Abstract
Previous animal studies have revealed significant involvement of genetics in nicotine intake; however, the extent of the genetic contribution to this behavior has not been well addressed. We report the first study of nine generations of selection for high and low voluntary nicotine intake in outbred Sprague–Dawley rats. Bidirectional mass selection resulted in progressively greater nicotine consumption in the high nicotine-preferring line but no decrease in nicotine intake in the low nicotine-preferring line across generations. Our estimated realized heritability for high voluntary nicotine intake is 0.26 vs close to zero for low voluntary nicotine intake. In contrast, we found no differences between the lines across generations for saccharine intake. These selected lines may provide useful animal models for identifying susceptibility and resistance genes and variants for controlling voluntary nicotine intake in rodents, although we recognize that more generations of selection of these two lines and independent replication of our selection for high and low nicotine-preferring lines are needed.
Keywords: Nicotine, Selection, Breeding, Realized heritability, Rat
Introduction
Tobacco smoking is a complex quantitative trait affected by both genetic and environmental factors. Many previous studies have revealed that significant differences exist among individuals in their response to nicotine (Portugal and Gould 2008; Arinami et al. 2000; Walton et al. 2001; Bierut 2011). Studies of molecular mechanisms and genetic differences in both humans and animals have shown that responsiveness to nicotine is greatly influenced by both genetic and non-genetic factors (Portugal and Gould 2008; Ho and Tyndale 2007; Hall et al. 2012; Chen et al. 2012). A significant body of data from family, twin, and adoption studies have provided evidence for heritability of various smoking-related phenotypes, including initiation, dependence, and cessation (Vink et al. 2005; Maes et al. 2004; Li 2006; Li 2003; Lessov et al. 2004a, 2004b; Lessov et al. 2004a, 2004b). Although genome-wide linkage and association studies in humans have revealed a number of candidate genes and variants related to nicotine addiction, results from various studies differ greatly, depending on many factors, especially genetic and phenotypic heterogeneity.
Animal research has played a significant role in understanding the genetic and environmental factors affecting vulnerability to nicotine dependence. Many animal studies have been conducted in an attempt to identify candidate genes and their contributions to nicotine intake behavior (Agatsuma et al. 2006; Pons et al. 2008; Levin et al. 2009; Siu et al. 2006; Zhu et al. 2007). Studies in rodents have indicated that both genetic and non-genetic factors affect nicotine-induced physiological and behavioral responses during the development of physical dependence on nicotine (Siu et al. 2006; Zhu et al. 2007; Faraday et al. 2005; Park et al. 2007; Koehl et al. 2000; Marks et al. 1983; Hatchell and Collins 1980; Leao et al. 2012). Differences in sensitivity to the reinforcing and rewarding effects of nicotine have been documented with drug self-administration in various mice and rat strains, indicating the impact of strain differences on vulnerability to nicotine intake under both limited and unlimited access models (Brower et al. 2002; Shoaib et al. 1997; Shram et al. 2008).
A voluntary oral nicotine self-administration paradigm presents an effective model for determining individual differences in nicotine intake behavior. With this model, animals can be exposed to oral nicotine choice 24 h a day for an extended period (Collins et al. 2012). Unlimited access, free choice, and a long experimental period allow animals to experience nicotine dependence phases according to their sustained nicotine consumption rates. When rats were given a free choice of oral nicotine, they can be classified as minimum or maximum nicotine preferring, depending on their voluntary consumption rates (Maehler et al. 2000; Nesil et al. 2011). However, relatively little is known about genetic variations in nicotine intake behavior in the progression of dependence. A recent mapping study in C57BL/6 J × C3H/HeJ F2 intercross mice showed that free choice of oral nicotine is likely to be influenced by at least four quantitative trait loci (QTL) (Li et al. 2007). Moreover, exposure to oral nicotine alters reward-related molecular signaling pathways (Brunzell et al. 2003).
Selection of a phenotype of interest has been used in agriculture for a long time (Falconer and Mackay 1996). When parents are selected for a phenotype of interest, the frequencies of genetic loci controlling the phenotype would be expected to change across generations. Such selection effects can be inherited from generation to generation through segregation of those loci controlling the trait being selected. In the past, mice and rats have been selected for sensitivity, tolerance, dependence, and preference for several addictive drugs, including alcohol, opiates, cocaine, and methamphetamine (Mardones and Segovia-Riquelme 1983; Mogil et al. 1995; Marley et al. 1998; Wheeler et al. 2009). Specifically, the genetic bases of responsiveness to nicotine exposure have been investigated in a few selectively bred mouse models (Schechter et al. 1995; Smolen et al. 1994). The findings from these studies confirm that nicotine has profound and lasting effects on dependence-related behavioral traits. However, to our knowledge, no attempt to select distinct responsiveness patterns to nicotine has been reported from an outbred stock.
Sprague–Dawley rats have a diverse genetic pool and are widely used in research on drugs of abuse. Drug-related bio-behavioral inheritance has been performed in selectively bred outbred rat strains. The results show that novelty-seeking and saccharine-intake behavior can be selected as high and low responders (Stead et al. 2006; Carroll et al. 2008). The primary objective of this present study was to select rats with high or low nicotine intake preference with the goal of determining the heritability of different nicotine responsiveness traits. Oral nicotine self-administration was used for identifying low and high nicotine-preferring rats, and bidirectional mass selection was applied in each generation to increase the frequency of desired trait-relevant alleles.
Materials and methods
Animals
All animal-related experimental protocols were approved by the Ege University ACUC committee. Twenty-four adult male and forty-two female outbred Sprague–Dawley rats were obtained from the Ege University Animal Breeding Facility and used in behavioral testing as the founding population for the selection experiment reported in this study (F0 generation). Animals were kept in same-sex groups with ad libitum access to food and water and were housed under standard laboratory conditions (20–22 °C, 12–12 h light–dark cycle) when they were not being used for breeding or testing. When females had litters, they were housed in large maternity cages. Pups were weaned from their mothers at postnatal day (PN) 24 and housed with their siblings. When being tested for oral nicotine intake, each rat was housed individually.
