Abstract
All-trans-retinoic acid (RA), a natural metabolite of retinol, carries out most of the biological activities of vitamin A and is required for normal growth, cell differentiation, and immune functions. In the present studies, THP-1 human monocytes were used to investigate the mechanisms by which RA may regulate progression through the G1/S phase of the cell cycle. Physiological concentrations of all-trans-RA reduced the levels of cyclin E mRNA by 6 h and reduced cyclin E protein in a dose- and time-dependent manner. Similar reductions were observed for the retinoic acid receptor RARα and RXRα proteins. Concomitantly, RA increased the level of the cyclin-dependent kinase inhibitor p27 (Kip-1). The levels of retinoblastoma mRNA and protein (pRb) were also increased, while the proportion of hyperphosphorylated (phosphoserine 807/811) pRb was markedly reduced. Overall, RA increased the functionality of pRb as an inhibitor of cell cycle progression. Furthermore, RA reduced the binding activity of the transcription factor E2F to its core DNA element. Retinoic acid-induced changes in cell cycle-related proteins occurred in 4–6 h, including reduced cyclin E expression in bromodeoxyuridine (BrdU)-labeled cells, before the onset of cell differentiation as indicated by an increase in the percentage of G1 phase cells and a reduction in S phase cells at 24 h. The expression of CD11b, a cell surface marker of macrophage-like differentiation was increased by RA, as was phagocytic activity. The multiple effects of RA on cell cycle progression may help to explain its well-documented ability to induce the differentiation of THP-1 cells, and thereby to enhance macrophage-like immune functions.
Keywords: Cyclin E, Retinoblastoma protein, p27, CD11b, Gene expression, Retinoic acid
Introduction
Retinoic acid (RA), a potent metabolite of vitamin A, acts as a growth and differentiation factor in many tissues. In vitamin A adequacy, RA is present in plasma at nanomolar concentrations, and thus circulating leukocytes are continuously exposed to low levels of RA [1]. Most of the biological actions of RA are mediated by two families of nuclear retinoid receptors [2]. The binding of RA to nuclear transcription factors of the RA receptor (RAR) and retinoid X receptor (RXR) families enables these receptors to induce the transcriptional activation of a wide range of retinoid-responsive genes [2,3]. The ability of retinoids to regulate cell growth and promote cell differentiation has been established in numerous cell culture and animal models. Conversely, in several models of carcinogenesis, a deficiency of vitamin A has been shown to promote tumor development [4,5]. Due to the antiproliferative and apoptosis-inducing effects of retinoids, both natural isomers of RA and various synthetic retinoids have been used therapeutically in human diseases such as leukemia and other cancers [6–10]. Retinoids are also required for adequate innate and adaptive immune responses to agents of infectious disease [11,12]. Although the requirement for RA to maintain normal immune competence is well documented, the mechanisms of its action in the immune system are still not well understood. One way in which RA may stimulate immunity is by inducing the maturation/differentiation of myeloid and lymphoid cells.
Cell growth and differentiation are tightly controlled processes that coordinately maintain normal tissue homeostasis. The decision of cells to differentiate is commonly made in the G1 phase of the cell cycle, and the induction of differentiation is believed to require cell cycle arrest [13,14]. However, despite a general under-standing that retinoids often induce cells to cease or slow their rate of proliferation and to assume a more mature phenotype, there is still little specific knowledge of how RA can induce the arrest of the cell cycle, and coordinately, activate a program of cell-type specific differentiation. In the present study, we have used THP-1 cells exposed to physiological concentrations of all-trans-RA to investigate the regulation of key factors involved in cell cycle progression, especially the G1/S transition, and cell differentiation. THP-1 cells, a human monoblastic cell line originating from a patient with acute myeloid leukemia defined as AML-M5, monoblastic subtype [15], are conducive to studies of cell cycle regulation and differentiation because they maintain distinct lineage (monocytic) markers, closely resemble human monocytes [15–17], and are induced under the influence of retinoids or other diet-derived agents such as 1,25-dihydroxyvitamin D [18,19] as well as phorbol diester [16] to differentiate toward more mature macro-phage-like cells [19]. These changes are typically associated with a loss of proliferative capacity [16]. THP-1 cells provides a good model for the differentiation and maturation of monocytes to macrophages, which are essential for the development of both innate and adaptive immunity (for review, see Ref. [20]). The results obtained in the present studies with THP-1 cells provide evidence that RA can rapidly induce alterations in the level or functional status of key cell cycle-related proteins including cyclin E, p27 (p27/Kip-1), retinoblastoma (pRb), and the transcription factor E2F, resulting in cell cycle arrest in the G1 phase of the cell cycle. Proliferation was reduced in RA-treated cells, while the expression of CD11b, a cell surface marker of macrophage differentiation [21], and phagocytic activity was increased. The ability of RA to regulate cell cycle progression and to promote the differentiation of monocytic cells into macrophages could be one of the pathways by which RA enhances innate and adaptive immune responses in vivo.
Materials and methods
Materials
All-trans-RA (Sigma, St. Louis, MO) was prepared as a 2-mM stock in ethanol and stored at −20°C before being diluted to the desired concentration in medium in each experiment. Recombinant human tumor necrosis factor (TNFα) was from R&D Systems (Minneapolis, MN). The antibodies used in our experiments are introduced in Immunoblot analysis of cell cycle-related proteins.
Cells and culture conditions
The THP-1 cell line, obtained from the ATCC (Rockville, MD) was used in these experiments as a model of cell growth regulation and monocytic differentiation (15–17). THP-1 cells were routinely propagated in RPMI 1640 supplemented with 10% vol/vol heat-inactivated fetal bovine serum (FBS), 5 × 10−5 M β–mercaptoethanol, 100 units/ml penicillin and 100 µg/ml streptomycin, all from Life Technologies, Inc. (Carlsbad, CA). As the characters of the cell can change markedly after 25 passages [17], we routinely discard and use freshly recovered cells after 15 passages. For specific experiments, cells were grown in the same medium with reduced FBS (defined as low serum conditioning), a standard means of synchronizing cells in the G1 phase of the cell cycle [22]. THP-1 cells did not thrive in serum-free medium or with 1% FBS, but viability was very good in cells that were sequentially incubated for 24 h in RPMI 1640 medium containing 5% FBS, followed by incubation for 24 h in medium containing 2% FBS. At this time (designated time 0), the medium was replaced with RPMI 1640 containing either 10% FBS or 2% FBS, and the desired final concentration of all-trans-RA was added. The vehicle, ethanol, did not exceed 0.01%. Cells were then incubated at 37°C in a humidified atmosphere of air/5% CO2 for the times indicated in the figures. Cell viability was monitored by trypan blue dye exclusion (0.4% Trypan blue solution, Sigma) at the time of harvesting. Viability was consistently >96%.
RT-PCR
Total RNA was isolated using an RNeasy Mini Kit (Qiagen, Valencia, CA). A quantitative RT-PCR assay was conducted in which [α-33P]dATP was incorporated directly into the PCR products during amplification, followed by electrophoresis of the 33P-labeled PCR products on a polyacrylamide gel, as described in detail previously [23]. Primers used for cyclin E were: forward primer, 5′-ATGGAGGTGTGTGAAGTCTATAA, reverse primer, 5′-ACATTCAGCCAGGACACAATGGTC, giving rise to a PCR product of 279 bp. PCR was performed at 94°C for 30 s, 62°C for 1 min, and 72°C for 30 s. The housekeeping gene GAPDH was amplified for the same samples to verify the equality and integrity of RNA [23].
