously unknown mechanism by which ribosomes regulate a deleterious enzyme.
Keywords: Escherichia coli, Protein Stability, Ribonuclease, Ribosomes, RNA Metabolism
Abstract
Ribonucleases play an important role in RNA metabolism. Yet, they are also potentially destructive enzymes whose activity must be controlled. Here we describe a novel regulatory mechanism affecting RNase R, a 3′ to 5′ exoribonuclease able to act on essentially all RNAs including those with extensive secondary structure. Most RNase R is sequestered on ribosomes in growing cells where it is stable and participates in trans-translation. In contrast, the free form of the enzyme, which is deleterious to cells, is extremely unstable, turning over with a half-life of 2 min. RNase R binding to ribosomes is dependent on transfer-messenger RNA (tmRNA)-SmpB, nonstop mRNA, and the modified form of ribosomal protein S12. Degradation of the free form of RNase R also requires tmRNA-SmpB, but this process is independent of ribosomes, indicating two distinct roles for tmRNA-SmpB. Inhibition of RNase R binding to ribosomes leads to slower growth and a massive increase in RNA degradation. These studies indicate a previously unknown role for ribosomes in cellular homeostasis.
Introduction
Ribonucleases (RNases) are important participants in essentially every aspect of RNA metabolism (1–5). Yet they are also destructive enzymes that potentially could cause serious problems with a cell complement of RNA. As a consequence, we would expect that cells have evolved mechanisms to protect RNA against such possible dangers, although at present very little information is available in this area. As possible general mechanisms, one can envision strategies in which the RNA is protected by its structure, sequestration, or location in the cell. Alternatively, the RNases themselves could have evolved to be highly specific for a limited number of substrates or they could be subject to strict regulation. In a cell such as Escherichia coli, with at least 20 RNases, it would not be surprising if multiple mechanisms come into play.
Our laboratory has been exploring one highly unusual regulatory process that affects the 3′ to 5′ exoribonuclease, RNase R (6, 7). Because RNase R is processive and is able to digest all RNAs, even those with extensive secondary structure (8–12), it has the potential to be an extremely destructive enzyme. In fact, overexpression of RNase R is known to be deleterious to cells (7). Hence, it is important that the activity of RNase R be carefully controlled, and this is accomplished by regulating the protein stability. RNase R is very unstable in growing E. coli but is stable in stationary phase cells (13, 14). Its instability is determined by the acetylation of a single lysine residue, Lys-544, which stimulates binding of the trans-translation factors, tmRNA2 and SmpB, to the C-terminal region of the RNase (15, 16). The binding of tmRNA-SmpB stabilizes association of the proteases, HslUV or Lon, with the N-terminal region of RNase R resulting in proteolysis (17). Because stationary phase RNase R is not acetylated due to the absence of the acetylating enzyme (18), tmRNA and SmpB bind poorly, and as a result RNase R is completely stable (16).
tmRNA-SmpB binding to its C-terminal region is also required for RNase R to associate with ribosomes for its participation during the process of trans-translation (19, 20). Thus, it was of considerable importance to determine whether RNase R instability might be a consequence of its role in trans-translation and to determine what role ribosomes might play in the instability of RNase R. We show here that RNase R is largely bound to ribosomes in growing cells and that it is dependent on the amount of nonstop mRNA. Interestingly, this binding actually stabilizes RNase R. Replacement of tmRNA with a mutant form of the RNA that still promotes proteolysis of RNase R but inhibits its binding to ribosomes results in RNase R becoming much more unstable than usual. We conclude that it is the free form of RNase R that is the substrate for proteolysis and that its instability is independent of its participation in trans-translation. We also find that unbound RNase R is the form of the enzyme that inappropriately degrades cellular RNAs and thereby is deleterious to cells. Most importantly, these data indicate that ribosomes are able to regulate the stability and action of a deleterious bacterial protein.
EXPERIMENTAL PROCEDURES
Materials
Antibody against RNase R was prepared and purified as described previously (7, 15). Anti-FLAG M2 mAb was from Sigma. Anti-rabbit and anti-mouse IgG HRP conjugate were obtained from Santa Cruz Biotechnology. [γ-32P]ATP, l-[14C]alanine, and [3H]uridine were purchased from PerkinElmer Life Sciences and GE Healthcare. RNeasy mini kit was from Qiagen. Protease inhibitor mixture was purchased from Calbiochem. Nickel-nitrilotriacetic acid His-bind resin was obtained from Novagen. M-MLV reverse transcriptase and RNasin were from Promega. Purification of tmRNA, SmpB, and RNase R was described previously (15, 16). All other materials were reagent grade.
Bacterial Strains and Growth Conditions
All strains used were derivatives of E. coli K12 strain MG1655(Seq)rph+ and were previously described (15–18). The rimO insertion mutant allele was provided by Dr. Michael Brad Strader, United States Food and Drug Administration (21). The rimO and rna (encoding RNase I) mutant genes were each introduced into wild type or indicated mutant strains by phage P1-mediated transduction using P1vir.
Site-directed mutagenesis of tmRNA G3 to A in the chromosome was performed with oligo T1 (Table 1) using a previously described protocol (15). Aspartic acid 278 of RNase R was changed to asparagine with oligo R1 (Table 1). Recombinants were selected by PCR with primers T2 and T3 or with R2 and R3 (Table 1), and the resulting gene mutations were confirmed by DNA sequencing (22).
TABLE 1.
