Abstract
Vitamin A supplementation for infants and young children is recommended by WHO/UNICEF for countries with a high prevalence of vitamin A deficiency, and vitamin A is often administered at immunization contacts. Using a rat model, we tested whether supplementation with vitamin A or other retinoids at the time of neonatal immunization has prospective benefit in terms of preventing postweaning vitamin A deficiency and promoting antibody responses to T-cell dependent (TD) antigens administered at the neonatal stage and at the young adult stage. Rats were treated orally on postnatal d 6–8 with oil (placebo control), vitamin A, retinoic acid, or a combination of both (VARA) (n ≥ 12/group), and immunized with tetanus toxoid (TT) on d 7. The primary anti-TT response was measured on d 21, after which weanling rats were fed the vitamin A-deficient diet until ~ 10 wk. At 8 wk, rats were immunized again with TT to determine the recall response, and with a novel TD antigen, keyhole limpet hemocyanin (KLH), to assess the adult primary response. None of the supplements affected the plasma titer of anti-TT immunoglobulinG (IgG) on d 21 (P = 0.25). However, neonatal-age supplementation with vitamin A or VARA at the young adult stage resulted in: >5 times higher anti-TT IgG recall response (P < 0.01); 5- and 9-times higher anti-KLH primary IgM and IgG responses, respectively (P < 0.05), and plasma retinol in the normal range (~1.0 µmol/L vs. ~0.35 µmol/L in retinoic acid-treated and control groups, P < 0.0001). We conclude that early-life supplementation with vitamin A or VARA can prospectively benefit the primary and recall antibody responses to TD antigens administered at the young adult stage, which may involve the maintenance of normal plasma retinol levels.
Introduction
Vitamin A (retinol) and its active metabolites are essential for normal innate immunity and the development of humoral immune responses to vaccines and pathogens (1,2). Vitamin A is an important, cost-effective micronutrient for improving health and survival in a population <5 y of age (3). Randomized clinical trials have shown that vitamin A supplementation administered to children and infants at risk of vitamin A deficiency reduces young-child mortality by 22–30% (4–6). Vitamin A is also effective in reducing morbidity due to measles (7). These effects of vitamin A are thought to be attributable to its ability to enhance immune responses, which thereby leads to reduced severity of infectious diseases and an increased likelihood of survival (1,8,9). As a result of the demonstrated protective effect of vitamin A, children in countries where vitamin A deficiency is most prevalent are receiving supplements of vitamin A along with immunization against poliovirus, tuberculosis, measles, diphtheria, pertussis, and tetanus, based on policies established by WHO/UNICEF (10,11). Whereas the primary goal of vitamin A supplementation is to prevent vitamin A deficiency (5), there is also interest in whether vitamin A affects the outcome of vaccine-induced immune responses (11). Several animal studies showed that giving vitamin A or its active metabolite, retinoic acid (RA),5 along with vaccination, often increases the humoral immune response (2). However, studies of the antibody response of young children who have received vitamin A at the time of vaccination have been inconsistent, and most studies have failed to demonstrate an impact on morbidity (12) or increased antibody production following primary immunization [see (2,9) for reviews]. It has been suggested that in a number of these studies the sample size was too small, and/or the vitamin A status of the children may have been adequate even without vitamin A supplementation (2).
Regardless of whether neonatal vitamin A supplementation promotes the initial antibody response following primary immunization, this treatment may still be advantageous if it delays the onset of vitamin A deficiency and thereby improves the secondary, or recall, response elicited by subsequent immunization with the same antigen or exposure to the pathogen targeted by the vaccine. Based on this reasoning, we designed the present immunization studies using a neonatal rat model to test 2 main hypotheses. First, we hypothesized that vitamin A supplementation given to neonates concurrent with immunization, whether or not it enhances the neonatal primary antibody response, will increase the recall response at a young adult age. This hypothesis is relevant to human vaccination because an effective recall response is crucial for boosting vaccine-induced antibodies that are crucial for neutralizing the cognate pathogen during episodes of infection. We used tetanus toxoid (TT) as the antigen because it is 1) a thymus-dependent (TD) antigen requiring the involvement of T-helper (Th) cells; 2) widely used as a recall antigen for assessing immunocompetence in humans; 3) capable of priming neonatal-aged rats (13) and mice (14) for a secondary antibody response; and 4) highly relevant clinically. A recent survey in 193 countries estimated that infection with Clostridium tetani is still one of the major causes of neonatal death (15). Second, we tested the related hypothesis that vitamin A supplementation during the neonatal period will benefit the primary immunization response to a novel TD antigen administered to young adult rats. It is possible that providing vitamin A to neonates, if it prevents vitamin A deficiency, might enhance immune responses at a later age. This hypothesis is relevant for determining whether improving the vitamin A status of neonates is beneficial with respect to enhancing immunity to new vaccines or pathogens encountered later in childhood. To our knowledge, this situation has not yet been evaluated in human clinical studies of vitamin A supplementation. In the present study, we treated neonates with vitamin A (all-trans-retinyl palmitate) alone and with RA alone. Each of these forms of vitamin A has been shown to increase TD antibody responses in mouse and rat models. We also treated a group of neonates with the combination of vitamin A and RA [VARA (16)]. This combination has been shown to increase vitamin A storage in the lungs of neonatal rats and mice (16–18), but its immunological effects are not known.
Materials and Methods
Animals and diets
The animal protocols were approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University. Sprague-Dawley rat dams (virus antibody-free) were either purchased from Charles River Breeding Laboratory as lactating rats with 12–14 female pups, or they were mated with males of the same strain in our animal facility. All rats were housed in a rodent facility at 22°C with a 12-h light-dark cycle and given free access to food and drinking water. Dams were fed AIN-93G diet (19) modified to lack vitamin A (20). This protocol reduces transfer of vitamin A in milk to the nursling pups (21); however, neither the dams nor pups were vitamin A-deficient at this stage. After weaning (see below), rats were fed either vitamin A-deficient diet (Expt. 1 and 2) or the same diet containing 4 µg of retinol, in the form of retinyl palmitate, per gram of diet (Research Diets) to maintain adequate vitamin A status during growth.