The total numbers of animals tested in generations F1–F9 were 33, 73, 65, 39, 23, 38, 28, 104, and 71, respectively. Of these animals, 20, 42, 40, 10, 11, 9, 17, 71, and 32 were tested for the high nicotine-preferring line; and 13, 31, 25, 29, 12, 29, 11, 33, and 39 were tested for the low nicotine-preferring line in the F1–F9 generations.
Measurement of voluntary nicotine intake
Oral nicotine self-administration was determined as described earlier (Nesil et al. 2011). Briefly, rats were given access to either nicotine in water [Sigma, (−) nicotine hydrogen tartrate] or water using a two bottle free-choice method. The concentration of nicotine was 10 mg/L during the initial 2 weeks. At the beginning of the third week, the nicotine concentration was raised to 20 mg/L and kept at this amount for the rest of the experiments. Nicotine exposure lasted 6 weeks. Saccharine sodium (10 mg/L) was added to both the nicotine and water bottles to mask the bitter taste of nicotine.
The nicotine consumption and body weight of each animal were monitored weekly. The nicotine consumption rate was calculated as milligrams per kilogram averaged over the last 3 weeks of drug administration.
Selective breeding strategy
Animals in the F0 population were exposed to oral nicotine self-administration for 6 weeks. At the end of this period, the highest and lowest nicotine consumers among both females and males were selected to generate high and low nicotine-preferring offspring, respectively. Rats were separated from their siblings and singly housed 1 week before nicotine self administration began. In the F1, F2, and F4 generations, rats were exposed to the oral nicotine choice at 20, 5, and 18 weeks of age, respectively. In the remaining F3 and F5–F9 generations, nicotine self administration was initiated at 12 weeks of age. Starting nicotine exposure at 5 weeks in the F2 generation was a mistake by the animal technician.
The offspring from both breeding groups were exposed to oral nicotine self-administration for 6 weeks with individual housing until the F6 generation. By the F6 generation, rats became aggressive because of the single-housing conditions; therefore, they began to receive nicotine and water in their sibling group cages for 3 weeks, as they were 10–12 weeks old. After 3 weeks of nicotine choice in sibling group cages, they were again housed individually and exposed to nicotine or water for an additional 3 weeks. This change was helpful in overcoming the aggressive behavior.
During the bidirectional mass selection, inbreeding was always avoided because of concern about reduced viability. In each generation, two or more breeding animals were used for each preferring group. Promoting outbreeding and selection of the trait in each of the preferring groups, one male from the highest or lowest nicotine-preferring group mated with one or more of the best females consuming a high or low amount of nicotine, respectively. When more than one sustained and extreme acceptable phenotype was available, breeders were chosen from as many families as possible.
Calculation of selection differential, response to selection, divergent, and realized heritability
The selection differential (SD) of each generation was calculated as the difference between the mean nicotine consumption rate of the parents and that of the animals chosen from a particular generation. The response to selection (R) was calculated as the difference between two consecutive generational means. The realized heritability (h2) was calculated by fitting a linear regression to the generation means and cumulative SD over the selection of generations (Falconer 1996). For the divergence heritability, the values of response and cumulative SD in each generation were used. Response values were obtained from mean differences between high and low nicotine-preferring lines in individual generations. Divergent heritability was estimated by regression of response on cumulative SD with the values for generations F1–F9. The inbreeding coefficient was calculated as described by Wright (1922).
Data analysis
The Ward cluster method (Ward 1963) was used to group rats according to their preferences for nicotine consumption in the F0 generation, which yielded high, medium, and low nicotine-preferring groups. As determined by the objective of this study, only rats from the high and low nicotine-preferring groups were used as a founding population for subsequent selection of high and low nicotine-preferring lines. ANOVAs followed by post hoc Bonferroni correction were used to analyze the statistical significance of nicotine and saccharine consumption rates between generations in each line and between lines. Significance was set at p < 0.05.
