Abstract
Retinoic acid (RA), produced from vitamin A (VA, retinol), is required for normal lung development and postnatal lung maturation. The concentration of retinyl ester (RE), the major storage form of retinol, decreases in the lungs in the perinatal period. Previously, we tested VARA, a nutrient-metabolite combination of VA and RA (10:1 molar ratio), on lung RE formation in postnatal rats and showed that the components of VARA acted synergistically to increase lung RE, as compared with the effects of equal amounts of VA and RA given alone. In this study, we first determined the equivalency of orally administered VARA in comparison to a standard oral supplement of VA, with respect to lung and liver RE storage. In a dose-dilution study, VARA was 4 times as effective as the standard dose of VA (VARA-25% did not differ from VA-100%). The synergistic effect of VARA was selective for the lungs, compared with the liver, in which VA and VARA had equal effects. Secondly, we tested whether the 2 components of VARA must be coadministered to exert their synergistic effect on lung RE content. RA and VA and were administered separately and together as VARA. Although RA alone had no effect on lung RE in this 24-h experiment, RA synergized with VA administered either 12 h before RA or 12 h after RA, as well as when coadministered as VARA. We infer that VA and RA are both limiting for lung RE formation in neonates. Given the importance of bioactive retinoids in cell differentiation and lung development, assuring an adequate lung RE content postnatally could be of benefit for lung maturation.
Introduction
Retinoids produced from vitamin A (VA,5 retinol) are required for normal lung development (1–3). During the embryonic period of organogenesis, retinoic acid (RA) regulates factors involved in stromal cell–parenchymal cell interactions that, in turn, are required for branching morphogenesis and angiogenesis (2–6). The lungs continue to develop in the postnatal period. RA has been shown to promote alveolar septation, a process essential for increasing the gas-exchange surface area of the lungs. Moreover, RA has shown potential for ameliorating lung injury, as demonstrated in glucocorticoid-treated neonatal rats and mice (7–10) and in other models of lung injury (8,11). Vitamin A, given orally or intramuscularly as retinol, improves the outcome for premature infants of low birth weight, who are at risk for bronchopulmonary dysplasia and are more likely to have low levels of plasma retinol (12–17).
Retinyl esters (RE) are the major storage form of VA in most tissues. The concentration of RE in the lungs is highest a few days before birth and then declines by postnatal d (P) 3 (18), near the beginning of the alveolar stage of lung development [from P4 to P14 in rats and mice and from 36 wk gestation up to ~2 y in humans (6)]. Previous studies have shown that VA supplementation of pregnant rats can increase lung RE in their offspring (19). However, direct oral supplementation of the offspring may be more efficient. Recently, we compared lung RE levels in neonatal rats supplemented orally from P5 to P7 with VA alone, its metabolite RA alone, or a nutrient-metabolite combination, which we refer to as VARA, that contained VA and RA mixed in a molar ratio of 10:1 (20). In those neonates given VARA, the concentration of RE in the lungs was increased synergistically, several times more than by the same amount of VA alone. Other studies in adult rats treated by repeated intraperitoneal doses of RA (21) or fed a high level of RA in the diet (22) have also shown that RA increases the storage of VA in the lungs. However, in our studies, the RA dose was much lower and the effect of VARA on lung RE was apparent after the first dose (20). Additionally, a metabolic experiment showed that the uptake of labeled retinol in the VARA mixture was increased 6 h after oral dosing (20). However, our previous study was not designed to make a quantitative comparison between VARA and VA, with respect to RE formation. Thus, the first objective of this study was to determine the equivalency of VARA and RA by directly comparing the RE response, in the lungs and liver of postnatal rats, to a series of concentrations of VARA, which we compared with a standard dose of VA.
