Summary
Nemaline myopathy (NM) is a congenital myopathy with an estimated incidence of 1∶50,000 live births. It is caused by mutations in thin filament components, including nebulin, which accounts for about 50% of the cases. The identification of NM cases with nonsense mutations resulting in loss of the extreme C-terminal SH3 domain of nebulin suggests an important role of the nebulin SH3 domain, which is further supported by the recent demonstration of its role in IGF-1-induced sarcomeric actin filament formation through targeting of N-WASP to the Z-line. To provide further insights into the functional significance of the nebulin SH3 domain in the Z-disk and to understand the mechanisms by which truncations of nebulin lead to NM, we took two approaches: (1) an affinity-based proteomic screening to identify novel interaction partners of the nebulin SH3 domain; and (2) generation and characterization of a novel knockin mouse model with a premature stop codon in the nebulin gene, eliminating its C-terminal SH3 domain (NebΔSH3 mouse). Surprisingly, detailed analyses of NebΔSH3 mice revealed no structural or histological skeletal muscle abnormalities and no changes in gene expression or localization of interaction partners of the nebulin SH3 domain, including myopalladin, palladin, zyxin and N-WASP. Also, no significant effect on peak isometric stress production, passive tensile stress or Young's modulus was found. However, NebΔSH3 muscle displayed a slightly altered force–frequency relationship and was significantly more susceptible to eccentric contraction-induced injury, suggesting that the nebulin SH3 domain protects against eccentric contraction-induced injury and possibly plays a role in fine-tuning the excitation–contraction coupling mechanism.
Key words: Nebulin, Nemaline myopathy, Skeletal muscle, Z-line, Sarcomere
Introduction
Nemaline myopathy (NM) is a slowly progressive or non-progressive congenital myopathy characterized by muscle weakness and hypotonia as well as the presence of rod-shaped structures called nemaline bodies in affected muscle fibers. The disease is the most common of the non-dystrophic congenital myopathies and affects roughly 1 in 50,000 live births (North et al., 1997). NM is both clinically and genetically heterogeneous and, so far, mutations in seven different genes, most encoding components of the thin filament, have been shown to be causative for NM, with mutations in the nebulin gene accounting for about 50% of the cases (Lehtokari et al., 2006; Pelin et al., 1999; Wallgren-Pettersson et al., 2002; Wallgren-Pettersson et al., 2011).
Nebulin is a giant sarcomeric protein (500–900 kDa) that binds along the thin filament in skeletal muscle with its C-terminus anchored in the Z-line at the actin barbed end and its N-terminus oriented towards the actin pointed end (Castillo et al., 2009; Millevoi et al., 1998; Wang and Wright, 1988). The majority of the protein is composed of repeating modules, which interact with thin filament components along the length of the thin filament, whereas its N- and C-terminal regions contain unique sequences that bind to proteins at the actin filament pointed end and the Z-line, respectively. Based on in vivo and in vitro studies, nebulin has recently been implicated in various important processes, including stabilization of thin filaments (Bang et al., 2006; Castillo et al., 2009; Gokhin and Fowler, 2013; Littlefield and Fowler, 2008; Pappas et al., 2010; Witt et al., 2006), myofibrillar force generation (Bang et al., 2006; Gokhin et al., 2009; Ochala et al., 2011; Ottenheijm et al., 2010; Ottenheijm et al., 2009), regulation of the actomyosin interaction (Bang et al., 2009; Castillo et al., 2009; Ochala et al., 2011; Ottenheijm et al., 2011), sarcoplasmic Ca2+ handling (Ottenheijm et al., 2008; Witt et al., 2006), maintenance of sarcomeric integrity during muscle contraction (Bang et al., 2006), as well as Z-line alignment, width and integrity (Bang et al., 2006; Pappas et al., 2010; Tonino et al., 2010; Witt et al., 2006). However, although recent studies have provided new insights into the role of nebulin in skeletal muscle, its function in the Z-line remains poorly understood.
The Z-line is a multiprotein complex, which defines the boundaries between sarcomeres and plays a pivotal role in skeletal muscle structure and function, including sarcomere assembly and organization, muscle force generation and transmission, mechanosensing, signaling and sarcolemmal membrane integrity (reviewed by Frank et al., 2006; Luther, 2009; Sheikh et al., 2007). Within the Z-line, nebulin C-terminal modules interact with the intermediate filament desmin (Bang et al., 2002; Tonino et al., 2010) as well as the actin barbed-end capping protein CapZ (Pappas et al., 2008). Furthermore, the extreme C-terminal end of nebulin contains a serine-rich region with several predicted phosphorylation sites preceding an Src homology 3 (SH3) domain. SH3 domains are composed of ∼60 amino acid residues arranged into two tightly packed anti-parallel β-sheets, forming a β-barrel (Musacchio et al., 1992). SH3 domains are present in numerous intracellular or membrane-associated proteins and are involved in a variety of cellular processes, including transduction of biochemical signals, assembly of multiprotein complexes and formation of cytoskeletal linkages. SH3 domains generally bind to proline-rich sequences capable of forming a polyproline II helix conformation with the minimal consensus site PxxP (Mayer, 2001; Musacchio et al., 1992). However, recently the ability of SH3 domains to bind to unconventional sites rich in positively charged residues (for example [R/K]xx[K/R]) has been reported (Jia et al., 2005), complicating SH3 target prediction. The SH3 domain of nebulin is highly conserved between species and has been studied in detail by nuclear magnetic resonance (NMR) spectroscopy (Politou et al., 1998; Politou et al., 2002). We previously demonstrated the binding of the nebulin SH3 domain to the striated muscle-specific protein myopalladin and its ubiquitously expressed homologue palladin, which are both linked to α-actinin in the Z-line and play important roles in the organization of the actin cytoskeleton (Bang et al., 2001; Goicoechea et al., 2008; Ma and Wang, 2002). In addition, the nebulin SH3 domain can bind to zyxin (Li et al., 2004), a component of focal adhesions associated with α-actinin (Crawford et al., 1992) as well as Ena/VASP and cysteine-rich protein (CSRP) family members, involved in actin cytoskeletal organization (Louis et al., 1997; Reinhard et al., 1995). The nebulin SH3 domain has also been shown to bind to two different regions of titin, a giant sarcomeric protein (∼3.7 MDa) that functions as a template for the assembly and organization of sarcomeric components and serves as a molecular spring, responsible for myofibrillar passive stiffness and maintenance of structural integrity (reviewed by Kontrogianni-Konstantopoulos et al., 2009). One SH3-binding site is located in a proline-rich region within the Zis1 region of titin corresponding to the localization of the nebulin SH3 domain in the Z-line (Labeit et al., 2006; Witt et al., 2006). Furthermore, additional binding sites are present within the I-band region of titin in the PEVK region, which contains multiple tandemly arranged copies of polyproline sequences with affinity for the nebulin SH3 domain (Ma et al., 2006; Ma and Wang, 2002). As the titin PEVK region is located in the I-band, distant from the nebulin SH3 domain in the Z-line, the physiological significance of this interaction is unclear, particularly because SH3 domains can nonspecifically bind to proline-rich sequences. Therefore, a potential physiological interaction would have to be transitional and potentially occur during myofibrillogenesis, the significance of which would be unclear since nebulin is dispensable for myofibrillogenesis (Bang et al., 2006; Witt et al., 2006).
Intriguingly, Takano et al., recently demonstrated that the nebulin SH3 domain can bind and recruit neuronal Wiscott-Aldrich syndrome protein (N-WASP) to the Z-line upon insulin-like growth factor 1 (IGF-1)-induced phosphoinositide 3-kinase (PI3K)–Akt activation, inhibiting glycogen synthase kinase 3β (GSK3β), which prevents the interaction by phosphorylating nebulin at two serine sites within its serine-rich region (Takano et al., 2010). Through this mechanism, N-WASP was shown to cooperate with nebulin in promoting actin nucleation and elongation independently of ARP2/3 through which N-WASP is known to stimulate actin nucleation in nonmuscle cells (Rohatgi et al., 1999). The relevance of this mechanism in vivo was demonstrated by in vivo knockdown of N-WASP, which prevented IGF-1-induced incorporation of actin into the sarcomere and resulted in a reduced muscle cross-sectional area (CSA) independent of IGF-1 (Takano et al., 2010). Based on these results, the authors proposed that the IGF-1–Akt signaling pathway stimulates myofibrillogenesis and muscle hypertrophy through this novel pathway.
In addition to these studies indicating an important role of the nebulin SH3 domain in skeletal muscle, the functional importance of the nebulin C-terminal region is illustrated by the identification of NM-causing nebulin mutations, resulting in truncations of nebulin (Gurgel-Giannetti et al., 2002; Pelin et al., 1999). Therefore, to dissect the functional role of the nebulin C-terminal SH3 domain in the Z-disk and provide insights into the mechanisms by which truncations of nebulin lead to human NM, we took two approaches: (1) an affinity-based proteomic study to identify novel direct and indirect binding partners of the nebulin SH3 domain; and (2) characterization of a novel nebulin knockin mouse model with a premature stop codon, resulting in deletion of its C-terminal SH3 domain (NebΔSH3 mouse). Surprisingly, NebΔSH3 mice exhibited no histological or structural skeletal muscle abnormalities and no significant effects on peak isometric stress production, passive tensile stress, or Young's modulus were found. However, NebΔSH3 muscle exhibited a slight depression in maximum stress production at lower frequencies and was more vulnerable to eccentric contraction-induced injury, suggesting that the nebulin SH3 domain plays a role in protecting the muscle against eccentric contraction-induced injury and possibly in modulating the calcium sensitivity of the contractile apparatus.
