Abstract
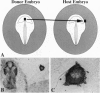
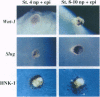
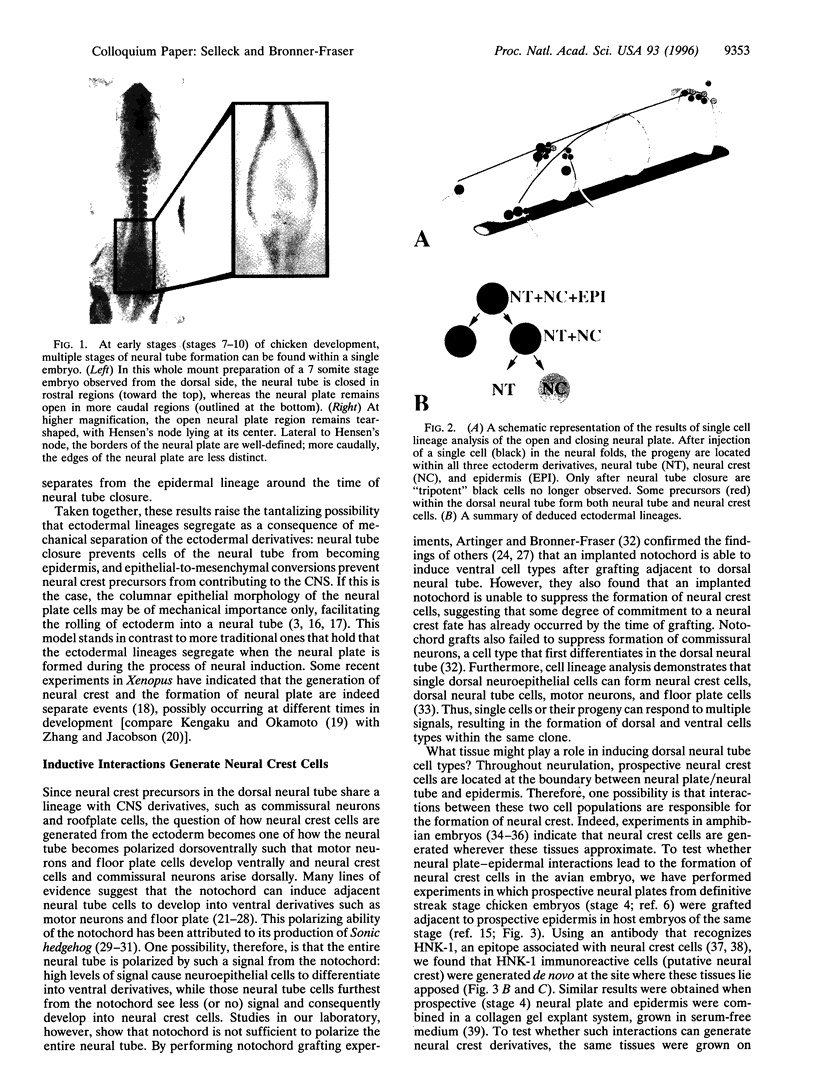
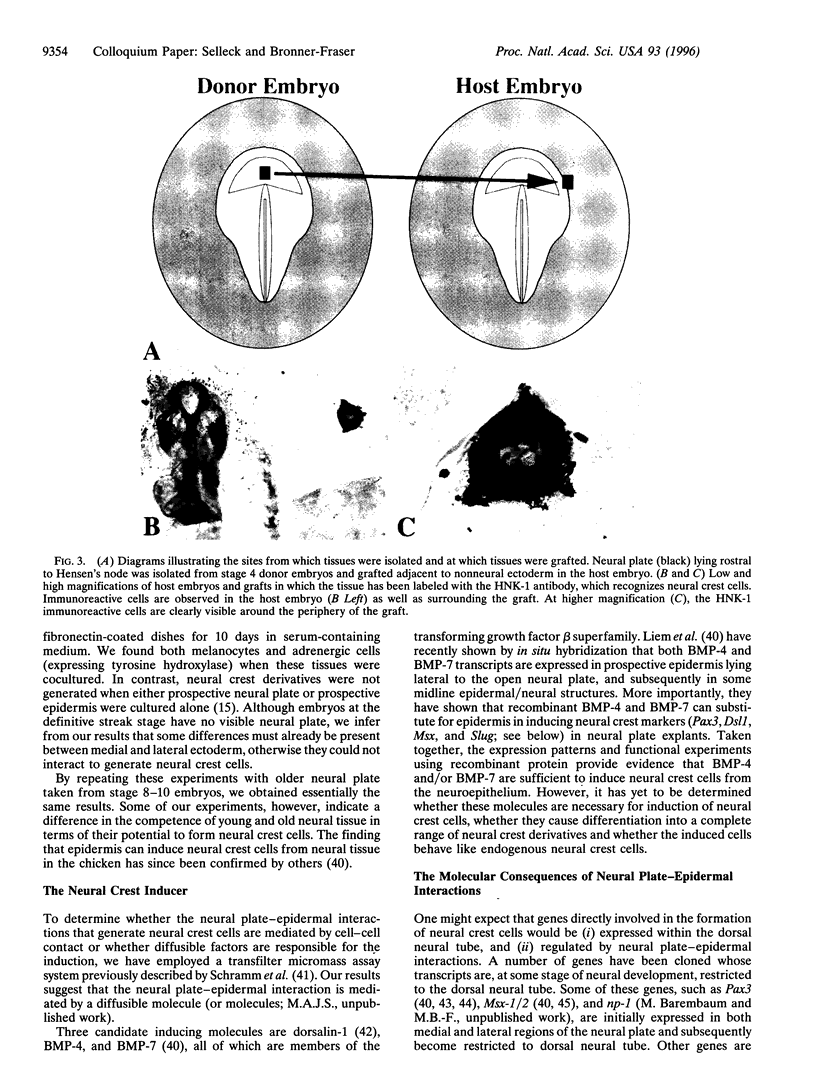
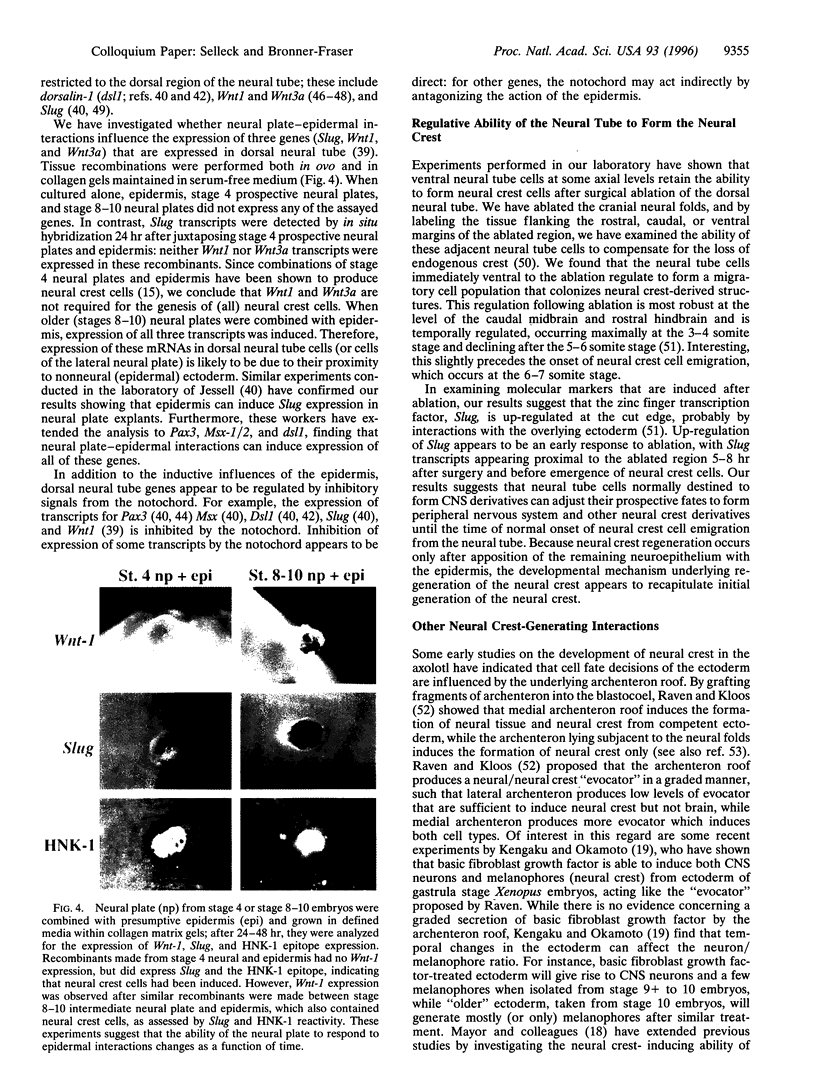
Neural crest cells arise from the ectoderm and are first recognizable as discrete cells in the chicken embryo when they emerge from the neural tube. Despite the classical view that neural crest precursors are a distinct population lying between epidermis and neuroepithelium, our results demonstrate that they are not a segregated population. Cell lineage analyses have demonstrated that individual precursor cells within the neural folds can give rise to epidermal, neural crest, and neural tube derivatives. Interactions between the neural plate and epidermis can generate neural crest cells, since juxtaposition of these tissues at early stages results in the formation of neural crest cells at the interface. Inductive interactions between the epidermis and neural plate can also result in "dorsalization" of the neural plate, as assayed by the expression of the Wnt transcripts characteristic of the dorsal neural tube. The competence of the neural plate changes with time, however, such that interaction of early neural plate with epidermis generates only neural crest cells, whereas interaction of slightly older neural plate with epidermis generates neural crest cells and Wnt-expressing cells. At cranial levels, neuroepithelial cells can regulate to generate neural crest cells when the endogenous neural folds are removed, probably via interaction of the remaining neural tube with the epidermis. Taken together, these experiments demonstrate that: (i) progenitor cells in the neural folds are multipotent, having the ability to form multiple ectodermal derivatives, including epidermal, neural crest, and neural tube cells; (ii) the neural crest is an induced population that arises by interactions between the neural plate and the epidermis; and (iii) the competence of the neural plate to respond to inductive interactions changes as a function of embryonic age.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artinger K. B., Bronner-Fraser M. Notochord grafts do not suppress formation of neural crest cells or commissural neurons. Development. 1992 Dec;116(4):877–886. doi: 10.1242/dev.116.4.877. [DOI] [PubMed] [Google Scholar]
- Artinger K. B., Fraser S., Bronner-Fraser M. Dorsal and ventral cell types can arise from common neural tube progenitors. Dev Biol. 1995 Dec;172(2):591–601. doi: 10.1006/dbio.1995.8038. [DOI] [PubMed] [Google Scholar]