Experimental design, immunization, and blood collection
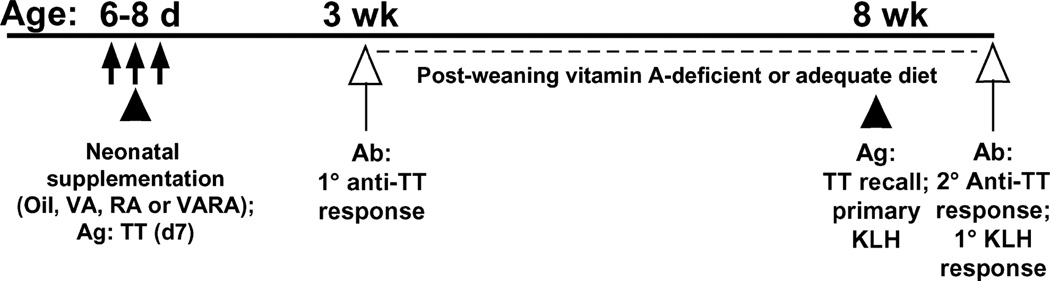
Rat pups within litters were randomly assigned to 4 different supplementation groups: control (vehicle only), vitamin A, RA, and VARA (Fig. 1). The preparation of the doses was previously described (16). Neonates were treated with either canola oil (placebo control, n = 15), vitamin A (50 nmol of retinyl palmitate per mL oil, n = 14), all-trans-RA (5 nmol/mL oil, n = 12), and an exact combination, VARA containing retinyl palmitate and RA in a 10:1 ratio [(16), n = 20]. The dose of vitamin A only and the amount of vitamin A in VARA represent, on a per-kg basis (16), an amount of retinol similar to the dose of 15mgof retinol (25,000IU) recommended for infants (11,22) and scaled to body weight (16). The dose of RA given alone and the amount of RA present in VARA were equal, and both were based on a dose of 500 µg RA/kg that, when previously injected i.p. to 4–14 d–old neonatal rats, improved lung septation (23). Because RA and VARA were administered orally, the amount of RA was increased 25% (16). The doses were all given on a weight-adjusted basis by weighing each pup daily and delivering 0.4 µL of the dose preparation per gram of body weight directly into the mouth using a small micropipette. Each neonate received its assigned dose 3 times, on d 6, d 7 (the day of primary immunization with TT), and d 8 (Fig. 1).
FIGURE 1.
Schematic of study design. VA, vitamin A, Ab, antibody; Ag, antigen; 1°, primary; 2° secondary. Closed arrows indicate days of neonatal supplementation (d 6–8), closed triangles indicate times of immunization, and open upward arrows indicate times of blood collection. The dashed line indicates the duration of the experimental diet, either vitamin A-deficient or vitamin A-adequate.
The main study comprised the pooled results of 2 separately conducted experiments (n = 61 rats), all of which were fed vitamin A-deficient diet after weaning. Each of the 2 experiments included all 4 neonatal supplementation groups described above and were of similar design except that rats in Expt. 1 were injected i.p. with TT (lot 297; Connaught Laboratories), 10 µg in 300 µL of sterile saline on d 7 for primary immunization, and again with TT (100 µg in 1 mL sterile saline at 8 wk) for secondary immunization; whereas rats in Expt. 2 (Fig. 1) were immunized with TT (10 µg on d 7) for primary immunization, again with TT (100 µg at 8 wk) for secondary immunization, and with keyhole limpet hemocyanin (KLH) [150 µg (24) at 8 wk] for primary adult-age immunization.6 As an additional control to Expt. 1, a parallel experiment was conducted with 24 rats administered neonatal treatments exactly as in Expt. 1 (n = 6/group), but were fed vitamin A-adequate diet in the postweaning period (beginning at age 21 d); these rats were reimmunized with TT as 8-wk–old young adults.
To assess the primary anti-TT response, ~0.2 mL of heparinized blood was collected from the retro-orbital sinus of rats sedated by isofluraneoxygen inhalation or from the tail vein. Seven to 10 d after the second immunization with TT, rats were killed by carbon dioxide inhalation and heparinized blood was collected from the vena cava. The separated plasma was stored at −20°C prior to assay for anti-TT and anti-KLH antibodies.
Plasma anti-TT and anti-KLH antibody determinations
Anti-TT immunoglobulin G (IgG) and anti-KLH immunoglobulin M (IgM) and IgG responses were quantified by ELISA, as previously described for TT (25,26), and slightly modified for KLH (24). Briefly, wells of polystyrene plates (Immulon 4HBX 96-well flat bottom plates, Thermo Electron) were coated with 100 µL of TT [10 mg/L in 0.15 mol/L Tris-HCl buffer (TB buffer), pH 7.6] or KLH (10 µg/mL in NaHCO3 buffer, pH 9.5). After overnight incubation, the plates were washed and incubated for 1 h with blocking solution [1% bovine serum albumin (BSA) in 0.15 mol/L TB buffer for TT-coated plates, or 1% BSA in saline for KLH-coated plates]. After blocking, 100 µL of six 2-fold serial dilutions of rat plasma samples were added to the wells and incubated at 4°C overnight. The plate was washed and then incubated with 100 µL of appropriately diluted horseradish peroxidase-conjugated sheep anti-rat IgG (Binding Site), or goat anti-rat IgM (Southern Biotech) at room temperature for 1 h after washing 3 times, 100 µL of 3,3′,5,5′-tetramethylbenzidine substrate (BD Biosciences) was added to each well for color development, and subsequently 50 µL of 1 mol/LH3PO4 was added to stop the reaction. Absorbance was read at 450 and 570 nm on a dual channel microplate reader. As an internal control, each plate contained a serially diluted standard plasma sample obtained from vitamin A-adequate adult rats on 10 d after the secondary immunization with either 100 µg TT or 150 µg of KLH. The titers of anti-TT IgG, anti-KLH IgM, and anti-KLH IgG were calculated by comparing the measurements in a linear dose-response range to the serially diluted pooled serum run as a standard on each plate. One unit was defined as the dilution fold that produced 50% of the maximal optical density for the standard sample.