Oligonucleotides used
| Oligos | Sequence (5′-3′) |
|---|---|
| A1 | GAGGCTAGCATGAGCAAGAGCACCGCTGAG |
| A2 | GCACTCGAGTTATTGCAATTTCGCGCTGACCC |
| C1 | CAGCCATGGTACACCATCACCATCACCATATGAGCACAAAAAAGAAACCATTAACACAAGAGCAGCTTG |
| C2 | CTGAAGCTTAAAAAAGCCCGCTCATTAGGCGGGCTGCACTAACCGCTTCATACATCTCGTAGATTTCTCTGGCGAT |
| C3 | CGTGCGTCCTCAAGCTGCTC |
| R1 | GCCGCGTTTTTTCTCGCAGTAAACTGCATCGTCAAAGTTACGGGCGTCTTCGCCATCAATGGTGACCAGCGGTAAATCGC |
| R2 | ATTGATGGCGAAGACGCCCGTA |
| R3 | TAGAGGTTATGCAACTCTTCGAG |
| R4 | ATTGATGGCGAAGACGCCCGTG |
| S1 | TCCACCTGCCATTCGCGCTC |
| S2 | AACCTGGTTCAGCGACCATCG |
| T1 | GGCGCTCATAAATCTGGTATACTTACCTTTACACATTGGAGCTGATTCTGGATTCGACGGGATTTGCGAAACCCAA |
| T2 | GTATACTTACCTTTACACATTGGA |
| T3 | TTCCTACATCCTCGGTACTACA |
| T4 | TGACCTCTCTTGATCCCCGTCCTA |
| T5 | AGCCATATGATGTCTAAAGAAAAGTTTGAACGTAC |
| T6 | CGAGGATCCTTAGCTCAGAACTTTTGCTACAAC |
Cells were grown at 37 °C in liquid culture in YT medium or M9-glucose medium. Antibiotics, when present, were at the following concentrations: kanamycin, 50 μg/ml; ampicillin, 100 μg/ml; chloramphenicol, 34 μg/ml. Exponential phase cells were collected at an A550 of ∼0.3, and cells grown overnight were used as stationary phase samples.
Ribosome Isolation
Exponential and stationary phase wild type or indicated mutant strain cells were disrupted in lysis buffer (50 mm Tris-HCl, pH 7.5, 300 mm NH4Cl, 20 mm MgCl2, 2 mm β-mercaptoethanol, and 1 mg/ml DNase I) containing protease inhibitor mixture as described (15). The suspension was centrifuged at 30,000 × g for 10 min to remove cell debris. The supernatant fraction was further centrifuged at 100,000 × g for 90 min, and the resulting supernatant and pellet fractions were used to detect the amount of unbound and ribosome-bound RNase R or tmRNA, respectively (23).
RNase R and tmRNA Cellular Concentration Measurement
Wild type cells were grown to an A550 of ∼0.3 and collected by centrifugation. The concentration of RNase R and tmRNA were determined as previously described (24, 25) using Western blotting and Northern blotting, respectively.
Induction of c1 mRNA
The coding sequence for the N-terminal domain of the bacteriophage c1 gene followed by the strong trp operon transcriptional terminator (26) was amplified by PCR with primers C1 and C2 (Table 1) using λ DNA as template. The PCR product was purified and digested with NcoI and HindIII and then cloned into the corresponding sites on pBAD24 (nonstop mRNA). Two tandem stop codons were inserted before the trpA terminator to generate the stop reporter construct as previously described (20). In each construct, six histidine codons were introduced before the c1 gene, which enables the capture and enrichment of ribosomes translating the c1 message using affinity chromatography on a nickel column (20). Wild type and smpB mutant strains harboring the constructs were grown in YT medium with 0.2% glucose (to inhibit leakage expression of c1) to an A550 of ∼0.3 and collected by centrifugation. The cell pellet was washed once in YT medium and then grown in the same medium containing 0.02% arabinose for the indicated times to induce expression of c1 mRNA (27).
Measurement of RNase R Half-life
Cells were grown in YT medium to an A550 of ∼0.3. A portion of the culture was collected for the zero time point, and chloramphenicol was added to the remaining culture at 200 μg/ml. Cells were collected at the indicated times, lysed by sonication (13), and assayed by immunoblotting to determine the amount of RNase R remaining.
Western Blot Analysis
Proteins were resolved on either 8% (for detection of RNase R) or 12% (for detection of SmpB) SDS-PAGE and subjected to immunoblotting. RNase R and recombinant FLAG-SmpB were detected by purified RNase R antibody (1:10,000 dilution) and anti-FLAG M2 mAbs (1:1000 dilution), respectively. Underexposed films were used for quantitation by Quantity One (Bio-Rad).
Northern Blot and RT-PCR Analyses
RNA was extracted from cells with the RNeasy mini kit according to the manufacturer's protocol and separated by electrophoresis on 6 or 12% polyacrylamide, 7.5 m urea gels. Prehybridization and hybridization was performed as described previously (28). Oligos C3 and T4 (Table 1) were used to probe c1 mRNA and tmRNA, respectively.
For RT-PCR, 2 μg of total RNA was used for first-strand cDNA synthesis by M-MLV in the presence of primer R3 (for rnr) or S1 (for smpB). Partial sequences of rnr and smpB were amplified with primers R3 and R4 or primers S1 and S2 (Table 1), respectively. PCR conditions were as follows: 3 min at 94 °C followed by 30 cycles of 30 s at 94 °C, 30 s at 60 °C, and 1 min at 72 °C. PCR products were resolved on 1.5% agarose gels and visualized by ethidium bromide staining (29).
In Vitro Proteolysis Assay
HslUV-mediated in vitro degradation of RNase R was performed as described (17). Briefly, 0.1 nm RNase R was mixed with 0.01 nm HslU6 and 0.05 nm HslV12 in proteolysis buffer ( 25 mm HEPES-KOH, pH 7.6, 5 mm KCl, 20 mm MgCl2, 0.032% Nonidet P-40, 10% glycerol, 4 mm ATP, 50 mm creatine phosphate, and 80 μg/ml creatine kinase) in the presence of 0.1 nm tmRNA and SmpB. The mixtures (100 μl) were incubated at 37 °C, and samples were taken at various time points for quantitation of RNase R remaining.
To determine the effect of ribosomes on RNase R degradation in vitro, 0.1 nm RNase R was incubated with 0.1 nm Ala-tmRNA, 0.1 nm SmpB, 0.5 nm EF-Tu, and 0.2 nm GTP in proteolysis buffer in the presence of 0.5 nm “stop” or “nonstop” ribosomes at 37 °C for 10 min. HslU6 (0.01 nm) and 0.05 nm HslV12 were then added, and the mixtures were incubated at 37 °C for the indicated times. RNase R remaining was detected as described above.
Purification of Ala-tRNA Synthetase and EF-Tu
The alaS and tufB genes were PCR-amplified with primers A1 and A2 and primers T5 and T6 (Table 1), respectively. The PCR products were purified and digested with NheI and XhoI or with NdeI and BamHI and then cloned into the corresponding sites on pET28a and pET15b, respectively. Purification of His-tagged Ala-tRNA synthetase and EF-Tu was carried out according to the manufacturer's protocol.
