Abstract
Although it is understood that plasma retinol concentration is not proportional to the concentration of vitamin A stored in liver, plasma retinol still is often used as an indicator of vitamin A status. An aim of vitamin A supplementation strategies is to maintain plasma retinol concentration in a range considered adequate, generally >1.05 µmol/L in humans, with some adjustment for age. In the present study in rats, we addressed the following question: Does lung vitamin A increase postnatally, as is observed in rats fed a vitamin A–adequate diet, if plasma retinol is maintained at ~1 µmol/L by supplementation at neonatal age, but the weaning diet is deficient in vitamin A? We treated rats on postnatal d 6, 7, and 8 with placebo (oil), vitamin A, retinoic acid (RA), and a nutrient-metabolite combination of vitamin A and RA, VARA, after which tissues were analyzed on d 9. Other rats treated identically as neonates were fed a vitamin A–deficient diet from 3–9 wk of age, and in parallel, another group of rats was fed a vitamin A–adequate diet. Although supplementation with vitamin A or VARA elevated liver and lung retinyl esters (RE) on d 9 (P < 0.0001), and prevented the fall in plasma retinol to <1 µmol/L by 9 wk of age, when the diet was vitamin A–deficient, lung RE fell to 28% of the concentration present in the lungs of rats fed the vitamin A–adequate diet (P < 0.0001). We infer that the lungs depend, at least in part, on the uptake of dietary vitamin A, probably from chylomicrons, to develop RE stores in the postweaning growth period.
Introduction
Vitamin A supplementation is now recognized as an effective means to prevent xerophthalmia and reduce morbidity and mortality in infants and young children (1–5). WHO/UNICEF has recommended that vitamin A supplements be given to infants and young children in at-risk populations as a safeguard against an inadequate dietary intake of vitamin A (6,7). Over the years, the concept has developed that providing vitamin A periodically, typically every 4–6 mo and in the range of 15–30 mg retinol for infants and young children up to 11 mo of age, and 60 mg for children 12 mo and older [see discussion in (8,9)], is an adequate strategy, because no further reduction in morbidity and mortality were observed with 4-mo vs. 6-mo intervals (8). It is believed that with these doses, enough vitamin A is stored in the liver to maintain plasma retinol at a sufficient level [generally considered ≥1.05 µmol/L (30 µg/dL) in children (10,11)], for the time between dosings. An underlying premise of this strategy is that as long as plasma retinol remains at or above an accepted level, target tissues that use vitamin A will receive an adequate supply of retinol from the circulation. The relationship between liver vitamin A storage and plasma retinol concentration has been studied for a number of years, being first deduced from analysis of human and animal plasma and liver tissue specimens (12), and later studied in vivo using isotope dilution methods, including some studies in which both plasma retinol and liver retinol were directly sampled (13,14). Additionally, numerous studies of plasma and liver retinol levels have been conducted in rats, in which dietary vitamin Awas also controlled (15–18). Nonetheless, there is still a paucity of information concerning the relationship between dietary vitamin A intake, plasma retinol concentration, and the levels of vitamin A in tissues other than the liver. Particularly, it has not been directly demonstrated that an adequate concentration of plasma retinol alone is sufficient to maintain tissue retinol levels under circumstances in which dietary vitamin A intake is low or absent.
This study of lung retinol levels was part of an experiment on the effects of vitamin A supplementation in the neonatal period on measures of immunocompetence early in life and at young adult age in rats that had been fed a vitamin A–deficient diet after weaning (19). In this study, neonatal-age rats nursed by vitamin A–adequate dams were supplemented with vitamin A alone and with the principal active metabolite of retinol, retinoic acid (RA),5 as well as the combination of both, VARA, consisting of a 10:1 ratio of vitamin A and RA. This combination was tested because it had been shown previously to synergistically augment lung retinyl ester (RE) formation in neonatal-age rats (20). The lungs are of special interest because vitamin A is important in the development of the lungs, both pre-and postnatally, in humans as well as many animal species, including rats and mice (21). Clinically, low plasma retinol is associated with increased risk of bronchopulmonary dysplasia, and vitamin A may improve outcome (22–24). At birth, tissue retinol levels are low (25). An investigation of the ontogeny of lung and liver vitamin A concentrations in the perinatal period, conducted in rats where the dams were vitamin A adequate and the weanling rats were fed a diet with adequate vitamin A, showed that RE and retinol concentrations in the lungs are higher in fetal rats, fall before birth, and remain low up to weaning (26). The sources of retinoids taken up by the lungs are not well understood. Retinol circulates in plasma bound to retinol-binding protein (RBP), which is maintained at nearly constant levels over a wide range of liver retinol concentrations (12,15), while after meals containing vitamin A, RE, and, to a lesser extent, retinol are present in the circulation in chylomicrons, chylomicron remnants, and other lipoproteins to which some RE may transfer (27).
In this study, we analyzed total retinol concentrations in the liver, plasma, and lungs of rats with and without neonatal vitamin A supplementation and after weaning onto a vitamin A–deficient diet, in comparison to rats fed a vitamin A–adequate diet. The results provide evidence that plasma retinol alone, even ~1 µmol/L, may not be adequate for increasing lung total retinol concentrations in the postweaning period, if the diet is inadequate in vitamin A. From these results, we infer that the direct input of vitamin A from the diet to the lungs may be important, regardless of whether liver vitamin A storage is sufficient to maintain a normal concentration of plasma retinol.