Plasma retinol analysis
Plasma total retinol concentrations were quantified after saponification using reverse-phase HPLC with UV detection, as previously described (27).
Statistical analysis
Data are expressed as means ± SEM. When variances among groups were unequal, the antibody titer values were transformed logarithmically (log10) to obtain a normal distribution. Differences among the 4 supplementation groups of rats fed vitamin A-deficient diet (Expt. 1 and 2 combined), and similarly for the study of vitamin A–adequate rats, were determined by 2-way ANOVA with vitamin A and RA as factors. Group means were compared by 1-way ANOVA and Least Significant Difference test. Linear regression was used to test associations. Statistical analyses were performed using the statistical program SuperAnova (Abacus Software). Significant difference was determined at P ≤ 0.05.
Results
Neonatal supplementation did not affect early-life growth
Neonatal supplementation with oil, vitamin A, RA, and VARA was well tolerated. Body weights, which were measured every week throughout the study, did not differ among the 4 groups before or after they were fed vitamin A-deficient diet (P > 0.1).
Neonatal supplementation did not increase the primary anti-tetanus IgG response
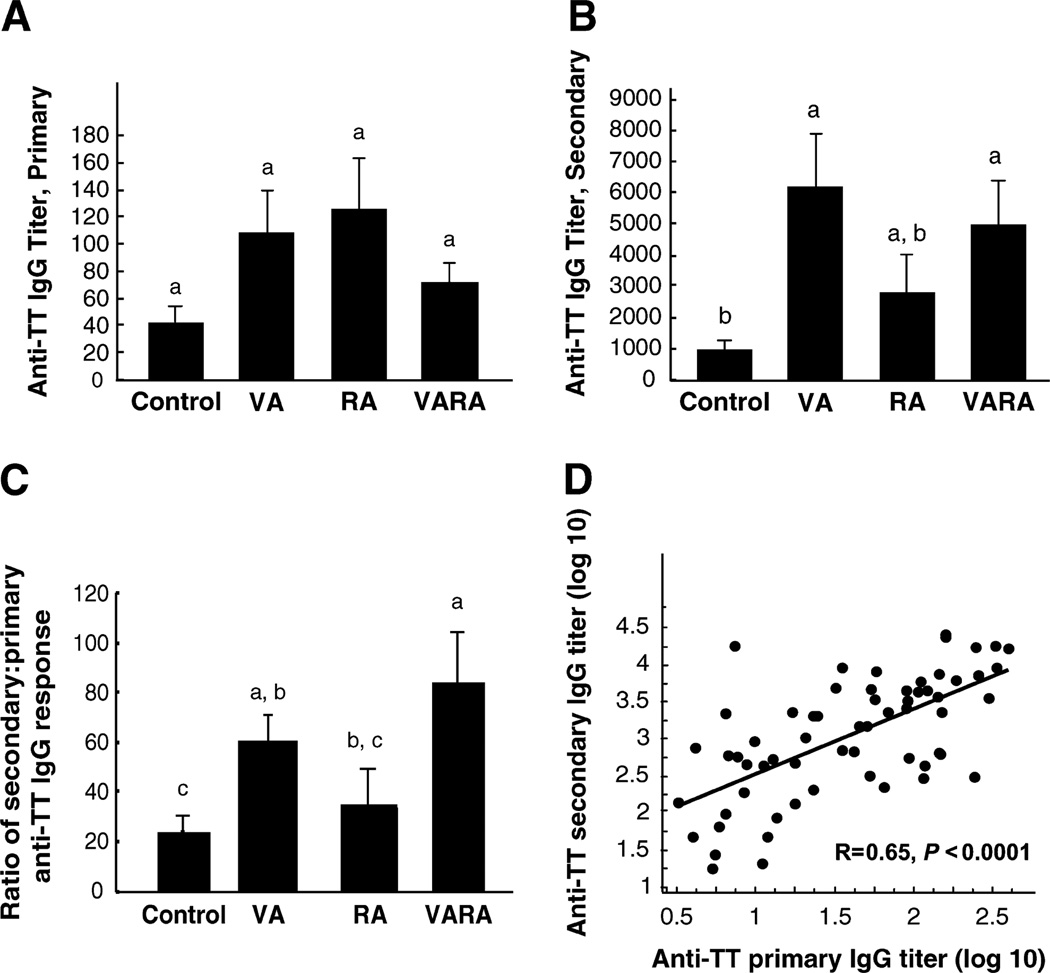
The primary anti-TT IgG response was measured 14 d after priming and did not differ (P = 0.25), although the mean responses of vitamin A, RA, and VARA-supplemented pups were all higher than that of the control group (Fig. 2A).
FIGURE 2.
Neonatal supplementation with vitamin A or VARA increases the secondary antibody response to TT in rats fed vitamin A-deficient diet after weaning. Values are means ± SEM, n = 12/group (A, B, and C). Groups with superscripts with different letters differ, P < 0.01 (B and C). Primary anti-TT IgG titer assayed 14 d after primary immunization with TT on d 7 (A). Secondary (recall) anti-TT IgG titer (B). Ratio of secondary to primary anti-TT IgG titer (C). Correlation between the primary and secondary anti-TT IgG titer, tested for all rats, n = 61, (D).
Neonatal supplementation with vitamin A and VARA increased the anti-tetanus IgG recall response in vitamin A-deficient young adult rats
Vitamin A-deficient young adult rats that received vitamin A or VARA as neonates produced a secondary anti-TT IgG response that was 5.6-times and 5.3-times higher, respectively, compared with the control group (P < 0.01; Fig. 2B). Neonatal supplementation with RA resulted in an intermediate response [P > 0.05 compared with control, vitamin A, and VARA groups (Fig. 2B)]. To estimate the booster effect of reimmunization, the ratio of the secondary anti-TT IgG to primary anti-TT IgG was calculated for each supplementation group (Fig. 2C). The ratio was higher for the vitamin A and VARA groups compared with the control group (both P < 0.01). For all rats, the primary and secondary IgG anti-TT titers were correlated (r = 0.70, P < 0.0001; Fig. 2D).