The second objective of this study was to determine whether the components of VARA must be coadministered to synergize in increasing lung RE formation. We consider it likely that the VA and RA components of VARA, even when given together, would be absorbed from the intestine by different routes. Dietary RE must be hydrolyzed in the lumen or on the brush border before the uptake of retinol by enterocytes, and new REs formed within the enterocytes are incorporated into chylomicrons [reviewed in (23,24)]. Chylomicrons are initially secreted into mesenteric lymphatic ducts. After chylomicrons enter the venous circulation, most of the triacylglycerol in the chylomicron lipid core is rapidly hydrolyzed by lipoprotein lipase and, during this lipolytic process, a minor fraction of newly absorbed RE is apparently taken up by lipoprotein lipase-containing tissues (25–27). However, most of the newly absorbed REs, contained in the core of chylomicron remnants, are rapidly taken up by liver parenchymal cells. In the liver, RE are rapidly hydrolyzed, after which the retinol is either secreted into plasma on retinol-binding protein or reesterified and stored within hepatic stellate cells (28). In contrast to retinol, most RA is absorbed via the portal circulation, bound to albumin, and then removed very rapidly from plasma into numerous tissues; however, RA is not stored to any significant extent (29,30). In adult rats, >95% of an i.v. dose of 3H-RA disappeared from plasma within 3–5 min (31,32), and the oxidative metabolism of 3H-RA was also rapid (32). Considering these intrinsic differences in the absorption and turnover of VA and RA, our second experiment was designed to test the idea that the components of VARA, even if delivered separately, might still recapitulate the synergistic effect of VARA in increasing RE in the lungs.
Materials and Methods
Dose preparation
Vitamin A, in the form of all-trans-retinyl palmitate, and all-trans-RA were purchased from Sigma-Aldrich. Vitamin A was first prepared as a 2× concentrated stock solution in canola oil at 0.1 mmol/g oil, and RA was prepared as a 2× concentrated stock solution at 0.01 mmol/g, as previously described (20). These stock solutions were then mixed 1:1 by weight to form the oral dose referred to as VARA-100% (0.05 mmol VA and 0.005 mmol RA/g dose). Similarly, the standard VA dose (0.05 mmol VA/g) was prepared by diluting 2× VAwith oil. For Expt. 1, VARA-100% was further diluted by weight with canola oil to form the doses referred to as VARA-50%, VARA-25% and VARA-12.5%. For Expt. 2, a dose of RA only was also prepared (0.005 mmol RA/g). The doses were stored at 4°C and protected from light and air.
Animals and experimental designs
Animal procedures were approved by the Institutional Animal Use and Care Committee of The Pennsylvania State University. Vitamin A–adequate Sprague-Dawley rats (obtained from Charles River) were mated in our animal facility. In Expt. 1 (equivalency study), pups were assigned at random to 6 treatments (n = 4/group) and were treated on P5, 6, and 7 with either oil (Control), the standard dose of VA, VARA-100%, VARA-50%, VARA-25%, or VARA-12.5%. The volume of each dose was ~0.4 μL/g body wt, adjusted daily after weighing, and delivered with a micropipette directly into the rat pup’s mouth.
In Expt. 2 (coadministration study), the components of VARA were administered to 9- to 10-d-old rat pups, weighing ~20 g, either separately, 12-h apart, or together in a 24-h experiment. The first dose was administered at 0 h, the second dose at 12 h, and the experiment ended at 24 h. Those pups that were treated with VA or RA received the same amount of these individual components as were given in the VARA-100% treatment. VARA itself was delivered either once as a single dose at 0 h, or as the same dose divided into halves (half at 0 h and half at 12 h). The treatment schedule was designed so that all pups received the same volume of oil and the same amount of VA and RA as in VARA, regardless of the order of treatment.
At the end of each experiment, pups were killed with carbon dioxide and weighed. Blood was collected from the vena cava, and the lungs and liver were removed, trimmed and weighed, and then frozen in liquid nitrogen and stored at −80°C prior to retinoid analysis.
Retinoid analysis
Lung and liver tissues were prepared for analysis of RE and retinol by extracting weighed portions of each organ overnight in 20 or more volumes of chloroform:methanol, 2:1 v:v (33). After washing the extracts, they were dried under argon and reconstituted in a small volume of chloroform:methanol, 1:1, to which a known amount of an internal standard of trimethylmethoxyphenyl-retinol was added. The samples from Expt. 1 were then analyzed by HPLC for retinol and REs, as described previously (20,34). The eluate was monitored at 325 nm with a photodiode array detector, and the areas of the peaks for TMMP-retinol, unesterified retinol, and RE were integrated. Results are shown as RE (sum of retinyl palmitate, oleate, and stearate) + retinol/g tissue, where retinol was almost always <5% of the total. Lung tissue samples from Expt. 2 were extracted similarly, but the chloroform:methanol extract was then dried under argon, saponified, and total retinol extracted after the hydrolysis of REs was determined by HLPC. Total retinol by this faster method was comparable to the sum of retinol plus REs, as was checked for several samples. Because ~95% of the total retinol was esterified before saponification, it is referred to as RE in the text.
