Abstract
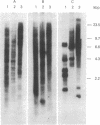
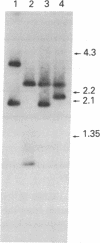
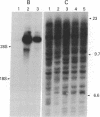
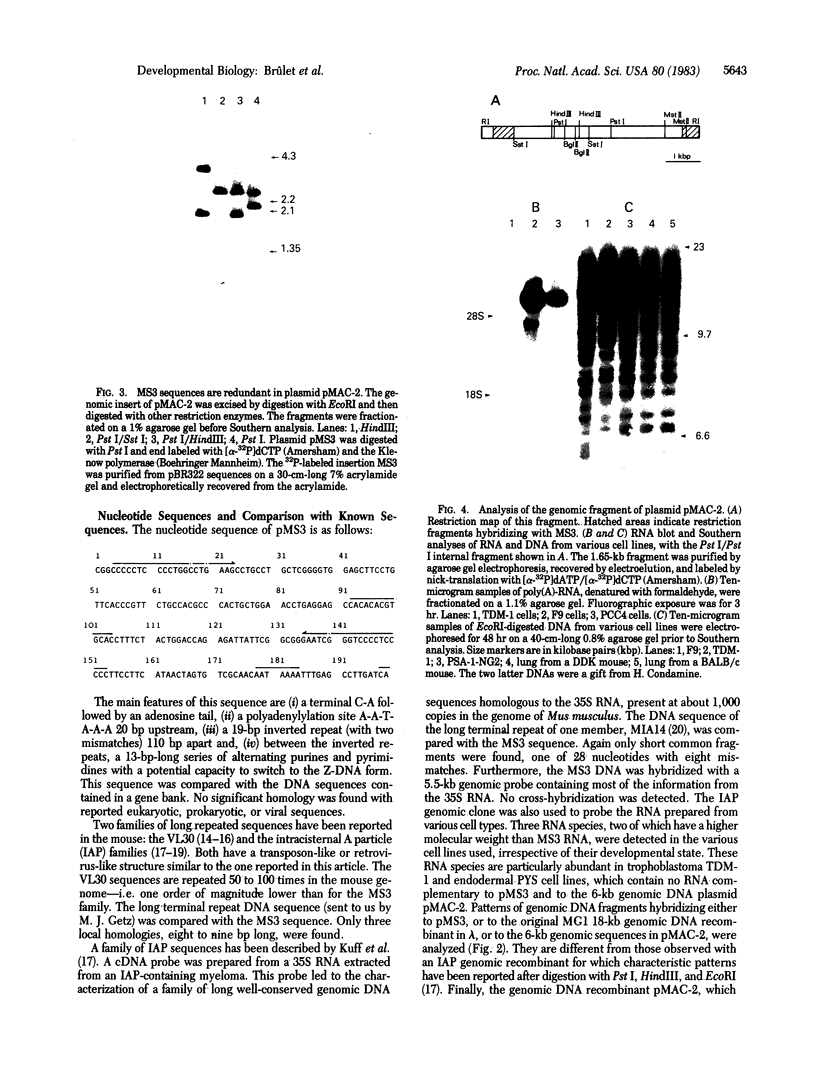
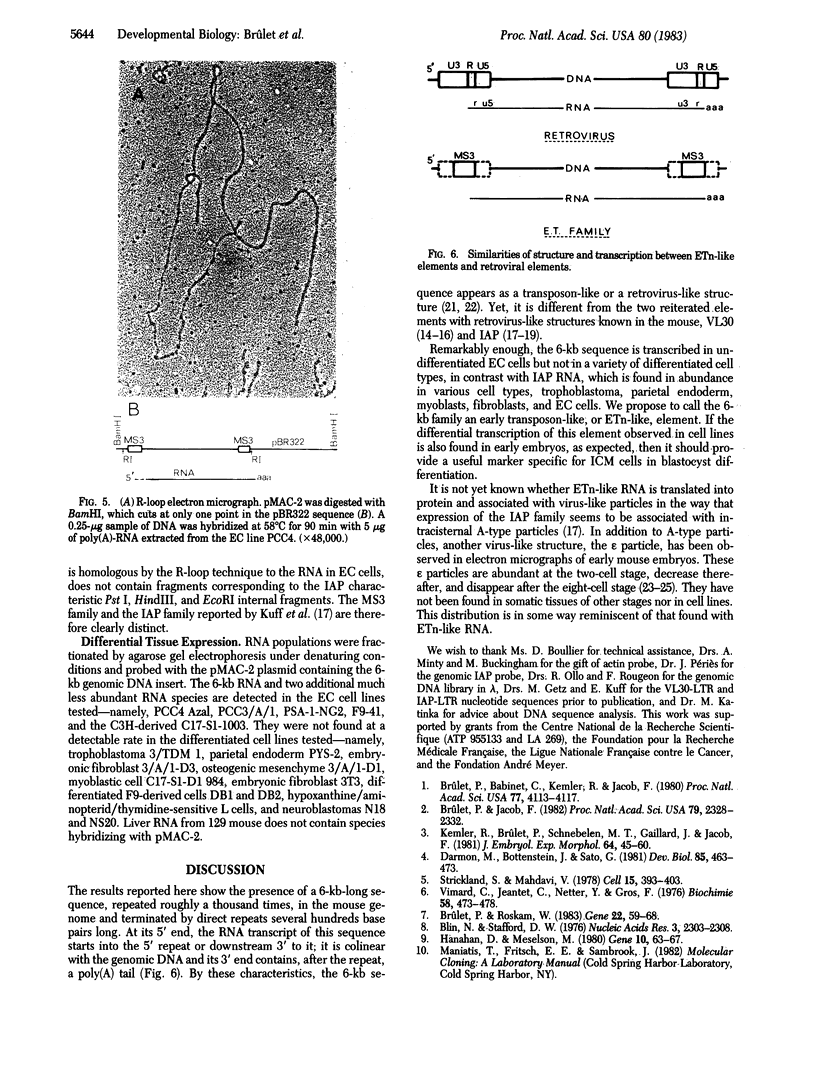
Another family of long moderately repetitive and dispersed sequences has been identified in the mouse genome. These sequences have a transposon-like structure. A 6-kilobase RNA transcript is detected in undifferentiated embryonal carcinoma cell lines but not in any of the differentiated cell types tested. By R-loop formation, the RNA is colinear with a DNA fragment from a randomly selected genomic clone.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brûlet P., Babinet C., Kemler R., Jacob F. Monoclonal antibodies against trophectoderm-specific markers during mouse blastocyst formation. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4113–4117. doi: 10.1073/pnas.77.7.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brûlet P., Jacob F. Molecular cloning of a cDNA sequence encoding a trophectoderm-specific marker during mouse blastocyst formation. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2328–2332. doi: 10.1073/pnas.79.7.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brûlet P., Roskam W. G. Improved method for cloning DNA complementary to minor mRNAs: preparation of a hybridization probe from purified mRNAs encoding intermediate filament proteins. Gene. 1983 Apr;22(1):59–68. doi: 10.1016/0378-1119(83)90064-1. [DOI] [PubMed] [Google Scholar]
- Chase D. G., Pikó L. Expression of A- and C-type particles in early mouse embryos. J Natl Cancer Inst. 1973 Dec;51(6):1971–1975. doi: 10.1093/jnci/51.6.1971. [DOI] [PubMed] [Google Scholar]