Immunoblot analysis of cell cycle-related proteins
The procedure for Western blot analysis was essentially as described previously [23]. Briefly, THP-1 protein lysates were routinely made in the presence of proteinase inhibitor and phosphatase inhibitor. Fifty micrograms of protein was separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a nitro-cellulose membrane (Bio-Rad). The membrane was blocked with 5% nonfat milk in TBST (10 mM Tris– HCl, pH 8.0, 150 mM NaCl, and 0.05% Tween-20) and then probed with one of the following appropriately diluted primary antibodies: anti-cyclin E, anti-p27, anti-RXRα, anti-RARα, anti-actin (Santa Cruz Biotechnology), and anti-pRb (PhosphoPlus kit from Cell Signaling Technology, Beverly, MA), followed by incubation with a corresponding secondary antibody coupled to horse-radish peroxidase (Amersham, Piscataway, NJ). Protein bands were visualized using the SuperSignal chemiluminescence system (Pierce Chemical, Rockford, IL). Equal protein loading in each lane was confirmed by Ponceau S staining and anti-actin Western blot analysis afterwards.
Electrophoretic mobility shift assay
Nuclear protein was extracted from THP-1 cells following a procedure we have described previously [23]. For each electrophoretic mobility shift assay (EMSA) reaction, 5 µg of nuclear protein was preincubated with 10 mM Hepes, 100 mM KCl, 0.05 mM EDTA, 2.5 mM MgCl2, and 0.5 µg of ssDNA for 10 min at room temperature. Then, an appropriate [γ-32P]ATP end-labeled probe (10,000 cpm) of either an E2F (E2F-1 from Santa Cruz Biotechnology) or Sp-1 consensus element was added for an additional 30 min. For competition assays, a 25-fold excess of an unlabeled oligonucleotide containing either consensus E2F (5-ATTTAAGTTTCGCGCCCTTTCTCA) or mutant E2F (5-ATTTAAGTTTCGATCCCTTTCTCA), or consensus Sp-1 binding sites (Santa Cruz Biotechnology), was added to the preincubation mixture for 10 min and then incubated with the appropriate labeled probe for 30 min at room temperature. The reaction mixtures were then loaded onto a 5% native polyacrylamide gel. After electrophoresis, the gel was dried and subjected to autoradiography.
Cell cycle analysis and determination of intracellular cyclin E level
Analysis of cell cycle phase distribution was performed according to an established method [24] with modifications. Briefly, THP-1 cells were first subjected to low serum conditioning. Then at time 0, medium containing either 2% or 10% FBS and the indicated concentration of RA was added to cells for the times indicated in the figures. The harvested cells were washed once in cold PBS, and fixed in cold 70% ethanol at 4°C for at least 4 h. Cells were then washed once again with PBS and stained with propidium iodide/RNase A solution (BD Pharmingen, San Diego, CA) for 1 h. Stained cells were subjected to analysis immediately by flow cytometry using a FACS Scan in the Center for Quantitative Cell Analysis of the Pennsylvania State University, and the results were analyzed using MacCycle software (Phoenix Flow Systems, San Diego, CA). As for triple staining, THP-1 cells cultured in medium with 10% FBS without low serum conditioning were paused with 30 µM of bromodeoxyuridine (BrdU) for 1 h and then cells were washed and incubated in the presence and absence of RA in medium with 10% FBS. At end of the culture, cells were fixed and stained for intracellular cyclin E protein, BrdU labeling, and cellular DNA as described [25]. Cyclin E was determined by indirect staining of anti-cyclin E antibody followed by FITC-conjugated anti-mouse secondary antibody, BrdU was detected by direct staining with PE-conjugated anti-BrdU antibody (BD Pharmingen), and DNA staining was performed with To-pro 3 (Molecular Probes, Eugene, OR), which was analyzed by MacCycle software.
Cell surface CD11b expression
For determination of CD11b expression, THP-1 cells (5 × 105 cells/analysis) were washed twice with PBS and stained with phycoerythrin (PE)-conjugated anti-CD11b polyclonal antibody (BD Biosciences, San Jose, CA) for 30 min at 4°C. The stained cells were washed twice with PBS containing 1% bovine serum albumin and 0.1% sodium azide and fixed with 1% paraformaldehyde in PBS. The proportion of cells expressing CD11b and the fluorescence intensity of CD11b were measured by flow cytometry, using an isotype-matched control antibody for background subtraction.
[3H]Thymidine incorporation
THP-1 cells were cultured in RPMI 1640 with 5% FBS for 24 h, and then cells were changed to medium with 2% FBS and seeded in a 96-well plate at 2 × 104 cells for 24 h. Retinoic acid and/or FBS were then added to cells, making the final serum concentration as 10% and of RA as 10 and 100 nM, respectively. After 20 h, 0.5 µCi of [3H]thymidine was added to each well for another 4 h. Cells were harvested and the incorporation of [3H]thymidine was determined using an LKB Beta-Plate liquid scintillation detector.
Phagocytosis assay
Phagocytosis by THP-1 cells was measured by the uptake of opsonized Escherichia coli (E. coli-fluorescein BioParticle, Molecular Probes) according to the manufacturer’s instructions. THP-1 cells was plated and treated with RA as stated above. At end of the incubation period, fluorescein-labeled E. coli K-12 BioParticle was added to cells for another 2 h of incubation at 37°C. Then 0.25% trypan blue was added immediately to the cells for 1 min to quench the surface-bound fluorescence, and cells were washed twice with PBS and subjected to staining with PE-labeled anti-CD11b, followed by two-color flow cytometric analysis.
Statistical analysis
The data are presented as the mean ± SEM. Super-ANOVA software (Abacus, Berkeley CA) was used for analysis; P < 0.05 was considered significant.
Results
Regulation of cell cycle-related gene expression by RA in THP-1 cells
To investigate mechanisms involved in the regulation of cell growth and differentiation by RA, we used THP-1 cells, an established model of monocyte–macrophage differentiation [15,16,19]. We initially focused on cyclin E, the regulatory subunit of the cyclin E/cyclin dependent kinase (CDK)-2 holoenzyme because it is one of the rate-determining factors for cells to progress through the G1/S checkpoint in late G1 phase of cell cycle [26,27]. Moreover, in a preliminary study using microarray analysis, we had observed an elevation of cyclin E gene expression in retinoid-deficient spleen tissue and a reduction to the control level after animals were repleted with RA (Q. Chen and A. C. Ross, unpublished observations). Thus, it was of interest to determine whether RA also regulates the expression of cyclin E in RA-treated THP-1 cells. First, quantitative RT-PCR analysis was performed to detect the level of cyclin E mRNA in THP-1 cells after low serum conditioning, and after the addition of 2% or 10% FBS in the absence and presence of RA for 24–48 h. As shown in Fig. 1A, RA reduced the level of cyclin E mRNA dose-dependently in THP-1 cells. Cyclin E mRNA was markedly reduced even at low concentrations of RA (1 and 10 nM), similar to those present in human serum [1]. A time course study (Fig. 1B) shows that 6 h after the change of medium from 2% to 10% FBS, which is known to release cells from G1 arrest [22], RA completely abrogated the increase in cyclin E mRNA due to 10% FBS. The significant reduction in cyclin E mRNA in RA-treated cells persisted for 48 h.
Fig. 1.