Results
Oral nicotine self-administration over nine generations of selective breeding
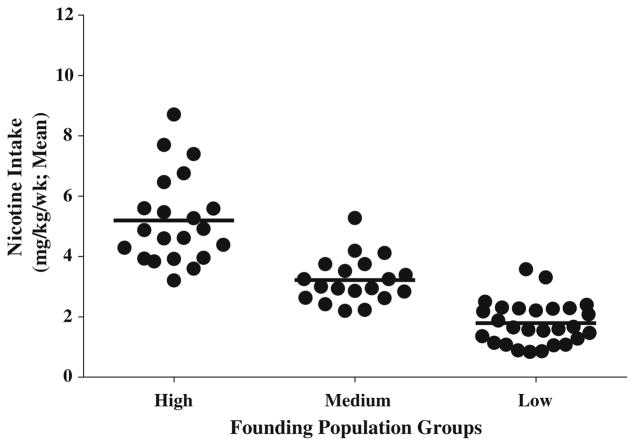
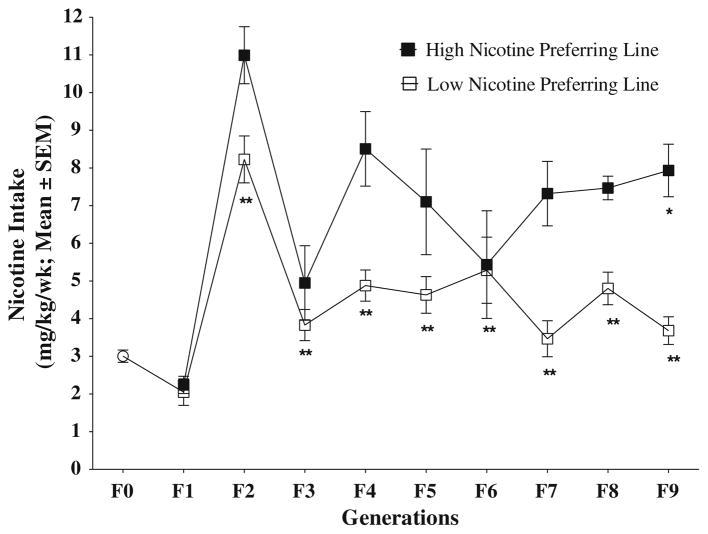
To generate high and low nicotine-preferring lines, oral nicotine self-administration was performed in 66 rats for 3 weeks. Based on the average nicotine consumption over this period, we grouped these animals into three groups (high: 4.02 ± 0.27; medium: 3.01 ± 0.25, low: 2.19 ± 0.18 mg of nicotine/kg, respectively; p < 0.05) using the Ward cluster method (Ward 1963). Figure 1 shows average nicotine intake of each rat in the three groups. From the high and low nicotine-intake groups, we selected 3 male and 4 female rats based on their nicotine consumption and used them as parents for breeding high and low nicotine-preferring lines in the F1 generation. In the ninth generation, the average nicotine consumption was 7.93 ± 0.69 mg/kg in the high nicotine-preferring rats and 3.60 ± 0.36 for the low-preferring rats (Fig. 2). A univariate ANOVA with average nicotine consumption over 3 weeks as the dependent variable and generation (0–9) and nicotine preference as the independent factors revealed significant effects of generations (F(9,514) = 28.255; p < 0.05) and nicotine preference (F(1,514) = 53.262; p < 0.05), as well as a significant interaction between preference and generation (F(9,514) = 25.881; p < 0.05). Post hoc Bonferroni testing showed that F0 generation differed significantly from all generations except F1 in each line (Fig. 2).
Fig. 1.
High, medium, and low nicotine-consuming groups in F0 generation. Rats had free access to nicotine in water or plain water provided in two bottles for 6 weeks starting at 12 weeks of age. Each point shows the average weekly nicotine consumption of individual animals in the three groups over the last 3 weeks of exposure. Only rats with the highest and lowest nicotine consumption from the high and low groups were used for breeding of high and low nicotine-preferring lines in the F1 generation
Fig. 2.
Voluntary nicotine intake over nine generations of selection. Rats had free access to nicotine in water or plain water provided in two bottles 24 h/day. The figure shows the average ± SEM of each generation’s nicotine consumption during the last 3 weeks in the high and low nicotine-preferring lines. Different from F0, F1, F2, F3, * p < 0.05 in high nicotine-preferring line. Different from F2, ‡ p <0.05 in low nicotine-preferring line (post hoc Bonferroni tests). Generation different from F0, ** p < 0.05 (post hoc Bonferroni tests)
One-way ANOVA using the average nicotine consumption as a dependent variable and the generation as the factor revealed a significant difference between generations in both high (F(9,262) = 15.826; p < 0.05) and low (F(9,196) = 12.088; p < 0.05) nicotine-preferring lines. Post hoc Bonferroni testing revealed that nicotine consumption in the F9 generation was significantly higher than in the high nicotine-preferring line of generations F0–F3. Nicotine intake in the F9 generation by the low nicotine-preferring line was significantly different only from that in the F2 generation. When average nicotine consumption of each generation was regressed on generation number, the regression coefficient of the high and low nicotine-preferring lines was 0.234 ± 0.32 (p = 0.49) and −0.06 ± 0.23 (p = 0.77), respectively.
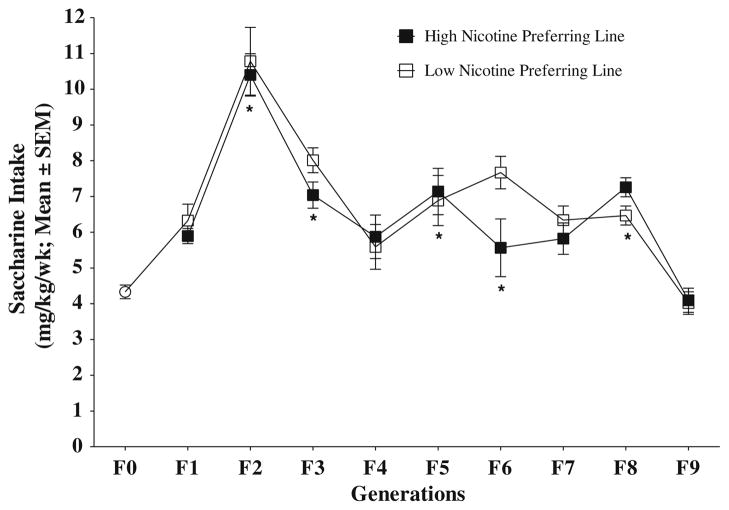
To determine the effect of selective breeding on saccharine consumption, we also analyzed the average saccharine intake over the nine generations for each line (Fig. 2). A Univariate ANOVA was performed with average saccharine consumption over 3 weeks as the dependent variable and generation and nicotine preference as the independent variable. Our results revealed a significant difference between generations (F(9,502) = 32.996; p <0.05) but not significant in saccharine consumption and interaction between generation and saccharine consumption. Post hoc Bonferroni testing showed that saccharine consumption varied; the F0 generation had lower consumption than the F2, F3, F5, F6, and F8 generations, and no difference was observed between the F0 generation and the F1, F4, F7, and F9 generations. These results indicate that high and low nicotine-preferring lines consumed similar amounts of saccharine during the whole selection period.