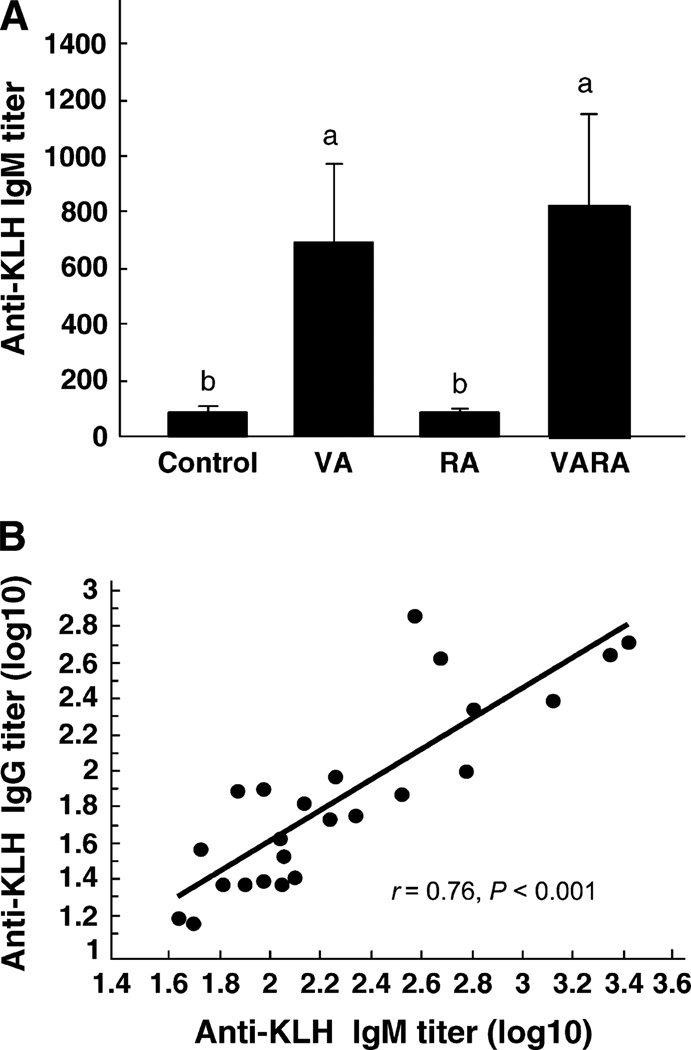
Neonatal supplementation with VA or VARA increased primary anti-KLH response in vitamin A-deficient young adult rats
The primary anti-KLH IgM titers of rats that had been supplemented as neonates with vitamin A or VARA were 8- and 9-times higher, respectively, compared with the control group (Fig. 3A, P ≤ 0.05), whereas anti-KLH IgG was 8- and 5-times higher, respectively (data not shown). Neonatal supplementation with RA did not affect the primary anti-KLH response in vitamin A-deficient young adult rats. The primary anti-KLH IgM and anti-KLH IgG titers were correlated (r = 0.88, P < 0.001, not shown). Although TT and KLH are independently immunogenic antigens, the correlation between the primary anti- KLH IgG response and the secondary anti-TT IgG, measured at the same time, was significant (Fig. 3B, r = 0.56, P ≤ 0.01). However, the primary anti-KLH IgG response at young adult age was not correlated with the primary anti-TT IgG response of the same rats at neonatal age (r = 0.35, P > 0.1, not shown).
FIGURE 3.
Vitamin A supplementation at the neonatal age increases the young-adult–age primary response to a novel T-cell dependent antigen, KLH, in rats fed vitamin A-deficient diet. The KLH antibody response [anti-KLH IgM (A) and anti-KLH IgG, not shown] was measured 10 d after primary immunization at 8 wk of age (A). Values are means ± SEM, n = 6/group. Groups with superscripts with different letters differ, P < 0.05. Correlation of the primary anti-KLH IgG response with the secondary anti-TT IgG, both at young adult age (B).
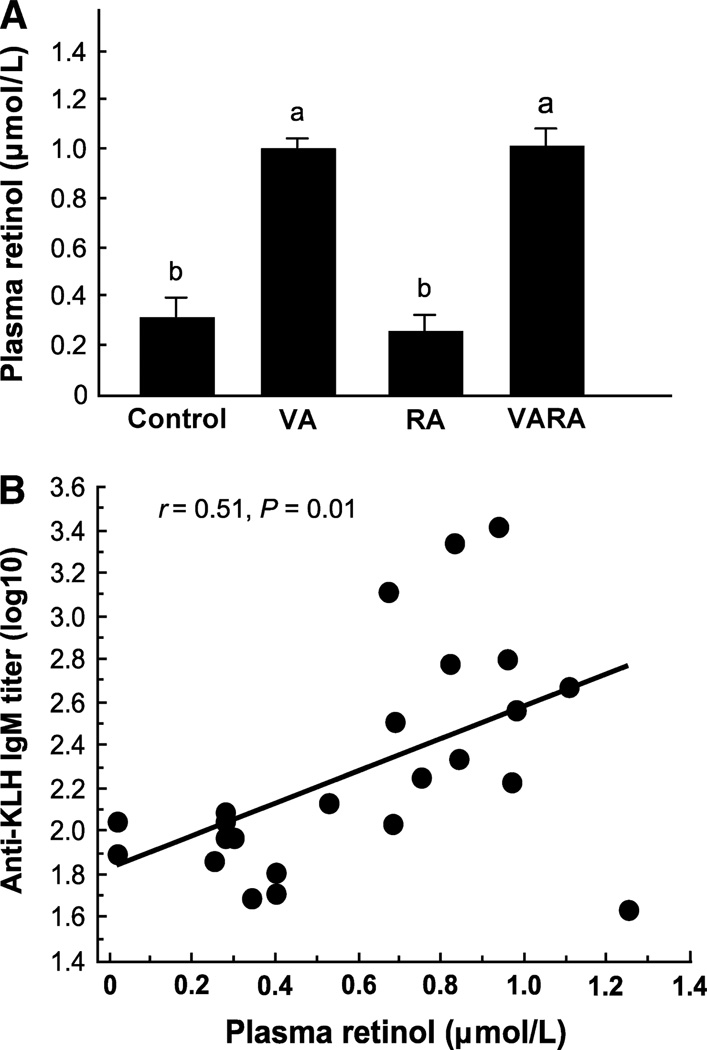
Neonatal supplementation with vitamin A and VARA maintained plasma retinol levels in young adult rats fed vitamin A-deficient diet
When rats were supplemented as neonates with either vitamin A or VARA, their plasma retinol concentration remained at ~1 µmol/L even though they had consumed vitamin A-deficient diet from weaning to 10 wk of age (Fig. 4A, P < 0.0001 vs. control group). Retinoic acid-treated rats had the same low plasma retinol (~0.35 µmol/L) as control-treated vitamin A-deficient rats. The adult age primary anti-KLH IgM titer (log10) was associated with plasma retinol concentration (r = 0.51, P < 0.01, Fig. 4B), as were the primary anti-KLH IgG (r = 0.56, P < 0.01) and secondary anti-TT IgG titers (r = 0.31, P < 0.05) (data not shown).