Materials and Methods
Animals and diets
All procedures with animals were approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University. The neonates were the female offspring or cross-fostered pups of Sprague-Dawley rat dams that were purchased (Charles River) each with 12–14 female pups, 4–5 d old. The rats were housed in a rodent facility at 22°C with a 12-h light-dark cycle, with free access to food and drinking water. Because the dams had been fed a commercial stock diet by the supplier, they were therefore vitamin A adequate, but they were fed a modified vitamin A–deficient AIN-93G diet (28) after arrival to reduce the transfer of vitamin A in milk to the nursling pups (29) and shorten the onset of vitamin A deficiency. The pups were randomized into 4 groups for supplementation on postnatal d 6, 7, and 8, with 1 of 4 doses: oil, vitamin A, RA, or VARA, which are described in the next section. Tissues from some pups were collected on d 9 for determination of tissue retinol immediately after neonatal treatment. Other pups were treated identically with canola oil as vehicle, vitamin A, RA, or VARA on d 6, 7, and 8. These rats stayed with their dams until they were weaned at 3 wk of age and fed a vitamin A–deficient diet until they were 9 wk old. In parallel, a separate group of rats, referred to as vitamin A–adequate age-matched rats, was treated with vehicle when they were neonates and fed the same diet with an adequate level of vitamin A [4mg of retinol, in the form of retinyl palmitate, per kg of diet, (Research Diets)] to maintain normal vitamin A status during growth. Most of the rats in the 9-wk study were also immunized on d 7 and again 7–10 d before the end of the study with tetanus toxoid, as described previously [see Fig. 1A within reference (19)]. This immunization component of this study has been fully described and, because this procedure is not expected to have had any effect on tissue retinol levels, it will not be considered further. All rats had free access to food and water throughout the study.
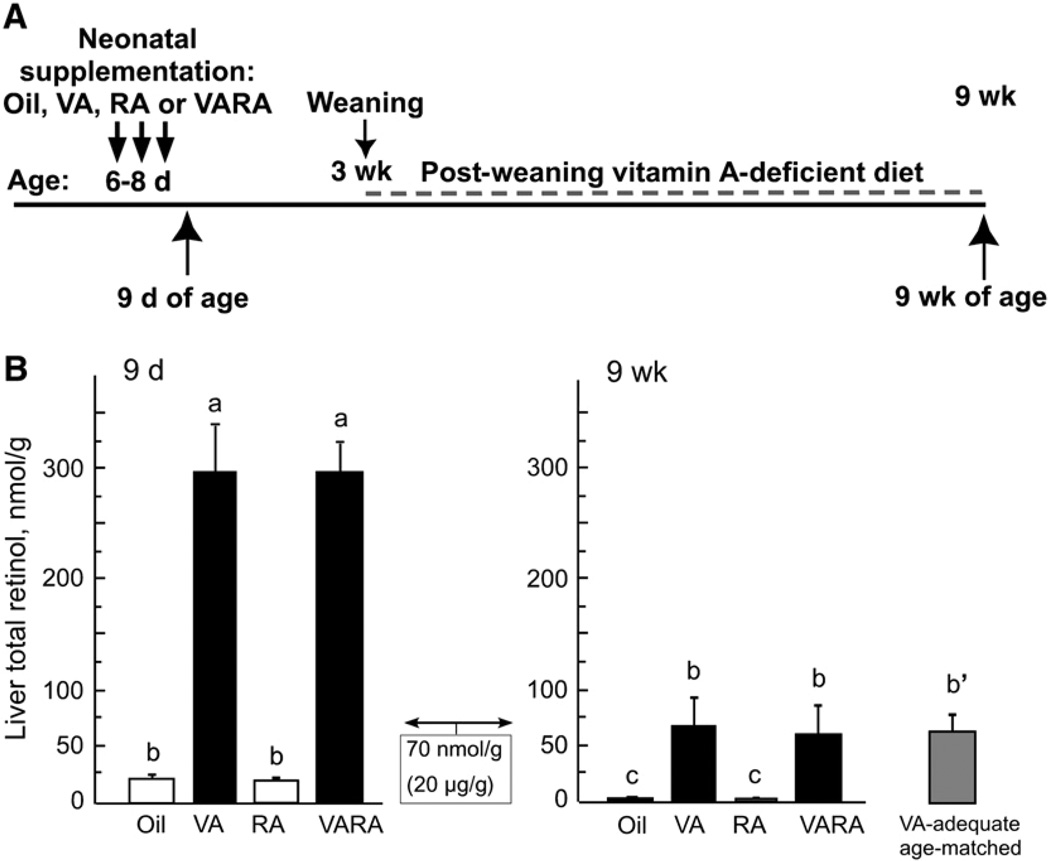
FIGURE 1.
Design of study (A) and liver total retinol at 9 d and 9 wk of age (B) in rats treated as neonates with oil, vitamin A (VA), RA, or VARA. The box showing 70 nmol/g (20 µg/g) represents a value considered to indicate sufficient liver retinol storage in adult humans (see text). Values are means ± SE, n = 4 (9 d) and 6 (9 wk). Means without a common letter differ, P < 0.0001. The reference group of 9-wk-old VA–adequate rats marked b′ did not differ from the 9-wk groups marked b, but was not compared with the 9-d-old rats.
Supplementation and tissue collection
Rat pups within litters were divided into the following 4 supplementation groups: oil (placebo control containing vehicle only), vitamin A, RA, and VARA (Fig. 1A). The dosages were selected to resemble a dose of retinol used previously in human studies of vitamin A supplementation (30,31), and a dose of RA used in rat studies lung septation (32); the rationale for each dose has been fully described (33). The vitamin A dose preparation contained 50 nmol of retinyl palmitate per mL and the RA dose preparation contained 5 nmol of all-trans-RA per mL, each prepared in oil. The VARA dose preparation contained the same amounts of retinyl palmitate and RA as above and, therefore, the retinol to RA ratio was 10:1 (33). Each pup was weighed daily and administered 0.4 µL/g body weight of the assigned dose, which was delivered orally directly into the mouth by micropipette. One set of rats (n = 4/group) was killed on postnatal d 9 to determine the immediate effects on supplementation on liver, plasma, and lung total retinol levels. The other rats, (n = 6/group), remained with their mothers until 3 wk of age and were then fed the vitamin A–deficient diet until they were 9 wk old. The vitamin A–adequate age-matched reference group was treated with oil as neonate and fed the vitamin A–adequate diet after weaning.