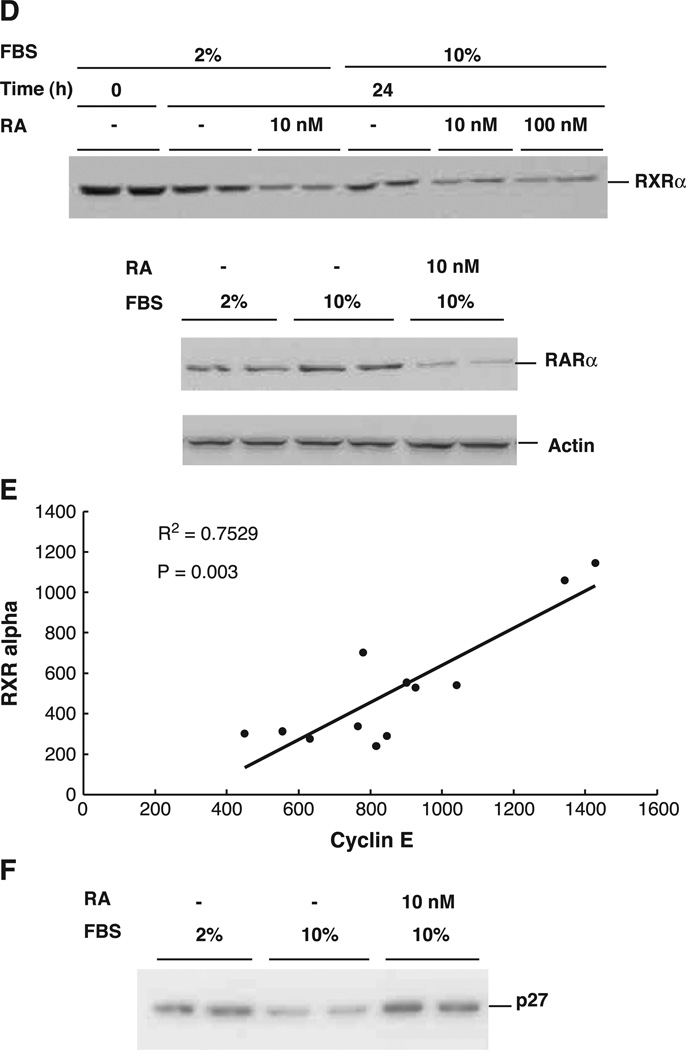
Regulation of cyclin E gene expression in THP-1 cells by retinoic acid. THP-1 cells were plated in RPMI 1640 medium with 5% serum for 24 h and then with 2% FBS for another 24 h. Serum was added to cells at a final concentration of 10% and RA was added to cells at the indicated concentrations. Total RNA was isolated at the end of each treatment and subjected to RT-PCR analysis. (A) Expression level of the cyclin E gene in the absence or presence of RA at different concentrations for 24 h. The level is represented by the ratio of treated cells over the control without RA. (B) Expression of cyclin E in the presence of RA for different times. The numbers shown in graphs A and B are the mean ± SD of three independent experiments. (C) Western blot analysis showing the levels of cyclin E in the presence (10 nM) and absence of RA. The image represents one of three independent experiments performed in duplicate. Same samples were also subjected to the Western blot analysis with anti-actin antibody and the result is shown underneath. (D) Western blot analysis of the same THP-1 cell protein lysates as described in (C) using antibodies specific to RXRα and RARα. (E) Graph showing the correlation between the protein levels of cyclin E and RXRα, both regulated by RA. The axes are arbitrary density units. (F) Western blot analysis of p27 protein in the presence and absence of RA. The actin bands were shown underneath as internal control.
The relative level of cyclin E protein was determined by immunoblot analysis in THP-1 cells cultured under low serum conditions and after incubation with 10% FBS in the absence and presence of RA, as described above. Whereas the level of cyclin E protein was reduced after incubation with 2% serum for 24 h, the addition of 10% FBS for 24 h resulted in an increase of cyclin E protein (Fig. 1C). Similar to the results observed for cyclin E mRNA, physiological concentrations of RA reduced the level of cyclin E protein in cells cultured with 2% FBS, and antagonized the increase of cyclin E protein in cells stimulated with 10% FBS. Thus, the results obtained for cyclin E mRNA and protein in THP-1 cells confirmed the regulation of cyclin E gene expression we had observed in whole tissue by RNA microarray analysis. These results suggest that RA may regulate the growth and differentiation of THP-1 cells at least in part by reducing the availability of cyclin E, the regulatory subunit of the cyclin E/CDK2 complex that is required for the G1/S cell cycle transition.
While the effects of RA undoubtedly involve interaction with nuclear retinoid receptors, some of these receptors may themselves be regulated during the cell cycle. As shown in Fig. 1D, under the same conditions described above for cyclin E, there was a consistent reduction in RXRα and RARα proteins in RA-treated cells. The levels of RXRα and cyclin E proteins were significantly correlated (R2 = 0.75, P = 0.003, Fig. 1E).
The activity of the cyclin E/CDK2 holoenzyme is also regulated by a group of cyclin-dependent kinase inhibitory proteins, among which p27 has been shown to play a critical role in the negative control of the G1/S cell cycle transition [26,27]. Therefore, the level of p27 protein was determined by immunoblot analysis in THP-1 cells cultured in the presence and absence of RA. As shown in Fig. 1F, RA enhanced the accumulation of p27 protein. Together with the results for cyclin E, these data imply that RA can regulate the progression of THP-1 cells through the cell cycle via a reciprocal regulation of the abundance of cyclin E, which is reduced by RA, and of its CDK inhibitor, p27, which is increased by RA.
Because the cyclin E/CDK complex is a major regulator of pRb protein at the late G1 phase of the cell cycle [28], and the serine 807/811 residue of pRb is the target of cyclin E/CDK2 phosphorylation [29], we performed additional experiments to determine the level and phosphorylation status of pRb in RA-treated THP-1 cells. Protein extracts were subjected to Western blot analysis using detection antibodies specific for pRb protein and for the S807/811-phosphorylated form of pRb. As shown in Fig. 2, the level of pRb was increased in THP-1 cells cultured in 10% FBS and was further increased by RA. The level of the phosphorylated pRb (S807/811) was also increased after culture in 10% FBS (Fig. 2A). However, in contrast to pRb, phosphorylated Rb was markedly reduced in cells incubated with RA. The increase in pRb and concomitant reduction in phosphorylated pRb were evident in cells treated with as little as 10 nM RA for times as short as 6 h, and both differences were even greater when the dose of RA and time of exposure were increased. Due to these reciprocal changes, the ratio of total pRb to phosphorylated pRb was increased >5-fold in cells treated with RA for 24 h. In addition, the level of pRb mRNA was significantly increased in THP-1 cells incubated with 10 nM of RA (data not shown). These data provide strong evidence that RA can enhance the functionality of this cell cycle inhibitor both by increasing the total amount of pRb and by reducing its phosphorylation status, which in turn may regulate the cell’s capacity for growth.
Fig. 2.
Regulation of retinoblastoma gene expression and protein phosphorylation by retinoic acid in THP-1 cells. THP-1 cells were treated as stated above, cellular protein was subjected to Western blot analysis. (A) A representative Western blot analysis showing the expression levels of pRb and pRb phosphorylated at position S807/811. Fifty micrograms of protein from THP-1 cell treated with/without RA was subjected to Western blot analysis. These images represent one of three independent experiments with consistent results. (B) Graph illustrating the ratio of total pRb protein compared to phosphorylated pRb, normalized to the ratio in control cells in medium with 2% FBS, at 24 h of incubation, based on data shown in (A).
pRb is known to actively repress cell proliferation through its binding with other important factors that are required for cell cycle progression [30]. One critical repressive complex is formed by the binding of pRb with E2F. The E2F family of transcription factors is known to regulate several genes required for DNA replication as well as cyclin D1 and cyclin E [31] that must be transcribed before the S phase of the cell cycle. Therefore, we next determined the level of E2F function in THP-1 cells cultured in the presence and absence of RA. When THP-1 cells were cultured in the presence of 10% FBS without RA, the binding of nuclear proteins to an E2F DNA element, detected by EMSA, was increased (Fig. 3, left panel). The addition of RA to THP-1 cells resulted in a down-regulation of the nuclear-binding complex. Binding specificity was demonstrated by the full competition of a 25-fold excess of unlabeled E2F consensus element, whereas a mutated element with two nucleotide substitutions in the core binding region (see Materials and methods) did not compete. By comparison, binding of the constitutively expressed transcription factor Sp-1 (Fig. 3, right panel) was not regulated by RA. These results demonstrate the integrity of the nuclear proteins in both control and RA-treated cells. The observation that RA can specifically reduced E2F binding activity, which may be caused by the enhanced functionality of pRb described above, suggests that the formation of an active suppressor complex by pRb/E2F could explain, at least in part, the reduced levels of cyclin E mRNA and protein shown in Fig. 1.