- Baroffio A., Dupin E., Le Douarin N. M. Clone-forming ability and differentiation potential of migratory neural crest cells. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5325–5329. doi: 10.1073/pnas.85.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler K., Edlund T., Jessell T. M., Yamada T. Control of cell pattern in the neural tube: regulation of cell differentiation by dorsalin-1, a novel TGF beta family member. Cell. 1993 May 21;73(4):687–702. doi: 10.1016/0092-8674(93)90249-p. [DOI] [PubMed] [Google Scholar]
- Bortier H., Vakaet L. C. Fate mapping the neural plate and the intraembryonic mesoblast in the upper layer of the chicken blastoderm with xenografting and time-lapse videography. Dev Suppl. 1992:93–97. [PubMed] [Google Scholar]
- Bronner-Fraser M., Fraser S. E. Cell lineage analysis reveals multipotency of some avian neural crest cells. Nature. 1988 Sep 8;335(6186):161–164. doi: 10.1038/335161a0. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M., Fraser S. Developmental potential of avian trunk neural crest cells in situ. Neuron. 1989 Dec;3(6):755–766. doi: 10.1016/0896-6273(89)90244-4. [DOI] [PubMed] [Google Scholar]
- Cohen A. M., Konigsberg I. R. A clonal approach to the problem of neural crest determination. Dev Biol. 1975 Oct;46(2):262–280. doi: 10.1016/0012-1606(75)90104-9. [DOI] [PubMed] [Google Scholar]
- Dickinson M. E., Selleck M. A., McMahon A. P., Bronner-Fraser M. Dorsalization of the neural tube by the non-neural ectoderm. Development. 1995 Jul;121(7):2099–2106. doi: 10.1242/dev.121.7.2099. [DOI] [PubMed] [Google Scholar]
- Echelard Y., Epstein D. J., St-Jacques B., Shen L., Mohler J., McMahon J. A., McMahon A. P. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993 Dec 31;75(7):1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Erickson C. A., Perris R. The role of cell-cell and cell-matrix interactions in the morphogenesis of the neural crest. Dev Biol. 1993 Sep;159(1):60–74. doi: 10.1006/dbio.1993.1221. [DOI] [PubMed] [Google Scholar]
- Fraser S. E., Bronner-Fraser M. Migrating neural crest cells in the trunk of the avian embryo are multipotent. Development. 1991 Aug;112(4):913–920. doi: 10.1242/dev.112.4.913. [DOI] [PubMed] [Google Scholar]
- Gallera J. Primary induction in birds. Adv Morphog. 1971;9:149–180. doi: 10.1016/b978-0-12-028609-6.50008-x. [DOI] [PubMed] [Google Scholar]