Tissue collection and retinol analysis
At d 9 or wk 9, rats were killed by carbon dioxide inhalation, blood was collected into heparinized syringes for preparation of plasma, and the liver and lungs were dissected, weighed, and then rapidly frozen for storage prior to analysis (34). Plasma total retinol concentration was determined after saponification using a reverse-phase HPLC method with detection at 325 nm and trimethylmethoxyphenol retinol as an internal standard (20). Liver and lung tissue was subjected to total lipid extraction, followed by purification of the lipid extract, and either analysis of individual RE and retinol by HPLC, or after saponification of the total lipid extract and the quantification of total retinol. The sum of the individual RE constituted ~95% of the total retinol in the liver and lungs (33) and, because the results for total retinol were similar without and after saponification, we analyzed most samples as total retinol after saponification.
Statistical analysis
Data are expressed as means ± SEM. When variances among groups were unequal, the data were transformed (log10) before statistical analysis. Differences among the 8 main groups (4 supplementation groups at 2 ages) were analyzed by 2-way ANOVA, followed by Fisher’s protected least significant difference test and least squares means test to compare all groups. The vitamin A–adequate reference group was compared by ANOVA to the other 9-wk-old rats (SuperAnova, Abacus Software).A difference of P < 0.05 was considered significant.
Results
Liver vitamin A increased after vitamin A and VARA supplementation in neonatal rats and declined on a vitamin A–deficient diet in young adult rats
Rats were treated on the schedule shown in Fig. 1A. In 9-d control rats (Fig. 1B), liver retinol was <20 nmol/g, well below the value of 70 nmol/g liver [20 µg retinol/g, which is often referenced as a value below which liver reserves are inadequate in humans (10,11)]. However, this value is typical for the newborns of vitamin A–adequate rat dams (35). Supplementation with vitamin A alone or as a component of VARA increased liver total retinol to nearly 300 nmol/g. Supplementation with RA alone, or as a component of VARA, had no effect on liver retinol, because RA cannot be converted to retinol; these data are in agreement with our previous results (20). When the rats were 9 wk old, having been fed the vitamin A–deficient diet for 6 wk, liver retinol was ≤2 nmol/g in the oil and RA groups. In the groups treated with vitamin A and VARA as neonates, the values were lower than they were immediately after supplementation, but they were still at or slightly above 70 nmol/g. Overall, liver retinol fell in all groups of rats fed the vitamin A–deficient diet (P < 0.0001 for 9 d vs. 9 wk), but it was higher in rats that had been supplemented as neonates with vitamin A or VARA, compared with oil or RA (P < 0.0001).
Plasma retinol was maintained by neonatal supplementation with vitamin A or VARA in rats fed vitamin A–deficient diet after weaning
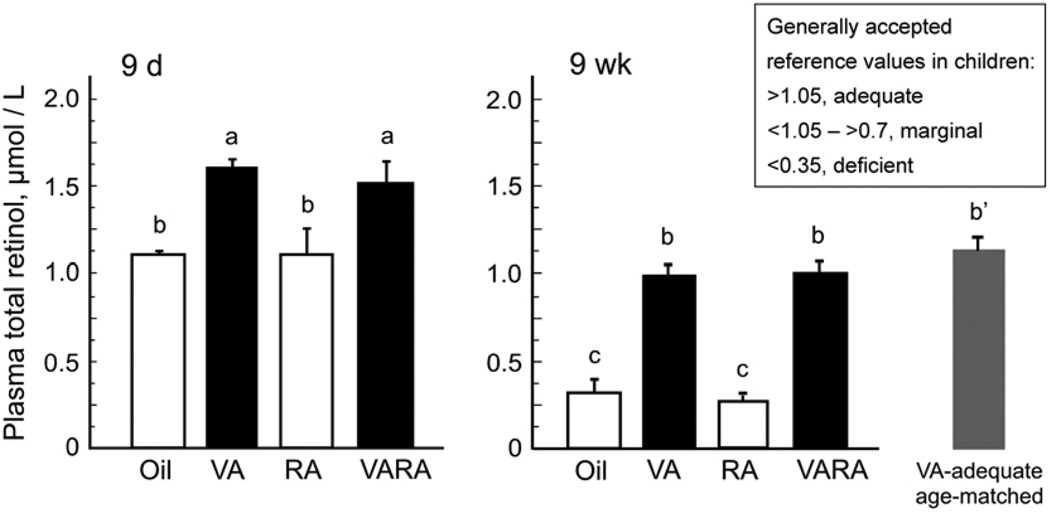
Plasma retinol was determined in the same 9-d- and 9-wk-old rats (Fig. 2). Values representative of vitamin A status in children (11) are included in the figure for comparison to the determined plasma retinol concentrations in our rats. At 9 d of age, plasma retinol was >1.0 µmol/L in all groups (Fig. 2A). The higher concentrations in the vitamin A and VARA groups at this age are likely the result of some residual unprocessed lipoprotein-associated RE from the last supplement existing in the circulation at this time. At 9 wk of age, after 6 wk on the vitamin A–deficient diet, plasma retinol had fallen in the oil and RA groups whereas, in contrast, plasma retinol was still >1 µmol/L in the groups that received vitamin A and VARA as neonates, similar to same-aged rats that were fed the vitamin A–adequate diet after weaning (Fig. 2B).