Fig. 3.
Regulation of E2F DNA binding activity by retinoic acid. THP-1 cells were treated with low serum conditioning and medium was replaced with 2% or 10% serum in the presence or absence of RA for 24 h. Nuclear protein was isolated and tested for binding activity with the 32P-labeled E2F consensus element. The left panel is the EMSA showing the formation of E2F protein–DNA binding complex, which appears as a diffuse complex, probably due to multiple interacting components. A 25-fold excess unlabeled E2F or mutated E2F DNA elements were used in the competition assay with the nuclear protein from 10% FBS-treated cells to demonstrate the specificity of the complex. The right panel shows the complex formed on a 32P-labeled Sp-1 consensus element incubated with the same nuclear protein extracts used in the left panel.
RA induces the arrest of THP-1 cells in the G1 phase of the cell cycle, associated with the development of macrophage function
Having observed thus far that RA can regulate the expression and function of cyclin E, p27, pRb, and E2F, all of which are important cell cycle regulators for the G1 to S phase transition [28], we next tested whether cell cycle progression is inhibited. Cells were treated as in previous experiments, as indicated in Fig. 4, and profiles of DNA were obtained using propidium iodide staining and flow cytometry to indicate the distribution of cells in various stages of the cell cycle. During growth in low serum conditioning (2% FBS), an average of 54% of THP-1 cells contained an amount of DNA consistent with their being in the G1 phase of the cell cycle (Fig. 4A). After the introduction of 10% serum for 24 h, the percentage of G1 phase cells was significantly reduced, while the percentage of S phase cells was increased (P < 0.001), indicating progression of the cell cycle. However, the combined addition of RA and 10% serum to cells consistently resulted in a higher percentage of G1 phase cells and a lower percentage of S phase cells (P < 0.001, Fig. 4A). Fig. 4B shows the percentage of S phase cells in the presence and absence of RA for up to 72 h of incubation in the presence of 10% FBS. At each time, the percentage of S phase cells was significantly reduced by RA (P < 0.001). The observed accumulation of cells in the G1 phase of the cell cycle and the reduction in S-phase cells are indicative of blockage at the G1/S checkpoint [32]. To further assess S phase cell function, [3H]-thymidine incorporation was determined as an indicator of cell proliferation (Fig. 4C). Similar to the results for cyclin E, the inclusion of 10% FBS stimulated [3H]-thymidine incorporation, whereas the addition of 10 or 100 nM RA reduced [3H]-thymidine incorporation by 30%. These data further support the hypothesis that RA regulates THP-1 cell proliferation by inhibiting the progression from the G1 phase to the DNA-synthetic phase of the cell cycle.
Fig. 4.
Regulation of THP-1 cell cycle progression by retinoic acid. THP-1 cells were treated as stated in Fig. 3. Cells were stained with propidium iodide/RNase A solution and the cell cycle profile was assessed by determining DNA content per cell. (A) THP-1 cell distribution during the cell cycle in the presence of RA at different concentrations. Cells treated with RA for 24 h are illustrated. Mean ± SD of 4 experiments conducted in triplicate. (B) The percentage of S phase cells in the presence and absence of 100 nM RA. The values shown in the graph are the mean ± SD of four independent experiments performed in triplicate; * P < 0.05. (C) [3H]Thymidine incorporation by THP-1 cells. After the addition of RA and 10% serum for 24 h, cells were pulse labeled with [3H]thymidine for the last 4 h and then harvested for scintillation counting. The reduction due to RA was calculated relative to the results for cells in medium with 10% FBS, set as 1.00. N = 5 samples per treatment; * P < 0.05. (D and E) Regulation of cellular cyclin E level and cell cycle progression by RA. THP-1 cells cultured in medium with 10% FBS (unsynchronized cells) were pulsed with BrdU for 1 h. Cells were then washed and cultured with and without RA and harvested after 4 and 24 h of RA treatment. Intracellular cyclin E protein (fluorescence) in total cells and BrdU-positive cells at 4 h (D), and the cell cycle profile (% of G1 and S-phase cells) (E), were determined by flow cytometry. Data represent two independent experiments with triplicate samples (mean ± SD). * P < 0.01.
To further study the association of cyclin E gene expression and cell cycle progression, a triple staining experiment was performed. To assure that the results shown above were not due to the synchronization process, we used THP-1 cells cultured in medium with 10% FBS (unsynchronized cells) in this experiment. THP-1 cells were first pulsed with BrdU for 1 h. Washed cells were then cultured in the presence and absence of 10 nM RA for 4 and 24 h. After harvesting, cellular cyclin E protein and cell cycle profile as well as BrdU labeling were analyzed by three-channel flow cytometry. As shown in Fig. 4D, the level of cyclin E protein was reduced by 4 h after RA treatment (Fig. 4D). The reduction was not only observed in the total cell population, but it was also observed in the BrdU-labeled cells which represent the S phase cells actively going through the cell cycle 1 h before RA treatment. For BrdU-labeled cells, cyclin E was reduced by 45% (P < 0.01) by RA at 4 h. However, the accumulation of G1 phase cells and the reduction of S phase cells by RA were detected only at later times, such as 24 h, by To-pro staining (Fig. 4E). The reduction in the level of cyclin E in these unsynchronized cells preceded the changes in cell cycle profile, confirming our suggestion that cyclin E plays an early role in RA-induced cell cycle regulation.