- Cole M. D., Ono M., Huang R. C. Terminally redundant sequences in cellular intracisternal A-particle genes. J Virol. 1981 May;38(2):680–687. doi: 10.1128/jvi.38.2.680-687.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmon M., Bottenstein J., Sato G. Neural differentiation following culture of embryonal carcinoma cells in a serum-free defined medium. Dev Biol. 1981 Jul 30;85(2):463–473. doi: 10.1016/0012-1606(81)90277-3. [DOI] [PubMed] [Google Scholar]
- Foster D. N., Schmidt L. J., Hodgson C. P., Moses H. L., Getz M. J. Polyadenylylated RNA complementary to a mouse retrovirus-like multigene family is rapidly and specifically induced by epidermal growth factor stimulation of quiescent cells. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7317–7321. doi: 10.1073/pnas.79.23.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Cohn R. H., Kedes L. H., Davidson N. Positions of sea urchin (Strongylocentrotus purpuratus) histone genes relative to restriction endonuclease sites on the chimeric plasmids pSp2 and pSp17. Biochemistry. 1977 Apr 5;16(7):1504–1512. doi: 10.1021/bi00626a040. [DOI] [PubMed] [Google Scholar]
- Howk R. S., Troxler D. H., Lowy D., Duesberg P. H., Scolnick E. M. Identification of a 30S RNA with properties of a defective type C virus in murine cells. J Virol. 1978 Jan;25(1):115–123. doi: 10.1128/jvi.25.1.115-123.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. T., Jr, Calarco P. G. Evidence for the cell surface expression of intracisternal A particle-associated antigens during early mouse development. Dev Biol. 1981 Mar;82(2):388–392. doi: 10.1016/0012-1606(81)90462-0. [DOI] [PubMed] [Google Scholar]
- Kaback D. B., Angerer L. M., Davidson N. Improved methods for the formation and stabilization of R-loops. Nucleic Acids Res. 1979 Jun 11;6(7):2499–2317. doi: 10.1093/nar/6.7.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R., Brûlet P., Schnebelen M. T., Gaillard J., Jacob F. Reactivity of monoclonal antibodies against intermediate filament proteins during embryonic development. J Embryol Exp Morphol. 1981 Aug;64:45–60. [PubMed] [Google Scholar]