Statistics
Data are presented as the means ± SEM. When variances among groups were unequal, the data were log10 transformed before statistical analysis by t test or ANOVA, followed by least squares means test (SuperANOVA software, Abacus). Differences with P < 0.05 were considered significant.
Results
Dose dilution to determine equivalency of VARA and VA in neonatal lungs
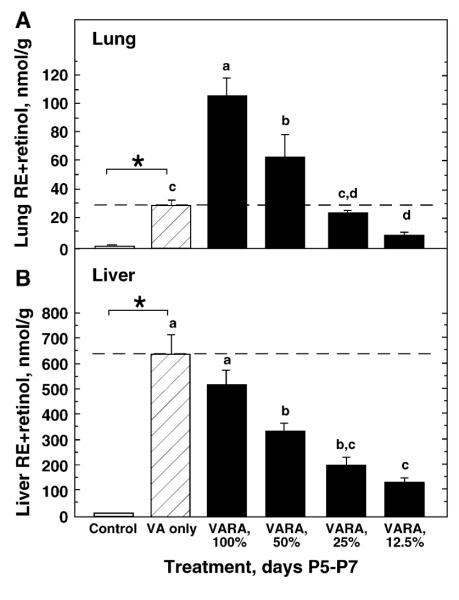
In Expt. 1, neonates were treated for 3 d with vehicle, the standard VA dose (VA only), VARA-100% containing the same amount of VA as the standard VA dose, and 3 dilutions of VARA (Fig. 1A). Growth did not differ by treatment (data not shown). Lung RE differed between the control group and the VA-only group, as expected. Compared with VA only, both VARA-100% and VARA-50% increased lung RE significantly. VA-only and VARA-25% increased lung RE equally. Although the difference between VA only and VARA-12.5% was not significant by ANOVA and least squares means test (P = 0.087), the mean for VARA-12.5% was only one-third the mean for VA only, and this difference was significant by unpaired t test (P < 0.0001). Therefore, we do not consider VARA-12.5% to be equivalent to the standard dose of VA. From this experiment, VARA was 4 times as effective as VA alone in increasing RE in the lungs.
Figure 1.
VARA diluted to 25% is as effective as a standard dose of VA in increasing lung RE in postnatal rats. Concentrations of RE + retinol were measured in lungs (A) and liver (B) of 8-d-old rats treated on P5–7 with oil (Control), a standard dose of VA only, VARA-100% containing the same amount of VA, and 3 dilutions of VARA (VARA-50%, VARA-25%, and VARA-12.5%). The dashed line marks the RE+retinol concentration produced by the standard dose of VA alone. Values are means ± SEM, n = 4. *Control and VA groups differed, P < 0.05 (t test). Means for all groups other than the control without a common letter differ, P < 0.05 (ANOVA and LSM test). In (B), statistical analyses were performed on log 10-transformed data.
In the liver, VARA-100% and the standard VA dose both increased RE nearly 50-fold above the nonsupplemented control group. However, VARA and VA did not differ from each other (P = 0.205). Liver RE concentrations were increased above the control level by all VARA dilutions, in nearly direct proportion to the VA content of VARA.
Effect of VARA components delivered separately or coadministered on lung RE concentration
In Expt. 2, the components of VARA-100% were delivered separately, 12 h apart, as well as together (Fig. 2). Treatment with RA alone, regardless of the time interval (either oil first, RA second, or RA first, oil second), did not differ from the oil-oil (control) group. When the treatment included VA, whether alone or with RA, lung RE was significantly higher than in the control group, with the exception of oil first, then VA, at 12 h. When RA was administered first, before VA, lung RE was significantly higher than when oil was administered before VA. Likewise, when VA was administered first, before RA, lung RE was also significantly increased. These treatments did not differ from oil and VARA combined. The highest mean increase was obtained by administering the VARA dose as a divided dose, half at 0 h and half at 12 h. Overall, coadministration of VA and RA was not essential for their synergy. Still, coadministration as a divided dose of VARA was the most effective, increasing lung RE to a level ~10 times that in the control group, and 2.5–3 times that in the 2 groups treated with VA only, within this 24-h study.