- Goulding M. D., Chalepakis G., Deutsch U., Erselius J. R., Gruss P. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J. 1991 May;10(5):1135–1147. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M. D., Lumsden A., Gruss P. Signals from the notochord and floor plate regulate the region-specific expression of two Pax genes in the developing spinal cord. Development. 1993 Mar;117(3):1001–1016. doi: 10.1242/dev.117.3.1001. [DOI] [PubMed] [Google Scholar]
- Griffith C. M., Sanders E. J. Differentiation of the chick embryo floor plate. Anat Embryol (Berl) 1991;184(2):159–169. doi: 10.1007/BF00942747. [DOI] [PubMed] [Google Scholar]
- Hollyday M., McMahon J. A., McMahon A. P. Wnt expression patterns in chick embryo nervous system. Mech Dev. 1995 Jul;52(1):9–25. doi: 10.1016/0925-4773(95)00385-e. [DOI] [PubMed] [Google Scholar]
- Kengaku M., Okamoto H. Basic fibroblast growth factor induces differentiation of neural tube and neural crest lineages of cultured ectoderm cells from Xenopus gastrula. Development. 1993 Dec;119(4):1067–1078. doi: 10.1242/dev.119.4.1067. [DOI] [PubMed] [Google Scholar]
- Krauss S., Concordet J. P., Ingham P. W. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993 Dec 31;75(7):1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Liem K. F., Jr, Tremml G., Roelink H., Jessell T. M. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995 Sep 22;82(6):969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Mayor R., Morgan R., Sargent M. G. Induction of the prospective neural crest of Xenopus. Development. 1995 Mar;121(3):767–777. doi: 10.1242/dev.121.3.767. [DOI] [PubMed] [Google Scholar]
- Mitani S., Okamoto H. Inductive differentiation of two neural lineages reconstituted in a microculture system from Xenopus early gastrula cells. Development. 1991 May;112(1):21–31. doi: 10.1242/dev.112.1.21. [DOI] [PubMed] [Google Scholar]
- Moury J. D., Jacobson A. G. Neural fold formation at newly created boundaries between neural plate and epidermis in the axolotl. Dev Biol. 1989 May;133(1):44–57. doi: 10.1016/0012-1606(89)90295-9. [DOI] [PubMed] [Google Scholar]
- Moury J. D., Jacobson A. G. The origins of neural crest cells in the axolotl. Dev Biol. 1990 Oct;141(2):243–253. doi: 10.1016/0012-1606(90)90380-2. [DOI] [PubMed] [Google Scholar]
- Newgreen D. F., Gooday D. Control of the onset of migration of neural crest cells in avian embryos. Role of Ca++-dependent cell adhesions. Cell Tissue Res. 1985;239(2):329–336. doi: 10.1007/BF00218011. [DOI] [PubMed] [Google Scholar]
- Newgreen D. F., Minichiello J. Control of epitheliomesenchymal transformation. I. Events in the onset of neural crest cell migration are separable and inducible by protein kinase inhibitors. Dev Biol. 1995 Jul;170(1):91–101. doi: 10.1006/dbio.1995.1198. [DOI] [PubMed] [Google Scholar]