Selection differential (SD)
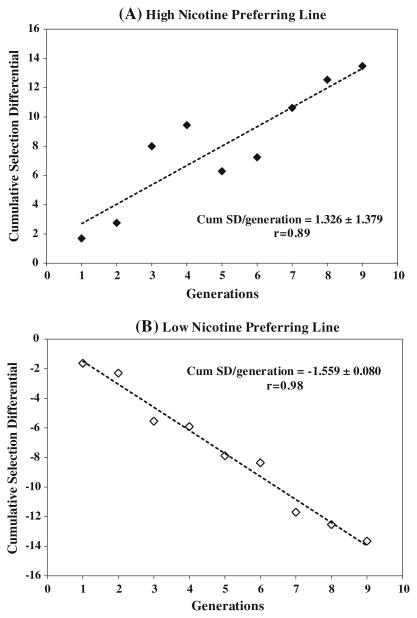
Figure 3a, b shows the accumulated SD over the nine generations of selection. There was an asymmetrical SD between high and low nicotine-preferring lines. The regression coefficient of cumulative SD per generation was 1.32 ± 1.37 (p = 0.009) in the high nicotine-preferring line and −1.55 ± 0.08 (p = 0.00004) in the low nicotine-preferring line. Progressively increased upward SD pressure led to improvement of nicotine consumption in the high nicotine-preferring line in each generation (Fig. 4). In addition, regression of cumulative SD over the generations confirmed the effective of the selection method for producing a distinctive phenotypic trait.
Fig. 3.
Upward and downward selection differential for voluntary nicotine intake during nine generations of selection. In (a), the highest nicotine consumers were selected, whereas in (b), the lowest nicotine consumers were selected according to their last 3 week average nicotine consumption. Cumulative selection differential values of high and low nicotine-preference lines are regressed on generation numbers
Fig. 4.
Saccharine intake over nine generations of selection in high and low nicotine-preferring lines. Each point shows the average ± SEM of each generation’s saccharine consumption during the last three weeks in each line. Generation different from F0, * p <0.05 (post hoc Bonferroni tests)
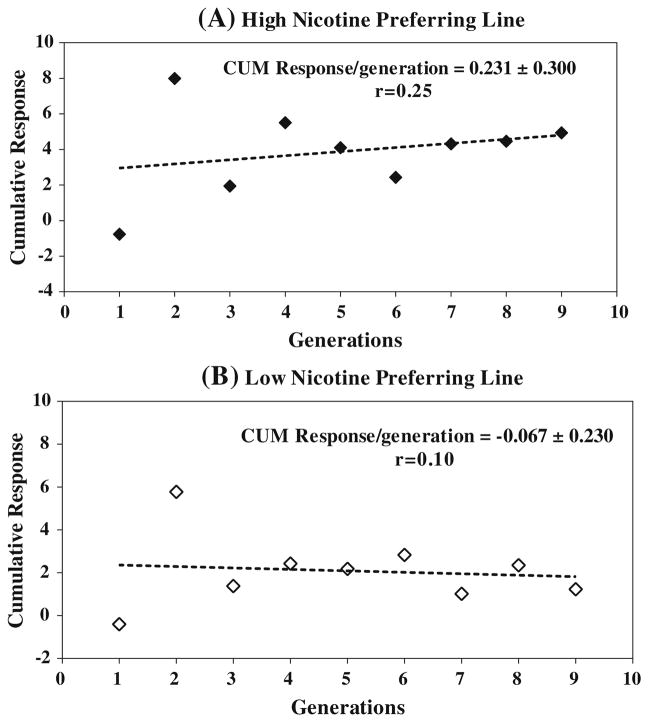
Response to selection
Cumulative response data revealed that the response to selection occurred in a positive direction in the high nicotine-preferring line. The low nicotine-preferring line did not show any clear response to nicotine intake even under a strong downward SD (Fig. 5a, b). By the F9 generation, the cumulative response per generation was 0.231 ± 0.30 (p = 0.62) for the high nicotine-preferring line and −0.0677 ± 0.23 (p = 0.77) for the low nicotine-preferring line. This suggests that upward selection pressure on the high nicotine-preferring rats increased the desirable high nicotine intake trait over the nine generations, and downward selection pressure did not trigger a decreased response to voluntary oral nicotine intake.
Fig. 5.
Responses related to selection differential. Difference between two consecutive generational means plotted against the cumulative selection differential in high (a) and low (b) nicotine-preferring lines
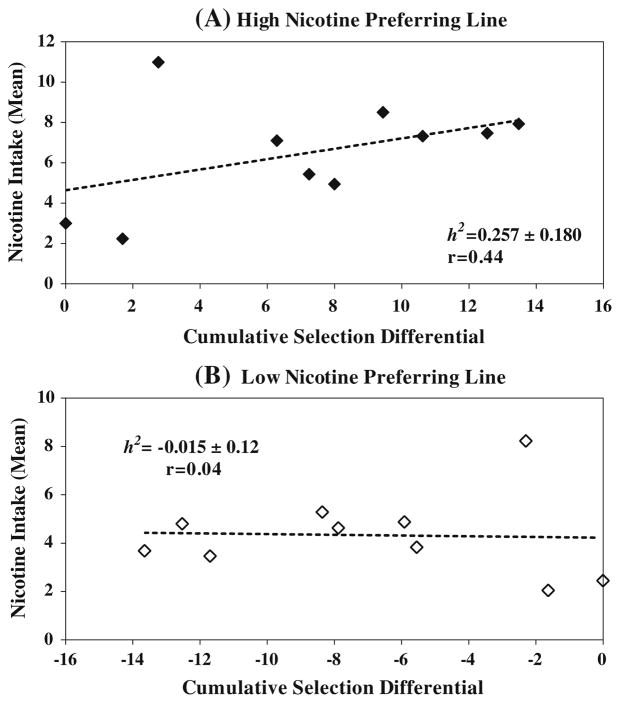
Realized heritability
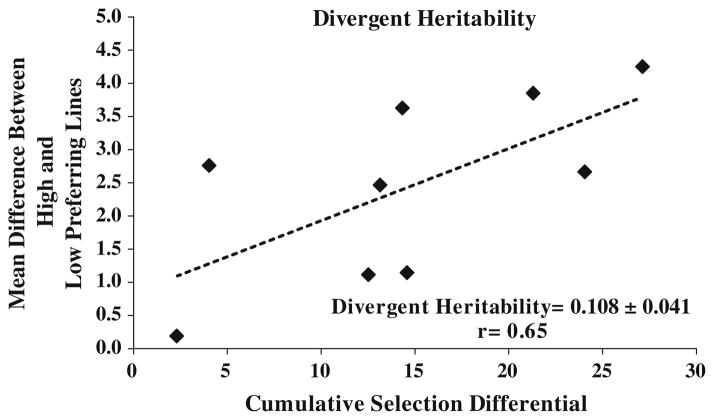
The realized heritability for the high and low nicotine-preferring line was estimated to be 0.26 ± 0.18 and −0.014 ± 0.12, respectively (Fig. 6). To eliminate potential environmental influence, realized heritability was also estimated in terms of inter-strain divergence. Each generational mean difference between the high and low nicotine-preferring lines was regressed on deviation of their cumulative SD values (Fig. 7). The slope of linear regression showed progressive divergence during the selective breeding.