Results
Identification of nebulin-SH3-domain-binding proteins using a proteomic approach
In a search for interaction partners of the nebulin SH3 domain using the yeast two-hybrid system, we previously identified a novel protein, myopalladin (Bang et al., 2001). However, since the nebulin SH3 domain was partly autoactivating as bait, giving rise to a large amount of false positives, which complicated the identification of real interaction partners, this approach was not optimal. Therefore, we applied a proteomic approach based on the pulldown of proteins associated with the nebulin SH3 domain, followed by liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis. As illustrated in supplementary material Fig. S1A, 6×His-glutamine-S-transferase-3C-protease recognition site (H6-GST-3C)-tagged nebulin SH3 domain was purified on a nickel column and incubated with the lysate of differentiated C2C12 myoblasts, which had been pre-cleared by incubation with the H6-GST-3C tag. The protein mixture was immobilized on a nickel column, and after extensive washing, bound proteins were eluted and subsequently identified by excision of polypeptide bands from an SDS-PAGE gel (supplementary material Fig. S1B), followed by in-gel trypsin digestion and LC-MS/MS analysis (Table 1). A pulldown using the H6-GST-3C tag alone was performed in parallel to allow for exclusion of proteins unspecifically bound to the H6-GST-3C tag or the nickel column (supplementary material Fig. 1B). Many of the identified proteins were Z-line proteins, including myopalladin and zyxin, which are already known interaction partners of the nebulin SH3 domain, thus validating the approach (Bang et al., 2001; Li et al., 2004).
Table 1. Proteins identified by pulldown with H6-GST-3C-tagged nebulin SH3 domain.
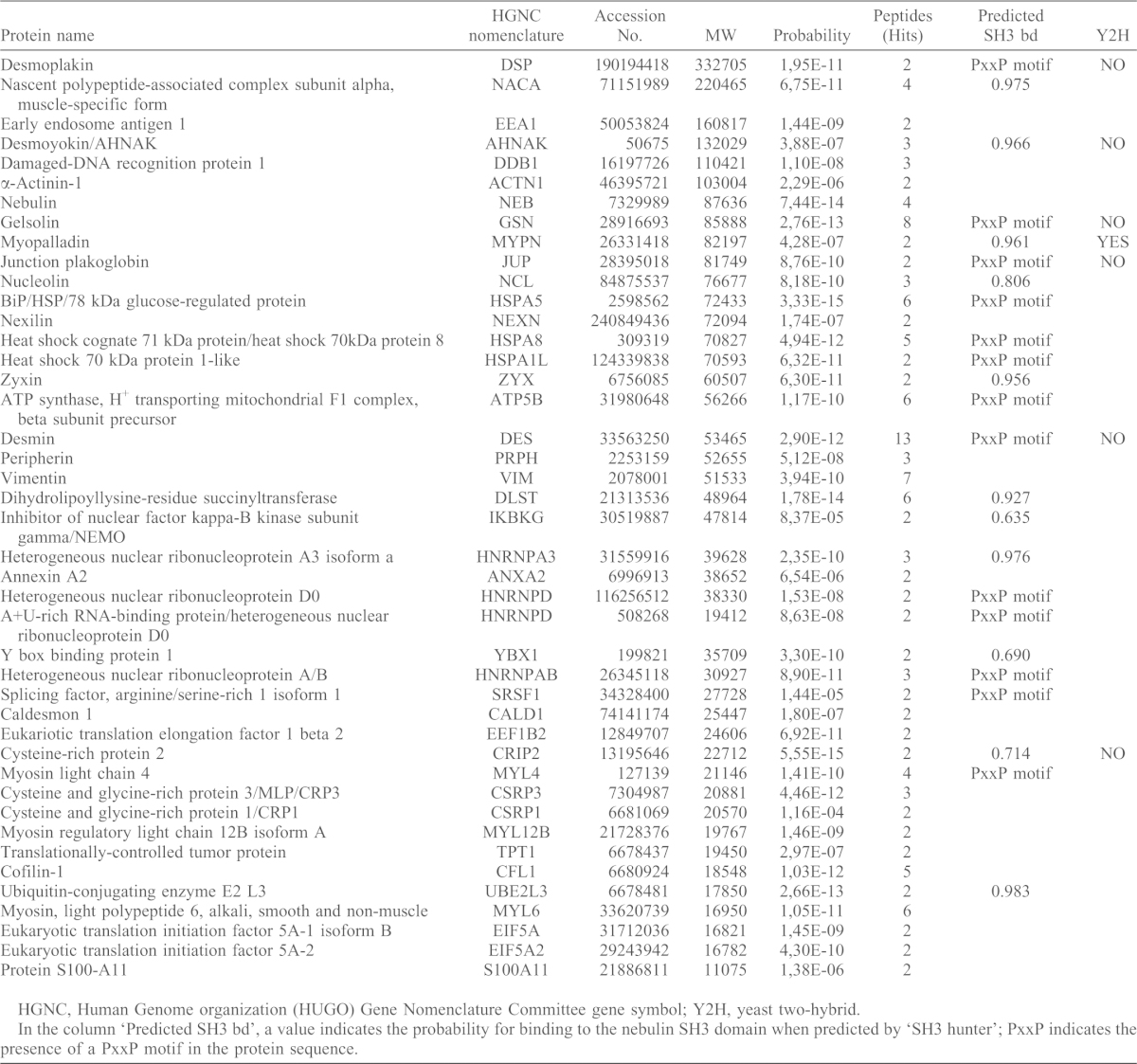
HGNC, Human Genome organization (HUGO) Gene Nomenclature Committee gene symbol; Y2H, yeast two-hybrid.
In the column ‘Predicted SH3 bd’, a value indicates the probability for binding to the nebulin SH3 domain when predicted by ‘SH3 hunter’; PxxP indicates the presence of a PxxP motif in the protein sequence.
Search for potential nebulin SH3 consensus sites and test of potential direct interactions
To determine whether any of the identified proteins are direct interaction partners of the nebulin SH3 domain, the online program SH3 Hunter was used to search for potential consensus sites for the nebulin SH3 domain in the identified proteins using both the default and the PxxP peptide filter (Table 1). Based on these results, potential interactions between the nebulin SH3 domain and either full-length or predicted SH3-binding regions of selected candidate proteins were tested in the yeast two-hybrid system by cloning the potential interaction partners in the bait vector and the nebulin SH3 domain in the prey vector, thereby avoiding the issue with autoactivation of the nebulin SH3 domain when used as bait. Interaction with full-length or selected regions of the following proteins containing predicted SH3-binding sites were tested: AHNAK/desmoyokin, gelsolin, junction plakoglobin, desmin and cysteine rich protein 2 (CRIP2) as well as myopalladin as a positive control. However, while the interaction with myopalladin was confirmed, none of the tested proteins appeared to directly bind to nebulin (Table 1). As non-conventional SH3-binding peptides rich in R/K have been identified (Jia et al., 2005), we also tested the ability of a repetitive KxPK-motif-containing sequence in AHNAK to bind to the nebulin SH3 domain, but also this failed to bind to the nebulin SH3 domain. Thus, the identified proteins are likely to be indirectly bound to nebulin.
Generation of a protein association network between identified proteins
To test whether the identified proteins may be indirectly associated with the nebulin SH3 domain, we used STRING 9.05 (Search Tool for the Retrieval of Interacting Genes/Proteins) for the generation of a protein association network (Jensen et al., 2009; Szklarczyk et al., 2011). Furthermore, we manually included a few additional known interactions, which were not retrieved by STRING, i.e. Zyxin–CSRP1/3 (Louis et al., 1997) and MYPN–filamentous actin (F-actin; our unpublished results). As shown in supplementary material Fig. S2, more than two thirds of the proteins identified by LC-MS/MS could be connected into a network of proteins that are either directly or indirectly associated with the nebulin SH3 domain through interaction with its known binding partners, myopalladin, palladin and zyxin (Bang et al., 2001; Li et al., 2004), or their interaction partners, which among others include α-actinin and filamentous-actin (F-actin). Therefore, although actin was not among the identified proteins, F-actin was included in the interaction network. In addition to the proteins that are part of the interaction network, many nuclear proteins with no known link to nebulin or the Z-line were identified, the significance of which is not clear. However, the fact that the majority of the identified proteins are Z-line proteins and can be linked together through known interactions, suggests that most of the identified proteins are true interactors (direct or indirect) of the nebulin SH3 domain.
Generation of a mouse line with a premature stop codon in the last exon (exon 166) of nebulin, resulting in deletion of its SH3 domain
To study the function of the nebulin SH3 domain in the Z-line and understand the mechanism by which truncations of nebulin cause nemaline myopathy, we generated a mouse line in which the nebulin SH3 domain is deleted. Briefly, a targeting construct was generated in which residue I7097 encoded by the last exon of nebulin was replaced by stop codons in all reading frames, preventing the translation of the nebulin SH3 domain, as well as a neomycin (neo) cassette flanked by FLPase Recognition Target (FRT) sites (Fig. 1A). Targeted embryonic stem (ES) cells were identified by Southern blot analysis (Fig. 1B) and injected into blastocysts from C57/BL6 mice to generate chimeric mice. The resulting heterozygous mutant mice were crossed with FLPase deleter mice to remove the neo gene and subsequently mated to generate homozygous mutant mice (designated NebΔSH3), as determined by PCR. The correct targeting of the nebulin gene was confirmed by RT-PCR analysis on total skeletal muscle RNA followed by sequencing (Fig. 1C) as well as by western blot analysis using an antibody against the nebulin SH3 domain (Fig. 1D). In addition, using antibodies against nebulin modules M160–164 and M161–165, truncated nebulin protein was found to be expressed to the same level in NebΔSH3 mice as that of wild-type (WT) nebulin protein in WT littermate controls (Fig. 1D). To rule out the possibility that upregulation of nebulette, the cardiac-specific homologue of nebulin, might compensate for the absence of the nebulin SH3 domain, we tested its protein expression level, but found no expression of nebulette in skeletal muscle either in the presence or absence of the nebulin SH3 domain (Fig. 1E).
Fig. 1.