Aminoacylation of tmRNA in Vitro
tmRNA (50 pm) was incubated with 2.5 mm Ala-tRNA synthetase in 100 mm Tris-HCl, pH 8.0, 10 mm KCl, 5 mm MgCl2, 2 mm ATP, 10 mm dithioerythritol, and 30 mm l-[14C]alanine for 1 h at 37 °C (30). The RNAs were then extracted with the RNeasy mini kit.
Stalled Ribosome Enrichment
smpB mutant strain cells containing the stop or nonstop c1 construct were grown in YT medium with 0.2% glucose to an A550 of ∼0.3. The cells were then washed once in YT medium and grown in the same medium containing 0.02% arabinose for 20 min to induce expression of c1 mRNA. Isolation and enrichment of ribosomes containing the translational products of the stop or nonstop c1 gene via nickel column chromatography were performed as previously described (20).
Growth Competition
Equal amounts of wild type (KanR) and K544R, ΔC, or D278N mutant strain cells, based on absorbance, were mixed and diluted into YT medium. The culture was incubated at 37 °C with constant shaking at 200 rpm. Growth was monitored by A550 measurements. After 3 h the culture still in exponential phase growth was diluted 1:1000 into fresh YT medium. This represents 1 cycle (3 h) of exponential growth competition. Such cycles were repeated three times. Before each cycle, 100 μl of culture was taken, diluted, and plated onto YT plates with or without kanamycin. The cfu for each strain was determined at the beginning and end of each cycle. Calculation of the strain doubling time was carried out as previously described (14).
Analysis of Acid-soluble Material Derived from Cellular RNAs
A single colony of wild type, K544R, or the ΔC mutant strain was inoculated into 2 ml of M9, 0.2% glucose, 0.5% casamino acids medium. After overnight growth, 100 μl of each culture was inoculated into 100 ml of M9, 0.2% glucose, 0.5% casamino acids supplemented with 1 mCi/ml [3H]uridine and 0.1 mm uridine (31). Cultures were grown at 37 °C to an A550 of ∼0.2 and collected by centrifugation. The cell pellet was washed once in M9 salts and inoculated into 100 ml of M9, 0.2% glucose, 0.5% casamino acids with 0.1 mm uridine to prevent reincorporation of radioactive material. A550 readings were taken to monitor growth. At an A550 of ∼0.4, 500-μl portions were removed from the culture and treated with 250 μl of 4 m formic acid (32). After 15 min on ice, samples were centrifuged at maximum speed for 15 min in a Fisher benchtop microcentrifuge at 4 °C. Half of the supernatant fraction was removed and neutralized with 1 m Tris. Ten milliliters of scintillation fluid was added, and samples were counted in a scintillation counter to determine acid-soluble radioactivity (33). The remaining cells were harvested for total RNA isolation.
Measurement of c1 Nonstop mRNA Decay
Wild type cells harboring the c1 nonstop reporter plasmid were grown in YT medium in the absence of glucose to allow low level leakage expression of c1 mRNA. Cells were grown to an A550 of ∼0.3 or overnight, and rifampicin (0.45 mg/ml) was then added to inhibit further transcription (20). Samples were taken at the indicated time points, and total RNA was extracted. The reporter mRNA was probed with 32P-labeled oligo C3 (Table 1).
Co-immunoprecipitation Assay
Cells were ruptured by sonication in binding buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm DTT, 0.5% Nonidet P-40, 1 mm PMSF) containing protease inhibitor mixture. Anti-FLAG M2-agarose suspension (100 μl/200 ml of bacterial culture) was then added and incubated overnight with the crude extract at 4 °C. The beads were washed 3 times with 500 μl of binding buffer containing 0.5 m NaCl to remove nonspecific contaminants, and the bound proteins were eluted with FLAG peptide (1 mg/ml in binding buffer, 50 μl/200 ml of bacterial culture). FLAG-SmpB and RNase R in the eluant and the supernatant fraction were detected by Western blot analysis.
RESULTS
The unusual instability of RNase R in exponential phase cells is dependent on prior binding of tmRNA-SmpB to the C-terminal region of the RNase (15). tmRNA-SmpB binding to this region is also required for recruitment of RNase R to ribosomes for its role in nonstop mRNA removal during the process of trans-translation (20). However, despite this common feature, it is not known if the two processes are related or whether binding to ribosomes and participation in trans-translation plays any role in the very short half-life of RNase R. These questions are explored in the studies described below.
RNase R Is Bound to Ribosomes in Exponential Phase Cells
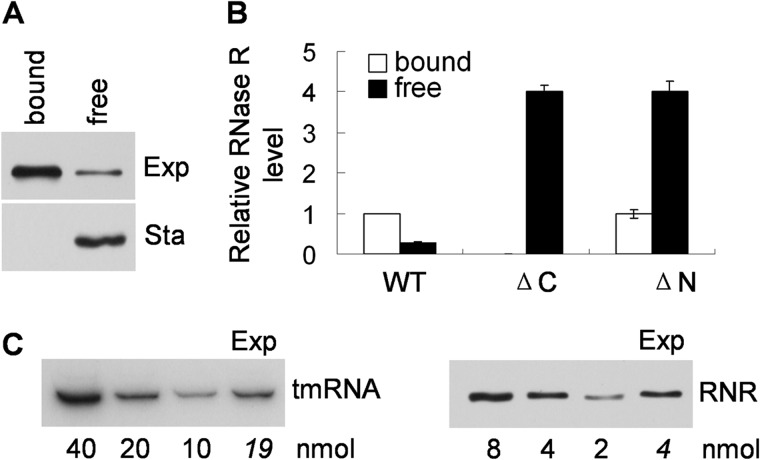
As a first step to examine whether involvement in trans-translation might affect RNase R stability, we determined how much RNase R is actually bound to ribosomes. Based on this analysis, we found that ∼80% of RNase R is associated with ribosomes in exponential phase cells; in contrast, no RNase R is bound to ribosomes in stationary phase (Fig. 1A). The difference between exponential and stationary phase cells is not unexpected given that tmRNA-SmpB binding to RNase R is part of the process and these factors bind poorly to the stationary phase enzyme (16, 20). Moreover, truncation of the C-terminal region of RNase R, which eliminates tmRNA-SmpB binding and also stabilizes the protein (15), completely prevents association of RNase R with ribosomes (Fig. 1B). On the other hand, truncation of the N-terminal region, which inhibits protease binding and thereby also stabilizes RNase R (17), does not alter its association with ribosomes (Fig. 1B). However, these latter data suggest that the amount of RNase R that is able to bind to ribosomes normally is limited as despite its large increase due to stabilization in the N-terminal truncation mutant, none of the additional RNase R is bound (Fig. 1B).