FIGURE 2.
Plasma retinol at 9 d and 9 wk of age in rats treated as neonates with oil, vitamin A (VA), RA, or VARA. At 9 wk the value for a group of same-aged rats fed the vitamin A–adequate diet is also shown. The box shows values that have been used as indicators of vitamin A status in humans (see text). Values are means ± SE, n = 4 (9 d) and 6 (9 wk). Means without a common letter differ, P < 0.05. The reference group of 9-wk-old VA–adequate rats marked b′ did not differ from the 9-wk groups marked b, but was not compared with the 9-d-old rats.
Retinol concentration in the lungs was not maintained by neonatal-age supplementation with vitamin A or VARA when rats were fed a vitamin A–deficient diet after weaning
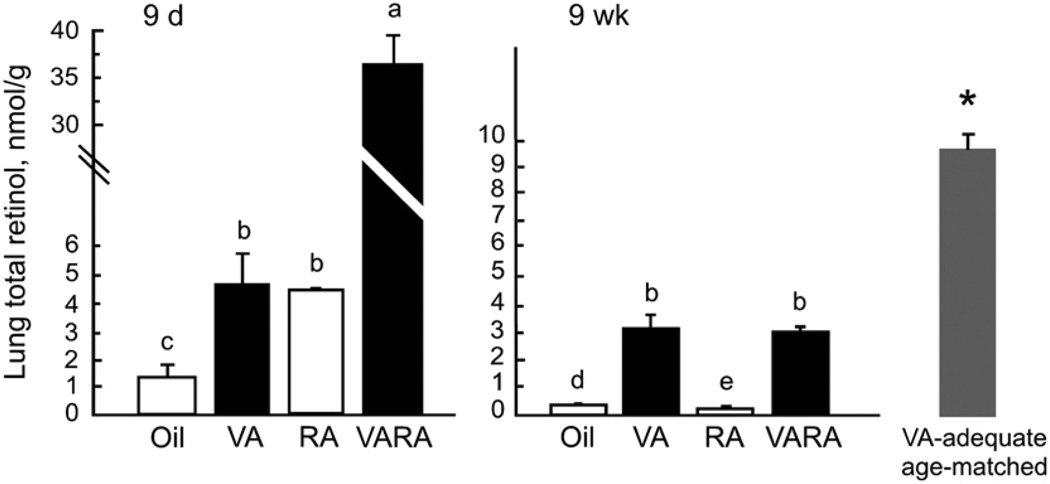
Lung total retinol, determined in 9-d-old rats after supplementation with vitamin A, RA, or VARA, showed the same pattern we reported previously for similarly treated neonatal rats (20). Whereas the concentration in the control group was low (~1.5 nmol/g), it was significantly increased by vitamin A and RA, each given alone, while VARA containing the same amount of vitamin A combined with the same amount of RA as in the single doses produced a strongly synergistic increase (Fig. 3A). When lung total retinol concentrations were determined at 9 wk of age, values had fallen below 1 nmol/g in the oil and RA groups (Fig. 3B). Lung total retinol was significantly higher in rats that had received vitamin A or VARA, but these values were 72% lower than those of the 9-wk-old rats that had been treated with oil as neonates and fed the vitamin A–adequate diet after weaning.
FIGURE 3.
Lung total retinol at 9 d and 9 wk of age in rats treated as neonates with oil, vitamin A (VA), RA, or VARA. At 9 wk the value for a group of same-aged rats fed vitamin A–adequate diet is also shown. No reference values are shown, as none have been established for lung. Values are means ± SE, n = 4 (9 d) and 6 (9 wk). Means without a common letter differ, P < 0.0001. The reference group of 9-wk-old VA–adequate rats marked * differed from the other 9-wk groups (P < 0.001), but was not compared with the 9-d-old rats.
Discussion
The main observation from this study is that even when vitamin A supplementation is sufficient to maintain plasma retinol in the normal range (~1 µmol/L) in the absence of dietary vitamin A, this may not provide retinol to vitamin A–requiring target tissues, such as the lungs, in an amount or manner that is equivalent to that delivered by a vitamin A–adequate diet. The results of our study confirmed previous work showing that physiological levels of lung and liver retinol are low at birth (36), even though plasma retinol is not necessarily low in the offspring of vitamin A–adequate rat dams (35,37; Fig. 2A). Thus, neonates have little in the way of tissue retinol reserves on which to draw, and thus they appear to be highly dependent on obtaining adequate vitamin A from mother’s milk, supplements, or, later on, from their postweaning diet, to build tissue reserves. The increase in retinol in the lungs of neonatal rats supplemented with vitamin A alone (Fig. 3A), implies that the amount of retinol derived from milk (neonatal diet) may be limiting. The increase in lung retinol, most of which was RE [see (33)], after supplementation with vitamin A and RA alone, and the synergistic increase in lung RE after treatment with VARA, may be due to the higher induction of lecithin:retinol acyltransferase (LRAT) in the lungs compared with the liver, as lung LRAT was previously shown to be increased severalfold above control levels by RA of adult rats (38). However, further studies are needed to determine the mechanism underlying the synergistic effect of VARA in the lungs. In any case, VARA supplementation in the neonatal period was very effective in increasing lung RE rapidly (20,33,39; Fig. 3A), and this form of vitamin A supplementation might have utility in the treatment of bronchopulmonary dysplasia and other lung disorders, as we have suggested previously (39).