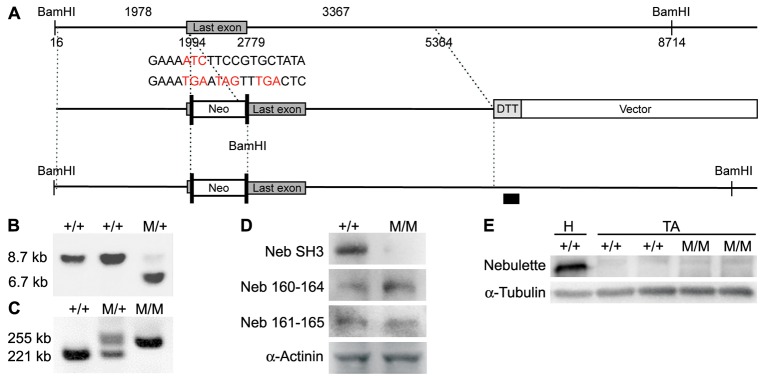
Generation of the mouse line in which residue I7097 of nebulin was replaced with stop codons in all reading frames, resulting in deletion of the nebulin SH3 domain (NebΔSH3 mice). (A) A restriction map of the relevant genomic region of nebulin (top), targeting construct (middle) and the mutated locus after recombination (lower). Neo, neomycin resistance gene; DTA, diphtheria toxin A chain. Long boxes represent FRT sites and the black box represents the probe used for Southern blot analysis. (B) Detection of WT and targeted alleles by Southern blot analysis on BamHI-digested electroporated ES cells using the probe shown in A. The 8.7 and 6.7 kb bands represent the WT and the targeted allele, respectively. (C) RT-PCR analysis on total RNA isolated from TA muscle using the genotyping primers, confirming the correct targeting of the nebulin gene. (D) Western blot analysis of TA muscle from WT and homozygous NebΔSH3 mice using antibodies against the nebulin SH3 domain, nebulin M160–164 and nebulin M161–165. α-Actinin antibody was used as loading control. (E) Western blot analysis of heart (H) and TA muscle from WT and homozygous NebΔSH3 mice using antibodies against nebulette. α-Tubulin antibody was used as loading control. M, mutant allele.
No histological or ultrastructural changes in NebΔSH3 mice
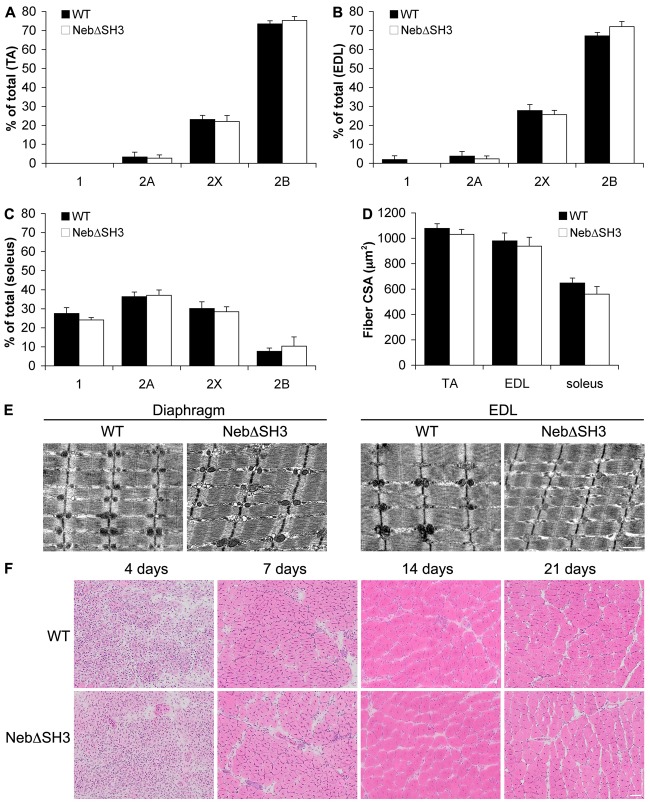
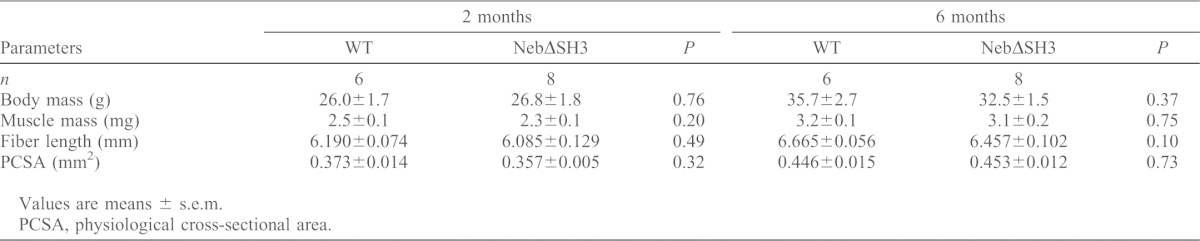
NebΔSH3 mice were born in normal Mendelian ratios, were viable and fertile, and had a normal life span. Furthermore, they did not exhibit any gross musculoskeletal defects, such as spine deformity or altered body size (Table 2), and no obvious abnormalities in grooming, mobility or other behavioral characteristics were observed. Histological analyses of tibialis anterior (TA), extensor digitorum longus (EDL), and soleus muscle from WT and NebΔSH3 mice stained with Hematoxylin and Eosin or Picro Sirius Red showed no evidence of centralized nuclei, inflammatory cell infiltration, fibrosis, necrosis, heightened fiber size variability, altered fiber density or unusual fiber shape up to 1 year of age (data not shown). Furthermore, SDS-PAGE and densitometry for myosin heavy chain isoforms revealed no statistically significant changes in fiber type distribution (Fig. 2A–C), and no significant differences were found in total myosin heavy chain content, indicating that the degree and type of muscle activity is not modified in NebΔSH3 muscle. Moreover, fiber cross-sectional areas (CSA) were similar between WT and NebΔSH3 mice as determined by analysis of laminin-stained sections using a plugin for ImageJ (Fig. 2D). Transmission electron microscopy (TEM) studies of EDL and diaphragm muscle from 1-year-old WT and NebΔSH3 mice showed normal ultrastructural organization with well-organized sarcomeres and no evidence of Z-line dissolution, streaming or misalignment (Fig. 2E). Finally, to determine whether the nebulin SH3 domain is involved in regeneration, we studied the recovery of NebΔSH3 muscle following injection of cardiotoxin into the TA muscle. As demonstrated in Fig. 2F, no difference in regeneration rate was observed between WT and NebΔSH3 muscle. Thus, based on these analyses, we conclude that the nebulin SH3 domain is dispensable for normal myogenesis, fiber type specification and muscle cytoarchitecture.
Table 2. General architectural properties of fifth toe EDL muscles from 2- and 6-month-old WT and NebΔSH3 mice.
Values are means ± s.e.m.
PCSA, physiological cross-sectional area.
Fig. 2.
Baseline muscle characteristics in 2-month-old WT and NebΔSH3 muscle fibers. Fiber type distribution as determined by relative amounts of type 1, 2A, 2X and 2B myosin heavy chain isoforms in TA (A), EDL (B) and soleus (C) muscle from WT and NebΔSH3 muscle. (D) Fiber cross sectional area (CSA) in TA, EDL and soleus muscle. n = 6 (A–D). (E) TEM of EDL and diaphragm muscle from 1-year-old WT and NebΔSH3 mice. Scale bar: 1 µm. (F) Hematoxylin and Eosin staining 4, 7, 14 and 21 days after injection of cardiotoxin into the TA of 2-month-old WT and NebΔSH3 mice. Scale bar: 50 µm.
NebΔSH3 mice are more vulnerable to eccentric contraction-induced injury
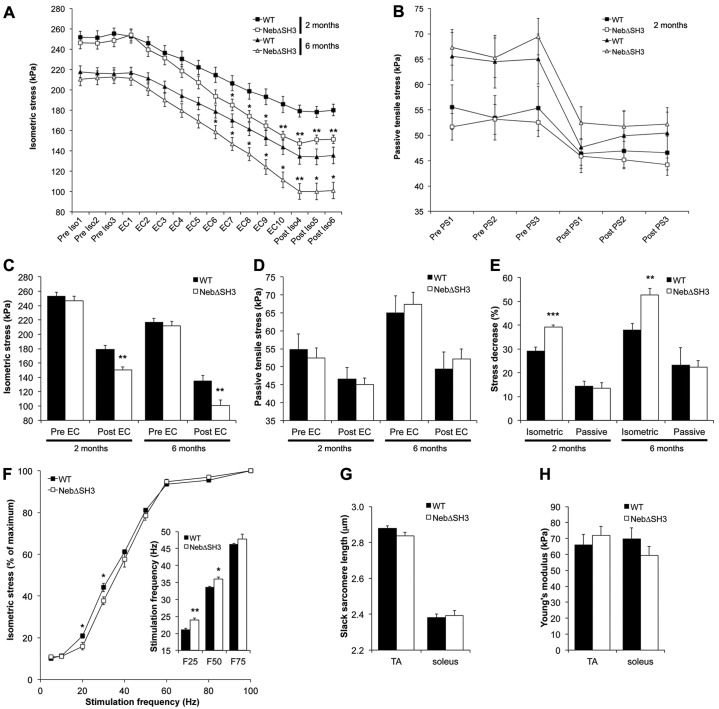
The architectural properties of the fifth toe EDL muscle, including mass, fiber length and physiological cross sectional area (PCSA) were identical in WT and NebΔSH3 muscle at both 2 and 6 months of age (Table 2), indicating that deletion of the nebulin SH3 domain does not affect muscle design. Isometric stress production and passive tensile stress before and after injury induced by cyclic eccentric contractions in the fifth toe EDL were measured in 2- and 6-month-old mice. As shown in Fig. 3A–C, both parameters were identical in WT and NebΔSH3 mice prior to eccentric exercise. However, while the decrease in passive load-bearing capacity in response to eccentric exercise was similar in WT and NebΔSH3 muscle (Fig. 3B,D,E), the reduction in isometric stress production was significantly greater in NebΔSH3 muscle compared with WT (P<0.01; Fig. 3A,C,E). Although isometric stress generation decreased with age and passive tensile stress increased with age, the reduction in both parameters in response to eccentric exercise was greater with age. In contrast, the greater decrease in isometric stress generation in NebΔSH3 muscle was independent of age (Fig. 3E). Taken together, our results show that the nebulin SH3 domain is not involved in determining peak isometric force and passive load bearing in muscle, but that NebΔSH3 muscle is more susceptible to eccentric contraction-induced injury than WT muscle, when ‘injury’ is defined as a reduction in isometric stress production across the eccentric exercise bout.
Fig. 3.