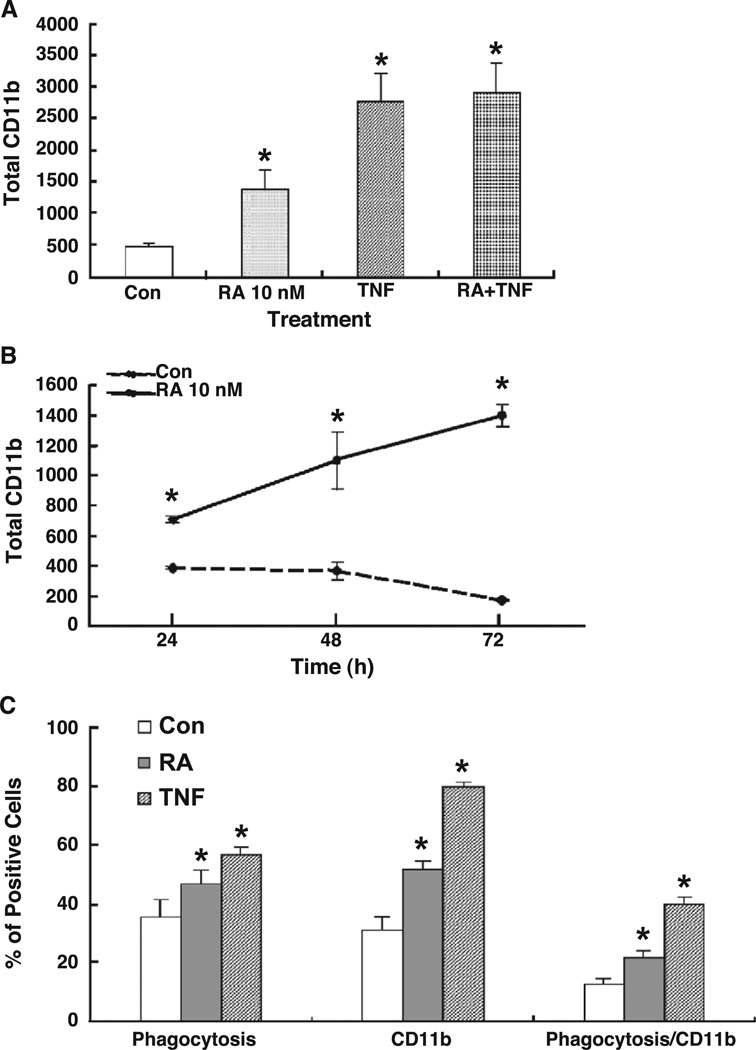
The expression of cell surface CD11b is known to be increased during monocyte differentiation and maturation, with the highest level on mature macrophages [21]. As the α subunit of the β2 integrin, CD11b forms a hetero-dimeric complex with CD18 on the surface of granulocytes and monocyte/macrophages that is important in a variety of adherence-related activities [33] and as a complement receptor which mediates the phagocytosis of opsonized particles [34]. To determine the state of THP-1 cell maturation after treatment with RA, cells exposed to 10 nM RA for 48 h were subjected to flow cytometry to determine the percentage of CD11b-positive cells and the level of CD11b expression per cell (fluorescence intensity). TNFα, known as an inducer of monocyte/macrophage maturation [35], was used as a positive control. Both RA and TNFα significantly induced the expression of CD11b, illustrated in Figs. 5A and 5B. The data shown illustrate total CD11b (the product of the percentage of positive cells and their mean fluorescence intensity); both of these parameters were significantly increased as the percentage of CD11b-positive cells rose from 40–50% in control cells to 80–90% in RA- or TNF-treated cells, while the average fluorescence intensity increased about 2- to 3-fold in RA- or TNF-treated cells (data not shown). The effect of the combination of RA plus TNFα was greater than either one alone, although less than additive.
Fig. 5.
CD11b expression level and phagocytosis activity in retinoic acid-treated THP-1 cells. THP-1 cells were plated in low serum condition and then treated with and without RA (10 nM) in the present of 10% FBS. (A) CD11b expression levels in THP-1 cells. Cells treated with and without RA for 48 h were subjected to flow cytometry after staining with an anti-CD11b antibody. The total expression level was calculated by multiplying the percentage of CD11b-positive cells by the average fluorescence intensity per cell. (B) CD11b level in THP-1 cells after treated for 24 to 72 h. (C) Phagocytic activity of RA-treated THP-1 cells. THP-1 cells were treated with or without RA for the times indicated before fluorescein-labeled E. coli bioparticles were added for an additional 2 h. After harvesting, the cells were subjected to CD11b staining and then analyzed by flow cytometry. The values shown are the mean ± SD of three independent experiments performed in triplicate. * P < 0.05.
To evaluate the functional activity of RA-treated THP-1 cells, phagocytosis was assessed using an opsonized fluorescein-tagged E. coli bioparticle to detect uptake into the cells. Phagocytosis was increased significantly (P < 0.05) by RA (10 nM), although somewhat less than by TNFα (Fig. 5C). When THP-1 cells were analyzed for both PE-labeled CD11b and fluorescein-tagged E. coli bioparticle uptake, the percentage of doubly stained cells was significantly higher after treatment with RA, indicating the attainment of both phenotypic and functional changes characteristic of a more mature stage of cell differentiation.
Discussion
The ability of all-trans-RA to induce differentiation in multiple cell lines [36,37] and to inhibit tumorigenesis in human and animal studies [38] has led to its clinical use either alone or in combination with other therapeutics in the treatment of several types of malignancy, including acute myelocytic leukemia [39] and certain solid tumors [9,10]. Unraveling the underlying mechanisms that lead to retinoid-induced cell differentiation is an essential task for researchers.
The present studies in the THP-1 cell model showed a time- and dose-dependent reduction in cyclin E mRNA and protein after the addition of physiological concentrations of RA to cell cultures (Figs. 1 and 4). Cyclin E, the regulatory subunit of the cyclin E/CDK2 complex, is synthesized in late G1 and is required for the transition from the G1 to the S phase of the cell cycle [40]. Expression of the cyclin E gene is regulated by the pRb-E2F pathway [26,27]. Mitogenic stimulations are known to induce E2F activity and then to promote cyclin E gene expression that, in turn, positively regulates cell cycle progression. The regulation of cyclin E by RA appears to be a strong feature in monocytic cells, such as THP-1, while in other cell models, the regulation of cyclin D was more predominant [32,37].
The other subunit of the complex, CDK2, is responsible for the enzymatic activity that phosphorylates target proteins, such as pRb, to regulate cell cycle-related functions. The activity of CDKs is controlled in part by families of CDK inhibitors, which are capable of blocking CDK activation. In RA-treated THP-1 cells, the level of the cyclin kinase inhibitor (CKI) p27, which belongs to a class of CKIs and can inhibit multiple cyclin-dependent kinases [40,41], was increased. Several mechanisms may be involved in the regulation of p27 expression [42–45], and at the present time, it is not known whether the increased expression of p27 protein in RA-treated cells is a direct effect of RA. It is possible that this increase is mediated indirectly by the effect of RA on cyclin E, since the cyclinE/CDK2 complex is known to destabilize and inhibit p27 function [46,47]. Similarly, the changes in the levels of RARα and RXRα protein, which correlated very well with cyclin E expression, could be regulated transcriptionally or post-transcriptionally [48,49]. Although we cannot rule out that the level of RARα was increased at an earlier time, our data do indicate that the maintenance of RARα or RXRα at an elevated or control level is not required for the down-regulation of cyclin E, which we observed as early as 6 h after addition of RA, or for the subsequent reduction in proliferation in RA-treated THP-1 cells. Moreover, consistent with our finding, several investigators have shown a reduction in RXRα and RARα protein levels in RA-treated cells in other cell models [48,49], suggesting that the reduced receptor level and the phosphorylation status play an indispensable role for the induction of G1 arrest and cell differentiation [49,50].
Because pRb is a critical regulatory protein in the G1/S cell cycle transition and is one of the target proteins of cyclin E/CDK2 [28], we proceeded to study the effect of RA on pRb function. Both the expression of pRb and its functionality in terms of its phosphorylation status are known to be important determinants of cell cycle progression [28,51], and the sequential phosphorylation of pRb by cyclin D/CDK4/6 and cyclin E/CDK2 is required for inactivation of pRb [52]. The major sites on pRb for phosphorylation by cyclin E/CDK2 are serine residues 807/811 which are in the C pocket of pRb [30]. This C pocket is also part of the large A/B pocket of pRb, which is important for the binding of E2F [30]. Retinoic acid reciprocally increased the level of pRb protein while reducing its phosphorylation status, as assessed using a specific antibody to detect total pRb and pRb phosphorylated on S807/811. Moreover, experiments at the mRNA level also showed that RA increased the expression of pRb (data not shown). It is known that pRb is a principal inhibitor of cell cycle progression due to its ability to interact with cellular target molecules, such as the E2F transcription factors whose transcriptional activity is actively repressed by pRb [53,54], or c-ABL whose tyrosine kinase activity is blocked [55]. As shown in Fig. 3, RA inhibited the formation of an E2F DNA binding complex, indicative of the existence of an active repressor, which most likely was formed by the association of pRb with E2F. The expression of pRb at higher levels in RA-treated cells would also be predicted to result in a higher proportion of cells in the G1 phase of the cell cycle and a lower proportion in S phase. As we have shown in Fig. 4, the addition of physiological concentrations of RA to THP-1 cells significantly slowed down the G1/S transition as indicated by an increase in G1 phase cells, a decrease in S phase cells, and a reduction in cell proliferation rate. The regulation of Rb by RA has been noted in several cell models including lymphocytes and monocytes [32,37,56,57] and therefore may be a common element of RA-induced growth suppression in cells of various lineages and stages of differentiation.