- Keshet E., Shaul Y., Kaminchik J., Aviv H. Heterogeneity of "virus-like" genes encoding retrovirus-associated 30S RNA and their organization within the mouse genome. Cell. 1980 Jun;20(2):431–439. doi: 10.1016/0092-8674(80)90629-7. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Smith L., Hawley R., Hozumi N., Shulman M. Intracisternal A-particle genes as movable elements in the mouse genome. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1992–1996. doi: 10.1073/pnas.80.7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Smith L. A., Lueders K. K. Intracisternal A-particle genes in Mus musculus: a conserved family of retrovirus-like elements. Mol Cell Biol. 1981 Mar;1(3):216–227. doi: 10.1128/mcb.1.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Shen-Ong G. L., Cole M. D. Differing populations of intracisternal A-particle genes in myeloma tumors and mouse subspecies. J Virol. 1982 May;42(2):411–421. doi: 10.1128/jvi.42.2.411-421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Function of the retrovirus long terminal repeat. Cell. 1982 Jan;28(1):3–5. doi: 10.1016/0092-8674(82)90367-1. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Structure, variation and synthesis of retrovirus long terminal repeat. Cell. 1981 Nov;27(1 Pt 2):1–3. doi: 10.1016/0092-8674(81)90353-6. [DOI] [PubMed] [Google Scholar]
- Vimard C., Jeantet C., Netter Y., Gros F. Changes in the sedimentation properties of acetycholinesterase during neuroblastoma differentiation. Biochimie. 1976;58(4):473–478. doi: 10.1016/s0300-9084(76)80258-1. [DOI] [PubMed] [Google Scholar]
- Yotsuyanagi Y., Szöllösi D. Early mouse embryo intracisternal particle: Fourth type of retrovirus-like particle associated with the mouse. J Natl Cancer Inst. 1981 Sep;67(3):677–685. [PubMed] [Google Scholar]