Biomechanical studies of skeletal muscle function in WT and NebΔSH3 mice. (A) Time course of isometric stress measured before (Pre Iso1–3), during (EC1–10) and after (Post Iso1–3) cyclic eccentric exercise of the fifth toe EDL muscle from 2- and 6-month-old WT and NebΔSH3 mice. Each symbol represents the mean ± s.e.m. of six mice per group. (B) Passive tensile stress (PS) at 15% passive stretch before (Pre PS1–3) and after (Post PS1–3) eccentric exercise. (C) Average maximum isometric stress production before and after eccentric exercise. (D) Average passive tensile stress at 15% stretch before and after eccentric exercise. (E) Magnitude of eccentric contraction-induced injury, defined as the percentage decrease in isometric stress and/or passive tensile stress after eccentric exercise. (F) Force–frequency relationship in the fifth toe EDL muscle from 2-month-old WT and NebΔSH3 mice. Inset: NebΔSH3 muscle requires higher stimulation frequency to achieve 25% and 50% of maximum isometric stress production (F25, F50). n = 6 (A–F). (G,H) Passive mechanical properties of single fibers from TA and soleus muscle from 2-month-old WT (n = 9) and NebΔSH3 (n = 9) mice. No significant differences in slack sarcomere length (G) or Young's modulus (H) between WT and NebΔSH3 fibers. *P<0.05, **P<0.01, ***P<0.001.
NebΔSH3 mice generate lower stress under isometric conditions at a given frequency
Isometric force was measured at different frequencies in the fifth toe EDL from 2-month-old WT and NebΔSH3 mice. At the lowest stimulation frequency of 5 Hz, muscle twitches did not fuse into tetanic contractions and identical twitch stresses were produced in WT and NebΔSH3 muscle. However, at frequencies of 20–30 Hz when twitches began to fuse into isometric tetani in both WT and NebΔSH3 muscle, NebΔSH3 muscle exhibited a slight, but statistically significant depression in isometric stress production. At frequencies greater than 30 Hz, isometric stress production was statistically indistinguishable in WT and NebΔSH3 muscle (Fig. 3F). Logistic regression analysis showed that the frequencies required to achieve 25% and 50% of maximum isometric stress were slightly elevated in NebΔSH3 muscle, but no difference was observed in the frequency required to achieve 75% of maximum isometric stress (Fig. 3F, inset). These data indicate that skeletal muscle lacking the nebulin SH3 domain exhibits slightly blunted sensitivity to electrical stimulation but only in a narrow range of frequencies.
The nebulin SH3 domain is dispensable for normal passive muscle mechanics
Passive mechanical testing of single fibers isolated from EDL and soleus muscle from 2-month-old WT and NebΔSH3 mice showed identical slack sarcomere lengths in both genotypes (Fig. 3G), and no differences were found in the elastic modulus (Young's modulus) as measured from the slope of the stress–strain curve when fibers were incrementally stretched in 250 µm steps (Fig. 3H). These observations provide further evidence that the nebulin SH3 domain is dispensable for normal passive muscle mechanics.
NebΔSH3 muscle exhibits no significant changes in gene expression
To study the potential effect of the nebulin SH3 domain on signaling leading to changes in gene expression, we performed microarray gene expression profiling on TA muscle from 2-month-old WT and NebΔSH3 mice. As shown in supplementary material Table S1, after adjusting for false discovery rate (Benjamini et al., 2001), no significant changes in gene expression were observed between WT and NebΔSH3 mice, including expression of genes encoding the other SH3-domain-containing proteins of the nebulin superfamily, LASP1 and nebulette. Thus, the nebulin SH3 domain does not influence gene expression, at least under basal unstressed conditions.
The nebulin SH3 domain does not affect the targeting of myopalladin, palladin, zyxin and N-WASP to the Z-line
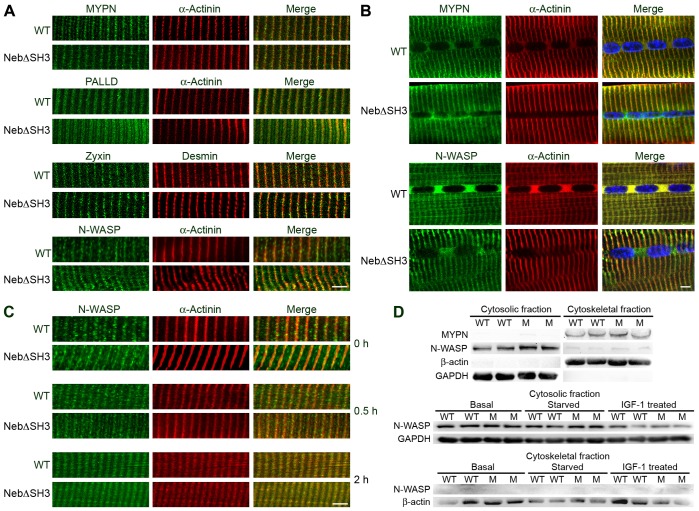
To determine the effect of the nebulin SH3 domain on the localization of its interaction partners in the Z-line, we performed immunostainings on longitudinal sections of TA muscle from WT and NebΔSH3 mice using antibodies against myopalladin, palladin, zyxin and N-WASP. As seen in Fig. 4A, the localization of these proteins in the Z-line was unaffected by the presence or absence of the nebulin SH3 domain as demonstrated by their colocalization with α-actinin or desmin. Furthermore, the absence of the nebulin SH3 domain did not affect the targeting of myopalladin and N-WASP to the Z-line during muscle regeneration following cardiotoxin-induced injury (Fig. 4B). Given the recent report demonstrating that N-WASP is targeted to the Z-line through interaction with the nebulin SH3 domain (Takano et al., 2010), we performed co-immunostainings for N-WASP and α-actinin on longitudinal muscle sections from WT and NebΔSH3 mice following 48 hours of starvation, as well as from prefasted mice administered with IGF-1 (0.5 and 2 hours after IGF-1 injection). As shown in Fig. 4C, N-WASP localized to the Z-line independent of starvation, IGF-1 treatment, or the presence or absence of the nebulin SH3 domain. These results were supported by western blot analyses on cytosolic and Triton-insoluble cytoskeletal fractions, which showed the presence of myopalladin in the cytoskeletal fraction, whereas N-WASP was predominantly found in the cytosolic fraction at levels that were unaffected by the presence or absence of the nebulin SH3 domain. Likewise, 48 hours of starvation or stimulation of PI3K–Akt signaling by IGF-1 injection of 48 hour prefasted WT or NebΔSH3 mice did not affect the levels or distribution of N-WASP between cytosolic and cytoskeletal fractions 2 hours after treatment (Fig. 4D). Thus, our data do not support a role of the nebulin SH3 domain in the targeting of N-WASP, myopalladin, palladin and zyxin to the Z-line.
Fig. 4.
The absence of the nebulin SH3 domain does not affect the localization of its interaction partners. (A) Immunostaining of cryosectioned WT and NebΔSH3 TA muscle with antibodies against myopalladin (MYPN), palladin (PALLD), zyxin and N-WASP, showing colocalization with α-actinin or desmin in the Z-line. (B) Immunostaining of cryosectioned WT and NebΔSH3 TA muscle during regeneration 14 days after injection of cardiotoxin, showing targeting of myopalladin and N-WASP to the Z-line in both WT and NebΔSH3 muscle. (C) Immunostaining for N-WASP of cryosectioned TA muscle from WT and NebΔSH3 mice after 48 hours of starvation and at different time points following administration of IGF-1 to 48-hour prefasted mice. All scale bars: 5 µm. (D) Top: western blot analysis for myopalladin and N-WASP on cytosolic and cytoskeletal fractions of TA muscle from WT and NebΔSH3 mice. Bottom: western blot analysis for N-WASP on fractionated TA muscle from WT and NebΔSH3 mice at basal conditions, after 48 hours of starvation, and 2 hours after administration of IGF-1 to prefasted mice.
Discussion
The aim of the present study was to dissect the functional role of the nebulin C-terminal SH3 domain in skeletal muscle. Using purified nebulin SH3 domain, we performed a pulldown on differentiated C2C12 muscle cells followed by protein identification through LC-MS/MS analysis. Unlike yeast two-hybrid screenings, which are limited to the identification of direct protein interactions, this approach allows for the identification of proteins both directly and indirectly connected to nebulin. As expected, the already known interaction partners myopalladin and zyxin were among the identified proteins (Bang et al., 2001; Li et al., 2004), validating the approach. The remaining identified proteins included Z-line proteins (CSRP1, CSRP3/MLP, CRIP2, nexilin, α-actinin 1), actin associated proteins (gelsolin, caldesmon, cofilin-1, nexilin, AHNAK/desmoyokin), intermediate filaments (desmin, peripherin, vimentin), desmosomal proteins (desmoplakin, junction plakoglobin), and nucleotide/ribosome-binding proteins (YB-1, HNRNPD, HNRNPA3, EEF1B2, EIF5A, SRSF1, NACA, DDB1, nucleolin). Using the online program SH3 hunter, we identified potential SH3 consensus sites in many of the identified proteins but when tested in the yeast two-hybrid system only myopalladin was confirmed as direct interaction partner of the nebulin SH3 domain. However, our STRING analysis (supplementary material Fig. S2) revealed that the majority of the identified proteins are indirectly connected to the nebulin SH3 domain either through direct interaction with its interaction partners myopalladin, palladin, zyxin, titin and N-WASP or indirectly through direct or indirect interaction with their binding partners, which include α-actinin [myopalladin (Bang et al., 2006), palladin (Bang et al., 2001; Crawford et al., 1992; Rönty et al., 2004) and zyxin (Crawford et al., 1992)], F-actin [myopalladin (our unpublished data) and palladin (Dixon et al., 2008)] and CSRP1/3 [zyxin (Louis et al., 1997)], which are each connected to many additional proteins. Strikingly, neither N-WASP nor any of its known interaction partners were among the identified proteins. Also, titin and palladin were not detected, which in the case of titin could be because of its large size, as separation on a 4–12% gel is not appropriate for its detection. Alternatively, these proteins might not be soluble in the lysis buffer or may have been retained in amounts too low for detection. For the larger identified proteins, such as AHNAK/desmoyokin, gelsolin and junction plakoglobin, in which only regions with predicted SH3-binding sites were tested in the yeast two-hybrid system, it is possible that non-conventional SH3-binding sites may be present in other parts of the proteins. In particular, AHNAK/desmoyokin contains repetitive sequences rich in positively charged residues, which might constitute potential SH3-binding sites (Jia et al., 2005). Another possibility is that SH3-binding domains may be masked through protein folding or that the binding affinities are too low to show positive binding in the yeast two-hybrid assay. Together, our pulldown approach revealed that the nebulin SH3 domain is part of a multiprotein complex in the Z-line connected to intermediate filaments and actin-organizing proteins as well as nuclear nucleotide- or ribosome-binding proteins and transcription factors.