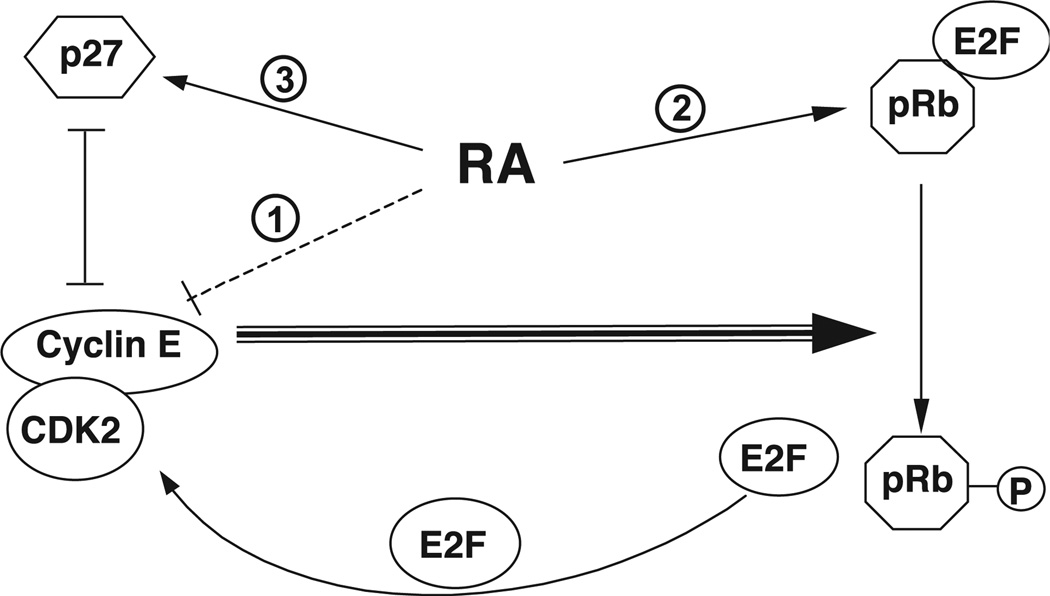
Taken together, the data from our experiments suggest a model in which RA can regulate cell growth through several, possibly concurrent, mechanisms (Fig. 6). When cells enter the cell cycle, as they are induced to do when cultured in higher concentrations of serum [22] or stimulated with specific mitogenic growth factors [58], cyclin E as the late G1 cyclin forms a functional complex with CDK2, which then catalyzes the further phosphorylation of pRb and leads to the activation of E2F. Among these factors, cyclin E is one of several crucial regulators of cell cycle progression at this point of cell cycle, and phosphorylation of pRb is a key event. In our studies, RA both decreased the amount of cyclin E and increased the amount of pRb, which could potentially restrain the cell cycle. The reduced level of hyperphosphorylated pRb in RA-treated cells also would be expected to result in a reduction in the activity of E2F which positively regulates the transcription of several cell growth-related genes, including the cyclin E gene, establishing a positive feedback loop. Thus, based on our results, we propose that RA affects cell cycle progression in THP-1 cells through several pathways: (i) by down-regulating the expression of cyclin E which can reduce the cyclin E/CDK2 complex activity and reduce the phosphorylation of pRb; (ii) by inducing the expression of pRb mRNA and protein while reducing the phosphorylation of pRb, changes which are expected to sequester the E2F transcription factor as a phosphorylated pRb/E2F complex and block the autoregulation of cyclin E gene expression; (iii) by regulating the accumulation of p27 protein, an inhibitor of cyclin E/CDK2 activity. This regulation could be a direct effect of RA on p27 or, as discussed above, the effect of RA on p27 could be indirect through the suppression of cyclin E/CDK activity, since elevated CDK2 activity has been shown to induce the proteolytic destruction of p27 [45,46]. The combined results of these multiple actions of RA could markedly increase the functionality of pRb and thereby reduce the rate of cell progression from the G1 to the S phase of the cell cycle.
Fig. 6.
A working model of retinoic acid-induced regulation of cell cycle progression in THP-1 cells. RA is proposed to regulate the differentiation of THP-1 cells through multiple pathways: (1) RA may reduce in the level of cyclin E, affecting the formation of cyclin E/CDK2 complex, and in turn reduces pRb phosphorylation; (2) RA may induce the expression of pRb mRNA and protein, enhancing the functionality of pRb in its negative regulation of cell growth; (3) RA may also affect the level of p27 protein, possibly directly or possibly as the indirect result of the suppression of cyclin E/CDK activity by RA. The combined results of these multiple actions markedly increase the functionality of pRb (increased ratio of Rb protein to hyperphosphorylated Rb as shown in Fig. 2), and reduces the rate of cell progression from the G1 to the S phase of the cell cycle.
The G1 phase of the cell cycle is a critical stage for cell differentiation and maturation [13,14]. CD11b, a cell surface marker of the myeloid cell lineage, is known to increase in expression as cells mature [21]. CD11b forms a functional complex with CD18 that mediates cell attachment and phagocytosis [34]. As shown in Fig. 5, the addition of RA not only induced the level of expression of CD11b, but also increased the phagocytic activity of THP-1 cells. These properties are required for the antigen-presenting activity of mature macrophages, and their up-regulation by RA suggests that similar changes in blood monocytes could be one mechanism through which retinoids enhance immunocompetence. Retinoic acid may also regulate cell cycle progression in other cells in the immune system, since in normal B lymphocytes, RA was shown to play a dual regulatory role by inhibiting the activation-induced cell proliferation by induction of the arrest in the G1 phase of the cell cycle [57], and at the same time, enhancing survival of resting B cells by preventing spontaneous apoptosis in vitro [59]. In a mouse model, RA was shown to promote the development of T helper 2 cells by acting on antigen presenting cells [60]. RA also increased the antigen-presenting activity of human epidermal Langerhans cells by inducing CD11c and MHC class II expression, leading to an increase in cutaneous immune responsiveness in human skin [61]. Our results in THP-1 cells further suggest that RA may induce monocytic cell differentiation and functional maturation with respect to CD11b-mediated complement functions, cell adhesion, and/or phagocytosis. All of these outcomes have the potential to increase immunocompetence and improve host defense mechanisms. In vivo, a deficiency of vitamin A and inadequate production of RA are likely to impair innate and adaptive immune responses through multiple mechanisms.
Acknowledgments
We thank Elaine Kunze and Susan Magargee for their helpful assistance with flow cytometry, and Denise Lackey for her participation in some of these experiments.
Grant support: this work was supported by NIH grant DK 41479 and funds from the Howard Heinz Endowment.