FIGURE 1.
RNase R binds to ribosomes. A, amount of ribosome bound and free RNase R in exponential (Exp) and stationary (Sta) phase wild type cells. The amount of RNase R in each fraction was determined by Western blotting. Note that a 5-fold higher amount of soluble protein was added for exponential phase cells than for stationary phase cells. B, amount of ribosome- bound and -free RNase R in exponential phase WT, C-terminal truncated (ΔC), and N-terminal truncated (ΔN) RNase R mutant strains. The data shown are the average of three independent experiments. The amount of ribosome bound RNase R in wild type cells was set at 1. Error bars indicate the S.E. C, quantification of tmRNA and RNase R in exponential phase cells. Measurement of tmRNA and RNase R (RNR) concentration by Northern blotting and Western blotting was carried out as described under “Experimental Procedures.” The data shown are from a representative experiment carried out a minimum of two times. The values for exponential phase tmRNA and RNR (Exp) were determined by the intensity of their respective bands relative to those of the standards and are shown in italics.
The finding that 80% of RNase R is bound to ribosomes raised questions about the stoichiometry of the process. Titration experiments using Western and Northern blot analyses revealed that tmRNA is present in cells at 5-fold higher levels than RNase R (Fig. 1C). Thus, there would be sufficient tmRNA (and SmpB) to bind to ribosomes all the RNase R present in the cell. Moreover, because it is already known that ribosomes are present at a 20-fold excess over tmRNA (24), we infer from these data that only ∼1% of cellular ribosomes undergo trans-translation and bind RNase R (see the next section).
RNase R Binding to Ribosomes Is Dependent on Nonstop mRNA
Inasmuch as RNase R removes nonstop mRNA during the process of trans-translation, we speculated that the amount of RNase R bound was limited by the number of ribosomes actually carrying nonstop mRNA and undergoing this process. To test this hypothesis, we elevated the amount of nonstop mRNA present. Bacteriophage λ c1 mRNAs containing either two tandem stop codons (stop mRNA) or lacking a stop codon (nonstop mRNA) were each overexpressed under the control of the ara promoter (Fig. 2A), and the amount of RNase R bound to ribosomes was determined. As shown in Fig. 2B, induction of stop c1 mRNA does not alter the amount of RNase R bound to ribosomes, whereas elevation of the c1 nonstop mRNA increases RNase R binding to 100%, including the increased amount of RNase R now present. When the same experiment is carried out in smpB mutant cells, no RNase R is bound to ribosomes (Fig. 2C). These data support the conclusion that RNase R binds only to the small population of ribosomes that have bound nonstop mRNA and, as a consequence, undergo trans-translation. In an smpB mutant strain, there is no trans-translation and no binding of RNase R to ribosomes.
FIGURE 2.
Overexpression of nonstop mRNA increases RNase R binding to ribosomes. A, total amount of RNase R (RNR), FLAG-SmpB, and tmRNA in cells overexpressing stop or nonstop c1 mRNA for 10 min determined by Western or Northern analysis. B, percent of RNase R binding to ribosomes in wild type cells overexpressing stop or nonstop c1 mRNA determined by Western blotting. The average of three independent experiments is shown. Error bars indicate the S.E. C, percent of ribosome-bound and free RNase R in smpB mutant cells overexpressing nonstop c1 mRNA determined by Western blotting. Average of three independent experiments is shown. Error bars indicate the S.E. D, amount of rnr and smpB mRNAs in cells overexpressing nonstop c1 mRNA. The culture was induced by arabinose for the indicated times, and total RNA was extracted. After reverse transcription, partial sequences of rnr and smpB were amplified with gene-specific primers, and the PCR products were detected by ethidium bromide staining. E, half-life of RNase R in cells overexpressing nonstop c1 mRNA. The time after nonstop mRNA induction is shown on the right, and the time after chloramphenicol (CM) addition is shown at the top. ind, induction.
As an interesting sidelight to this experiment we found that induction of nonstop mRNA also leads to elevation of both tmRNA and SmpB, amounting to as much as 4-fold after 10 min of induction (Fig. 2A). Although it is likely that the increase in tmRNA is a consequence of its increased transcription, RT-PCR analysis revealed that the elevation of SmpB also is due to increased smpB message. In contrast, there is no change in the amount of rnr message (Fig. 2D) despite elevation of the amount of RNase R (Fig. 2A). This latter effect is examined in detail in the next section.
Ribosome Binding Stabilizes RNase R
Induction of nonstop mRNA leads to an ∼4-fold increase in RNase R, whereas there was no change in the amount of the RNase upon induction of stop mRNA (Fig. 2A). Moreover, as shown in Fig. 2B, all of this additional RNase R is bound to ribosomes, indicating that the increased number of ribosomes carrying nonstop message provides sufficient additional ribosome binding sites to accommodate all the RNase R present.
However, what is the explanation for the elevation in RNase R that occurs upon induction of nonstop mRNA? As noted above, it is not due to an increase in rnr message. Rather, as shown in Fig. 2E, RNase R protein is stabilized concomitant with its increased ribosome binding (Fig. 2B). Thus, the half-life of RNase R increased from ∼10 min at 0 time of nonstop mRNA induction to the protein being completely stable by 20 min after induction. These data strongly suggest that it is the binding to ribosomes that stabilizes RNase R.
To examine this point in more detail, we made use of a strain carrying a mutant tmRNA (G3→A) that is unable to bind to ribosomes (23), and as a result, RNase R binding is eliminated as well. In wild type cells, essentially all tmRNA is ribosome bound, whereas in cells harboring this mutation, no tmRNA is ribosome-bound despite the fact that the RNA is present at normal levels (Fig. 3A). Although this mutant tmRNA cannot bind to ribosomes, it functions as well as WT tmRNA in an in vitro RNase R degradation system dependent on HslUV protease (Fig. 3B). Thus, although its function in trans-translation has been lost, its ability to participate in RNase R degradation is unaffected. In mutant cells containing this defective tmRNA, RNase R levels are reduced >80% (Fig. 3A), and the RNase R that remains is not bound to ribosomes (Fig. 3C). The decreased amount of RNase R is a consequence of its half-life shortening even more, from ∼10 min in WT cells to ∼2 min in the mutant strain (Fig. 3D). Based on these data, we conclude free RNase R is extremely unstable and that the protein is stabilized by binding to ribosomes.