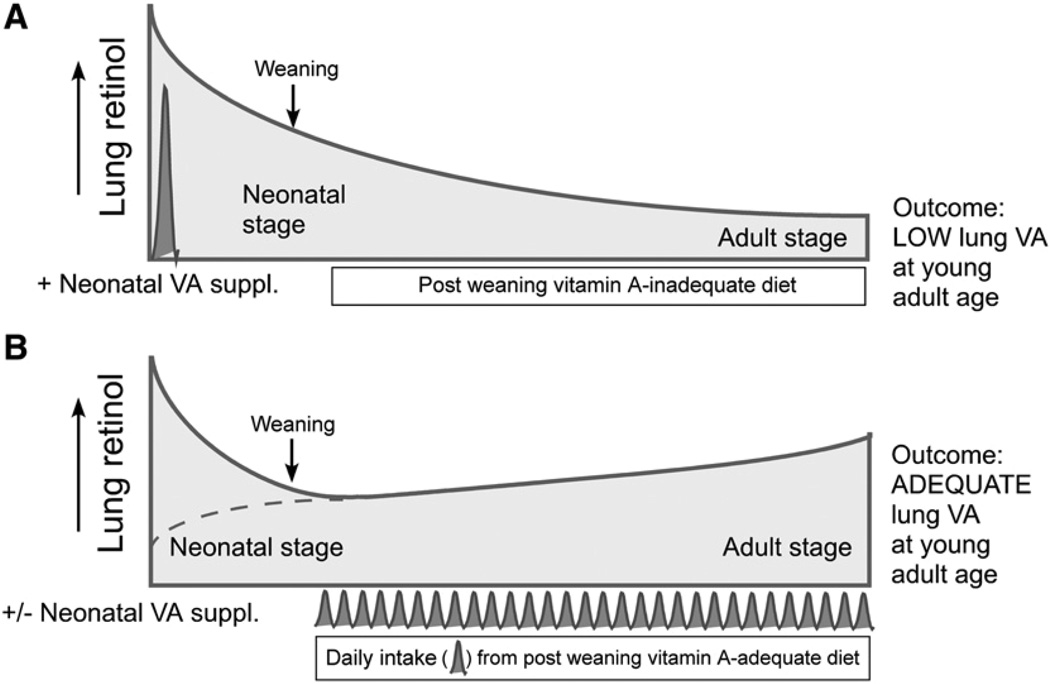
In our previous studies, after cessation of treatment with VARA, lung RE declined over the next 4 d, but the concentration still remained above that in the control group (20). In this study, nearly 8 wk had elapsed after the last treatment with VARA, and at that time there was no remaining evidence of the synergistic increase in lung total retinol seen at 9 d of age. These results imply that lung retinol had completely turned over in this interval and that only dietary vitamin A after weaning was likely to be a determinant of lung total retinol concentration at a young adult age. However, we believe that the most significant observation from these studies is that in the postweaning period, when dietary vitamin A was lacking, the neonatal supplementation treatments that preserved plasma retinol at a normal level did not prevent a >70% reduction in lung total retinol, as compared with the level in rats that were fed a vitamin A–adequate diet. We infer from these data that vitamin A from diet is necessary in the postweaning period to maintain lung retinol concentrations. A possible model for this effect is illustrated in Fig. 4. We speculate that in rats that received vitamin A, RA, or VARA as neonates, lung total retinol was elevated immediately after treatment (Fig. 4A). The doses of vitamin A and VARA are expected to rapidly and transiently increase the RE and retinol contents of chylomicrons, nearly in direct proportion to the amount of vitamin A ingested (40). Newly absorbed vitamin A, either due to its form as RE or to its presence in chylomicrons, might be an important contributor of vitamin A as RE and/or retinol to the lungs. When the diet was deficient in vitamin A, it is likely that only retinol released from storage in liver or other tissues and transported on RBP was able to supply retinol to target tissues. Apparently, dietary input, presumably as chylomicron-associated RE and retinol, is necessary to prevent the level of vitamin A in the lungs from falling to low values, such as those in our young adult rats treated with oil or RA and fed the vitamin A–deficient diet (Fig. 3B and Fig. 4A). Clearly, there was some residual effect of neonatal-age supplementation with vitamin A or VARA that could still be seen at the young adult age, as lung total retinol concentrations were severalfold higher in the vitamin A and VARA groups than in the control and RA-treated groups (Fig. 3B). But nevertheless, neonatal treatment with vitamin A or VARA was not equivalent to the effect of an adequate postweaning diet (Fig. 3B), even though plasma retinol levels were indistinguishable between 9-wk-old rats treated with vitamin A or VARA as neonates and the age-matched group fed a vitamin A–adequate diet (Fig. 2B). We hypothesize that the frequent intake of vitamin A-containing foods, through formation of RE- and retinol-containing chylomicrons and possibly other lipoproteins (41,42), provides a second source of vitamin A to the lungs (Fig. 4B), in addition to that provided by retinol bound to plasma RBP, and that this provides a substrate for the accumulation of vitamin A reserves in the lungs (and liver), as compared with those which accumulate when plasma retinol bound to RBP is the only form of retinol in the circulation. Further studies are needed to directly test the contribution of chylomicrons to lung vitamin A accumulation.
FIGURE 4.
Model of situations and outcomes resulting from neonatal supplementation with vitamin A or VARA, and a postweaning diet either lacking or adequate in vitamin A. (A) Supplementation with vitamin A or VARA would be expected to increase lung total retinol in the neonatal period, resulting in somewhat higher levels in the lungs at 9 wk, as compared with supplementation with oil or RA alone (see Fig. 3), but not to the level observed in rats fed vitamin A after weaning. (B) Regardless of neonatal vitamin A supplementation (dashed line without vitamin A or VARA; upper solid line with these treatments), the daily input of dietary vitamin A, mostly as RE, supports the gradual accumulation of vitamin A in the lungs, leading to the adult level.