Figure 2.
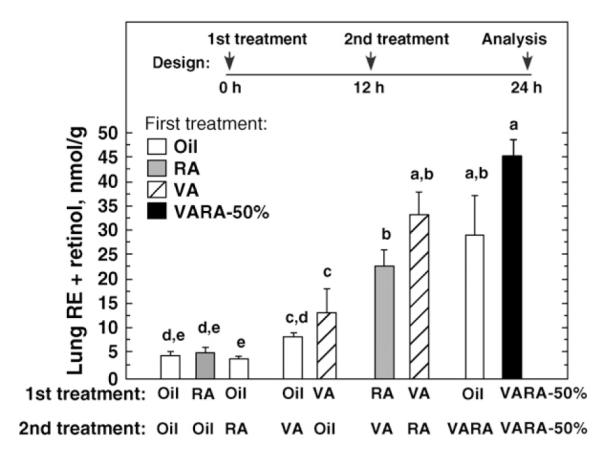
Rat lung RE is increased synergistically by VA and RA given separately or combined as VARA. The components of VARA were delivered separately or together, as shown on the X-axis. Lung RE+retinol was measured 24 h after the start of the experiment. The first treatment, at 0 h, is listed in the legend box and shown by the shading of the bars, and the second treatment, at 12 h, is shown on the X-axis. Values are means ± SEM, n = 3 or 4. Means without a common letter differ, P < 0.05 (ANOVA and LSM test). Data were log 10-transformed before statistical analysis.
Discussion
Nutrient-nutrient interactions usually involve 2 or more different nutrients. In this study, we examined an intranutrient interaction involving VA and its most active metabolite, RA. In previous work, we showed that VARA, a combination of VA and RA, acts synergistically to increase the concentration of RE in the lungs of neonatal rats (20). In our current studies, we investigated 2 questions: How much greater is the impact of retinol on lung RE formation when RA is coadministered in VARA? Do the 2 components of VARA need to be given as a single dose, or can they interact at the tissue level when administered separately? Our VARA combination was originally formulated based on amounts of VA (retinol, in the form of retinyl palmitate) and RA that have been used previously and independently for different purposes. Doses of 50,000 or 100,000 IU of VA are used, as recommended by the World Health Organization, to protect vulnerable infants and young children from VA deficiency for a period of 4–6 mo (35). Our standard VA dose for postnatal rats (as well as the amount of VA in VARA-100%) was based on a dose of 50,000 IU of VA in human infants, scaled down for rats on the basis of body weight (20). RA has been tested for its potential to promote lung maturation in neonatal rats. In those studies, 500 mg RA/kg (given i.p. daily from P3 to P14) was shown to improve alveolar septation in dexamethasone-treated and control rat pups (7,8). Additionally, a dose of 0.5 mg RA/kg is a common starter dose that is used therapeutically for treatment of leukemias and diseases of the skin (36). The preparation we have referred to as VARA-100% was the combination of the standard VA dose with the amount of RA that was used previously in neonatal rats, after we adjusted the latter dose slightly for oral administration (20). By combining these 2 independently selected doses, the resulting molar ratio of VA to RA in VARA was 10:1.