FIGURE 3.
Ribosome binding stabilizes RNase R. A, amount of total tmRNA, ribosome-bound tmRNA, and RNase R (RNR) in WT and a tmRNA mutant (Mut) strain. B, degradation of RNase R in vitro by HslUV protease in the presence of WT or Mut tmRNA. C, amount of RNase R binding to ribosomes in WT and tmRNA Mut strains. The data shown are the average of three independent experiments. The amount of ribosome bound RNase R in wild type cells was set at 1. Error bars indicate the S.E. D, half-life of full-length RNase R (RNR-FL) in WT and tmRNA Mut strains. The numbers below the lanes are the amount of RNase R remaining at each time point. CM, chloramphenicol. E, half-life of full-length RNase R (RNR-FL) in hslv/lon and hslv/lon/tmRNA Mut strains. F, half-life of C-terminal-truncated RNase R (RNR-ΔC) in WT and tmRNA Mut strains. G, half-life of N-terminal truncated RNase R (RNR-ΔN) in WT and tmRNA Mut strains. In all experiments RNase R was determined by Western blotting.
Further examination of the RNase R degradation process in tmRNA-mutant cells revealed that it is identical to that in WT cells. Thus, in both WT and tmRNA-mutant cells, RNase R proteolysis was dependent on the proteases HslUV and Lon (Fig. 3E). In the absence of these proteases, RNase R was stable in both cell types. Likewise, RNase R was stable in both cells when it lacked its C-terminal region (Fig. 3F), which is necessary for tmRNA-SmpB binding (15) or if it lacked its N-terminal region (Fig. 3G), which is necessary for protease binding (17). These data indicate that proteolysis of RNase R is an active process directed solely against the unbound form of RNase R. In fact, it is now clear that the 10-min half-life previously determined for RNase R in WT cells (14) is actually a composite measurement consisting of no turnover for the ribosome-bound population and a 2-min half-life for the free protein. The very short half-life of the unbound RNase suggests that in this state it may be deleterious to growing cells. Experiments to examine this point will be presented below.
Stabilization of RNase R Can Be Reproduced in Vitro
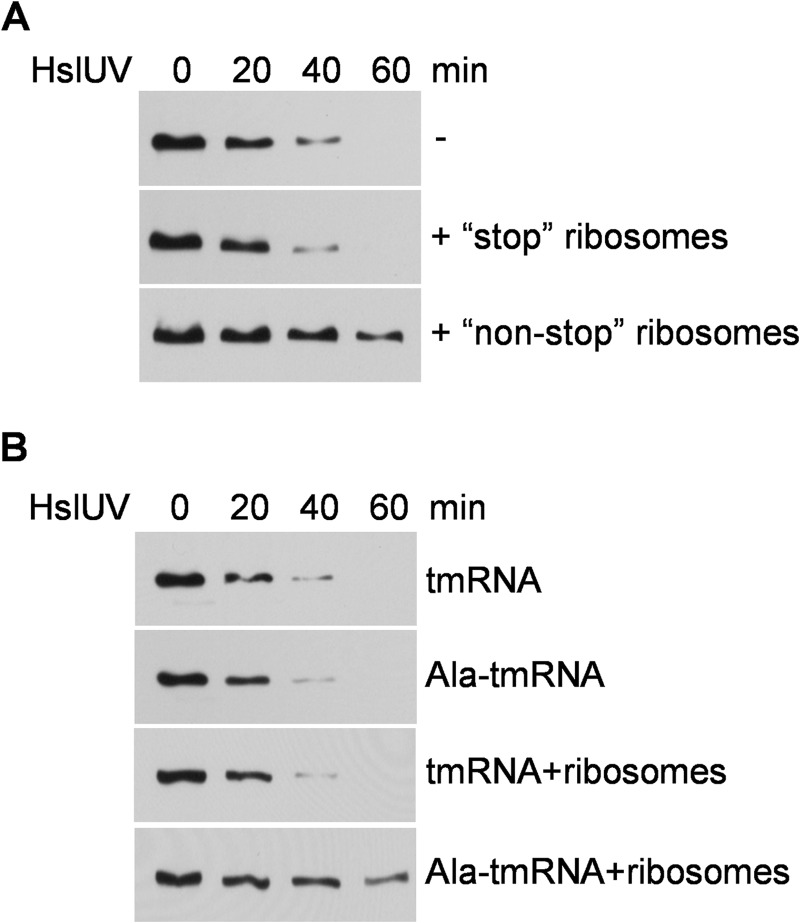
Additional evidence for the stabilization of RNase R on stalled ribosomes was obtained from in vitro experiments. In earlier work (17) we showed that proteolysis of RNase R by HslUV or Lon proteases could be reproduced in vitro. RNase R degradation in this system was dependent on acetylation of RNase R and on tmRNA-SmpB (17). To determine whether ribosomes affect the rate of RNase R degradation in this system, it was supplemented with Ala-tmRNA, SmpB, EF-Tu, and purified normal or stalled ribosomes isolated from cells overexpressing either stop or nonstop message (see “Experimental Procedures”). The data in Fig. 4A show that ribosomes carrying stop mRNA do not alter the rate of RNase R degradation, whereas nonstop ribosomes stabilize RNase R. These findings support the conclusion that RNase R is stabilized by binding to stalled ribosomes.
FIGURE 4.
Effect of ribosomes on RNase R degradation in vitro. A, proteolysis of RNase R by HslUV protease in the presence of ribosomes containing stop or nonstop mRNA carried out as described under “Experimental Procedures.”. B, proteolysis of RNase R by HslUV protease in the presence of tmRNA or tmRNA charged with Ala in the presence or absence of nonstop ribosomes. RNase R remaining was detected by Western blotting with RNase R-specific antibody.
