Abstract
It is known that chitosan oligosaccharides (COS) suppress LPS-induced vascular endothelial inflammatory response by mechanism involving NF-κB blockade. It remains unknown how COS inhibit NF-κB. We provided evidence both in cultured endothelial cells and mouse model supporting a new mechanism. Regardless of the endothelial cell types, the LPS-induced NF-κB-dependent inflammatory gene expression was suppressed by COS, which was associated with reduced NF-κB nucleus translocation. LPS enhanced O-GlcNAc modification of NF-κB/p65 and activated NF-κB pathway, which could be prevented either by siRNA knockdown of O-GlcNAc transferase (OGT) or pretreatment with COS. Inhibition of either mitogen-activated protein kinase or superoxide generation abolishes LPS-induced NF-κB O-GlcNAcylation. Consistently, aortic tissues from LPS-treated mice presented enhanced NF-κB/p65 O-GlcNAcylation in association with upregulated gene expression of inflammatory cytokines in vascular tissues; however, pre-administration of COS prevented these responses. In conclusion, COS decreased OGT-dependent O-GlcNAcylation of NF-κB and thereby attenuated LPS-induced vascular endothelial inflammatory response.
Keywords: Chitosan oligosaccharides, LPS (Lipopolysaccharides), Endothelial cells, O-GlcNAcylation, NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), Inflammatory response
1. Introduction
Vascular endothelial cells are key regulator of inflammatory response which provide an anti-inflammatory, anticoagulatory surface in the steady state and a controlled inflammatory response in injury (infection) state (Kadl & Leitinger, 2005). A properly regulated inflammatory response in these blood vessel-lining cells is crucial because it allows maintaining vascular homeostasis which usually becomes impaired over the course of inflammatory diseases including atherosclerotic cardiovascular diseases and diabetes. Bacterial Lipopolysaccharides (LPS) have a well-established role in the induction of inflammatory response through promoting the production of pro-inflammatory cytokines in many cell types (Raetz & Whitfield, 2002). It is also well recognized that LPS impair vascular endothelial function through aberrant inflammatory reactions (Bierhaus et al., 2000). Emerging evidence highlighted that LPS-induced vascular endothelial inflammatory response can be efficiently blocked by administration of chitosan oligosaccharides (COS) (Liu et al., 2011; Liu et al., 2010b).
COS are depolymerized products, as oligomers of D-glucosamine (Arvanitoyannis et al., 1998), from the naturally-occurring compounds chitin and chitosan through chemical and enzymatic hydrolysis. Biological activities of COS have been extensively studied due to their high solubility (Da Silva et al., 2010; Kim & Rajapakse, 2005), absorption (Eijsink et al., 2010) and biocompatibility (Du et al., 2009). Increasingly emerging evidence indicate that COS exhibit anti-inflammatory activities in experimental models in vitro (Liu et al., 2011; Liu et al., 2010b) and in vivo (Qiao et al., 2011), in addition to those of antitumor (Fernandes et al., 2012), antifungal (Hussain et al., 2012), antimicrobial (Malcata et al., 2010; Tavaria et al., 2012), and free radical scavenging (Kim et al., 2012) activities. As such, COS have in recent years been recommended as healthy food supplements in Asian countries due to these properties (Nam et al., 2007; Nishimura et al., 1984). Although it remains largely unknown exactly how COS exert these potential beneficial effects, studies with animals have observed an anti-inflammatory feature shared among various models treated with COS. These have included a rabbit model of breast capsular contracture (Marques et al., 2011), a mouse model of sepsis (Qiao et al., 2011), chemical-induced paw edema (Fernandes et al., 2010), asthma (Chung et al., 2012), and diet-induced obesity (Choi et al., 2012). Further in-depth studies with cultured cell models support the notion that the anti-inflammatory feature of COS may attribute to the suppression of NF-κB-dependent inflammatory gene expression. Indeed, pretreatments with COS significantly abolish the otherwise increased inflammatory cytokines by LPS, such as IL-1β (Pangestuti et al., 2011; Qiao et al., 2011), IL-6 (Liu et al., 2010b; Ma et al., 2011; Pangestuti et al., 2011; Yoon et al., 2007), IL-8 (Liu et al., 2011) and TNF-α (Ma et al., 2011; Pangestuti et al., 2011; Qiao et al., 2011; Yoon et al., 2007) in endothelial cells, as well as in macrophage and microglia. However, it has yet to be established how COS suppress NF-κB activation induced by LPS.