FIGURE 4.
Supplementation at the neonatal age maintains plasma retinol concentration of young adult rats fed vitamin A-deficient diet, which is correlated with the adult-stage antibody response. Plasma retinol was measured in young adult rats ≈9 wk of age (A). Values are means ± SEM, n = 12/group. Groups with superscripts with different letters differ, P ≤ 0.0001. Correlation of primary anti-KLH IgM titer at young adult age with plasma retinol (B).
Neonatal vitamin A supplementation did not affect the antibody response in rats fed vitamin A-adequate diet after weaning
In parallel with our study of rats fed vitamin A-deficient diet in the postweaning period, additional rats were treated, as neonates, in the same manner with oil, vitamin A, RA, or VARA, with neonatal immunization with TT, but were fed vitamin A–adequate diet from weaning to young adult age (10 wk). Under these dietary conditions, neonatal supplementation did not affect the primary or the secondary anti-TT IgG titers (Table 1), and all groups had mean plasma retinol concentrations >1 µmol/L.
TABLE 1.
Primary and secondary antibody response to tetanus toxoid and plasma retinol concentration of rats fed vitamin A-adequate diet1
| Anti-TT IgG titer2 | |||
|---|---|---|---|
| Neonatal treatment group |
Primary response |
Secondary response |
Plasma retinol,3 µmol/L |
| Control | 191 ± 65 | 2192 ± 1013 | 1.1 ± 0.1 |
| Vitamin A | 139 ± 54 | 1550 ± 718 | 1.1 ± 0.1 |
| RA | 346 ± 168 | 2752 ± 1222 | 1.0 ± 0.1 |
| VARA | 539 ± 149 | 3809 ± 1552 | 1.1 ± 0.1 |
Values are means ± SEM, n = 6/group.
Rats were immunized i.p. with TT on d 7 (primary response was measured 2 wk later), and reimmunized at 8 wk of age (secondary response was measured 7 d later).
Retinol was measured using plasma collected for the secondary response to TT.
Discussion
The effect of oral vitamin A supplementation on immune responses in children with mild vitamin A deficiency was previously investigated in a randomized, placebo-controlled clinical study in which two hundred thirty-six 3 to 6-y–old Indonesian children with or without mild xerophthalmia received 60 mg of retinol (as retinyl palmitate) or a placebo 2 wk before diphtheria-pertussis-tetanus (DPT) immunization (28). Whereas anti-TT IgG titers were higher in the vitamin A-supplemented group than the control group, they did not differ in children with pre-existing ocular signs of vitamin A deficiency when compared with children with normal eyes. However, in a small study of 50 Indian children 1 to 6 y of age, the administration of 100,000 IU or 200,000 IU of vitamin A, together with diphtheria and tetanus immunization, did not elicit a significant difference in antibody response to either toxoid after 4 wk, whereas the antibody response to diphtheria was reported to be higher in vitamin A-supplemented children than in control children (29). A small clinical trial of DPT immunization conducted in vitamin A-supplemented 6–17 wk–old infants in Bangladesh did not find a difference for the antibody response against tetanus and pertussis immunization, but it did find that the diphtheria antibody level was higher in the vitamin A-supplemented group than the placebo group (30). Other studies conducted in vaccination trials against polio virus, measles virus, and DPT vaccines also generally showed no significant difference, although trends favored a positive effect of vitamin A [reviewed in (2,9)].A lack of an effect of vitamin A in several of these human clinical trials may possibly be related to the small sample size of the studies, lack of demonstrated vitamin A deficiency in the children who were studied, or other factors (2).
In contrast, animal studies have provided a relatively consistent picture, generally showing that impaired antibody responses to several TD antigens in the vitamin A-deficient population were reversed by supplementation with vitamin A or RA, a metabolite that is known to widely promote cellular differentiation and to act as a regulator of many physiological processes (31). Moreover, oral supplementation with vitamin A or RA augmented antigen-specific antibody responses in normal, vitamin A-adequate rats, or mice (26,32,33). However, little is known about the effect of combined supplementation of vitamin A and RA on the immune response to vaccination.
Previous studies showed that vitamin A supplementation given concurrently with TT immunization in neonatal rats did not stimulate the primary anti-TT IgG response, although the rats were clearly primed by TT as manifested by a heightened antibody response to reimmunization at 40 d of age, compared with the primary response of 40-d–old naïve rats (13). However, when young rats were raised on vitamin A-deficient diet and immunized with TT for the first time on d 40 or later, the primary anti-TT response was significantly lower than that in vitamin A-adequate rats (34–36). The recall response measured at ~d 60 was also impaired, unless the rats were repleted with vitamin A beforehand, which rescued their ability to produce a recall response (35). These results showed that: 1) neonatal rats are responsive to TT, although their antibody titers are lower than in adults; 2) an adequate level of vitamin A is necessary to maintain a normal response to TT at young adult age; and 3) vitamin A supplementation can readily restore a normal response to TT in previously vitamin A-deficient young adult rats. But to date, and to our knowledge, no study has examined the effect of vitamin A supplementation at the neonatal age, concurrent with immunization, on the memory response to a TD antigen or on the primary antibody response to a novel TD antigen administered at young adult age. In the current study, we tested whether vitamin A or VARA administered to neonates at the time of TT immunization would prevent vitamin A deficiency and enhance the secondary response at the young adult age (~8 wk). We also tested their primary response to a novel TD antigen, KLH, first encountered at young adult age.