References
- 1.Soderlund MB, Sjoberg A, Svard G, Fex G, Nilsson-Ehle P. Biological variation of retinoids in man. Scand. J. Clin. Lab. Invest. 2002;62:511–519. doi: 10.1080/003655102321004521. [DOI] [PubMed] [Google Scholar]
- 2.Altucci L, Gronemeyer H. Nuclear receptors in cell life and death. Trends Endocrinol. Metab. 2001;12:460–468. doi: 10.1016/s1043-2760(01)00502-1. [DOI] [PubMed] [Google Scholar]
- 3.Wei LN. Retinoid receptors and their coregulators. Annu. Rev. Pharmacol. Toxicol. 2003;43:47–72. doi: 10.1146/annurev.pharmtox.43.100901.140301. [DOI] [PubMed] [Google Scholar]
- 4.Zile MH, Cullum ME, Roltsch IA, DeHoog JV, Welsch CW. Effect of moderate vitamin A supplementation and lack of dietary vitamin A on the development of mammary tumors in female rats treated with low carcinogenic dose levels of 7,12-dimethylbenz(a)anthracene. Cancer Res. 1986;46:3495–3503. [PubMed] [Google Scholar]
- 5.Ghosh J, Das S. A study on the effect of vitamin A deficiency and supplementation on tumorigenesis in mice. Neoplasma. 1988;35:41–49. [PubMed] [Google Scholar]
- 6.Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM, Liu MM, He KL, Niu C, Ma J, Zhang P, Zhang TD, Paul P, Naoe T, Kitamura K, Miller W, Waxman S, Wang ZY, de The H, Chen SJ, Chen Z. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- 7.Chao TY, Jiang SY, Shyu RY, Yeh MY, Chu TM. All-trans retinoic acid decreases susceptibility of a gastric cancer cell line to lymphokine-activated killer cytotoxicity. Br. J. Cancer. 1997;75:1284–1290. doi: 10.1038/bjc.1997.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagy L, Thomazy VA, Heyman RA, Davies PJ. Retinoid-induced apoptosis in normal and neoplastic tissues. Cell Death Differ. 1998;5:11–19. doi: 10.1038/sj.cdd.4400337. [DOI] [PubMed] [Google Scholar]
- 9.Todesco A, Carli M, Iacona I, Frascella E, Ninfo V, Rosolen A. All-trans retinoic acid and interferon-alpha in the treatment of a patient with resistant metastatic osteosarcoma. Cancer. 2000;89:2661–2666. doi: 10.1002/1097-0142(20001215)89:12<2661::aid-cncr20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Lippman SM, Lotan R, Schleuniger U. Retinoid-interferon therapy of solid tumors. Int. J. Cancer. 1997;70:481–483. doi: 10.1002/(sici)1097-0215(19970207)70:4<481::aid-ijc20>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 11.Ross AC. Vitamin A, retinoids and immune responses. In: Livrea MA, editor. Vitamin A and retinoids: an update of biological aspects and clinical applications. Basel/Switzerland: Birkhauser Verlag; 2000. [Google Scholar]
- 12.Semba RD. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc. Nutr. Soc. 1999;58:719–727. doi: 10.1017/s0029665199000944. [DOI] [PubMed] [Google Scholar]
- 13.Ohnuma S, Philpott A, Harris WA. Cell cycle and cell fate in the nervous system. Curr. Opin. Neurobiol. 2001;11:66–73. doi: 10.1016/s0959-4388(00)00175-6. [DOI] [PubMed] [Google Scholar]
- 14.Zhu L, Skoultchi AI. Coordinating cell proliferation and differentiation. Curr. Opin. Genet Dev. 2001;11:91–97. doi: 10.1016/s0959-437x(00)00162-3. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int. J. Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T, Tada K. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982;42:1530–1536. [PubMed] [Google Scholar]
- 17.Kremlev SG, Phelps DS. Effect of SP-A and surfactant lipids on expression of cell surface markers in the THP-1 monocytic cell line. Am. J. Physiol. 1997;272:L1070–L1077. doi: 10.1152/ajplung.1997.272.6.L1070. [DOI] [PubMed] [Google Scholar]
- 18.Defacque H, Dornand J, Commes T, Cabane S, Sevilla C, Marti J. Different combinations of retinoids and vitamin D3 analogs efficiently promote growth inhibition and differentiation of myelomonocytic leukemia cell lines. J. Pharmacol. Exp. Ther. 1994;271:193–199. [PubMed] [Google Scholar]
- 19.Ferret PJ, Soum E, Negre O, Wollman EE, Fradelizi D. Protective effect of thioredoxin upon NO-mediated cell injury in THP1 monocytic human cells. Biochem. J. 2000;346(Pt. 3):759–765. [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 21.Maciaszek JW, Coniglio SJ, Talmage DA, Viglianti GA. Retinoid-induced repression of human immunodeficiency virus type 1 core promoter activity inhibits virus replication. J. Virol. 1998;72:5862–5869. doi: 10.1128/jvi.72.7.5862-5869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orren DK, Petersen LN, Bohr VA. A UV-responsive G2 checkpoint in rodent cells. Mol. Cell Biol. 1995;15:3722–3730. doi: 10.1128/mcb.15.7.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Q, Ma Y, Ross AC. Opposing cytokine-specific effects of all trans-retinoic acid on the activation and expression of signal transducer and activator of transcription (STAT)-1 in THP-1 cells. Immunology. 2002;107:199–208. doi: 10.1046/j.1365-2567.2002.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu B, Kim ST, Lim DS, Kastan MB. Two molecularly distinct G(2)/M checkpoints are induced by ionizing irradiation. Mol. Cell. Biol. 2002;22:1049–1059. doi: 10.1128/MCB.22.4.1049-1059.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomasoni D, Lupi M, Brikci FB, Ubezio P. Timing the changes of cyclin E cell content in G1 in exponentially growing cells. Exp. Cell Res. 2003;288:158–167. doi: 10.1016/s0014-4827(03)00207-6. [DOI] [PubMed] [Google Scholar]
- 26.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 27.Sherr CJ. Cell cycle control and cancer. Harvey Lect. 2000;96:73–92. [PubMed] [Google Scholar]
- 28.Cooper S, Shayman JA. Revisiting retinoblastoma protein phosphorylation during the mammalian cell cycle. Cell Mol. Life Sci. 2001;58:580–595. doi: 10.1007/PL00000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitagawa M, Higashi H, Jung HK, Suzuki-Takahashi I, Ikeda M, Tamai K, Kato J, Segawa K, Yoshida E, Nishimura S, Taya Y. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 1996;15:7060–7069. [PMC free article] [PubMed] [Google Scholar]
- 30.Knudsen ES, Wang JY. Differential regulation of retinoblastoma protein function by specific Cdk phosphorylation sites. J. Biol. Chem. 1996;271:8313–8320. doi: 10.1074/jbc.271.14.8313. [DOI] [PubMed] [Google Scholar]
- 31.Ohtani K, DeGregori J, Nevins JR. Regulation of the cyclin E gene by transcription factor E2F1. Proc. Natl. Acad. Sci. U. S. A. 1995;92:12146–12150. doi: 10.1073/pnas.92.26.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dimberg A, Bahram F, Karlberg I, Larsson LG, Nilsson K, Oberg F. Retinoic acid-induced cell cycle arrest of human myeloid cell lines is associated with sequential down-regulation of c-Myc and cyclin E and posttranscriptional up-regulation of p27(Kip1) Blood. 2002;99:2199–2206. doi: 10.1182/blood.v99.6.2199. [DOI] [PubMed] [Google Scholar]