Fig. 6.
Differences in Realized Heritability: The realized heritability values of high (a) and low nicotine-preferring lines (b) were calculated from linear regression of between SD and F0–F9 means
Fig. 7.
Divergent heritability: heritability from inter-strain difference was calculated from the linear regression between cumulative selection differential and nicotine consumption mean differences between high and low nicotine-preferring lines in individual generations
Discussion
The present study demonstrates that selective breeding of rats for free choice of high nicotine intake resulted in increased nicotine intake across selection generations, with a realized heritability of 0.26 after nine generations of selection. In contrast, rats consuming low amounts of nicotine had no or a very low response, as demonstrated by a realized heritability of close to zero.
Selective breeding has been used for generating rodent models with various sensitivities to methamphetamine, opiates, or alcohol or related bio-behavioral traits (Carlson et al. 1996; Scibelli et al. 2011; Li et al. 1993). However, phenotypic selection for differences in nicotine-related physiological changes and behaviors has rarely been done. Genetic studies in mice revealed that the estimated heritability for nicotine-induced hypothermia (Marks et al. 1984), seizure sensitivity (Miner and Collins 1989), and locomotor activity (Smolen et al. 1994) was 0.3, 0.63 and 0.2, respectively. Although these studies indicate that nicotine-induced behavioral alterations can be selected across generations, the inbred or heterogeneous mice used in those studies provide limited information on selection potential for high and low nicotine intake in an outbred strain. Thus, the current report represents the first study demonstrating that free-choice nicotine-intake behavior can be improved in an outbred rat strain by selective breeding.
We used outbred Sprague–Dawley rats as the founding population to determine the selection potential for nicotine preference. There were several reasons for using an outbred stock. First, unlike inbred strains, outbred strains have a diverse genetic pool. This factor likely provides high mapping resolution and more accurate determination of the location of QTL. Second, the limitation on the number of animals involved in selection for each generation imposed by the availability of resources for the bidirectional mass selection of a phenotype of interest could lead loss of genetic variation and inbreeding depression. During selective breeding, the extent of genetic variation among outbred colonies provides an animal model that is more like human smokers. However, one concern about selective breeding with relatively small numbers of animals per generation is inbreeding depression (Charlesworth and Willis 2009). To address this concern, we avoided brother–sister mating during the selection of different voluntary nicotine-intake phenotypes because of the possibility of negative effects. In order to have a good understanding of inbreeding during the nine generations, we calculated the inbreeding coefficient and found that the average value per generation was 0.08 ± 0.006 in the high nicotine-preferring line and 0.06 ± 0.006 in the low-preferring line. We are continuing selective outbreeding; however, in future generations; if there is a need, we may change our breeding strategy to inbreeding in order to demonstrate a clear genetic contribution. Up to the F9 generation, however, we did not observe any clear reduction in fertility during selection, and there was no notable adverse effect on animal health or well-being related to selective breeding.
Selection of the parents based on their phenotypic trait performance significantly improves the expression of the trait through changing genetic variance underlying quantitative traits in their offspring. In the current study, continued bidirectional selective breeding for high and low nicotine-preferring lines resulted in progressively greater voluntary nicotine consumption rates in the high-preferring line but did not lead to decreased consumption in the low-preferring line. Although a constant increase of upward and downward selection pressure was applied to each line, bidirectional mass selection was successful only in the high voluntary nicotine-intake phenotype. By the ninth generation, the cumulative response to selection for voluntary nicotine intake per generation was 0.231 ± 0.30 for the high nicotine-preferring line and −0.067 ± 0.24 mg/nicotine/kg for the low-preferring line. This indicates that selection for high nicotine preference worked but did not for low-preferring phenotype according to our selection results. However, we must point out that such findings need to be replicated in independent selection experiments with the same approach used in this study, or at least a similar one. Considering the relatively small samples, we could not draw a convincing conclusion about sex differences in nicotine intake based on our existing data, but this is an important question to address in the future.
To determine the effect of selective breeding on saccharine consumption, we analyzed saccharine consumption for both lines. Although there was a difference in saccharine consumption rates over nine generations for both lines, we found no difference in saccharine consumption between the high and low nicotine-preferring lines. Further, we analyzed selection differential, response to selection, and realized and divergent heritability for saccharine consumption in both lines, which revealed no selection effect on saccharine consumption rates in either line.