There are 2 principal limitations to the interpretation of our data. First, there are no normative data for vitamin A in lung tissue to which to compare the values in our study, and no “optimal” level of lung vitamin A storage has been determined. However, we contend that our comparison with rats fed a vitamin A–adequate diet is a fair measure of the “normal” postweaning response of the lungs to vitamin A when it is available after uptake from diet. Secondly, we do not at this time have any evidence that the differences in lung retinol levels (see Fig. 3) are associated with physiological differences. Nonetheless, some data suggests that low lung retinol levels exist in pathophysiological contexts. It has been reported that vitamin A in adult rat lung is reduced by 60% by a 1-wk glucocorticoid treatment (43), and by 30% in the lung tissue of patients with lung cancer compared with levels in those without treatment (44). Given the demonstrated roles that retinoids play in regulating gene expression in the lungs and the extensive maturation of the lungs that occurs postnatally in rodents and humans alike [see (45,46) for reviews], and demonstrations that RA can reverse the impairment of lung septation due to glucocorticoid treatment in rat and mouse models (47,48), it seems plausible that adequate tissue RE and retinol concentrations could have an impact on these processes. Thus, we suggest that it is important to further investigate how dietary vitamin A and different vitamin A supplementation strategies, alone and combined, may affect retinol accumulation in the lungs, and to learn what tissue retinol levels may be “optimal” with respect to lung maturation and physiological functioning, both early and later on in life.
In conclusion, this study provides evidence that the lungs of young rats require a steady input of dietary vitamin A, presumably delivered by chylomicrons, to accumulate and/or maintain total retinol in the lungs during the postnatal period of rapid growth. In the absence of dietary vitamin A, a normal level of plasma retinol alone was not enough to support lung vitamin A storage in this period. These results suggest that high-dose vitamin A supplementation, even if it is adequate for maintaining plasma retinol over a period of time, may not be an optimal strategy for maintaining the health of peripheral tissues such as the lungs. Interestingly, there is increasing interest in using dietary, food-based approaches to provide adequate vitamin A to human populations. Such a strategy was advocated in the 1970s (49), and more recently (50,51). We believe our results add new biochemical evidence that a dietary approach could be very important for the establishment and maintenance of adequate vitamin A reserves in peripheral tissues such as the lungs. A diet-based approach may diversify the molecular forms of retinol in plasma, principally by providing RE formed during the intestinal metabolism of vitamin A and transported by lipoproteins. At present, little is known of the functionality of chylomicron RE, other than as an efficient means of storing or transporting retinol. A better understanding of its role in providing vitamin A to extrahepatic tissues is necessary to help connect dietary vitamin A intake, plasma retinol levels, tissue vitamin A concentration, and, possibly, health outcomes.
Acknowledgments
We thank Drs. Yifan Ma and Sandhya Sankaranarayan for their contributions to this study.
Footnotes
Supported by NIH DK-41479 and CA-90214.
Author disclosures: A. C. Ross and N. Li, no conflicts of interest.
Abbreviations used: RA, retinoic acid; RBP, retinol-binding protein; RE, retinyl ester; VARA, vitamin A combined with RA, 10:1 mol:mol.
Literature Cited
- 1.Schultink W. Use of under-five mortality rate as an indicator for vitamin A deficiency in a population. J Nutr. 2002;132:2881S–2883S. doi: 10.1093/jn/132.9.2881S. [DOI] [PubMed] [Google Scholar]
- 2.Semba RD. Vitamin A and infectious diseases. Vitamin A and retinoids: An update of biological aspects and clinical applications. In: Livrea MA, editor. Basel: Birkhèuser Verlag. 2000. pp. 97–108. [Google Scholar]
- 3.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 4.Villamor E, Fawzi WW. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18:446–464. doi: 10.1128/CMR.18.3.446-464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darlow BA, Graham PJ. Vitamin A supplementation for preventing morbidity and mortality in very low birthweight infants. Cochrane Database Syst Rev. 2002:CD000501. doi: 10.1002/14651858.CD000501. [DOI] [PubMed] [Google Scholar]
- 6.Expanded WHO. Programmes for the control of vitamin A deficiency: the role of the EPI in new initiatives for the 1990s. Geneva: World Health Organization; 1988. Programme on Immunization. [Google Scholar]
- 7.World Health Organization. Report of an Informal Consultation: WHO, Geneva, June 30-July 1, 1992. Geneva: WHO; 1993. Using Immunization Contacts to Combat Vitamin A Deficiency; pp. 1–17. WHO/ NVT/EPI/93.1 WHO. [Google Scholar]