In the current study, the primary anti-TT IgG response in rat pups was detectable; however, it was not significantly affected by the coadministration of vitamin A, RA, or VARA with TT immunization. This result is consistent with the majority of human clinical studies in which a benefit of vitamin A on antibody response was not observed following initial immunization. Previous studies have reported that the plasma retinol level of 7 to 8-d–old rats nursed by dams fed vitamin A-adequate diet, or dams switched after parturition to vitamin A-deficient diet, was >1 µmol/L, which is in the vitamin A-adequate range (17,37) and similar to the retinol concentrations for vitamin A-adequate rats in the present study (Table 1). Thus, the vitamin A status of the neonates at the time of primary immunization can be considered adequate. This may explain why we did not observe a significant effect of vitamin A supplementation on the primary anti-TT response. Retinoic acid alone also did not significantly increase the primary anti-TT IgG response in neonatal rats. Previously we reported that oral treatment with RA augmented the primary anti-TT response of neonatal mice (14); however, the amount of RA per g body wt · d−1 in mice was ~4 times higher and the treatment was continued for 6 d, compared with the 3-d treatment used in the present study in neonatal rats.
Nevertheless, when we assessed the memory response in young adult rats fed a vitamin A-deficient diet after weaning, it was significantly increased in the groups treated with vitamin A or VARA as neonates. Because RA alone did not affect the recall response, and vitamin A alone did not differ from VARA, it can be concluded that neonatally administered retinol (vitamin A) supplementation, but not RA supplementation, is responsible for the improved secondary antibody response of rats fed a vitamin A-deficient diet. Retinol can be stored as retinyl esters for later mobilization, in contrast to RA for which the half-life is only minutes to hours (38). Therefore, it is likely that the provision of retinol and the storage of retinyl esters for gradual mobilization of retinol and production of RA in vivo maintained the plasma and tissue vitamin A concentrations of the growing rats at a level sufficient for them to mount a strong immune response. We also observed that the booster effect of reimmunization with TT, evaluated by the ratio of the plasma titers of secondary anti-TT IgG to primary anti-TT IgG, was 3–4 times higher for the vitamin A and VARA groups than for the control group. Two explanations are possible: first, it may be that the value for the control group is low due to the development of vitamin A deficiency; or, second, it may be that vitamin A and VARA, given neonatally, promoted a secondary anti-TT IgG response at the young adult stage. We favor the first explanation, due to the observations that plasma retinol was low in the control group fed vitamin A-deficient diet (Fig. 4A), and this condition was shown in previous studies to result in a low primary anti-tetanus response (34–36). In contrast, the plasma retinol concentrations of young adult rats supplemented with vitamin A or VARA (Fig. 4A) were similar to that of neonatal rats (37) and of young adult rats fed vitamin A-adequate diet after weaning (Table 1). Moreover, the secondary anti-TT IgG response at young adult age was significantly correlated with plasma retinol concentration determined at this age. Overall, the results show that supplementing neonates with vitamin A or VARA can provide insurance against postweaning vitamin A deficiency. However, a benefit may not be detectable by measuring the neonatal-age primary antibody response. It nonetheless may be detectable later, at young adult age in the present studies, in terms of a stronger memory response to reimmunization and in the maintenance of a normal plasma retinol concentration, which is expected to benefit many, if not all, physiological systems.
The prospective benefit of neonatal supplementation with vitamin A or VARA was also evident at the young adult age in terms of the primary antibody response to the novel TD antigen, KLH. Both KLH and TT are protein antigens and therefore share a similar requirement for processing by antigen-presenting cells, activation of receptor-specific T helper cells, T-cell proliferation, and cytokine production. Previous studies in vitamin A-deficient children have shown T-cell abnormalities (39). Appropriate T-helper cell function is required for antigen-specific B-cell proliferation and plasma cell differentiation (40), which secrete IgM as well as other classes of Ig, predominantly IgG, in the case of TT and KLH. Immunization with KLH allowed us to investigate the immune function of vitamin A-deficient young adult rats and to determine whether neonatal supplementation could affect the antibody response to a novel antigen given a substantial period of time after neonatal supplementation. Young adult rats receiving vitamin A or VARA as neonates generated stronger primary anti-KLH IgM and IgG responses (Fig. 3), although their primary anti-TT response in the neonatal period did not differ significantly from that of control rats. A significant correlation between the primary anti-KLH IgG and the secondary anti-TT IgG, and between the primary antibody response to KLH and plasma retinol (Fig. 4B), further confirmed the positive effect of vitamin A or VARA supplementation at the neonatal stage on the antibody response later in life. These results support and strengthen the conclusion that a main benefit of neonatal vitamin A supplementation may occur prospectively, becoming manifest as an improvement in vitamin A status under conditions of a diet low in vitamin A and as a stronger immune response to later immunizations. Although caution needs to be taken in extrapolating the results of any animal study to humans, if a similar prospective benefit is produced by supplementing infants with vitamin A, then it may improve their recall response to antigens to which the child was previously vaccinated (as for TT in our model) or improve the primary response to a new antigen (as for KLH in our model) or to pathogens, a condition we did not test. Nonetheless, the effect of vitamin A in reducing mortality was observed mostly in the first 2 mo of life and in infants of low birth weight (41), and thus vitamin A also may have other beneficial effects early in life, whereas our study revealed a later benefit on antibody production in young adult-age rats. Our results may also suggest that prospective clinical studies of vitamin A supplementation with immunization, such as is promoted by the WHO/UNICEF Expanded Program on Immunization (10), could help to reveal whether vitamin A supplementation, given along with immunization to young children, is beneficial in improving the recall response to immunization or reducing mortality from infectious diseases. Such studies should ideally include an adequate sample size and a direct assessment of vitamin A status to validate the state of the study population and to determine, in the same subjects, whether neonatal vitamin A supplementation is effective in preventing the onset of vitamin A deficiency.