The mechanism by which VARA promotes lung RE formation to a greater extent than an equal amount of VA is not yet known, but because RA is not a substrate for RE synthesis, the RA component of VARA must be modifying the metabolism of the VA component. The combination of VA and RA could be useful for promoting RE formation in the lungs, and thus it was of interest to determine the equivalency of VARA as compared with VA, and to understand whether the 2 components of VARA must be coadministered. In the dose-dilution study (Expt. 1), the increase in lung RE produced by the standard dose of VA and undiluted VARA was compatible with our previous findings (20). The dilution curve fell nearly linearly, indicating that none of the doses used, including VARA-100%, were saturating with respect to lung RE formation. Thus, the capacity of neonatal lungs to store RE is much higher than is suggested by the usual RE concentration in the lungs of nonsupplemented neonates. The dose-response curve showed that the effect of VARA-25% was equal to that of the standard VA dose (Fig. 1A). The amount of VA in VARA-25% would be similar, scaled for the size of an infant or young child, to a supplement of 12,500 IU (3.75 mg retinol) of VA, and doses of 50,000 IU VA have been shown to be safe in infants (37,38). The RA component of VARA-25% equalled ~0.125 mg RA/kg, which is well below doses now used therapeutically (36). Overall, this study showed that RA, present at one-tenth the amount of VA in VARA, gave the retinol component of VARA a “4× boost” with respect to lung RE formation and retention.
In the liver, VARA-100% and the standard VA dose increased RE concentration equally (20). When VARA was diluted (Fig. 1B), the amount of VA stored in the liver fell nearly dose dependently. It is noteworthy that the VA concentration in the liver of nonsupplemented 8-d-old neonates, born to and nursed by VA-adequate dams, averaged <12 nmol/g (<5 μg retinol/g). By comparison, VA concentrations <20 μg retinol/g in adult livers are considered to indicate that VA stores are inadequate and are associated with low plasma retinol (39). Even the most diluted VARA preparation we tested (VARA-12.5%), increased liver VA to >105 nmol/g (>30 μg retinol/g). Although plasma retinol was normal in the control group (1.4 μmol/L), liver RE was very low in the absence of VA supplementation. However, liver RE was increased dose dependently by VA and by VARA alike. Therefore, the results suggest that supplementation with either VA or VARA would be helpful in protecting the neonate, or a prematurely born infant whose VA levels at birth are even lower, against a future shortfall in VA intake. Overall, our comparison of the response of the lungs and the liver to VARA shows that the “potency” of VARA is not fixed, but instead is tissue specific, as the regulation of RE formation appears to differ significantly for the lungs compared with the liver.
In Expt. 2, we determined whether the 2 components of VARA must be coadministered to act synergistically on lung RE formation. Due to known differences in their metabolism and routes of absorption from the intestine, we hypothesized that their synergy in the lungs might still be observed even if they were not coadministered. We also thought that RA might be more effective if it was given before VA, based on the notion that RA, which is well known to induce gene expression (40), might “prime” the lungs by up-regulating factors (presently unknown) that could, in turn, facilitate the uptake of retinol or its retention as RE in neonatal lungs. The results provided support for the main hypothesis, as all combinations of VA and RA, whether given simultaneously, VA before RA, or RA before VA, resulted in significantly higher concentrations of RE in the lungs, compared with the same amount of VA given alone (Fig. 2), and, therefore, concurrent administration of the 2 retinoids was not required for synergy between VA and RA. However, we were surprised that RA was at least as effective when it was administered after VA as when administered before VA. RA given after VA may have facilitated the uptake or retention by the lungs of retinol that had already been absorbed prior to treatment with RA. It is also possible that part of the VA itself was converted to RA, and therefore the total amount of RA from the oral supplement and from the metabolism of retinol given earlier could have been higher in this group. It is not known whether increasing the RA in VARA, for example by formulating VARA with a VA to RA ratio of 10:2, would further increase RE in the lungs.
Numerous nutritional factors are important to the growth and development of the lungs (41). Our results provide some insight into factors that are limiting for RE formation. RA alone did not increase lung RE, and therefore it did not synergize with “endogenous” retinol (present without VA supplementation) within the short time frame of this study. Yet RA did synergize with VA when VA was administered as a supplement. From this comparison, it can be inferred that the amount of VA that neonates obtain from milk is a limiting factor for RE in the lungs. We can also reason that the production of RA from VA by the neonate is another limiting factor, because supplementation with VA could not exert a maximal effect on lung RE concentration, unless exogenous RA was also administered. It is therefore likely that both factors, VA and RA, are limiting for lung RE formation in neonates. Overall, although coadministration was not essential for the synergy between VA and RA, coadministration was at least as effective, or more so.