Nonsense mutations in the nebulin gene, resulting in truncation and absence of the nebulin C-terminal SH3 domain have previously been found in patients with a typical form of nemaline myopathy. One case was a 6-year-old girl, who tested negative for the nebulin SH3 epitope by western blot analysis, whereas immunostainings confirmed the presence of nebulin epitopes at the N2 line region, modules M176–181 and the serine-rich region, suggesting the presence of a mutation in the 3′ part of the nebulin serine-rich region (Gurgel-Giannetti et al., 2002). Unfortunately, the causative mutation has not been identified. Another case was a child with a homozygous nonsense mutation in the ninth codon of exon 185, resulting in the loss of the last 134 amino acids, corresponding to the beginning of the nebulin C-terminal serine-rich region (Pelin et al., 1999). The absence of the nebulin SH3 domain was confirmed by immunohistochemical analysis.
To provide further insights into the role of the nebulin SH3 domain in vivo and the molecular mechanisms leading to nemaline myopathy, we generated a knockin mouse model with a stop codon at the 3′ end of the nebulin serine-rich region, resulting in specific deletion of the nebulin SH3 domain. An in vivo site-specific mutagenesis approach was chosen because the large size of nebulin (∼800 kDa) precludes the expression of full-length nebulin in current in vivo and in vitro expression systems. Furthermore, the knockin technology is the most reliable method for studying the effect of mutations without affecting gene expression and the surrounding genomic region. The correct targeting of the nebulin gene was confirmed by RT-PCR, sequencing and western blot analyses. Truncated and WT nebulin was expressed at similar levels and no compensation through upregulation of other members of the nebulin superfamily was found. Surprisingly, NebΔSH3 mice exhibited no histological and ultrastructural abnormalities at any developmental stage, and genome-wide expression analysis did not reveal any significant differences between NebΔSH3 and WT muscle (see supplementary material Table S1). Furthermore, peak isometric stress generation and passive mechanical properties were comparable between NebΔSH3 and WT mice, suggesting that the nebulin SH3 domain is not directly involved in force generation or that connections between other Z-line components compensate for its absence. However, the greater reduction in isometric force production in NebΔSH3 muscle in response to cyclic eccentric contractions compared to that of WT muscle, suggests that the nebulin SH3 domain plays a role in protecting the muscle from injury, perhaps by distributing the stress more effectively across the Z-disk. During eccentric contractions, muscles are forced to withstand loads substantially greater than those that they can actively create, and eccentric loads can exceed the mechanical tolerance threshold of skeletal muscle and, thus, produce tissue damage. Certain Z-line proteins, and in particular mechanotransducers (Knöll et al., 2002), may act as molecular scaffolds that stabilize the sarcomere during eccentric contractions. The observation that NebΔSH3 muscle is unusually vulnerable to eccentric contraction-induced injury indicates that the nebulin SH3 domain may have a scaffolding role, consistent with previous observations that the nebulin C-terminus is involved in defining the internal structure of the Z-disk (Pappas et al., 2008; Tonino et al., 2010). A role of nebulin in preventing damage to the sarcomere is also supported by our previous studies of nebulin knockout mice, which exhibited progressive myofibrilar disorganization during muscle use (Bang et al., 2006). The absence of histological and ultrastructural abnormalities in NebΔSH3 mice despite their heightened vulnerability to muscle injury could be explained by the fact that laboratory mice are sedentary and unlikely to be exposed to biomechanically challenging events, such as the eccentric exercise protocol that we applied ex vivo, i.e. a 15% active strain during maximal activation at 100 Hz. Therefore, increased susceptibility to eccentric contraction-induced injury or other biomechanical challenges should not be ruled out as a possible underlying cause for the muscle pathology observed in nemaline myopathy patients. Furthermore, it should be noted that the vulnerability of the NebΔSH3 mouse to muscle injury was independent of age, consistent with the commonly accepted characterization of nemaline myopathy as a nonprogressive disorder (North et al., 1997).
Comparison of force–frequency curves of NebΔSH3 and WT muscle revealed a slight, but statistically significant depression in maximum isometric stress production at lower frequencies of 20–30 Hz in NebΔSH3 muscle. This suggests a role of the nebulin SH3 domain in excitation–contraction coupling, either through slower Ca2+ release or faster Ca2+ uptake. Ablation of nebulin has previously been shown to result in a dramatic upregulation of sarcolipin, a sarcoplasmic reticulum Ca2+ ATPase (SERCA) inhibitor (Gokhin et al., 2009; Ottenheijm et al., 2008; Witt et al., 2006). However, no changes in sarcolipin mRNA levels were found in the gene expression analysis. Therefore, it remains unclear how the nebulin SH3 domain might affect excitation–contraction coupling.
Takano et al. recently demonstrated that N-WASP is present in the Z-line where it binds to the nebulin SH3 domain in a phosphorylation-dependent manner as GSK3β-mediated phosphorylation of the nebulin serine-rich region prevented the interaction (Takano et al., 2010). Correspondingly, N-WASP was shown to be diffusely distributed in muscle from fasted mice, but mobilized to the Z-line within 30 minutes after IGF-1 stimulation, activating PI3K–Akt and consequently inhibiting GSK3β. In contrast, we did not detect any influence of the nebulin SH3 domain on the recruitment of N-WASP to the Z-line during muscle regeneration and found that the localization of N-WASP in the Z-line and its distribution between the cytosolic and cytoskeletal fraction was unaffected by the presence or absence of the nebulin SH3 domain, or whether or not the mice had been exposed to starvation or IGF-1 treatment. Furthermore, in contrast to myopalladin, which was associated mainly with the cytoskeletal fraction, N-WASP was predominantly present in the cytosolic fraction and only detected at very low levels in the cytoskeletal fraction, suggesting that N-WASP is only loosely connected to the Z-line. Thus, our data do not support a role of the nebulin SH3 domain in the targeting of N-WASP to the Z-line. In line with this, we also did not find any reduction in fiber cross-sectional area or effect on muscle regeneration following cardiotoxin-induced injury in NebΔSH3 muscle compared with WT muscle, consistent with the previously reported normal embryonic development of nebulin knockout mice (Bang et al., 2006; Witt et al., 2006). Thus, the decreased muscle cross-sectional area in response to knockdown of N-WASP is unlikely to be mediated through its interaction with nebulin, which could also explain why the effect on fiber size was independent of IGF-1. Instead, in a recent study of skeletal-muscle-specific knockout embryos for N-WASP, Gruenbaum-Cohen et al. demonstrated that N-WASP is required for myogenic cell fusion, essential for the formation of functional muscle fibers (Gruenbaum-Cohen et al., 2012). Therefore, the reduced myoblast fiber size caused by N-WASP knockdown is likely to be related to its role in myoblast cell fusion.
We also tested the effect of the nebulin SH3 domain on the expression and localization of myopalladin, but found no difference in either localization or amount of myopalladin associated with the cytoskeletal fraction in the presence or absence of the nebulin SH3 domain. This is in contrast to the previously reported more diffuse Z-line localization of myopalladin in nebulin knockout mice compared to WT mice (Witt et al., 2006). However, our studies were performed on adult mice, whereas Witt et al. studied 10- to 15-day-old nebulin knockout mice, exhibiting severe growth deficit and muscle disorganization that may have secondarily affected the localization of myopalladin. The presence of myopalladin, palladin, zyxin and N-WASP in the Z-line in the absence of the nebulin SH3 domain might be ascribed to their binding to other Z-line proteins, such as α-actinin, which binds to myopalladin, palladin and zyxin (Bang et al., 2001; Crawford et al., 1992; Rönty et al., 2004). By contrast, there are no other known binding partners of N-WASP in the Z-line. Therefore, N-WASP may either bind to an as yet unidentified Z-line protein or, in the absence of the nebulin SH3 domain, bind to a protein to which it does not normally bind in WT muscle as a compensatory effect. This, however, seems unlikely given the fact that neither nebulette nor LASP1 were upregulated in NebΔSH3 skeletal muscle. Although the localization of all known interaction partners of the nebulin SH3 domain are localized in the Z-line independent from of the absence of the domain, it is possible that their missing connection to nebulin may cause weakness of the Z-line and be responsible for the higher susceptibility of NebΔSH3 mice to eccentric contraction-induced injury.
Based on the identification of nemaline myopathy patients with truncations in nebulin, resulting in deletion of its SH3 domain, it is surprising that the NebΔSH3 mouse model exhibits only a relatively weak phenotype. One explanation could be that the patients in which nebulin truncations have been identified contain additional unidentified mutations in the nebulin gene or other nemaline-myopathy-causing genes. In the patient with a nonsense mutation resulting in loss of both the serine-rich region and the SH3 domain, it is possible that the absence of the serine-rich region, and not the SH3 domain, is responsible for the development of the disease (Pelin et al., 1999). An alternative explanation could be the lower protein expression level of mutant nebulin in human patients due to a nonsense-mediated mRNA decay mechanism. Indeed, a reduction in nebulin expression was found in the patient specifically lacking the SH3 domain (Gurgel-Giannetti et al., 2002). We generated the NebΔSH3 mouse model by knocking in a nonsense codon in the last exon, thus avoiding nonsense-mediated mRNA decay and as demonstrated above truncated nebulin was expressed at the same level as WT nebulin. Finally, it is also possible that the consequence of nebulin SH3 domain deletion in mouse is not as detrimental as in human. This is, for example, the case for the well-characterized dystrophin-deficient mdx mouse model of Duchenne muscular dystrophy, which, in contrast to human patients, do not exhibit progressive muscle wasting because of its compensatory upregulation of utrophin and robust capacity for muscle regeneration (Watchko et al., 2002). Thorough sequence and quantitative protein analysis of the known NM-causing genes in the patient described by Gurgel-Giannetti et al. (Gurgel-Giannetti et al., 2002) or other patients who have been diagnosed negative for the nebulin SH3 epitope, would establish whether absence of the nebulin SH3 domain is indeed causative for nemaline myopathy in humans.
Materials and Methods
Expression and purification of proteins
Nebulin mouse cDNA residues 21699–21878 [GenBank accession number (acc.) NM_010889], encoding its C-terminal SH3 region, were cloned into the pETM-33 expression vector (Dümmler et al., 2005) for expression and purification of H6-GST-3C tag-fused protein using a nickel column (Bio-Rad). To generate polyclonal antibodies, mouse myopalladin residue 411–740 (acc. NM_182992) and mouse nebulin residue 21699-21878 were cloned into the pETM-14 expression vector for expression of H6-3C-tagged proteins. Polyclonal antibodies were generated and affinity purified by Eurogentec.