NF-κB is present as a dimer consisting of p65 (RelA) and p50 subunits in most cell types. This dimer is localized to the cytoplasm and binds the inhibitor IκB. Treatment with LPS or other activating agents stimulate IκB kinase, which phosphorylates IκB and thereby induces its degradation. The degradation of IκB leads to dissociation and translocation of NF-κB into the nucleus and activation of target genes. In this study, we sought to provide evidence to test a potentially novel hypothesis involving NF-κB modulation through which COS exerted their anti-inflammatory effects induced by LPS. The mechanism will be tested both in cultured cell and mouse models, which may represent COS as the anti-inflammatory agent in vascular endothelial cells.
2. Materials and methods
2.1. Chemicals and reagents
COS were prepared as previously described with degree of deacetylation over 95%, average molecular weight: <1,000 Da, and endotoxin free (limulus amebocyte lysate test) (Zhang et al., 1999). The weight percentages of COS were 3.7%, 16.1%, 28.8%, 37.2% and 14.2%, respectively, with DP (degree of polymerization) 2–6 in oligomixture. Antibodies against mitogen-activated protein kinase (MAPK), MAPK kinase (MEK), phosphorylated MAPK, O-GlcNAc transferase (OGT), and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA); antibodies against NF-κB/p65, Histone H3, and all peroxidase conjugated secondary antibodies were from Cell Signaling (Danvers, MA, USA); a goat anti-rabbit IgG conjugated to a fluorescent green dye Alexa Fluor 488 was from Invitrogen (Carlsbad, CA, USA); LPS (from E.coli 055:B5) was from Sigma-Aldrich (St. Louis, MO, USA); mito-TEMPO-H (mTempol) was from Enzo Life Sciences (Farmingdale, NY, USA); ST045849 was bought from TimTec LLC (Newark, DE, USA). Human control and OGT siRNA were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). NE-PER® Nuclear and Cytoplasmic Extraction Reagents were purchased from Thermo (Thermo, USA). Inhibitors SB203580 and PD98059 were bought from Fisher Scientific (Waltham, MA, USA). Ponceau S was obtained from Sigma (St. Louis, MO, USA). The NF-kB p50/p65 Transcription Factor Assay Kit was from Abcam (Cambridge, MA, USA).
2.2. Treatment of Mice
Male C57BL/6J mice, 10 weeks of age, 20–30g, were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were housed under controlled temperature (21°C) and lighting, with 12 hours of light and 12 hours of dark, and had free access to water and a standard mouse chow diet. Mice were handled in accordance with the protocols approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center (Oklahoma City, OK). The mice were randomly assigned to two major groups: the COS and non-COS treatment groups (COS-group: 1mg/ml in drinking water, 2 weeks; non-COS group: normal drinking water). Each major group was further divided into two subgroups treated either with LPS or vehicle (LPS from E.coli: 3 mg/kg, i.p.; vehicle: PBS). Body weight and food/water uptake were recorded at the start and before the endpoints of experiments. All mice were euthanized 24h after the acute injection of either LPS or vehicle and the aortic tissues were prepared as described previously and stored at −80 °C.