- 8.Ross DA, Kirkwood BR, Binka FN, Arthur P, Dollimore N, Morris SS, Shier RP, Gyapong JO, Smith PG. Child morbidity and mortality following vitamin A supplementation in Ghana: time since dosing, number of doses, and time of year. Am J Public Health. 1995;85:1246–1251. doi: 10.2105/ajph.85.9.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sommer A, West KP., Jr The duration of the effect of vitamin A supplementation. Am J Public Health. 1997;87:467–468. doi: 10.2105/ajph.87.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilch SM. Analysis of vitamin A data from the Health and Nutrition Examination Surveys. J Nutr. 1987;117:636–640. doi: 10.1093/jn/117.4.636. [DOI] [PubMed] [Google Scholar]
- 11.Underwood BA. Methods for assessment of vitamin A status. J Nutr. 1990;120:1459–1463. doi: 10.1093/jn/120.suppl_11.1459. [DOI] [PubMed] [Google Scholar]
- 12.Olson JA. Serum level of vitamin A and carotenoids as reflectors of nutritional status. J Natl Cancer Inst. 1984;73:1439–1444. [PubMed] [Google Scholar]
- 13.Furr HC, Amedee-Manesme O, Clifford AJ, Bergen HR, 3rd, Jones AD, Anderson DP, Olson JA. Vitamin A concentrations in liver determined by isotope dilution assay with tetradeuterated vitamin A and by biopsy in generally healthy adult humans. Am J Clin Nutr. 1989;49:713–716. doi: 10.1093/ajcn/49.4.713. [DOI] [PubMed] [Google Scholar]
- 14.Haskell MJ, Handelman GJ, Peerson JM, Jones AD, Rabbi MA, Awal MA, Wahed MA, Mahalanabis D, Brown KH. Assessment of vitamin A status by the deuterated-retinol-dilution technique and comparison with hepatic vitamin A concentration in Bangladeshi surgical patients. Am J Clin Nutr. 1997;66:67–74. doi: 10.1093/ajcn/66.1.67. [DOI] [PubMed] [Google Scholar]
- 15.Harrison EH, Blaner WS, Goodman DS, Ross AC. Subcellular localization of retinoids, retinoid-binding proteins, and acyl-CoA:retinol acyltransferase in rat liver. J Lipid Res. 1987;28:973–981. [PubMed] [Google Scholar]
- 16.Green MH, Green JB, Lewis KC. Variation in retinol utilization rate with vitamin A status in the rat. J Nutr. 1987;117:694–703. doi: 10.1093/jn/117.4.694. [DOI] [PubMed] [Google Scholar]
- 17.Dawson HD, Yamamoto J, Zolfaghari R, Rosales F, Dietz J, Shimada T, Li N-Q, Ross AC. Regulation of hepatic vitamin A storage in a rat model of controlled vitamin A status during aging. J Nutr. 2000;130:1280–1286. doi: 10.1093/jn/130.5.1280. [DOI] [PubMed] [Google Scholar]
- 18.Adams WR, Green MH. Prediction of liver vitamin A in rats by an oral isotope dilution technique. J Nutr. 1994;124:1265–1270. doi: 10.1093/jn/124.8.1265. [DOI] [PubMed] [Google Scholar]
- 19.Sankaranarayanan S, Ma Y, Bryson MC, Li NQ, Ross AC. Neonatal-age treatment with vitamin A delays postweaning vitamin A deficiency and increases the antibody response to T-cell dependent antigens in young adult rats fed a vitamin A-deficient diet. J Nutr. 2007;137:1229–1235. doi: 10.1093/jn/137.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross AC, Li NQ, Wu L. The components of VARA, a nutrient-metabolite combination of vitamin A and retinoic acid, act efficiently together and separately to increase retinyl esters in the lungs of neonatal rats. J Nutr. 2006;136:2803–2807. doi: 10.1093/jn/136.11.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thurlbeck WM. Postnatal growth of the lung and its significance in disease. Hum Pathol. 1978;9:492–493. doi: 10.1016/s0046-8177(78)80130-0. [DOI] [PubMed] [Google Scholar]
- 22.Shenai JP, Kennedy KA, Chytil F, Stahlman MT. Clinical trial of vitamin A supplementation in infants susceptible to bronchopulmonary dysplasia. J Pediatr. 1987;111:269–277. doi: 10.1016/s0022-3476(87)80086-0. [DOI] [PubMed] [Google Scholar]
- 23.Shenai JP, Rush MG, Stahlman MT, Chytil F. Vitamin A supplementation and bronchopulmonary dysplasia-revisited. J Pediatr. 1992;121:399–401. doi: 10.1016/s0022-3476(05)81793-7. [DOI] [PubMed] [Google Scholar]
- 24.Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, Stoll BJ, Lemons JA, Stevenson DK, et al. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 1999;340:1962–1968. doi: 10.1056/NEJM199906243402505. [DOI] [PubMed] [Google Scholar]
- 25.Dann WJ. The transmission of vitamin A from parents to young in mammals. Biochem J. 1932;26:1072–1080. doi: 10.1042/bj0261072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shenai JP, Chytil F, Stahlman MT. Liver vitamin A reserves of very low birth weight neonates. Pediatr Res. 1985;19:892–893. doi: 10.1203/00006450-198509000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Ross AC, Harrison EH. Vitamin A and Carotenoids. In: Zempleni J, Rucker RB, Suttie JW, McCormick DB, editors. Handbook of Vitamins. 4th ed. Boca Raton (FL): CRC Press; 2007. pp. 1–40. [Google Scholar]
- 28.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 29.Davila ME, Norris L, Cleary MP, Ross AC. Vitamin A during lactation: Relationship of maternal diet to milk vitamin A content and to the vitamin A status of lactating rats and their pups. J Nutr. 1985;115:1033–1041. doi: 10.1093/jn/115.8.1033. [DOI] [PubMed] [Google Scholar]
- 30.Humphrey JH, Rice AL. Vitamin A supplementation of young infants. Lancet. 2000;356:422–424. doi: 10.1016/S0140-6736(00)02541-1. [DOI] [PubMed] [Google Scholar]