- Newgreen D., Gibbins I. Factors controlling the time of onset of the migration of neural crest cells in the fowl embryo. Cell Tissue Res. 1982;224(1):145–160. doi: 10.1007/BF00217274. [DOI] [PubMed] [Google Scholar]
- Nieto M. A., Sargent M. G., Wilkinson D. G., Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994 May 6;264(5160):835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- Noden D. M. The control of avian cephalic neural crest cytodifferentiation. II. Neural tissues. Dev Biol. 1978 Dec;67(2):313–329. doi: 10.1016/0012-1606(78)90202-6. [DOI] [PubMed] [Google Scholar]
- Placzek M., Jessell T. M., Dodd J. Induction of floor plate differentiation by contact-dependent, homeogenetic signals. Development. 1993 Jan;117(1):205–218. doi: 10.1242/dev.117.1.205. [DOI] [PubMed] [Google Scholar]
- Roelink H., Augsburger A., Heemskerk J., Korzh V., Norlin S., Ruiz i Altaba A., Tanabe Y., Placzek M., Edlund T., Jessell T. M. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994 Feb 25;76(4):761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- Roelink H., Nusse R. Expression of two members of the Wnt family during mouse development--restricted temporal and spatial patterns in the developing neural tube. Genes Dev. 1991 Mar;5(3):381–388. doi: 10.1101/gad.5.3.381. [DOI] [PubMed] [Google Scholar]
- Rollhäuser-ter Horst J. Artificial neural crest formation in amphibia. Anat Embryol (Berl) 1979;157(1):113–120. doi: 10.1007/BF00315644. [DOI] [PubMed] [Google Scholar]
- Rosenquist G. C. Epiblast origin and early migration of neural crest cells in the chick embryo. Dev Biol. 1981 Oct 30;87(2):201–211. doi: 10.1016/0012-1606(81)90143-3. [DOI] [PubMed] [Google Scholar]
- Scherson T., Serbedzija G., Fraser S., Bronner-Fraser M. Regulative capacity of the cranial neural tube to form neural crest. Development. 1993 Aug;118(4):1049–1062. doi: 10.1242/dev.118.4.1049. [DOI] [PubMed] [Google Scholar]
- Schoenwolf G. C. Shaping and bending of the avian neuroepithelium: morphometric analyses. Dev Biol. 1985 May;109(1):127–139. doi: 10.1016/0012-1606(85)90353-7. [DOI] [PubMed] [Google Scholar]
- Schoenwolf G. C., Sheard P. Fate mapping the avian epiblast with focal injections of a fluorescent-histochemical marker: ectodermal derivatives. J Exp Zool. 1990 Sep;255(3):323–339. doi: 10.1002/jez.1402550309. [DOI] [PubMed] [Google Scholar]
- Schoenwolf G. C., Smith J. L. Mechanisms of neurulation: traditional viewpoint and recent advances. Development. 1990 Jun;109(2):243–270. doi: 10.1242/dev.109.2.243. [DOI] [PubMed] [Google Scholar]