- 33.Hickstein DD, Baker DM, Gollahon KA, Back AL. Identification of the promoter of the myelomonocytic leukocyte integrin CD11b. Proc. Natl. Acad. Sci. U. S. A. 1992;89:2105–2109. doi: 10.1073/pnas.89.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Cabec V, Carreno S, Moisand A, Bordier C, Maridonneau-Parini I. Complement receptor 3 (CD11b/CD18) mediates type I and type II phagocytosis during nonopsonic and opsonic phagocytosis, respectively. J. Immunol. 2002;169:2003–2009. doi: 10.4049/jimmunol.169.4.2003. [DOI] [PubMed] [Google Scholar]
- 35.Winston BW, Krein PM, Mowat C, Huang Y. Cytokine-induced macrophage differentiation: a tale of 2 genes. Clin. Invest. Med. 1999;22:236–255. [PubMed] [Google Scholar]
- 36.Lee KH, Chang MY, Ahn JI, Yu DH, Jung SS, Choi JH, Noh YH, Lee YS, Ahn MJ. Differential gene expression in retinoic acid-induced differentiation of acute promyelocytic leukemia cells, NB4 and HL-60 cells. Biochem. Biophys. Res. Commun. 2002;296:1125–1133. doi: 10.1016/s0006-291x(02)02043-0. [DOI] [PubMed] [Google Scholar]
- 37.Zhang D, Vuocolo S, Masciullo V, Sava T, Giordano A, Soprano DR, Soprano KJ. Cell cycle genes as targets of retinoid induced ovarian tumor cell growth suppression. Oncogene. 2001;20:7935–7944. doi: 10.1038/sj.onc.1204971. [DOI] [PubMed] [Google Scholar]
- 38.Zusi FC, Lorenzi MV, Vivat-Hannah V. Selective retinoids and rexinoids in cancer therapy and chemoprevention. Drug Discovery Today. 2002;7:1165–1174. doi: 10.1016/s1359-6446(02)02526-6. [DOI] [PubMed] [Google Scholar]
- 39.Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, Gu LJ, Wang ZY. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–572. [PubMed] [Google Scholar]
- 40.Bast RC, Kufe DW, Pollock RE, Weichselbaum RR, Holland JF, Emil FE, Gansler TS. Cancer Medicine. Section 1. Cancer Biology. Ontario: B.C. Decker; 2000. [Google Scholar]
- 41.Matsuo T, Thiele CJ. p27Kip1: a key mediator of retinoic acid induced growth arrest in the SMS-KCNR human neuroblastoma cell line. Oncogene. 1998;16:3337–3343. doi: 10.1038/sj.onc.1201830. [DOI] [PubMed] [Google Scholar]
- 42.Servant MJ, Coulombe P, Turgeon B, Meloche S. Differential regulation of p27(Kip1) expression by mitogenic and hypertrophic factors: Involvement of transcriptional and posttranscriptional mechanisms. J. Cell Biol. 2000;148:543–556. doi: 10.1083/jcb.148.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirano M, Hirano K, Nishimura J, Kanaide H. Transcriptional upregulation of p27(Kip1) during contact-induced growth arrest in vascular endothelial cells. Exp. Cell Res. 2001;271:356–367. doi: 10.1006/excr.2001.5384. [DOI] [PubMed] [Google Scholar]
- 44.Shirane M, Harumiya Y, Ishida N, Hirai A, Miyamoto C, Hatakeyama S, Nakayama K, Kitagawa M. Down-regulation of p27(Kip1) by two mechanisms, ubiquitin-mediated degradation and proteolytic processing. J. Biol. Chem. 1999;274:13886–13893. doi: 10.1074/jbc.274.20.13886. [DOI] [PubMed] [Google Scholar]
- 45.Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 46.Vlach J, Hennecke S, Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamura M, Matsuo T, Stauffer J, Neckers L, Thiele CJ. Retinoic acid decreases targeting of p27 for degradation via an N-myc-dependent decrease in p27 phosphorylation and an N-myc-independent decrease in Skp2. Cell Death Differ. 2003;10:230–239. doi: 10.1038/sj.cdd.4401125. [DOI] [PubMed] [Google Scholar]
- 48.Nomura Y, Nagaya T, Hayashi Y, Kambe F, Seo H. 9-cis-retinoic acid decreases the level of its cognate receptor, retinoid X receptor, through acceleration of the turnover. Biochem. Biophys. Res. Commun. 1999;260:729–733. doi: 10.1006/bbrc.1999.0969. [DOI] [PubMed] [Google Scholar]
- 49.Matsushima-Nishiwaki R, Okuno M, Adachi S, Sano T, Akita K, Moriwaki H, Friedman SL, Kojima S. Phosphorylation of retinoid X receptor alpha at serine 260 impairs its metabolism and function in human hepatocellular carcinoma. Cancer Res. 2001;61:7675–7682. [PubMed] [Google Scholar]
- 50.Wang J, Barsky LW, Shum CH, Jong A, Weinberg KI, Collins SJ, Triche TJ, Wu L. Retinoid-induced G1 arrest and differentiation activation are associated with a switch to cyclin-dependent kinase-activating kinase hypophosphorylation of retinoic acid receptor alpha. J. Biol. Chem. 2002;277:43369–43376. doi: 10.1074/jbc.M206792200. [DOI] [PubMed] [Google Scholar]
- 51.Connell-Crowley L, Harper JW, Goodrich DW. Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol. Biol. Cell. 1997;8:287–301. doi: 10.1091/mbc.8.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol. Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Payton M, Coats S, Cyclin E2. the cycle continues. Int. J. Biochem. Cell Biol. 2002;34:315–320. doi: 10.1016/s1357-2725(01)00137-6. [DOI] [PubMed] [Google Scholar]
- 54.Adams PD. Regulation of the retinoblastoma tumor suppressor protein by cyclin/cdks. Biochim. Biophys. Acta. 2001;1471:M123–M133. doi: 10.1016/s0304-419x(01)00019-1. [DOI] [PubMed] [Google Scholar]
- 55.Wang JY. Regulation of cell death by the Abl tyrosine kinase. Oncogene. 2000;19:5643–5650. doi: 10.1038/sj.onc.1203878. [DOI] [PubMed] [Google Scholar]
- 56.Yen A, Roberson MS, Varvayanis S, Lee AT. Retinoic acid induced mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase-dependent MAP kinase activation needed to elicit HL-60 cell differentiation and growth arrest. Cancer Res. 1998;58:3163–3172. [PubMed] [Google Scholar]
- 57.Naderi S, Blomhoff HK. Retinoic acid prevents phosphorylation of pRB in normal human B lymphocytes: regulation of cyclin E, cyclin A, and and p21(Cip1) Blood. 1999;94:1348–1358. [PubMed] [Google Scholar]
- 58.Reddy GP, Tiarks CY, Pang L, Wuu J, Hsieh CC, Quesenberry PJ. Cell cycle analysis and synchronization of pluripotent hematopoietic progenitor stem cells. Blood. 1997;90:2293–2299. [PubMed] [Google Scholar]
- 59.Lomo J, Smeland EB, Ulven S, Natarajan V, Blomhoff R, Gandhi U, Dawson MI, Blomhoff HK. RAR-, not RXR, ligands inhibit cell activation and prevent apoptosis in B-lymphocytes. J. Cell. Physiol. 1998;175:68–77. doi: 10.1002/(SICI)1097-4652(199804)175:1<68::AID-JCP8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 60.Hoag KA, Nashold FE, Goverman J, Hayes CE. Retinoic acid enhances the T helper 2 cell development that is essential for robust antibody responses through its action on antigen-presenting cells. J. Nutr. 2002;132:3736–3739. doi: 10.1093/jn/132.12.3736. [DOI] [PubMed] [Google Scholar]
- 61.Meunier L, Bohjanen K, Voorhees JJ, Cooper KD. Retinoic acid upregulates human Langerhans cell antigen presentation and surface expression of HLA-DR and CD11c, a beta 2 integrin critically involved in T-cell activation. J. Invest. Dermatol. 1994;103:775–779. doi: 10.1111/1523-1747.ep12413014. [DOI] [PubMed] [Google Scholar]