In conclusion, the results of this study demonstrate that vitamin A supplementation given at the neonatal stage can be an effective strategy for protecting against retinol deficiency at young adult age, and that a benefit with respect to antibody production against both recall and novel TD antigens may become evident prospectively, even when it is not apparent at neonatal age.
Footnotes
Supported by NIH DK-41479.
Author disclosures: S. Sankaranarayanan, no conflicts of interest; Y. Ma, no conflicts of interest; M. C. Bryson, no conflicts of interest; N-Q Li, no conflicts of interest; and A. C. Ross, no conflicts of interest.
Abbreviations used: IgG, immunoglobulin G; IgM, immunoglobulin M; KLH, keyhole limpet hemocyanin; RA, retinoic acid; TD, T-cell dependent; TT, tetanus toxoid; VARA, vitamin A combined with RA, 10:1 mol:mol.
The solution for the secondary immunization with TT and primary immunization with KLH also contained 15 µg of pneumococcal polysaccharide type III (42), to be reported on separately.
Literature Cited
- 1.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 2.Ross AC. Vitamin A supplementation and retinoic acid treatment in the regulation of antibody responses in vivo. In: Litwack G, editor. Vitamins and hormones. vol. 75. San Diego: Academic Press; 2007. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultink W. Use of under-five mortality rate as an indicator for vitamin A deficiency in a population. J Nutr. 2002;132:2881S–2883S. doi: 10.1093/jn/132.9.2881S. [DOI] [PubMed] [Google Scholar]
- 4.Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. Vitamin A supplementation and child mortality. A meta-analysis. JAMA J Am Med Assoc. 1993;269:898–903. [PubMed] [Google Scholar]
- 5.Sommer A, West KP., Jr . Vitamin A deficiency: health, survival, and vision. New York: Oxford University Press; 1996. [Google Scholar]
- 6.Beaton GH, Martorell R, Aronson KA, Edmonston B, McCabe G, Ross AC, Harvey B. Vitamin A supplementation and child morbidity and mortality in developing countries. Food Nutr Bull. 1994;15:282–289. [Google Scholar]
- 7.WHO/UNICEF. Vitamin A for measles. Lancet. 1987;2:1067–1068. [PubMed] [Google Scholar]
- 8.Semba RD. Vitamin A and infectious diseases. In: Livrea MA, editor. Vitamin A and retinoids: an update of biological aspects and clinical applications. Basel: Birkhèuser Verlag; 2000. pp. 97–108. [Google Scholar]
- 9.Villamor E, Fawzi WW. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18:446–464. doi: 10.1128/CMR.18.3.446-464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO/UNICEF. Integration of vitamin A supplementation with immunization: policy and program implication; Report of a meeting; 12–13 January 1998; New York. New York: UNICEF; 1998. [Google Scholar]
- 11.WHO/CHD Immunization-Linked Vitamin A Supplementation Study Group. Randomized trial to assess benefits and safety of vitamin A supplementation linked to immunization in early infancy. Lancet. 1998;352:1257–1263. [PubMed] [Google Scholar]
- 12.Semba RD, Munasir Z, Akib A, Melikian G, Permaesih D, Muherdiyantiningsih, Marituti S, Muhilal Integration of vitamin A supplementation with the expanded program on immunization: lack of impact on morbidity or infant growth. Acta Paediatr. 2001;90:1107–1111. doi: 10.1080/080352501317061477. [DOI] [PubMed] [Google Scholar]
- 13.Gardner EM, Ross AC. Immunologic memory is established in nursling rats immunized with tetanus toxoid, but is not affected by concurrent supplementation with vitamin A. Am J Clin Nutr. 1995;62:1007–1012. doi: 10.1093/ajcn/62.5.1007. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Ross AC. The anti-tetanus immune response of neonatal mice is augmented by retinoic acid combined with polyriboinosinic:polyribocytidylic acid. Proc Natl Acad Sci USA. 2005;102:13556–13561. doi: 10.1073/pnas.0506438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawn JE, Wilczynska-Ketende K, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. Int J Epidemiol. 2006;35:706–718. doi: 10.1093/ije/dyl043. [DOI] [PubMed] [Google Scholar]
- 16.Ross AC, Ambalavanan N, Zolfaghari R, Li NQ. Vitamin A combined with retinoic acid increases retinol uptake and lung retinyl ester formation in a synergistic manner in neonatal rats. J Lipid Res. 2006;47:1844–1851. doi: 10.1194/jlr.M600061-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Ross AC, Li N-q, Wu L. The components of VARA, a nutrient-metabolite combination of vitamin A and retinoic acid, act efficiently together and separately to increase retinyl esters in the lungs of neonatal rats. J Nutr. 2006;136:2803–2807. doi: 10.1093/jn/136.11.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James ML, Ross AC, Bulger A, Ambalavanan N. Synergistic effect of vitamin A and retinoic acid in reducing neonatal hyperoxic lung injury. 2005 http://www.abstracts2view.com/pas/. [Google Scholar]
- 19.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 20.Pasatiempo AMG, Abaza M, Taylor CE, Ross AC. Effects of timing and dose of vitamin A on tissue retinol concentrations and antibody production in the previously vitamin A-depleted rat. Am J Clin Nutr. 1992;55:443–451. doi: 10.1093/ajcn/55.2.443. [DOI] [PubMed] [Google Scholar]
- 21.Davila ME, Norris L, Cleary MP, Ross AC. Vitamin A during lactation: relationship of maternal diet to milk vitamin A content and to the vitamin A status of lactating rats and their pups. J Nutr. 1985;115:1033–1041. doi: 10.1093/jn/115.8.1033. [DOI] [PubMed] [Google Scholar]