Protein pulldown experiments and LC-MS/MS analysis
Differentiated C2C12 cells were washed twice with ice-cold 1× PBS and lysed for 30 minutes on ice in lysis buffer containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1 mM EDTA, 0.5% NP40, protease inhibitor cocktail (Roche) and phosphatase inhibitors (0.1 mM Na3VO4, 10 mM NaF). After centrifugation at 20,000 rpm for 20 minutes at 4°C, the supernatant was filtered through a 0.45 µm filter and the supernatant was pre-cleared by incubation with the H6-GST-3C tag on a nickel column for 2 hours at 4°C. Equal amounts of the flow-through were incubated with equal amounts of H6-GST-3C-tagged nebulin SH3 domain and H6-GST-3C tag overnight at 4°C, and were subsequently bound to nickel columns. Following washing with 10 column volumes of wash buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1 mM EDTA, 0.1% NP40, protease and phosphatase inhibitors), bound proteins were eluted with elution buffer (50 mM Na2PO4, 500 mM NaCl, 500 mM imidazol). After dialysis into 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1 mM EDTA, 1 mM DTT, the H6-GST-3C tag was cleaved off by incubation with PreScission protease (1∶200) overnight at 4°C. To eliminate the cleaved tag, digested samples were incubated with Ni-beads and the flow-through containing the nebulin SH3 domain and its interaction partners was collected. The obtained samples were separated on a NuPAGE 4–12% gel (Novex, Invitrogen) and analyzed by LC-MS/MS as previously described (Lalle et al., 2012), except that tandem mass spectra were matched against the NCBI database and identifications were made on the basis of at least two peptides. Proteins identified in both the H6-GST-3C-nebulin SH3 pulldown and the control pulldown using the H6-GST-3C tag alone were regarded as nonspecific. Functional protein annotation network analysis was performed on the list of identified proteins using STRING 9.05 software (http://string-db.org/) (Jensen et al., 2009; Szklarczyk et al., 2011) and the following analysis parameters: Homo sapiens and Mus musculus; confidence level, 0.400; active prediction methods: experiments, databases. Interactions were individually verified by PubMed searches, and experimentally validated interactions are indicated by thick lines in supplementary material Fig. S2.
Search for SH3 consensus sites in identified proteins and test of binding to the nebulin SH3 domain
To search for potential consensus sites for the nebulin SH3 domain in the identified proteins, the online program SH3 hunter (http://cbm.bio.uniroma2.it/SH3-Hunter/) was used (Ferraro et al., 2007). Both the peptide filters ‘default (class I, class II)’ and ‘PXXP’ were used and for proteins with predicted binding to the nebulin SH3 domain, the prediction score was recorded (Table 1). Interactions with proteins containing SH3 consensus sites were tested using the Matchmaker Gold yeast two-hybrid system (Clontech) following the manufacturer's protocol. Briefly, pGADT7-AD vector expressing the nebulin SH3 domain (residues 21699–21878; acc. NM_010889) were co-transfected into the Y2Hgold yeast strain together with pGBKT7 vectors containing full-length coding regions of junction plakoglobin (acc. NM_010593), desmin (acc. NM_010043) and CRIP2 (acc NM_024223) as well as mouse gelsolin residue 85–1704 (acc. NM_146120), desmoplakin residue 1139–2709 (acc. NM_023842) and desmoyokin/AHNAK residues 10020–10404 and 16080–17228 (acc. NM_009643).
Generation of nebulin SH3-deleted mice
Nebulin genomic DNA was isolated from a 129/SvJ mouse genomic DNA library (Stratagene) and used to generate a nebulin-targeting construct in which nebulin residue I7097 (acc. NM_010889) was replaced by an frt-neo-frt cassette and stop codons in all reading frames as illustrated in Fig. 3A. The targeting construct was verified by sequencing and linearized with NotI before electroporation into 129/SvJ-derived embryonic stem (ES) cells at the Transgenic Core Facility at the University of California San Diego (UCSD). 1000 G418-resistant ES clones were screened for homologous recombination by Southern blot analysis of BamHI-digested ES cell DNA using a 419 bp probe generated by PCR on mouse genomic DNA with nebulin-specific primers (forward, 5′-CCTGGGAGCAACCTGAGAATCTCC-3′; reverse, 5′-TAGCACCCAGCAACTCTACTTGGAGT-3′). Two homologous recombinant ES cell clones were identified and microinjected into C57BL/B6 blastocysts. Resulting male chimeras were bred with female Black Swiss mice to generate germline-transmitted heterozygous mice, which were identified by PCR analysis using mouse tail DNA and gene-specific primers (sense: 5′-GTGGTAGCTGCACACTGTTCTTTGTAAC-3′; reverse: 5′-GCACAGTGCCATACATCCAGCCTTC-3′). The heterozygous mutant mice were crossed with FLPase deleter mice to remove the neo gene and subsequently mated to generate homozygous mutant mice (designated as NebΔSH3). To confirm that the gene targeting was successful, the genotyping primers were used for RT-PCR analysis on total RNA from TA muscle of NebΔSH3 mice using Trizol reagent and the SuperScript III One step RT-PCR system (Invitrogen), giving rise to a 221 bp band from the WT allele and a 255 bp band from the mutant allele. The presence of stop codons in all three reading frames was confirmed by sequencing. Furthermore, western blot analysis using the novel antibody against the nebulin SH3 domain (1∶500) confirmed the absence of the nebulin SH3 domain. Additional western blot analyses were performed using antibodies against nebulin M160-164, nebulin M161-165 and nebulette N-terminus (all 1∶200; kindly provided by Dr Siegfried Labeit, Universitätsklinikum Mannheim, Germany). Antibodies against α-actinin (1∶1000; Sigma Aldrich no. A7811) and α-tubulin (1∶3000; Abcam no. 18251) were used as loading controls. All animal procedures were in full compliance with the guidelines approved by the UCSD Animal Care and Use Committee and the Italian Ministry of Health.
Mouse procedures
To study muscle regeneration following degeneration, 80 µl of 10 µM cardiotoxin (Sigma-Aldrich) was injected into the TA muscle of one leg and PBS was injected into the contralateral leg. Tissue was collected 4, 7, 14 and 21 days after injection. To study the effect of fasting and IGF-1 stimulation, mice were fasted for 48 hours, after which they were injected with IGF-1 (0.1 µg/g body weight) through the tail vein.
Histology, immunohistochemistry and transmission electron microscopy
10 µm transverse cryosections of mouse hind limb muscles (TA, EDL, soleus) were subjected to Hematoxylin and Eosin and Picro Sirius Red staining and fiber cross sectional area was measured by laminin immunohistochemistry and image analysis as previously described (Minamoto et al., 2007). For confocal microscopy, longitudinal cryosections of stretched TA muscle were stained as previously described (Zhang et al., 2008). The following primary antibodies were used: N-WASP [1∶100 (Takano et al., 2010)] myopalladin (1∶100; newly generated), palladin [no. 622 1∶200, kindly provided by Prof. Carol Otey (Goicoechea et al., 2010)], zyxin (1∶50, Transduction lab no. Z45420), α-actinin (1∶500; Sigma-Aldrich no. A7811) and desmin (1∶80; Abcam no. ab8592). Alexa-Fluor-488-conjugated or Alexa-Fluor-568-conjugated IgG secondary antibodies (Invitrogen) were diluted at 1∶2000. Confocal microscopy was performed using a Nikon A1R confocal microscope with a Plan Apochromat 60× 1.4 NA objective (Nikon Instruments). For TEM, diaphragm and stretched EDL muscle were fixed in 3% glutaraldehyde in Ringer’s solution and subsequently stained for 1 hour in 1% osmium tetroxide, dehydrated in a series of acetone washes, and embedded in Araldite epoxy resin (Agar Scientific) as previously described (Vydyanath et al., 2012). Ultrathin sections (90–100 nm) were cut with a Reichert Ultracut E ultramicrotome and subsequently stained with uranyl acetate and lead citrate. The sections were imaged with a JEOL 1200EX electron microscope operated at 100 kV.
SDS-PAGE and western blot analysis on the Triton-insoluble cytoskeletal fraction
Fiber type distribution in TA, EDL and soleus muscle was determined by measuring myosin heavy chain isoform levels as previously described (Barash et al., 2007). Subcellular fractionation was performed essentially as previously described (Dimauro et al., 2012) with the modification that the Triton-insoluble cytoskeletal fraction was obtained as the pellet after resuspension in NET buffer (20 mM HEPES pH 7.9, 1.5 mM MgCl2, 0.5 M NaCl, 0.2 mM EDTA, 20% glycerol, 1% Triton X-100, protease and protease inhibitors). The cytoskeletal fraction was resuspended and sonicated in RIPA buffer with 1% SDS (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% SDS, 1% Triton X-100, protease and protease inhibitors). Western blot analysis was performed using antibodies against myopalladin (1∶1000), N-WASP [1∶500 (Takano et al., 2010)], GAPDH (1∶2500; Cell Signaling Technology no. 14C10), and β-actin (1∶1000; Santa Cruz no. Sc-1615).
Gene expression microarray analysis
Gene expression microarray analysis was performed in triplicate on RNA extracted from TA muscle of 2-month-old NebΔSH3 mice using MouseWG-6 v2.0 Expression BeadChips from Illumina and an Illumina iScan system as described by the manufacturer. Raw data were background subtracted and normalized using the quantile normalization method (Lumi software package) (Du et al., 2008) and subsequently analyzed with GeneSifter (Geospiza) using P<0.05 and the Benjamini and Hochberg correction. Raw and normalized data are available in Gene Expression Omnibus (GEO; acc. GSE47801).