During the past decade, genetic mapping studies in animals demonstrated that individual differences in the inherited response to drugs of abuse can be linked to or associated with specific allelic forms of a gene or genes in QTL (Crabbe 2002). Both QTL and genes for alcohol, methamphetamine, and opioid addiction-related behavioral phenotypes have been determined in different selectively bred mouse lines (Bice et al. 2011; 2006; Hitzemann et al. 2008; Bergeson et al. 2001). However, few studies have examined the candidate QTL and genes whose expression can predict nicotine consumption and vulnerability to nicotine addiction. Gill and Boyle (2005) identified genes for the psychostimulant effects of nicotine on chromosomes 11, 12, 13,14, and 17 in AcB/BcA recombinant congenic strains. In addition to mapping nicotine effects on the genome, Li et al. (2007) reported that the free choice of oral nicotine consumption behavior in the F2 offspring from crossing of significantly high-preferring C57BL/6 mice with low-preferring C34 mice was strongly influenced by at least four QTL (Li et al. 2007). Although these studies were successful in mapping QTL associated with different addiction-related phenotypes, it also is desirable to map the vulnerability loci and genes in an animal model developed from an outbred stock. Considering that inbred stocks present a potential difficulty in determining QTL because of the significant reduction in genetic heterogeneity, utilization of an outbred strain may have advantages over the existing animal models for the study of the human nicotine-dependency phenotype.
Additional research on neurobehavioral mechanisms of the phenotypic differences in nicotine intake behavior will improve our understanding of the addictive properties of nicotine. A recent study by Chen et al. (2012) showed that control of the motivation to obtain nicotine is distinct from natural rewards in a panel of isogenic rat strains during adolescence. Although they did not predict the behavior of the F1 hybrids from the parental strain behavior, the results from this study indicate the impact of genetics on the vulnerability to nicotine-induced reward behavior in adolescent rats (Chen et al. 2012). All reinforcing drugs induce behavioral sensitization and contribute to strain differences in drug preference and sensitivity related to drugs of abuse. Scibelli et al. (2011) selectively bred two lines based on their response to methamphetamine-induced sensitization. Selective breeding for methamphetamine high and low sensitization resulted in divergent methamphetamine consumption in different lines and a negative genetic relation between sensitivity to drug stimulation and drug intake (Scibelli et al. 2011). These findings indicate the trans-generational genetic impact on behavioral and biological factors that lead to different drug-induced psychological and physiological alternations in methamphetamine consumption. Therefore, our selected high and low nicotine-preferring lines could be useful for evaluating differences in the vulnerability to nicotine preference and its related phenotypes. Future experiments with intravenous self-administration, conditioned place preference, and drug discrimination studies on these lines would provide a better view of the nicotine-preferring phenotype. Understanding the possible changes in brain biochemistry and connectivity will not only contribute to basic science but will have translational value and may aid in the development of new pharmaceutical agents.
In conclusion, we developed an animal model from an outbred stock having different nicotine-intake phenotypes to assessing the hereditary effect on vulnerability to nicotine intake behavior. Although both high and low nicotine-preference lines were generated by bidirectional mass selection, selection was successful only for the high nicotine-intake phenotype. On the other hand, these animals could be used as a control group for understanding the genetic modulations of the high nicotine-responsive phenotype. Thus, high nicotine-preferring line may present a useful animal model for identifying vulnerability genes and loci influencing the genetic susceptibility to high nicotine consumption.
Acknowledgments
We are grateful to Dr. Gonca Mola, Merve Evren, and Tuna Nesil and Muzeyyen Ugur for their assistance in data collection and to Professor Qin Zhang of China Agricultural University for calculating inbreeding coefficients for the study. The animal-related study was funded by the Ege University Research Fund (Grant 001 BAM 2006). The analysis of data and preparation of this report were supported in part by National Institutes of Health grant DA-012844 to MDL.
Contributor Information
Tanseli Nesil, Department of Psychiatry and Neurobehavioral Sciences, University of Virginia, 1670 Discovery Drive, Charlottesville, VA 22911, USA.
Lutfiye Kanit, Center for Brain Research, Ege University, Bornova, 35100 Izmir, Turkey.
Ming D. Li, Email: ml2km@virginia.edu, Department of Psychiatry and Neurobehavioral Sciences, University of Virginia, 1670 Discovery Drive, Charlottesville, VA 22911, USA
Sakire Pogun, Email: sakire.pogun@ege.edu.tr, Center for Brain Research, Ege University, Bornova, 35100 Izmir, Turkey.