This in vitro system also allowed us to compare the requirement for tmRNA-SmpB in RNase R degradation and in RNase R binding to ribosomes. As shown in Fig. 4B, both uncharged tmRNA and Ala-tmRNA work equally well in the degradation of RNase R by HslUV. In contrast, only charged Ala-tmRNA functions in binding RNase R to ribosomes based on its stabilization by ribosomes in the degradation system. These data confirm that RNase R binding to ribosomes in the in vitro system is by the trans-translation mechanism, which utilizes EF-Tu bound to charged tmRNA. Moreover, they reveal that despite the fact that tmRNA-SmpB participates in both RNase R degradation and RNase R binding to ribosomes, the two processes are different.
Catalytically Inactive RNase R Remains Bound to Ribosomes
To further examine the relation between trans-translation, ribosome binding, and RNase R stability, we made use of a catalytically inactive form of RNase R. This protein, in which Asp-278 has been converted to Asn, is essentially devoid of catalytic activity (10). In cells in which the mutant rnr gene replaces WT rnr, RNase R protein was elevated ∼4-fold (Fig. 5A) due to stabilization of the mutant protein (Fig. 5B). Analysis of the location of mutant RNase R revealed that it is all ribosome-bound (Fig. 5C). Mutant cells grow more slowly than WT based on competition experiments (36 versus 32 min) (Fig. 5D). These data show that inactive RNase R binds to ribosomes, but being unable to digest nonstop mRNA, it simply remained bound and was stabilized. Moreover, because ribosomes in these RNase R mutant cells can bind the 4-fold-elevated amount of RNase R, the population of ribosomes carrying nonstop mRNA must be increasing as new mRNA is synthesized, a small portion of which is nonstop message. Thus, if stalled ribosomes cannot be recycled by trans-translation, their numbers will increase until other recycling mechanisms come into play (34, 35). We also infer from these data that RNase R normally dissociates from ribosomes once its function in trans-translation has been completed, cycling on and off ribosomes as it is needed for removal of nonstop mRNA.
FIGURE 5.
Effect of binding of catalytically inactive RNase R to ribosomes. A, amount of RNase R (RNR) in WT and D278N mutant cells determined by Western blotting. B, half-life of RNase R in wild type and D278N mutant cells. Times after chloramphenicol (CM) addition are shown at the top. C, amount of RNase R binding to ribosomes in WT and D278N strains. The data shown are the average of three independent experiments. The amount of ribosome bound RNase R in wild type cells was set at 1. Error bars indicate the S.E. D, doubling time of WT and D278N strains determined by competition experiments.
RNase R Binding Is Dependent on Ribosomal Protein S12
Binding of RNase R to ribosomes is dependent on tmRNA-SmpB (this work and Ref. 20) and, as shown above, on nonstop mRNA. To more fully understand the binding process, it was of interest to identify the RNase R binding site on ribosomes. A recent proteomic analysis (21) revealed that ribosomal protein S12 interacts with RNase R. In addition, protein S12 contains a β-methylthioaspartic acid modification in a loop that binds RNase R. To determine whether this interaction was important to the binding of RNase R to ribosomes dependent on nonstop mRNA, we examined RNase R stability and its binding to ribosomes in a mutant strain in which the β-methylthioaspartic acid modification does not occur (rimO mutant). As shown in Fig. 6A, RNase R levels are greatly reduced in a rimO mutant strain, and this is due to a shortened RNase R half-life (Fig. 6B). As would be expected from the decreased stability of RNase R, its binding to ribosomes is decreased ∼80% (Fig. 6C). These data demonstrate that binding of RNase R to ribosomes is dependent on protein S12 and that the post-translational modification of residue Asp-88 is required for this binding.
FIGURE 6.
Effect of a rimO mutation on RNase R binding to ribosomes. A, amount of RNase R (RNR) in WT and rimO mutant cells determined by Western blotting. B, half-life of RNase R in wild type and rimO mutant cells. Time after chloramphenicol (CM) addition is shown at the top. C, amount of RNase R binding to ribosomes in WT and rimO strains. The data shown are the average of three independent experiments. The amount of ribosome bound RNase R in wild type cells was set at 1. Error bars indicate the S.E.
Unbound RNase R Is Deleterious to Growing Cells
Because E. coli has evolved such an extensive regulatory process to avoid the presence of free RNase R, it is likely that elevated levels of this enzyme are deleterious to growing cells. In fact, early studies in which RNase R was overexpressed indicated that too much RNase R slowed cell growth (7). To examine in more detail why unbound RNase R is problematic to cells, we made use of two strains that each contain a mutant form of RNase R. In one mutant, K544R, the Lys residue cannot be acetylated, and as a consequence, tmRNA-SmpB binds poorly to RNase R (16). In the second mutant, ΔC, the C-terminal region of RNase R has been deleted, also preventing tmRNA-SmpB binding (15). In both mutant strains, RNase R is stabilized, resulting in an ∼4-fold elevation (e.g. see Fig. 1B), and because tmRNA-SmpB binds poorly or not at all, the RNase R present cannot bind to ribosomes (Fig. 1B), providing a useful system to examine the effect of elevated, free RNase R.
Strains carrying these mutant RNase R alleles grow more slowly than the wild type (Fig. 7A), based on competition experiments. This is likely due to the inappropriate degradation of important RNA molecules resulting from elevation of the free form of RNase R. In support of this conclusion, the amount of acid-soluble material derived from pre-existing RNA increases ∼20-fold in each of the mutant strains compared with that in WT (Fig. 7B), amounting to breakdown of ∼10% of cellular RNA. This extremely high level of RNA degradation indicates that it extends beyond just mRNA to include stable RNAs as well. Thus, elevation of the amount of free RNase R is extremely deleterious to growing cells.
FIGURE 7.

Effect of elevated free RNase R on growing cells. A, doubling time of WT, K544R, and ΔC mutant strains determined by competition experiments. B, amount of acid-soluble radioactivity present in WT, K544R, and ΔC mutant strains. Degradation of RNAs was determined from the release of acid-soluble radioactivity presented as a percentage of the total radioactive RNA in the culture sample. The data shown are the average and S.D. based on three independent experiments.