In conclusion, VARA promoted RE formation in the lungs of neonatal rats 4 times more than an equal amount of VA. This enhancement was selective for the lungs, as liver RE storage was the same with VARA and VA alone. Neonates are born with very low reserves of VA (42), and VARA, even if diluted to VARA-25%, might be an effective treatment to stimulate the uptake and esterification of VA in the lungs, while still modestly increasing the storage of VA in the liver. Although a functional benefit of VARA has not yet been demonstrated, tissue RE reserves themselves are increasingly being recognized as a source of retinol that can generate bioactive retinoids in the lungs (43). Given the importance of bioactive retinoids, such as RA, in cell differentiation and lung development, lung RE could be an important and possibly limiting factor in the generation of retinoids needed for lung maturation in the postnatal period.
Footnotes
Supported by NIH CA-90214 and the Graduate Program in Nutrition.
Abbreviations used: P, postnatal d; RA, retinoic acid; RE, retinyl ester; VA, vitamin A; VARA, vitamin A-retinoic acid combination dose; VARA-100%, 50%, 25%, and 12.5%: full-, half-, quarter-, and eighth-concentrations of VARA, respectively.
Literature Cited
- 1.Chytil F. Retinoids in lung development. FASEB J. 1996;10:986–92. doi: 10.1096/fasebj.10.9.8801181. [DOI] [PubMed] [Google Scholar]
- 2.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev. 2000;92:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 3.Maden M, Hind M. Retinoic acid in alveolar development, maintenance and regeneration. Philos Trans R Soc Lond B Biol Sci. 2004;359:799–808. doi: 10.1098/rstb.2004.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuger L, Varani J, Mitra R, Jr., Gilbride K. Retinoic acid stimulates mouse lung development by a mechanism involving epithelial-mesenchymal interaction and regulation of epidermal growth factor receptors. Dev Biol. 1993;159:462–73. doi: 10.1006/dbio.1993.1256. [DOI] [PubMed] [Google Scholar]
- 5.McGowan SE. Contributions of retinoids to the generation and repair of the pulmonary alveolus. Chest. 2002;121:206S–8S. doi: 10.1378/chest.121.5_suppl.206s. [DOI] [PubMed] [Google Scholar]
- 6.Roth-Kleiner M, Post M. Similarities and dissimilarities of branching and septation during lung development. Pediatr Pulmonol. 2005;40:113–34. doi: 10.1002/ppul.20252. [DOI] [PubMed] [Google Scholar]
- 7.Massaro GD, Massaro D. Postnatal treatment with retinoic acid increases the number of pulmonary alveoli in rats. Am J Physiol. 1996;270:L305–L10. doi: 10.1152/ajplung.1996.270.2.L305. [DOI] [PubMed] [Google Scholar]
- 8.Massaro GD, Massaro D. Retinoic acid treatment partially rescues failed septation in rats and in mice. Am J Physiol Lung Cell Mol Physiol. 2000;278:L955–L60. doi: 10.1152/ajplung.2000.278.5.L955. [DOI] [PubMed] [Google Scholar]
- 9.Veness-Meehan KA, Pierce RA, Moats-Staats BM, Stiles AD. Retinoic acid attenuates O2-induced inhibition of lung septation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L971–80. doi: 10.1152/ajplung.00266.2001. [DOI] [PubMed] [Google Scholar]
- 10.Maden M. Retinoids have differing efficacies on alveolar regeneration in a dexamethasone treated mouse. Am J Respir Cell Mol Biol. 2006 doi: 10.1165/rcmb.2006-0029OC. in press. [DOI] [PubMed] [Google Scholar]
- 11.Kaza AK, Kron IL, Kern JA, Long SM, Fiser SM, Nguyen RP, Tribble CG, Laubach VE. Retinoic acid enhances lung growth after pneumonectomy. Ann Thorac Surg. 2001;71:1645–50. doi: 10.1016/s0003-4975(01)02478-x. [DOI] [PubMed] [Google Scholar]