- Schramm C. A., Reiter R. S., Solursh M. Role for short-range interactions in the formation of cartilage and muscle masses in transfilter micromass cultures. Dev Biol. 1994 Jun;163(2):467–479. doi: 10.1006/dbio.1994.1163. [DOI] [PubMed] [Google Scholar]
- Sechrist J., Nieto M. A., Zamanian R. T., Bronner-Fraser M. Regulative response of the cranial neural tube after neural fold ablation: spatiotemporal nature of neural crest regeneration and up-regulation of Slug. Development. 1995 Dec;121(12):4103–4115. doi: 10.1242/dev.121.12.4103. [DOI] [PubMed] [Google Scholar]
- Selleck M. A., Bronner-Fraser M. Origins of the avian neural crest: the role of neural plate-epidermal interactions. Development. 1995 Feb;121(2):525–538. doi: 10.1242/dev.121.2.525. [DOI] [PubMed] [Google Scholar]
- Selleck M. A., Scherson T. Y., Bronner-Fraser M. Origins of neural crest cell diversity. Dev Biol. 1993 Sep;159(1):1–11. doi: 10.1006/dbio.1993.1217. [DOI] [PubMed] [Google Scholar]
- Sieber-Blum M., Cohen A. M. Clonal analysis of quail neural crest cells: they are pluripotent and differentiate in vitro in the absence of noncrest cells. Dev Biol. 1980 Nov;80(1):96–106. doi: 10.1016/0012-1606(80)90501-1. [DOI] [PubMed] [Google Scholar]
- Sieber-Blum M. Commitment of neural crest cells to the sensory neuron lineage. Science. 1989 Mar 24;243(4898):1608–1611. doi: 10.1126/science.2564699. [DOI] [PubMed] [Google Scholar]
- Sieber-Blum M. Role of the neurotrophic factors BDNF and NGF in the commitment of pluripotent neural crest cells. Neuron. 1991 Jun;6(6):949–955. doi: 10.1016/0896-6273(91)90235-r. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Schoenwolf G. C. Cell cycle and neuroepithelial cell shape during bending of the chick neural plate. Anat Rec. 1987 Jun;218(2):196–206. doi: 10.1002/ar.1092180215. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Schoenwolf G. C. Notochordal induction of cell wedging in the chick neural plate and its role in neural tube formation. J Exp Zool. 1989 Apr;250(1):49–62. doi: 10.1002/jez.1402500107. [DOI] [PubMed] [Google Scholar]
- Stern C. D. The avian embryo: a powerful model system for studying neural induction. FASEB J. 1994 Jul;8(10):687–691. doi: 10.1096/fasebj.8.10.8050666. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Monsoro-Burq A. H., Bontoux M., Le Douarin N. M. A role for Quox-8 in the establishment of the dorsoventral pattern during vertebrate development. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10237–10241. doi: 10.1073/pnas.89.21.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker G. C., Aoyama H., Lipinski M., Tursz T., Thiery J. P. Identical reactivity of monoclonal antibodies HNK-1 and NC-1: conservation in vertebrates on cells derived from the neural primordium and on some leukocytes. Cell Differ. 1984 Aug;14(3):223–230. doi: 10.1016/0045-6039(84)90049-6. [DOI] [PubMed] [Google Scholar]