Biomechanical testing of muscle
Biomechanical testing was performed on the fifth toe EDL muscle essentially as previously described (Zhang et al., 2008). Briefly, passive mechanical properties were measured three times at 2 minute intervals by imposing a 15% fiber length (Lf) stretch at a rate of 0.7 Lf/second, and maximum isometric tension was measured three times at 2 minute intervals by applying a 400 msecond train of 0.3 msecond pulses delivered at 100 Hz while maintaining constant muscle length. Muscles underwent a series of 10 eccentric contractions at 2 minute intervals. For each contraction, the muscle was first maximally activated isometrically until tension stabilized (∼200 msecond) and then stretched by 15% Lf at a rate of 2 Lf/second. To generate force–frequency curves, muscles underwent successive 400 msecond trains of 0.3 msecond pulses at 5, 10, 20, 30, 40, 50, 60, 80 and 100 Hz, spaced 2 minutes apart. Isometric stresses were normalized to the maximum isometric stress measured at 100 Hz. Logistic regression was applied to the force–frequency data to compute the stimulation frequencies corresponding to 25%, 50% and 75% of maximum isometric stress production. Young's moduli of single fibers from TA and soleus muscles were measured as previously described (Zhang et al., 2008).
Statistical analysis
Data are presented as means ± s.e.m. The effect of genotype was determined using the unpaired Student's t-test. Simultaneous effects of genotype and another experimental variable were determined using two-way analysis of variance (ANOVA) with post-hoc Fisher's protected least-significant difference (PLSD) analysis. A P-value <0.05 was considered significant. Statistical analysis was performed in StatView (SAS, Cary, NC).
Supplementary Material
Acknowledgments
We thank Dr Ario de Marco (IFOM-IEO, Milan, Italy) for providing expression vectors and PreScission protease.
Footnotes
Author contributions
D.L.Y. maintained the NebΔSH3 mice and performed molecular and biochemical studies. C.M. performed protein purification, pulldown assays and yeast two-hybrid experiments. J.Z. generated the NebΔSH3 mouse line. D.S.G. performed the physiological experiments and contributed to the writing of the paper. A.C. conducted the gene expression analysis. G.C. and P.J.E. assisted with biochemical assays, clonings and yeast two-hybrid experiments. F.P. performed protein fractionation, western blots and histological analyses. M.C.F. carried out western blots, P.K. assisted with bioinformatics analysis of gene expression data. S.C. and M.C. performed LC-MS/MS and data analysis. K.T. generated the N-WASP antibody. T.E. provided the N-WASP antibody and contributed to the interpretation of data. P.L. performed TEM. R.L.L. assisted with the interpretation of physiological experiments and in writing of the manuscript. M.L.B. and J.C. conceived the project. J.C. designed, directed, and interpreted the studies in regard to the generation and characterizing of the NebΔSH3 mouse model as well as assisted in writing the paper. M.L.B. designed and directed the study, performed experiments, prepared the figures, and wrote the manuscript.
Funding
This work was supported by grants from the Italian Telethon Foundation [grant number TCP07006 to M.L.B.]; the Cariplo Foundation [grant number 2007.5812 to M.L.B.]; the Italian Ministry of Education, Universities and Research [grant number PRIN 2010-2011 2010R8JK2X_006 to M.L.B.]; the National Institutes of Health [grant numbers R01AR059334 and R01HL066100 to J.C., and P30 AR061303 and R24 HD050837 to RLL]; and the Department of Veterans Affairs to R.L. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.137026/-/DC1
References
- Bang M. L., Mudry R. E., McElhinny A. S., Trombitás K., Geach A. J., Yamasaki R., Sorimachi H., Granzier H., Gregorio C. C., Labeit S. (2001). Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J. Cell Biol. 153, 413–428 10.1083/jcb.153.2.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang M. L., Gregorio C., Labeit S. (2002). Molecular dissection of the interaction of desmin with the C-terminal region of nebulin. J. Struct. Biol. 137, 119–127 10.1006/jsbi.2002.4457 [DOI] [PubMed] [Google Scholar]
- Bang M. L., Li X., Littlefield R., Bremner S., Thor A., Knowlton K. U., Lieber R. L., Chen J. (2006). Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. J. Cell Biol. 173, 905–916 10.1083/jcb.200603119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang M. L., Caremani M., Brunello E., Littlefield R., Lieber R. L., Chen J., Lombardi V., Linari M. (2009). Nebulin plays a direct role in promoting strong actin-myosin interactions. FASEB J. 23, 4117–4125 10.1096/fj.09-137729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash I. A., Bang M. L., Mathew L., Greaser M. L., Chen J., Lieber R. L. (2007). Structural and regulatory roles of muscle ankyrin repeat protein family in skeletal muscle. Am. J. Physiol. 293, C218–C227 10.1152/ajpcell.00055.2007 [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Drai D., Elmer G., Kafkafi N., Golani I. (2001). Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 125, 279–284 10.1016/S0166-4328(01)00297-2 [DOI] [PubMed] [Google Scholar]
- Castillo A., Nowak R., Littlefield K. P., Fowler V. M., Littlefield R. S. (2009). A nebulin ruler does not dictate thin filament lengths. Biophys. J. 96, 1856–1865 10.1016/j.bpj.2008.10.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. W., Michelsen J. W., Beckerle M. C. (1992). An interaction between zyxin and alpha-actinin. J. Cell Biol. 116, 1381–1393 10.1083/jcb.116.6.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimauro I., Pearson T., Caporossi D., Jackson M. J. (2012). A simple protocol for the subcellular fractionation of skeletal muscle cells and tissue. BMC Res. Notes 5, 513 10.1186/1756-0500-5-513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. D., Arneman D. K., Rachlin A. S., Sundaresan N. R., Costello M. J., Campbell S. L., Otey C. A. (2008). Palladin is an actin cross-linking protein that uses immunoglobulin-like domains to bind filamentous actin. J. Biol. Chem. 283, 6222–6231 10.1074/jbc.M707694200 [DOI] [PubMed] [Google Scholar]
- Du P., Kibbe W. A., Lin S. M. (2008). lumi: a pipeline for processing Illumina microarray. Bioinformatics 24, 1547–1548 10.1093/bioinformatics/btn224 [DOI] [PubMed] [Google Scholar]
- Dümmler A., Lawrence A. M., de Marco A. (2005). Simplified screening for the detection of soluble fusion constructs expressed in E. coli using a modular set of vectors. Microb. Cell Fact. 4, 34 10.1186/1475-2859-4-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro E., Peluso D., Via A., Ausiello G., Helmer-Citterich M. (2007). SH3-Hunter: discovery of SH3 domain interaction sites in proteins. Nucleic Acids Res. 35 Web Server issue, W451–W454 10.1093/nar/gkm296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D., Kuhn C., Katus H. A., Frey N. (2006). The sarcomeric Z-disc: a nodal point in signalling and disease. J. Mol. Med. 84, 446–468 10.1007/s00109-005-0033-1 [DOI] [PubMed] [Google Scholar]
- Goicoechea S. M., Arneman D., Otey C. A. (2008). The role of palladin in actin organization and cell motility. Eur. J. Cell Biol. 87, 517–525 10.1016/j.ejcb.2008.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goicoechea S. M., Bednarski B., Stack C., Cowan D. W., Volmar K., Thorne L., Cukierman E., Rustgi A. K., Brentnall T., Hwang R. F. et al. (2010). Isoform-specific upregulation of palladin in human and murine pancreas tumors. PLoS ONE 5, e10347 10.1371/journal.pone.0010347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhin D. S., Fowler V. M. (2013). A two-segment model for thin filament architecture in skeletal muscle. Nat. Rev. Mol. Cell Biol. 14, 113–119 10.1038/nrm3510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhin D. S., Bang M. L., Zhang J., Chen J., Lieber R. L. (2009). Reduced thin filament length in nebulin-knockout skeletal muscle alters isometric contractile properties. Am. J. Physiol. 296, C1123–C1132 10.1152/ajpcell.00503.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum-Cohen Y., Harel I., Umansky K. B., Tzahor E., Snapper S. B., Shilo B. Z., Schejter E. D. (2012). The actin regulator N-WASp is required for muscle-cell fusion in mice. Proc. Natl. Acad. Sci. USA 109, 11211–11216 10.1073/pnas.1116065109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgel-Giannetti J., Bang M. L., Reed U., Marie S., Zatz M., Labeit S., Vainzof M. (2002). Lack of the C-terminal domain of nebulin in a patient with nemaline myopathy. Muscle Nerve 25, 747–752 10.1002/mus.10097 [DOI] [PubMed] [Google Scholar]
- Jensen L. J., Kuhn M., Stark M., Chaffron S., Creevey C., Muller J., Doerks T., Julien P., Roth A., Simonovic M. et al. (2009). STRING 8—a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 37 Database issue, D412–D416 10.1093/nar/gkn760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia C. Y., Nie J., Wu C., Li C., Li S. S. (2005). Novel Src homology 3 domain-binding motifs identified from proteomic screen of a Pro-rich region. Mol. Cell. Proteomics 4, 1155–1166 10.1074/mcp.M500108-MCP200 [DOI] [PubMed] [Google Scholar]
- Knöll R., Hoshijima M., Hoffman H. M., Person V., Lorenzen-Schmidt I., Bang M. L., Hayashi T., Shiga N., Yasukawa H., Schaper W. et al. (2002). The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell 111, 943–955 10.1016/S0092-8674(02)01226-6 [DOI] [PubMed] [Google Scholar]
- Kontrogianni-Konstantopoulos A., Ackermann M. A., Bowman A. L., Yap S. V., Bloch R. J. (2009). Muscle giants: molecular scaffolds in sarcomerogenesis. Physiol. Rev. 89, 1217–1267 10.1152/physrev.00017.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeit S., Lahmers S., Burkart C., Fong C., McNabb M., Witt S., Witt C., Labeit D., Granzier H. (2006). Expression of distinct classes of titin isoforms in striated and smooth muscles by alternative splicing, and their conserved interaction with filamins. J. Mol. Biol. 362, 664–681 10.1016/j.jmb.2006.07.077 [DOI] [PubMed] [Google Scholar]