- 12.Shenai JP, Kennedy KA, Chytil F, Stahlman MT. Clinical trial of vitamin A supplementation in infants susceptible to bronchopulmonary dysplasia. J Pediatr. 1987;111:269–77. doi: 10.1016/s0022-3476(87)80086-0. [DOI] [PubMed] [Google Scholar]
- 13.Shenai JP, Rush MG, Stahlman MT, Chytil F. Vitamin A supplementation and bronchopulmonary dysplasia–revisited. J Pediatr. 1992;121:399–401. doi: 10.1016/s0022-3476(05)81793-7. [DOI] [PubMed] [Google Scholar]
- 14.Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, Stoll BJ, Lemons JA, Stevenson DK, et al. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 1999;340:1962–8. doi: 10.1056/NEJM199906243402505. [DOI] [PubMed] [Google Scholar]
- 15.Darlow BA, Graham PJ. Vitamin A supplementation for preventing morbidity and mortality in very low birthweight infants. Cochrane Database Syst Rev. 2002;(4):CD000501. doi: 10.1002/14651858.CD000501. [DOI] [PubMed] [Google Scholar]
- 16.Ambalavanan N, Tyson JE, Kennedy KA, Hansen NI, Vohr BR, Wright LL, Carlo WA, Natl Inst Child Hlth Human Dev NRN Vitamin A supplementation for extremely low birth weight infants: Outcome at 18 to 22 months. Pediatrics. 2005;115:E249–E54. doi: 10.1542/peds.2004-1812. [DOI] [PubMed] [Google Scholar]
- 17.Ambalavanan N, Wu TJ, Tyson JE, Kennedy KA, Roane C, Carlo WA. Comparison of three vitamin A dosing regimens in extremely-low-birth-weight infants. J Pediatr. 2003;142:656–61. doi: 10.1067/mpd.2003.214. [DOI] [PubMed] [Google Scholar]
- 18.Shenai JP, Chytil F. Vitamin A storage in lungs during perinatal development in the rat. Biol Neonate. 1990a;57:126–32. doi: 10.1159/000243172. [DOI] [PubMed] [Google Scholar]
- 19.Shenai JP, Chytil F. Effect of maternal vitamin-A administration on fetal lung vitamin-A stores in the perinatal rat. Biol Neonate. 1990b;58:318–25. doi: 10.1159/000243286. [DOI] [PubMed] [Google Scholar]
- 20.Ross AC, Ambalavanan N, Zolfaghari R, Li N-q. Vitamin A combined with retinoic acid increases retinol uptake and lung retinyl ester formation in neonatal rats. J Lipid Res. 2006;47:1844–51. doi: 10.1194/jlr.M600061-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Barua AB, McGowan SE, Ivanoff KD, Goswami BC, Olson JA. Elevation of retinyl ester level in the lungs of rats following repeated intraperitoneal injections of retinoic acid or retinoyl glucuronide. Pulm Pharmacol Ther. 2004;17:113–9. doi: 10.1016/j.pupt.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Cifelli CJ, Green JB, Green MH. Dietary retinoic acid alters vitamin A kinetics in both the whole body and in specific organs of rats with low vitamin A status. J Nutr. 2005;135:746–52. doi: 10.1093/jn/135.4.746. [DOI] [PubMed] [Google Scholar]
- 23.Harrison EH. Mechanisms of digestion and absorption of dietary vitamin A. Annu Rev Nutr. 2005;25:87–103. doi: 10.1146/annurev.nutr.25.050304.092614. [DOI] [PubMed] [Google Scholar]
- 24.Ross AC, Harrison EH. Vitamin A and carotenoids. In: McCormick DB, Rucker RR, Suttie JW, Janos Zempleni J, editors. Handbook of Vitamins. 4th ed. CRC Press; Boca Raton: 2006. In press. [Google Scholar]
- 25.Hagen E, Myhre AM, Smeland S, Halvorsen B, Norum KR, Blomhoff R. Uptake of vitamin A in macrophages from physiologic transport proteins: role of retinol-binding protein and chylomicron remnants. J Nutr Biochem. 1999;10:345–52. doi: 10.1016/s0955-2863(99)00013-3. [DOI] [PubMed] [Google Scholar]
- 26.van Bennekum AM, Kako Y, Weinstock PH, Harrison EH, Deckelbaum RJ, Goldberg IJ, Blaner WS. Lipoprotein lipase expression level influences tissue clearance of chylomicron retinyl ester. J Lipid Res. 1999;40:565–74. [PubMed] [Google Scholar]