2.3. Endothelial cells and the treatment with siRNA duplex
The endothelial cells: bovine aortic endothelial cells (BAEC) and human vascular endothelial cells (EA.hy926) were from ATCC (Manassas, VA). BAEC were grown in endothelial cells basal medium (EBM; Lonza, Walkersville, MD) containing 5% FBS, penicillin (100 U/ml), streptomycin (100 μg/ml) and growth factor; EA.hy926 cells were cultured with Dulbecco’s modified Eagle medium (DMEM; ATCC, Manassas, VA) containing 10% FBS, penicillin (100 U/ml) and streptomycin (100 μg/ml). All cells were incubated at 37°C in a humidified atmosphere of 5% CO2. In all experiments, cells used were between passages the third and eighth grown to 70 – 80% confluence as previously reported (Liu et al., 2012a; Liu et al., 2012b). Transfection of control or OGT siRNA was performed based on protocols provided by Santa Cruz Biotechnology (Santa Cruz, CA) as described previously (Liu et al., 2012a; Liu et al., 2012b). All cells were incubated in a humidified atmosphere of 5% CO2 + 95% O2 at 37°C.
2.4. Immunofluorescent staining of endothelial cells for NF-κB protein
The cultured endothelial cells were subjected to immunofluorescent staining with a commercial immune-staining kit including ProLong® Gold and SlowFade® Gold Antifade obtained from Life Technologies (Carlsbad, CA) as described previously (Liu et al., 2012a). Briefly, cells cultured on glass cover-slips were treated with experimental reagents and fixed with 4% paraformaldehyde for 30 min and permeabilized with 0.3% Triton X-100 for 25 min at RT. After blocking with 1% goat serum for 10 min, cells were incubated with a polyclonal antibody against NF-κB p65 overnight at 4°C, a goat anti-rabbit IgG conjugated with a fluorescent green dye Alexa Fluor 488 was used as the secondary antibody and cells were permanently mounted with DAPI. Fluorescent signals were captured and analyzed with fluorescence microscopy (Olympus, Japan).
2.5. Western blot analysis and immunoprecipitation
Protein samples were detected by Western blot. Western blotting and band densitometry were performed as previously reported (Liu et al., 2012a; Liu et al., 2012b). Briefly, the treated vascular endothelial cells were washed with ice-cold PBS and lysed in RIPA lysis buffer (50 mM Tris with pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulphate and 0.05 mM EDTA) for 15 min on ice, and cell lysate was centrifuged at 12,000 g for 10 min at 4 °C. The supernatant was collected and stored at −80 °C. Protein content was determined with a BCA (bicinchoninic acid) assay kit from Pierce (Pierce, USA). Levels of studied proteins were determined by Western blot analysis with their respective antibodies. Briefly, total cell lysate was boiled in 5×loading buffer (125 mM Tris•HCl, pH 6.8, 10% SDS, 8% dithiothreitol, 50% glycerol and 0.5% bromchlorphenol blue) for 10 min. Equal amount of proteins (50 μg) was subjected to 8–12% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% skim milk in PBS with 0.1% Tween 20 (PBST) for 1 h, and incubated with primary antibodies overnight at 4 °C. Antibodies were detected by means of HRP-conjugated secondary antibody for 1 h at room temperature. Immunoreactive bands were visualized with enhanced chemiluminescence reagents (ECL) and densitometric analysis was performed with PDI Imageware System (Bio-Rad, Hercules, CA, USA). Cultured cells and aortic tissues were homogenized on ice in cell-lysis buffer. For immunoprecipitation, cell lysates were incubated with antibodies overnight at 4 °C. Antibody-protein complex was precipitated with protein A/G Sepharose beads (Santa Cruz Biotechnology, Santa Cruz, CA).