- Lalle M., Camerini S., Cecchetti S., Sayadi A., Crescenzi M., Pozio E. (2012). Interaction network of the 14-3-3 protein in the ancient protozoan parasite Giardia duodenalis. J. Proteome Res. 11, 2666–2683 10.1021/pr3000199 [DOI] [PubMed] [Google Scholar]
- Lehtokari V. L., Pelin K., Sandbacka M., Ranta S., Donner K., Muntoni F., Sewry C., Angelini C., Bushby K., Van den Bergh P. et al. (2006). Identification of 45 novel mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Hum. Mutat. 27, 946–956 10.1002/humu.20370 [DOI] [PubMed] [Google Scholar]
- Li B., Zhuang L., Trueb B. (2004). Zyxin interacts with the SH3 domains of the cytoskeletal proteins LIM-nebulette and Lasp-1. J. Biol. Chem. 279, 20401–20410 10.1074/jbc.M310304200 [DOI] [PubMed] [Google Scholar]
- Littlefield R. S., Fowler V. M. (2008). Thin filament length regulation in striated muscle sarcomeres: pointed-end dynamics go beyond a nebulin ruler. Semin. Cell Dev. Biol. 19, 511–519 10.1016/j.semcdb.2008.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis H. A., Pino J. D., Schmeichel K. L., Pomiès P., Beckerle M. C. (1997). Comparison of three members of the cysteine-rich protein family reveals functional conservation and divergent patterns of gene expression. J. Biol. Chem. 272, 27484–27491 10.1074/jbc.272.43.27484 [DOI] [PubMed] [Google Scholar]
- Luther P. K. (2009). The vertebrate muscle Z-disc: sarcomere anchor for structure and signalling. J. Muscle Res. Cell Motil. 30, 171–185 10.1007/s10974-009-9189-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K., Wang K. (2002). Interaction of nebulin SH3 domain with titin PEVK and myopalladin: implications for the signaling and assembly role of titin and nebulin. FEBS Lett. 532, 273–278 10.1016/S0014-5793(02)03655-4 [DOI] [PubMed] [Google Scholar]
- Ma K., Forbes J. G., Gutierrez-Cruz G., Wang K. (2006). Titin as a giant scaffold for integrating stress and Src homology domain 3-mediated signaling pathways: the clustering of novel overlap ligand motifs in the elastic PEVK segment. J. Biol. Chem. 281, 27539–27556 10.1074/jbc.M604525200 [DOI] [PubMed] [Google Scholar]
- Mayer B. J. (2001). SH3 domains: complexity in moderation. J. Cell Sci. 114, 1253–1263 [DOI] [PubMed] [Google Scholar]
- Millevoi S., Trombitas K., Kolmerer B., Kostin S., Schaper J., Pelin K., Granzier H., Labeit S. (1998). Characterization of nebulette and nebulin and emerging concepts of their roles for vertebrate Z-discs. J. Mol. Biol. 282, 111–123 10.1006/jmbi.1998.1999 [DOI] [PubMed] [Google Scholar]
- Minamoto V. B., Hulst J. B., Lim M., Peace W. J., Bremner S. N., Ward S. R., Lieber R. L. (2007). Increased efficacy and decreased systemic-effects of botulinum toxin A injection after active or passive muscle manipulation. Dev. Med. Child Neurol. 49, 907–914 10.1111/j.1469-8749.2007.00907.x [DOI] [PubMed] [Google Scholar]
- Musacchio A., Gibson T., Lehto V. P., Saraste M. (1992). SH3 – an abundant protein domain in search of a function. FEBS Lett. 307, 55–61 10.1016/0014-5793(92)80901-R [DOI] [PubMed] [Google Scholar]
- North K. N., Laing N. G., Wallgren-Pettersson C. The ENMC International Consortium and Nemaline Myopathy(1997). Nemaline myopathy: current concepts. J. Med. Genet. 34, 705–713 10.1136/jmg.34.9.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochala J., Lehtokari V. L., Iwamoto H., Li M., Feng H. Z., Jin J. P., Yagi N., Wallgren-Pettersson C., Pénisson-Besnier I., Larsson L. (2011). Disrupted myosin cross-bridge cycling kinetics triggers muscle weakness in nebulin-related myopathy. FASEB J. 25, 1903–1913 10.1096/fj.10-176727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenheijm C. A., Fong C., Vangheluwe P., Wuytack F., Babu G. J., Periasamy M., Witt C. C., Labeit S., Granzier H. (2008). Sarcoplasmic reticulum calcium uptake and speed of relaxation are depressed in nebulin-free skeletal muscle. FASEB J. 22, 2912–2919 10.1096/fj.07-104372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenheijm C. A., Witt C. C., Stienen G. J., Labeit S., Beggs A. H., Granzier H. (2009). Thin filament length dysregulation contributes to muscle weakness in nemaline myopathy patients with nebulin deficiency. Hum. Mol. Genet. 18, 2359–2369 10.1093/hmg/ddp168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenheijm C. A., Hooijman P., DeChene E. T., Stienen G. J., Beggs A. H., Granzier H. (2010). Altered myofilament function depresses force generation in patients with nebulin-based nemaline myopathy (NEM2). J. Struct. Biol. 170, 334–343 10.1016/j.jsb.2009.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenheijm C. A., Lawlor M. W., Stienen G. J., Granzier H., Beggs A. H. (2011). Changes in cross-bridge cycling underlie muscle weakness in patients with tropomyosin 3-based myopathy. Hum. Mol. Genet. 20, 2015–2025 10.1093/hmg/ddr084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas C. T., Bhattacharya N., Cooper J. A., Gregorio C. C. (2008). Nebulin interacts with CapZ and regulates thin filament architecture within the Z-disc. Mol. Biol. Cell 19, 1837–1847 10.1091/mbc.E07-07-0690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas C. T., Krieg P. A., Gregorio C. C. (2010). Nebulin regulates actin filament lengths by a stabilization mechanism. J. Cell Biol. 189, 859–870 10.1083/jcb.201001043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelin K., Hilpelä P., Donner K., Sewry C., Akkari P. A., Wilton S. D., Wattanasirichaigoon D., Bang M. L., Centner T., Hanefeld F. et al. (1999). Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc. Natl. Acad. Sci. USA 96, 2305–2310 10.1073/pnas.96.5.2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politou A. S., Millevoi S., Gautel M., Kolmerer B., Pastore A. (1998). SH3 in muscles: solution structure of the SH3 domain from nebulin. J. Mol. Biol. 276, 189–202 10.1006/jmbi.1997.1521 [DOI] [PubMed] [Google Scholar]
- Politou A. S., Spadaccini R., Joseph C., Brannetti B., Guerrini R., Helmer-Citterich M., Salvadori S., Temussi P. A., Pastore A. (2002). The SH3 domain of nebulin binds selectively to type II peptides: theoretical prediction and experimental validation. J. Mol. Biol. 316, 305–315 10.1006/jmbi.2001.5312 [DOI] [PubMed] [Google Scholar]
- Reinhard M., Jouvenal K., Tripier D., Walter U. (1995). Identification, purification, and characterization of a zyxin-related protein that binds the focal adhesion and microfilament protein VASP (vasodilator-stimulated phosphoprotein). Proc. Natl. Acad. Sci. USA 92, 7956–7960 10.1073/pnas.92.17.7956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R., Ma L., Miki H., Lopez M., Kirchhausen T., Takenawa T., Kirschner M. W. (1999). The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221–231 10.1016/S0092-8674(00)80732-1 [DOI] [PubMed] [Google Scholar]
- Rönty M., Taivainen A., Moza M., Otey C. A., Carpén O. (2004). Molecular analysis of the interaction between palladin and alpha-actinin. FEBS Lett. 566, 30–34 10.1016/j.febslet.2004.04.006 [DOI] [PubMed] [Google Scholar]
- Sheikh F., Bang M. L., Lange S., Chen J. (2007). “Z”eroing in on the role of Cypher in striated muscle function, signaling, and human disease. Trends Cardiovasc. Med. 17, 258–262 10.1016/j.tcm.2007.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D., Franceschini A., Kuhn M., Simonovic M., Roth A., Minguez P., Doerks T., Stark M., Muller J., Bork P. et al. (2011). The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 39 Database issue, D561–D568 10.1093/nar/gkq973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano K., Watanabe-Takano H., Suetsugu S., Kurita S., Tsujita K., Kimura S., Karatsu T., Takenawa T., Endo T. (2010). Nebulin and N-WASP cooperate to cause IGF-1-induced sarcomeric actin filament formation. Science 330, 1536–1540 10.1126/science.1197767 [DOI] [PubMed] [Google Scholar]
- Tonino P., Pappas C. T., Hudson B. D., Labeit S., Gregorio C. C., Granzier H. (2010). Reduced myofibrillar connectivity and increased Z-disk width in nebulin-deficient skeletal muscle. J. Cell Sci. 123, 384–391 10.1242/jcs.042234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vydyanath A., Gurnett C. A., Marston S., Luther P. K. (2012). Axial distribution of myosin binding protein-C is unaffected by mutations in human cardiac and skeletal muscle. J. Muscle Res. Cell Motil. 33, 61–74 10.1007/s10974-012-9286-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallgren-Pettersson C., Donner K., Sewry C., Bijlsma E., Lammens M., Bushby K., Giovannucci Uzielli M. L., Lapi E., Odent S., Akcoren Z. et al. (2002). Mutations in the nebulin gene can cause severe congenital nemaline myopathy. Neuromuscul. Disord. 12, 674–679 10.1016/S0960-8966(02)00065-2 [DOI] [PubMed] [Google Scholar]
- Wallgren-Pettersson C., Sewry C. A., Nowak K. J., Laing N. G. (2011). Nemaline myopathies. Semin. Pediatr. Neurol. 18, 230–238 10.1016/j.spen.2011.10.004 [DOI] [PubMed] [Google Scholar]
- Wang K., Wright J. (1988). Architecture of the sarcomere matrix of skeletal muscle: immunoelectron microscopic evidence that suggests a set of parallel inextensible nebulin filaments anchored at the Z line. J. Cell Biol. 107, 2199–2212 10.1083/jcb.107.6.2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watchko J. F., O'Day T. L., Hoffman E. P. (2002). Functional characteristics of dystrophic skeletal muscle: insights from animal models. J. Appl. Physiol. 93, 407–417 [DOI] [PubMed] [Google Scholar]
- Witt C. C., Burkart C., Labeit D., McNabb M., Wu Y., Granzier H., Labeit S. (2006). Nebulin regulates thin filament length, contractility, and Z-disk structure in vivo. EMBO J. 25, 3843–3855 10.1038/sj.emboj.7601242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Bang M. L., Gokhin D. S., Lu Y., Cui L., Li X., Gu Y., Dalton N. D., Scimia M. C., Peterson K. L. et al. (2008). Syncoilin is required for generating maximum isometric stress in skeletal muscle but dispensable for muscle cytoarchitecture. Am. J. Physiol. 294, C1175–C1182 10.1152/ajpcell.00049.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.