- 31.Randomized trial to assess benefits and safety of vitamin A supplementation in early infancy. WHO/CHD Immunization Linked Vitamin A Supplementation Study Group. Lancet. 1998;352:1257–1263. [PubMed] [Google Scholar]
- 32.Massaro GD, Massaro D. Postnatal treatment with retinoic acid increases the number of pulmonary alveoli in rats. Am J Physiol. 1996;270:L305–L310. doi: 10.1152/ajplung.1996.270.2.L305. [DOI] [PubMed] [Google Scholar]
- 33.Ross AC, Ambalavanan N, Zolfaghari R, Li N-q. Vitamin A combined with retinoic acid increases retinol uptake and lung retinyl ester formation in neonatal rats. J Lipid Res. 2006;47:1844–1851. doi: 10.1194/jlr.M600061-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Cifelli CJ, Ross AC. Chronic vitamin A status and acute repletion with retinyl palmitate are determinants of the distribution and catabolism of all-trans-retinoic acid in rats. J Nutr. 2007;137:63–70. doi: 10.1093/jn/137.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davila ME, Norris L, Cleary MP, Ross AC. Vitamin A during lactation: relationship of maternal diet to milk vitamin A content and to the vitamin A status of lactating rats and their pups. J Nutr. 1985;115:1033–1041. doi: 10.1093/jn/115.8.1033. [DOI] [PubMed] [Google Scholar]
- 36.Shenai JP, Chytil F. Vitamin A storage in lungs during perinatal development in the rat. Biol Neonate. 1990a;57:126–132. doi: 10.1159/000243172. [DOI] [PubMed] [Google Scholar]
- 37.Akohoue SA, Green JB, Green MH. Dietary vitamin A has both chronic and acute effects on vitamin A indices in lactating rats and their offspring. J Nutr. 2006;136:128–132. doi: 10.1093/jn/136.1.128. [DOI] [PubMed] [Google Scholar]
- 38.Zolfaghari R, Ross AC. Lecithin:retinol acyltransferase expression is regulated by dietary vitamin A and exogenous retinoic acid in the lung of adult rats. J Nutr. 2002;132:1160–1164. doi: 10.1093/jn/132.6.1160. [DOI] [PubMed] [Google Scholar]
- 39.Ross AC, Ambalavanan N. Retinoic acid combined with vitamin A synergizes to increase retinyl ester storage in the lungs of newborn and dexamethasone-treated neonatal rats. Neonatology. 2007;92:26–32. doi: 10.1159/000100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrison EH. Mechanisms of digestion and absorption of dietary vitamin A. Annu Rev Nutr. 2005;25:87–103. doi: 10.1146/annurev.nutr.25.050304.092614. [DOI] [PubMed] [Google Scholar]
- 41.Blomhoff R, Skrede B, Norum KR. Uptake of chylomicron remnant retinyl ester via the low density lipoprotein receptor: Implications for the role of vitamin A as a possible preventive for some forms of cancer. J Intern Med. 1990;228:207–210. doi: 10.1111/j.1365-2796.1990.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 42.Sauvant P, Mekki N, Charbonnier M, Portugal H, Lairon D, Borel P. Amounts and types of fatty acids in meals affect the pattern of retinoids secreted in human chylomicrons after a high-dose preformed vitamin A intake. Metab Clin Exp. 2003;52:514–519. doi: 10.1053/meta.2003.50082. [DOI] [PubMed] [Google Scholar]
- 43.Georgieff MK, Radmer WJ, Sowell AL, Yeager PR, Blaner WS, Gunter EW, Johnson DE. The effect of glucocorticosteroids on serum, liver, and lung vitamin A and retinyl ester concentrations. J Pediatr Gastroenterol Nutr. 1991;13:376–382. doi: 10.1097/00005176-199111000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Redlich CA, Chung JS, Cullen MR, Blaner WS, van Bennekum AM, Berglund L. Effect of long-term beta-carotene and vitamin A on serum cholesterol and triglyceride levels among participants in the carotene and retinol efficacy trial (CARET) Atherosclerosis. 1999;143:427–434. doi: 10.1016/s0021-9150(98)00303-7. [DOI] [PubMed] [Google Scholar]
- 45.Zachman RD, Grummer MA. Retinoids and lung development. In: Zachman RD, Grummer MA, editors. Contemporary Endocrinology: Endocrinology of the lung: Development and surfactant synthesis. Totowa (NJ): Humana Press, Inc.; 2000. pp. 161–179. [Google Scholar]
- 46.Roth-Kleiner M, Post M. Similarities and dissimilarities of branching and septation during lung development. Pediatr Pulmonol. 2005;40:113–134. doi: 10.1002/ppul.20252. [DOI] [PubMed] [Google Scholar]
- 47.Massaro GD, Massaro D. Retinoic acid treatment partially rescues failed septation in rats and in mice. Am J Physiol Lung Cell Mol Physiol. 2000;278:L955–L960. doi: 10.1152/ajplung.2000.278.5.L955. [DOI] [PubMed] [Google Scholar]
- 48.Hind M, Maden M. Retinoic acid induces alveolar regeneration in the adult mouse lung. Eur Respir J. 2004;23:20–27. doi: 10.1183/09031936.03.00119103. [DOI] [PubMed] [Google Scholar]
- 49.Bauernfeind JC, Newmark H, Birn M. Vitamins A and E nutrition via intramuscular or oral route. Am J Clin Nutr. 1974;27:234–253. doi: 10.1093/ajcn/27.3.234. [DOI] [PubMed] [Google Scholar]
- 50.Trowbridge FL, Harris SS, Cook J, Dunn JT, Florentino RF, Kodyat BA, Mannar MG, Reddy V, Tontisirin K, et al. Coordinated strategies for controlling micronutrient malnutrition: a technical workshop. J Nutr. 1993;123:775–787. doi: 10.1093/jn/123.4.773. [DOI] [PubMed] [Google Scholar]
- 51.Underwood BA, Smitasiri S. Micronutrient malnutrition: Policies and programs for control and their implications. Annu Rev Nutr. 1999;19:303–324. doi: 10.1146/annurev.nutr.19.1.303. [DOI] [PubMed] [Google Scholar]