2.6. Detection of O-GlcNAc-modified proteins in cell lysates and mouse aortic homogenates
The cell lysates or tissue homogenates were prepared in lysis buffer containing 25 mM HEPES (pH 7.0), 1 mM EDTA, 1 mM EGTA, 1% NP-40, 0.1% SDS, 1% protease inhibitor cocktail (Sigma, St Louis, MO), 1% phosphatase inhibitor cocktail, and 1–100 μM PUGNAc (O-GlcNAcase inhibitor). Proteins modified by O-linked GlcNAc were pulled down by using an Agarose bound Wheat Germ Agglutinin (WGA) Kit (Vector Laboratories, Burlingame, CA) as described previously (Zachara et al., 2011). To confirm the WGA approach, an anti-O-GlcNAc antibody (CTD110.6) (Sigma, St Louis, MO) was used to replace WGA for immunoprecipitation. The enriched proteins were subjected to Western blot analysis using antibodies of interest.
2.7. Inflammatory cytokine gene expression analysis
The total RNA extraction from cultured cells and lung tissues was achieved with Total RNA Kit I (#R683401, Omega Bio-Tek, Norcross, GA), reverse transcribed into cDNA, and the resulting cDNA was subjected to quantitative polymerase chain reaction (PCR) as previously described [40]. Forward and reverse specific primer sequences were: MCP-1 (human) (Meier et al., 2012), forward: 5′-CAA TAG GAA GAT CTC AGT GC AGA GG-3′; reverse: 5′-GGA ATC CTG AAC CCA CTT CTG C-3′. E-selectin (human) (Heo et al., 2013), forward: 5′-GCT CTG CAG CTC GGA CAT -3′; reverse: 5′-GAA AGT CCA GCT ACC AAG GGA AT -3′. β-actin (human) (Liu et al., 2012b), forward: 5′-ACG GCA TCG TCA CCA ACT G-3′; reverse: 5′-GAG CCA CAC GCA GCT CAT T-3′. MCP-1 (mouse) (Jin et al., 2012), forward: 5′-CCT GGA TCG GAA CCA AAT GA-3′; reverse: 5′-CGG GTC AAC TTC ACA TTC AAA G-3′. E-selectin (mouse) (Sato et al., 2011), forward: 5′-CAT GGC TCA GCT CAA CTT-3′; reverse: 5′-GCA GCT CAT GTT CAT CTT-3′. GAPDH (mouse) (Pan et al., 2012), forward: 5′-TCC ATG ACA ACT TTG GCA TTG-3′; reverse: 5′-CAG TCT TCT GGG TGG CAG TGA-3′. iScript TM cDNA Synthesis Kit (170–8891), iQ TM SYBR Green Supermix (170–8880), and the Real Time Detection System were obtained from Bio-Rad (Hercules, CA). PCR specific primers were synthesized by Sigma (St. Louis, MO). PCR product was analyzed by running 6 μl of each reaction mixture on a 1.5% agarose gel containing 1% GoldView™. Band intensity was quantified with ImageJ system (NIH, USA) and presented as a percentage of GAPDH expression.
2.8. Statistics
Values are expressed as mean ± SEM. Statistical comparisons were performed using SPSS 10.0 package (SPSS Inc., Chicago, IL, USA). One-way ANOVA and Student’s t-test followed by a Bonferroni correction were carried out to determine statistical significance. Differences were considered significant at P<0.05.