DISCUSSION
The studies described here provide evidence that bacterial ribosomes can regulate the stability and thus the overall amount and activity of an important but potentially destructive enzyme. Specifically, we found that (a) RNase R, an enzyme involved in structured RNA degradation, is largely sequestered on ribosomes in growing cells, (b) binding of RNase R to ribosomes is dependent on tmRNA-SmpB and nonstop mRNA, (c) ribosome-bound RNase R is completely stable, whereas the free form of the enzyme turns over with an extremely short half-life of ∼2 min, (d) increased levels of the free form of RNase R lead to inappropriate degradation of cellular RNAs and a slower growth rate, and (e) tmRNA-SmpB is required for both association of RNase R with ribosomes and for RNase R instability, but the two processes are independent, and the latter process involves only the free form of the enzyme. Thus, RNase R instability is not a result of its participation in trans-translation. These findings and others presented earlier (13–18) indicate that RNase R is subject to extensive regulation to prevent unwanted action on RNA molecules in growing cells. This complex regulatory system serves as a detailed example of how cells deal with the presence of a potentially destructive, intracellular enzyme.
The cellular localizations and stabilities of exponential phase and stationary phase RNase R are dramatically different. Exponential phase RNase R is largely ribosome-bound, and its free form turns over rapidly. In contrast, RNase R in stationary phase cells is entirely in the free form, yet the protein is completely stable due to the fact that it is not acetylated (16). The absence of acetylation eliminates tmRNA-SmpB binding, and as a consequence, RNase R does not associate with ribosomes nor does it turn over. These facts suggest that RNase R serves different physiological roles during the two phases of growth and that it does so in different locations. In exponential phase cells, RNase R is known to participate in degradation of nonstop message (19, 20) and of messages containing REP sequences (9). Both of these processes occur on ribosomes, and it would not be surprising if they utilized similar mechanisms in which RNase R is bound to ribosomes at the same position and is dependent on tmRNA-SmpB in both cases.
Another known function of RNase R in exponential phase cells is in the maturation of the 3′ end of 16 S rRNA (36). This reaction also occurs on ribosomes, but in this case the substrate is a preribosomal particle. We suspect that in this situation the binding of RNase R to ribosomes is different. Rather than binding near the A-site dependent on tmRNA-SmpB, RNase R would bind directly at the 3′ terminus of the RNA precursor consistent with its known mode of action. Moreover, the binding would not be dependent on the presence of an mRNA as they are not present on a ribosome precursor particle.
In stationary phase cells RNase R is completely unbound, enabling it to act on any accessible RNA molecules. Although we have shown here that such action is deleterious in growing cells, it apparently is necessary in stationary phase cells in which overall RNA degradation accelerates (1, 33). The fact that RNase R is not associated with ribosomes in stationary phase cells also indicates that the process of trans-translation does not operate under these conditions. Supporting this conclusion is the observation that the nonstop message is ∼5× more stable in stationary phase than in exponential phase cells (data not shown). Thus, any stalled ribosomes that form in stationary phase either are removed by other mechanisms (34, 35) or they simply accumulate as there are sufficient functional ribosomes present to carry out the lowered level of protein synthesis operating in this growth phase. It is also likely that a similar scenario operates under cold shock conditions. Because RNase R is not acetylated under such conditions, it would not be bound to ribosomes and would be available to degrade all accessible RNA molecules even in cells growing in the cold. Presumably, increased degradation of RNA by RNase R would not present a problem for the cell as there would be excess RNA over that needed due to the downshift resulting from lowered temperatures.
The data presented here clearly indicate that even though the processes of RNase R instability and RNase R binding to ribosomes for trans-translation both make use of tmRNA-SmpB as essential cofactors, the two processes are independent. Thus, the degradation process acts only on free RNase R and can use either tmRNA or Ala-tmRNA, whereas trans-translation occurs only on ribosomes and requires Ala-tmRNA. This dichotomy raises the interesting question of why the RNase R degradation process evolved to make use of tmRNA-smpB as a required component (15–17). We suspect it is due to the fact that RNase R is always bound to tmRNA-SmpB in growing cells. In a co-immunoprecipitation experiment with crude extracts, the pulldown of FLAG-tagged SmpB, expressed at normal levels, immunoprecipitates all the RNase R present in the extract (data not shown). As a consequence, the utilization of already bound SmpB to help associate proteases with RNase R (17) would be efficient. Most importantly, these findings suggest that RNase R remains bound to tmRNA-SmpB throughout the trans-translation process and even when it dissociates from ribosomes after trans-translation is completed. This conclusion has important implications for the detailed mechanism of the trans-translation process and will need to be taken into account in future studies of the process.
Acknowledgments
We thank Dr. Kenneth E. Rudd and Dr. Michael Brad Strader for bacterial strains. We also thank Dr. Arun Malhotra, Dr. Kenneth E. Rudd, and members of the laboratory for critical comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant GM16317.
- tmRNA
- transfer-messenger RNA.