- 27.Ross AC, Pasatiempo AM, Green MH. Chylomicron margination, lipolysis, and vitamin A uptake in the lactating rat mammary gland: implications for milk retinoid content. Exp Biol Med (Maywood) 2004;229:46–55. doi: 10.1177/153537020422900106. [DOI] [PubMed] [Google Scholar]
- 28.Blomhoff R, Green MH, Green JB, Berg T, Norum KR. Vitamin A metabolism: new perspectives on absorption, transport and storage. Physiol Rev. 1991;71:951–90. doi: 10.1152/physrev.1991.71.4.951. [DOI] [PubMed] [Google Scholar]
- 29.Blaner WS, Olson JA. Retinol and retinoic acid metabolism. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry and Medicine. Raven Press; New York: 1994. pp. 229–55. [Google Scholar]
- 30.Napoli JL. Enzymology and biogenesis of retinoic acid. In: Livrea MA, editor. Vitamin A and retinoids: An update of biological aspects and clinical applications. 1st ed Birkhèuser Verlag; Basel: 2000. pp. 17–27. [Google Scholar]
- 31.Kurlandsky SB, Gamble MV, Ramakrishnan R, Blaner WS. Plasma delivery of retinoic acid to tissues in the rat. J Biol Chem. 1995;270:17850–7. doi: 10.1074/jbc.270.30.17850. [DOI] [PubMed] [Google Scholar]
- 32.Cifelli CJ, Ross AC. All-trans-retinoic acid distribution and metabolism in vitamin A-marginal rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G195–202. doi: 10.1152/ajpgi.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 34.Zachman RD, Kakkad B, Chytil F. Perinatal rat lung retinol (vitamin A) and retinyl palmitate. Pediatr Res. 1984;18:1297–9. doi: 10.1203/00006450-198412000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Ross DA. Recommendations for vitamin A supplementation. J Nutr. 2002;132:2902S–6S. doi: 10.1093/jn/132.9.2902S. [DOI] [PubMed] [Google Scholar]
- 36.Zouboulis CC. Retinoids - Which dermatological indications will benefit in the near future? Skin Pharmacol Appl Skin Physiol. 2001;14:303–15. doi: 10.1159/000056361. [DOI] [PubMed] [Google Scholar]
- 37.Agoestina T, Humphrey JH, Taylor GA, Usman A, Subardja D, Hidayat S, Nurachim M, Wu L, Friedman DS, West JKP, Sommer A. Safety of one 52-mmol (50 000 IU) oral dose of vitamin A administered to neonates. Bull W H O. 1994;72:859–68. [PMC free article] [PubMed] [Google Scholar]
- 38.Humphrey JH, Agoestina T, Juliana A, Septiana S, Widjaja H, Cerreto MC, Wu LSF, Ichord RN, Katz J, West KP., Jr. Neonatal vitamin A supplementation: effect on development and growth at 3 y of age. Am J Clin Nutr. 1998;68:109–17. doi: 10.1093/ajcn/68.1.109. [DOI] [PubMed] [Google Scholar]
- 39.Olson JA. Serum level of vitamin A and carotenoids as reflectors of nutritional status. J Natl Cancer Inst. 1984;73:1439–44. [PubMed] [Google Scholar]
- 40.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 41.Atkinson SA. Special nutritional needs of infants for prevention of and recovery from bronchopulmonary dysplasia. J Nutr. 2001;131:942S–6S. doi: 10.1093/jn/131.3.942S. [DOI] [PubMed] [Google Scholar]
- 42.Ross AC. Introduction to vitamin A: a nutritional and life cycle perspective. In: Packer L, Obermüller-Jevic U, Kraemer K, Sies H, editors. Carotenoids and Retinoids Molecular Aspects and Health Issues. AOCS Press; Champaign, IL: 2005. pp. 23–41. [Google Scholar]
- 43.McGowan SE, Doro MM, Jackson SK. Endogenous retinoids increase perinatal elastin gene expression in rat lung fibroblasts and fetal explants. Am J Physiol. 1997;273:L410–6. doi: 10.1152/ajplung.1997.273.2.L410. [DOI] [PubMed] [Google Scholar]