- 22.Humphrey JH, Rice AL. Vitamin A supplementation of young infants. Lancet. 2000;356:422–424. doi: 10.1016/S0140-6736(00)02541-1. [DOI] [PubMed] [Google Scholar]
- 23.Massaro GD, Massaro D. Postnatal treatment with retinoic acid increases the number of pulmonary alveoli in rats. Am J Physiol Lung Cell Mol Physiol. 1996;270:L305–L310. doi: 10.1152/ajplung.1996.270.2.L305. [DOI] [PubMed] [Google Scholar]
- 24.Bellinger DL, Stevens SY, Rajan ST, Lorton D, Madden KS. Aging and sympathetic modulation of immune function in Fischer 344 rats: effects of chemical sympathectomy on primary antibody response. J Neuroimmunol. 2005;165:21–32. doi: 10.1016/j.jneuroim.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Kinoshita M, Ross AC. Vitamin A status and immunoglobulin G subclasses in rats immunized with tetanus toxoid. FASEB J. 1993;7:1277–1282. doi: 10.1096/fasebj.7.13.8405813. [DOI] [PubMed] [Google Scholar]
- 26.Ma Y, Chen Q, Ross AC. Retinoic acid and polyriboinosinic: polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J Immunol. 2005;174:7961–7969. doi: 10.4049/jimmunol.174.12.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross AC. Separation and quantitation of retinyl esters and retinol by high-performance liquid chromatography. Methods Enzymol. 1986;123:68–74. doi: 10.1016/s0076-6879(86)23010-4. [DOI] [PubMed] [Google Scholar]
- 28.Semba RD, Muhilal, Scott AL, Natadisastra G, Wirasasmita S, Mele L, Ridwan E, West KP, Jr, Sommer A. Depressed immune response to tetanus in children with vitamin A deficiency. J Nutr. 1992;122:101–107. doi: 10.1093/jn/122.1.101. [DOI] [PubMed] [Google Scholar]
- 29.Bhaskaram P, Arun Jyothi S, Visweswara Rao K, Narasinga Rao BS. Effects of subclinical vitamin A deficiency and administration of vitamin A as a single large dose on immune function in children. Nutr Res. 1989;9:1017–1025. [Google Scholar]
- 30.Rahman MM, Mahalanabis D, Hossain S, Wahed MA, Alvarez JO, Siber GR, Thompson C, Santosham M, Fuchs GJ. Simultaneous vitamin A administration at routine immunization contact enhances antibody response to diphtheria vaccine in infants younger than six months. J Nutr. 1999;129:2192–2195. doi: 10.1093/jn/129.12.2192. [DOI] [PubMed] [Google Scholar]
- 31.De Luca LM. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J. 1991;5:2924–2933. [PubMed] [Google Scholar]
- 32.Cui D, Moldoveanu Z, Stephensen CB. High-level dietary vitamin A enhances T-helper type 2 cytokine production and secretory immunoglobulin A response to influenza A virus infection in BALB/c mice. J Nutr. 2000;130:1132–1139. doi: 10.1093/jn/130.5.1132. [DOI] [PubMed] [Google Scholar]
- 33.DeCicco KL, Youngdahl JD, Ross AC. All-trans-retinoic acid and polyriboinosinic: polyribocytidylic acid in combination potentiate specific antibody production and cell-mediated immunity. Immunology. 2001;104:341–348. doi: 10.1046/j.1365-2567.2001.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasatiempo AMG, Kinoshita M, Taylor CE, Ross AC. Antibody production in vitamin A-depleted rats is impaired after immunization with bacterial polysaccharide or protein antigens. FASEB J. 1990;4:2518–2527. doi: 10.1096/fasebj.4.8.2110538. [DOI] [PubMed] [Google Scholar]
- 35.Kinoshita M, Pasatiempo AMG, Taylor CE, Ross AC. Immunological memory to tetanus toxoid is established and maintained in the vitamin A-depleted rat. FASEB J. 1991;5:2473–2481. doi: 10.1096/fasebj.5.10.2065894. [DOI] [PubMed] [Google Scholar]
- 36.Arora D, Ross AC. Antibody response against tetanus toxoid is enhanced by lipopolysaccharide or tumor necrosis factor-alpha in vitamin A-sufficient and -deficient rats. Am J Clin Nutr. 1994;59:922–928. doi: 10.1093/ajcn/59.4.922. [DOI] [PubMed] [Google Scholar]
- 37.Ross AC, Ambalavanan N. Retinoic acid combined with vitamin A synergizes to increase retinyl ester storage in the lungs of newborn and dexamethasone-treated neonatal rats. Neonatalogy. 2007;X doi: 10.1159/000100083. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adamson PC, Balis FM, Smith MA, Murphy RF, Godwin KA, Poplack DG. Dose-dependent pharmacokinetics of all-trans-retinoic acid. J Natl Cancer Inst. 1992;84:1332–1335. doi: 10.1093/jnci/84.17.1332. [DOI] [PubMed] [Google Scholar]
- 39.Semba RD, Muhilal, Ward BJ, Griffin DE, Scott AL, Natadisastra G, West KP, Jr, Sommer A. Abnormal T-cell subset proportions in vitamin A-deficient children. Lancet. 1993;341:5–8. doi: 10.1016/0140-6736(93)92478-c. [DOI] [PubMed] [Google Scholar]
- 40.Abbas AK, Burstein HJ, Bogen SA. Determinants of helper T cell-dependent antibody production. Semin Immunol. 1993;5:441–447. doi: 10.1006/smim.1993.1050. [DOI] [PubMed] [Google Scholar]
- 41.Rahmathullah L, Tielsch JM, Thulasiraj RD, Katz J, Coles C, Devi S, John R, Prakash K, Sadanand AV, et al. Impact of supplementing newborn infants with vitamin A on early infant mortality: community based randomised trial in southern India. BMJ. 2003;327:254–257. doi: 10.1136/bmj.327.7409.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasatiempo AMG, Taylor CE, Ross AC. Vitamin A status and the immune response to pneumococcal polysaccharide: effects of age and early stages of retinol deficiency in the rat. J Nutr. 1991;121:556–562. doi: 10.1093/jn/121.4.556. [DOI] [PubMed] [Google Scholar]