References
- Agatsuma S, Lee M, Zhu H, Chen K, Shih JC, Seif I, Hiroi N. Monoamine oxidase A knockout mice exhibit impaired nicotine preference but normal responses to novel stimuli. Hum Mol Genet. 2006;15(18):2721–2731. doi: 10.1093/hmg/ddl206. [DOI] [PubMed] [Google Scholar]
- Arinami T, Ishiguro H, Onaivi ES. Polymorphisms in genes involved in neurotransmission in relation to smoking. Eur J Pharmacol. 2000;410(2–3):215–226. doi: 10.1016/s0014-2999(00)00816-5. [DOI] [PubMed] [Google Scholar]
- Bergeson SE, Helms ML, O’Toole LA, Jarvis MW, Hain HS, Mogil JS, Belknap JK. Quantitative trait loci influencing morphine antinociception in four mapping populations. Mamm Genome. 2001;12(7):546–553. doi: 10.1007/s003350020022. [DOI] [PubMed] [Google Scholar]
- Bice PJ, Foroud T, Carr LG, Zhang L, Liu L, Grahame NJ, Lumeng L, Li TK, Belknap JK. Identification of QTLs influencing alcohol preference in the high alcohol preferring (HAP) and low alcohol preferring (LAP) mouse lines. Behav Genet. 2006;36(2):248–260. doi: 10.1007/s10519-005-9019-6. [DOI] [PubMed] [Google Scholar]
- Bice PJ, Lai D, Zhang L, Foroud T. Fine mapping quantitative trait loci that influence alcohol preference behavior in the high and low alcohol preferring (HAP and LAP) mice. Behav Genet. 2011;41(4):565–570. doi: 10.1007/s10519-010-9414-5. [DOI] [PubMed] [Google Scholar]
- Bierut LJ. Genetic vulnerability and susceptibility to substance dependence. Neuron. 2011;69(4):618–627. doi: 10.1016/j.neuron.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower VG, Fu Y, Matta SG, Sharp BM. Rat strain differences in nicotine self-administration using an unlimited access paradigm. Brain Res. 2002;930(1–2):12–20. doi: 10.1016/s0006-8993(01)03375-3. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Russell DS, Picciotto MR. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6 J mice. J Neurochem. 2003;84(6):1431–1441. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- Carlson KR, Saulnier-Dyer CM, Moolten MS. Selective breeding for oral opioid acceptance or rejection in rats. Pharmacol Biochem Behav. 1996;53(4):871–876. doi: 10.1016/0091-3057(95)02099-3. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol. 2008;19(5–6):435–460. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Willis JH. The genetics of inbreeding depression. Nat Rev Genet. 2009;10(11):783–796. doi: 10.1038/nrg2664. [DOI] [PubMed] [Google Scholar]
- Chen H, Hiler KA, Tolley EA, Matta SG, Sharp BM. Genetic factors control nicotine self-administration in isogenic adolescent rat strains. PLoS ONE. 2012;7(8):e44234. doi: 10.1371/journal.pone.0044234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC, Pogun S, Nesil T, Kanit L. Oral nicotine self-administration in rodents. J addict res ther S2. 2012 doi: 10.4172/2155-6105.S2-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC. Genetic contributions to addiction. Annu Rev Psychol. 2002:53435–462. doi: 10.1146/annurev.psych.53.100901.135142. [DOI] [PubMed] [Google Scholar]
- Falconer D. A system architecture for broadband millimeter-wave access to an ATM LAN. IEEE Pers Commun. 1996;3(4):36–41. [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. Prentice Hall; New York: 1996. [Google Scholar]
- Faraday MM, Blakeman KH, Grunberg NE. Strain and sex alter effects of stress and nicotine on feeding, body weight, and HPA axis hormones. Pharmacol Biochem Behav. 2005;80(4):577–589. doi: 10.1016/j.pbb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Gill KJ, Boyle AE. Genetic basis for the psychostimulant effects of nicotine: a quantitative trait locus analysis in AcB/BcA recombinant congenic mice. Genes Brain Behav. 2005;4(7):401–411. doi: 10.1111/j.1601-183X.2005.00116.x. [DOI] [PubMed] [Google Scholar]
- Hall FS, Markou A, Levin ED, Uhl GR. Mouse models for studying genetic influences on factors determining smoking cessation success in humans. Ann N Y Acad Sci. 2012:124839–70. doi: 10.1111/j.1749-6632.2011.06415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchell PC, Collins AC. The influence of genotype and sex on behavioral sensitivity to nicotine in mice. Psychopharmacology. 1980;71(1):45–49. doi: 10.1007/BF00433251. [DOI] [PubMed] [Google Scholar]
- Hitzemann R, Malmanger B, Belknap J, Darakjian P, McWeeney S. Short-term selective breeding for high and low prepulse inhibition of the acoustic startle response; pharmacological characterization and QTL mapping in the selected lines. Pharmacol Biochem Behav. 2008;90(4):525–533. doi: 10.1016/j.pbb.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MK, Tyndale RF. Overview of the pharmacogenomics of cigarette smoking. Pharmacogenomics J. 2007;7(2):81–98. doi: 10.1038/sj.tpj.6500436. [DOI] [PubMed] [Google Scholar]
- Koehl M, Bjijou Y, Le Moal M, Cador M. Nicotine-induced locomotor activity is increased by preexposure of rats to prenatal stress. Brain Res. 2000;882(1–2):196–200. doi: 10.1016/s0006-8993(00)02803-1. [DOI] [PubMed] [Google Scholar]
- Leao RM, Cruz FC, Marin MT, da Planeta CS. Stress induces behavioral sensitization, increases nicotine-seeking behavior and leads to a decrease of CREB in the nucleus accumbens. Pharmacol Biochem Behav. 2012;101(3):434–442. doi: 10.1016/j.pbb.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Swan GE, Ring HZ, Khroyan TV, Lerman C. Genetics and drug use as a complex phenotype. Subst Use Misuse. 2004a;39(10–12):1515–1569. doi: 10.1081/ja-200033202. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Martin NG, Statham DJ, Todorov AA, Slutske WS, Bucholz KK, Heath AC, Madden PA. Defining nicotine dependence for genetic research: evidence from Australian twins. Psychol Med. 2004b;34(5):865–879. doi: 10.1017/s0033291703001582. [DOI] [PubMed] [Google Scholar]
- Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, Avery J, Nicholson J, Rose JE. Nicotinic alpha7- or beta2-containing receptor knockout: effects on radial-arm maze learning and long-term nicotine consumption in mice. Behav Brain Res. 2009;196(2):207–213. doi: 10.1016/j.bbr.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD. The genetics of smoking related behavior: a brief review. Am J Med Sci. 2003;326(4):168–173. doi: 10.1097/00000441-200310000-00003. [DOI] [PubMed] [Google Scholar]
- Li MD. The genetics of nicotine dependence. Curr Psychiatry Rep. 2006;8(2):158–164. doi: 10.1007/s11920-006-0016-0. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, Doolittle DP. Selective breeding for alcohol preference and associated responses. Behav Genet. 1993;23(2):163–170. doi: 10.1007/BF01067421. [DOI] [PubMed] [Google Scholar]
- Li XC, Karadsheh MS, Jenkins PM, Brooks JC, Drapeau JA, Shah MS, Lautner MA, Stitzel JA. Chromosomal loci that influence oral nicotine consumption in C57BL/6 J × C3H/HeJF2 intercross mice. Genes Brain Behav. 2007;6(5):401–410. doi: 10.1111/j.1601-183X.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- Maehler R, Dadmarz M, Vogel WH. Determinants of the voluntary consumption of nicotine by rats. Neuropsychobiology. 2000;41(4):200–204. doi: 10.1159/000026660. [DOI] [PubMed] [Google Scholar]
- Maes HH, Sullivan PF, Bulik CM, Neale MC, Prescott CA, Eaves LJ, Kendler KS. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychol Med. 2004;34(7):1251–1261. doi: 10.1017/s0033291704002405. [DOI] [PubMed] [Google Scholar]
- Mardones J, Segovia-Riquelme N. Thirty-two years of selection of rats by ethanol preference: UChA and UChB strains. Neurobehav Toxicol Teratol. 1983;5(2):171–178. [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Genetics of nicotine response in four inbred strains of mice. J Pharmacol Exp Ther. 1983;226(1):291–302. [PubMed] [Google Scholar]
- Marks MJ, Miner L, Burch JB, Fulker DW, Collins AC. A diallel analysis of nicotine-induced hypothermia. Pharmacol Biochem Behav. 1984;21(6):953–959. doi: 10.1016/s0091-3057(84)80079-9. [DOI] [PubMed] [Google Scholar]
- Marley RJ, Arros DM, Henricks KK, Marley ME, Miner LL. Sensitivity to cocaine and amphetamine among mice selectively bred for differential cocaine sensitivity. Psychopharmacology. 1998;140(1):42–51. doi: 10.1007/s002130050737. [DOI] [PubMed] [Google Scholar]
- Miner LL, Collins AC. Strain comparison of nicotine-induced seizure sensitivity and nicotinic receptors. Pharmacol Biochem Behav. 1989;33(2):469–475. doi: 10.1016/0091-3057(89)90532-7. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Marek P, Flodman P, Spence MA, Sternberg WF, Kest B, Sadowski B, Liebeskind JC. One or two genetic loci mediate high opiate analgesia in selectively bred mice. Pain. 1995;60(2):125–135. doi: 10.1016/0304-3959(94)00098-Y. [DOI] [PubMed] [Google Scholar]
- Nesil T, Kanit L, Collins AC, Pogun S. Individual differences in oral nicotine intake in rats. InNeuropharmacology. 2011:189–201. doi: 10.1016/j.neuropharm.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Belluzzi JD, Han SH, Cao J, Leslie FM. Age, sex and early environment contribute to individual differences in nicotine/acetaldehyde-induced behavioral and endocrine responses in rats. Pharmacol Biochem Behav. 2007;86(2):297–305. doi: 10.1016/j.pbb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28(47):12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Genetic variability in nicotinic acetylcholine receptors and nicotine addiction: converging evidence from human and animal research. Behav Brain Res. 2008;193(1):1–16. doi: 10.1016/j.bbr.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter MD, Meehan SM, Schechter JB. Genetic selection for nicotine activity in mice correlates with conditioned place preference. Eur J Pharmacol. 1995;279(1):59–64. doi: 10.1016/0014-2999(95)00139-c. [DOI] [PubMed] [Google Scholar]
- Scibelli AC, McKinnon CS, Reed C, Burkhart-Kasch S, Li N, Baba H, Wheeler JM, Phillips TJ. Selective breeding for magnitude of methamphetamine-induced sensitization alters methamphetamine consumption. Psychopharmacology. 2011;214(4):791–804. doi: 10.1007/s00213-010-2086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology. 1997;129(1):35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Li Z, Le AD. Age differences in the spontaneous acquisition of nicotine self-administration in male Wistar and Long-Evans rats. Psychopharmacology. 2008;197(1):45–58. doi: 10.1007/s00213-007-1003-9. [DOI] [PubMed] [Google Scholar]
- Siu EC, Wildenauer DB, Tyndale RF. Nicotine self-administration in mice is associated with rates of nicotine inactivation by CYP2A5. Psychopharmacology. 2006;184(3–4):401–408. doi: 10.1007/s00213-006-0306-6. [DOI] [PubMed] [Google Scholar]
- Smolen A, Marks MJ, DeFries JC, Henderson ND. Individual differences in sensitivity to nicotine in mice: response to six generations of selective breeding. Pharmacol Biochem Behav. 1994;49(3):531–540. doi: 10.1016/0091-3057(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006;36(5):697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Vink JM, Willemsen G, Boomsma DI. Heritability of smoking initiation and nicotine dependence. Behav Genet. 2005;35(4):397–406. doi: 10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- Walton R, Johnstone E, Munafo M, Neville M, Griffiths S. Genetic clues to the molecular basis of tobacco addiction and progress towards personalized therapy. Trends Mol Med. 2001;7(2):70–76. doi: 10.1016/s1471-4914(01)01915-3. [DOI] [PubMed] [Google Scholar]
- Ward JH. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58(301):236–244. [Google Scholar]
- Wheeler JM, Reed C, Burkhart-Kasch S, Li N, Cunningham CL, Janowsky A, Franken FH, Wiren KM, Hashimoto JG, Scibelli AC, Phillips TJ. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 2009;8(8):758–771. doi: 10.1111/j.1601-183X.2009.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Lee M, Agatsuma S, Hiroi N. Pleiotropic impact of constitutive fosB inactivation on nicotine-induced behavioral alterations and stress-related traits in mice. Hum Mol Genet. 2007;16(7):820–836. doi: 10.1093/hmg/ddm027. [DOI] [PubMed] [Google Scholar]