- Vincent M., Thiery J. P. A cell surface marker for neural crest and placodal cells: further evolution in peripheral and central nervous system. Dev Biol. 1984 Jun;103(2):468–481. doi: 10.1016/0012-1606(84)90334-8. [DOI] [PubMed] [Google Scholar]
- WATTERSON R. L., GOODHEART C. R., LINDBERG G. The influence of adjacent structures upon the shape of the neural tube and neural plate of chick embryos. Anat Rec. 1955 Aug;122(4):539–559. doi: 10.1002/ar.1091220405. [DOI] [PubMed] [Google Scholar]
- Weston J. A. Sequential segregation and fate of developmentally restricted intermediate cell populations in the neural crest lineage. Curr Top Dev Biol. 1991;25:133–153. doi: 10.1016/s0070-2153(08)60414-7. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bailes J. A., McMahon A. P. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987 Jul 3;50(1):79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]
- Yamada T., Placzek M., Tanaka H., Dodd J., Jessell T. M. Control of cell pattern in the developing nervous system: polarizing activity of the floor plate and notochord. Cell. 1991 Feb 8;64(3):635–647. doi: 10.1016/0092-8674(91)90247-v. [DOI] [PubMed] [Google Scholar]
- Zhang J., Jacobson A. G. Evidence that the border of the neural plate may be positioned by the interaction between signals that induce ventral and dorsal mesoderm. Dev Dyn. 1993 Feb;196(2):79–90. doi: 10.1002/aja.1001960202. [DOI] [PubMed] [Google Scholar]
- van Straaten H. W., Hekking J. W., Beursgens J. P., Terwindt-Rouwenhorst E., Drukker J. Effect of the notochord on proliferation and differentiation in the neural tube of the chick embryo. Development. 1989 Dec;107(4):793–803. doi: 10.1242/dev.107.4.793. [DOI] [PubMed] [Google Scholar]
- van Straaten H. W., Hekking J. W., Thors F., Wiertz-Hoessels E. L., Drukker J. Induction of an additional floor plate in the neural tube. Acta Morphol Neerl Scand. 1985 Oct;23(2):91–97. [PubMed] [Google Scholar]
- van Straaten H. W., Hekking J. W., Wiertz-Hoessels E. J., Thors F., Drukker J. Effect of the notochord on the differentiation of a floor plate area in the neural tube of the chick embryo. Anat Embryol (Berl) 1988;177(4):317–324. doi: 10.1007/BF00315839. [DOI] [PubMed] [Google Scholar]