REFERENCES
- 1. Deutscher M. P. (2003) Degradation of stable RNA in bacteria. J. Biol. Chem. 278, 45041–45044 [DOI] [PubMed] [Google Scholar]
- 2. Deutscher M. P. (2006) Degradation of RNA in bacteria. Comparison of mRNA and stable RNA. Nucleic Acids Res. 34, 659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrade J. M., Pobre V., Silva I. J., Domingues S., Arraiano C. M. (2009) The role of 3′-5′ exoribonucleases in RNA degradation. Prog. Mol. Biol. Transl. Sci. 85, 187–229 [DOI] [PubMed] [Google Scholar]
- 4. Carpousis A. J., Luisi B. F., McDowall K. J. (2009) Endonucleolytic initiation of mRNA decay in Escherichia coli. Prog. Mol. Biol. Transl. Sci. 85, 91–135 [DOI] [PubMed] [Google Scholar]
- 5. Deutscher M. P. (2009) Maturation and degradation of ribosomal RNA in bacteria. Prog. Mol. Biol. Transl. Sci. 85, 369–391 [DOI] [PubMed] [Google Scholar]
- 6. Zuo Y., Deutscher M. P. (2001) Exoribonuclease superfamilies. Structural analysis and phylogenetic distribution. Nucleic Acids Res. 29, 1017–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng Z. F., Deutscher M. P. (2002) Purification and characterization of the Escherichia coli exoribonuclease RNase R. Comparison with RNase II. J. Biol. Chem. 277, 21624–21629 [DOI] [PubMed] [Google Scholar]
- 8. Cheng Z. F., Zuo Y., Li Z., Rudd K. E., Deutscher M. P. (1998) The vacB gene required for virulence in Shigella flexneri and Escherichia coli encodes the exoribonuclease RNase R. J. Biol. Chem. 273, 14077–14080 [DOI] [PubMed] [Google Scholar]
- 9. Cheng Z. F., Deutscher M. P. (2005) An important role for RNase R in mRNA decay. Mol. Cell 17, 313–318 [DOI] [PubMed] [Google Scholar]
- 10. Vincent H. A., Deutscher M. P. (2006) Substrate recognition and catalysis by the exoribonuclease RNase R. J. Biol. Chem. 281, 29769–29775 [DOI] [PubMed] [Google Scholar]
- 11. Vincent H. A., Deutscher M. P. (2009) The roles of individual domains of RNase R in substrate binding and exoribonuclease activity. The nuclease domain is sufficient for digestion of structured RNA. J. Biol. Chem. 284, 486–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vincent H. A., Deutscher M. P. (2009) Insights into how RNase R degrades structured RNA. Analysis of the nuclease domain. J. Mol. Biol. 387, 570–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen C., Deutscher M. P. (2005) Elevation of RNase R in response to multiple stress conditions. J. Biol. Chem. 280, 34393–34396 [DOI] [PubMed] [Google Scholar]
- 14. Chen C., Deutscher M. P. (2010) RNase R is a highly unstable protein regulated by growth phase and stress. RNA 16, 667–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liang W., Deutscher M. P. (2010) A novel mechanism for ribonuclease regulation. Transfer-messenger RNA (tmRNA) and its associated protein SmpB regulate the stability of RNase R. J. Biol. Chem. 285, 29054–29058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liang W., Malhotra A., Deutscher M. P. (2011) Acetylation regulates the stability of a bacterial protein. Growth stage-dependent modification of RNase R. Mol. Cell 44, 160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liang W., Deutscher M. P. (2012) Transfer-messenger RNA-SmpB protein regulates ribonuclease R turnover by promoting binding of HslUV and Lon proteases. J. Biol. Chem. 287, 33472–33479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liang W., Deutscher M. P. (2012) Post-translational modification of RNase R is regulated by stress-dependent reduction in the acetylating enzyme Pka (YfiQ). RNA 18, 37–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Richards J., Mehta P., Karzai A. W. (2006) RNase R degrades nonstop mRNAs selectively in an SmpB-tmRNA-dependent manner. Mol. Microbiol. 62, 1700–1712 [DOI] [PubMed] [Google Scholar]
- 20. Ge Z., Mehta P., Richards J., Karzai A. W. (2010) Nonstop mRNA decay initiates at the ribosome. Mol. Microbiol. 78, 1159–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strader M. B., Hervey W. J., 4th, Costantino N., Fujigaki S., Chen C. Y., Akal-Strader A., Ihunnah C. A., Makusky A. J., Court D. L., Markey S. P., Kowalak J. A. (2013) A coordinated proteomic approach for identifying proteins that interact with the E. coli ribosomal protein S12. J. Proteome Res. 12, 1289–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Datsenko K. A., Wanner B. L. (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Komine Y., Kitabatake M., Inokuchi H. (1996) 10S RNA is associated with 70 S ribosome particles in Escherichia coli. J. Biochem. 119, 463–467 [DOI] [PubMed] [Google Scholar]
- 24. Moore S. D., Sauer R. T. (2005) Ribosome rescue. tmRNA tagging activity and capacity in Escherichia coli. Mol. Microbiol. 58, 456–466 [DOI] [PubMed] [Google Scholar]
- 25. Sundermeier T. R., Karzai A. W. (2007) Functional SmpB-ribosome interactions require tmRNA. J. Biol. Chem. 282, 34779–34786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keiler K. C., Waller P. R., Sauer R. T. (1996) Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science 271, 990–993 [DOI] [PubMed] [Google Scholar]
- 27. Guzman L. M., Belin D., Carson M. J., Beckwith J. (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177, 4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liang W., Li C., Liu F., Jiang H., Li S., Sun J., Wu X., Li C. (2009) The Arabidopsis homologs of CCR4-associated factor 1 show mRNA deadenylation activity and play a role in plant defence responses. Cell Res. 19, 307–316 [DOI] [PubMed] [Google Scholar]
- 29. Liang W. X., Song L. M., Tian G. Z., Li H. F., Fan Z. F. (2006) The genomic sequence of Wisteria vein mosaic virus and its similarities with other potyviruses. Arch. Virol. 151, 2311–2319 [DOI] [PubMed] [Google Scholar]
- 30. Komine Y., Kitabatake M., Yokogawa T., Nishikawa K., Inokuchi H. (1994) A tRNA-like structure is present in 10Sa RNA, a small stable RNA from Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 91, 9223–9227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zundel M. A., Basturea G. N., Deutscher M. P. (2009) Initiation of ribosome degradation during starvation in Escherichia coli. RNA 15, 977–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cohen L., Kaplan R. (1977) Accumulation of nucleotides by starved Escherichia coli cells as a probe for the involvement of ribonucleases in ribonucleic acid degradation. J. Bacteriol. 129, 651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Basturea G. N., Zundel M. A., Deutscher M. P. (2011) Degradation of ribosomal RNA during starvation. Comparison to quality control during steady-state growth and a role for RNase PH. RNA 17, 338–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chadani Y., Ono K., Ozawa S., Takahashi Y., Takai K., Nanamiya H., Tozawa Y., Kutsukake K., Abo T. (2010) Ribosome rescue by Escherichia coli ArfA (YhdL) in the absence of trans-translation system. Mol. Microbiol. 78, 796–808 [DOI] [PubMed] [Google Scholar]
- 35. Chadani Y., Ono K., Kutsukake K., Abo T. (2011) Escherichia coli YaeJ protein mediates a novel ribosome-rescue pathway distinct from SsrA- and ArfA-mediated pathways. Mol. Microbiol. 80, 772–785 [DOI] [PubMed] [Google Scholar]
- 36. Sulthana S., Deutscher M. P. (2013) Multiple exoribonucleases catalyze maturation of the 3′ terminus of 16S ribosomal RNA (rRNA). J. Biol. Chem. 288, 12574–12579 [DOI] [PMC free article] [PubMed] [Google Scholar]