3. Results
3.1. COS block LPS-induced inflammatory cytokine gene expression in endothelial cells
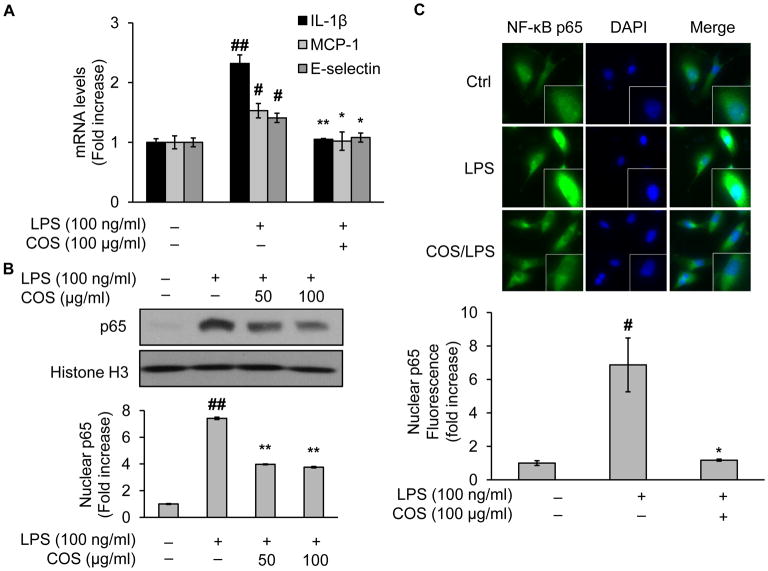
To prepare a cell model in which LPS elicits inflammatory responses, we first monitored the NF-κB dependent inflammatory cytokine gene expression in LPS-treated human vascular endothelial cells (EA.hy926). In this regard, we performed RT-PCR to detect LPS-altered mRNA expression levels of representative inflammatory cytokines. As depicted in Fig. 1A, in the presence of LPS, mRNA levels of respectively IL-1β, MCP-1, and E-selectin were significantly increased compared with untreated controls (Fig. 1A). When pretreated with COS, the increase of mRNA levels was dramatically attenuated (Fig. 1A). These data were consistent with those reported in other endothelial cells (Liu et al., 2011; Liu et al., 2010b), suggesting that the effect of COS was independent of endothelial cell type.
Fig. 1.
COS inhibit NF-κB nucleus translocation and inflammatory cytokine gene expression in LPS-treated endothelial cells. EA.hy926 or BAEC were pretreated with COS (up to 100 μg/ml) for 4 h followed by LPS (100 ng/ml) incubation for 4 h. Then the cells were subjected to the assessment of (A) mRNA levels of inflammatory cytokines including IL-1β, MCP-1, and E-selectin, using RT-PCR as described in detail in Materials and Methods (Inflammatory cytokine gene expression analysis) in EA.hy926, and in BAEC: (B) Western blotting of NF-κB in the nucleus fraction prepared by NE-PER® Nuclear and Cytoplasmic Extraction Reagents, and (C) immunofluorescent staining of NF-κB or DAPI with a kit including ProLong® Gold and SlowFade® Gold Antifade. The secondary antibody was conjugated with a fluorescent green dye (Alexa Fluor 488). Nuclear p65 fluorescence was quantified. All images shown are representative of three independent experiments. Data are expressed as means ± SEM. ##P<0.01 compared to the vehicle-treated control group; *P<0.05, **P<0.01 compared to the LPS-only group.
3.2. COS inhibit NF-κB nucleus translocation in LPS-treated endothelial cells
NF-κB is a fairly well studied transcriptional factor which is implicated both in health and disease in humans and experimental animals. It is widely recognized that increased NF-κB nucleus translocation contributes to LPS-elevated gene expression of inflammatory cytokines in immune cells (e.g., macrophages) as well as vascular endothelial cells. To further confirm the anti-inflammatory capacity of COS, we examined intracellular locations of NF-κB in LPS-treated endothelial cells (BAEC, a well established endothelial cell model). As expected, nuclear translocation of NF-κB was enhanced by LPS, evidenced by increased Western blotting of NF-κB in nuclear extraction from LPS-treated vs vehicle-treated control cells (Fig. 1B). However, NF-κB protein staining in the nucleus was greatly reduced when cells were pretreated with COS (Fig. 1B). Similar results were obtained in cells subjected to immunohistochemical analysis which confirmed similar alterations in NF-κB nuclear translocation (Fig. 1C). Given the importance of NF-κB in the regulation of inflammatory gene expression, these results built an appropriate cell model for the rest of the study to evaluate LPS-mediated inflammatory response in endothelial cells.
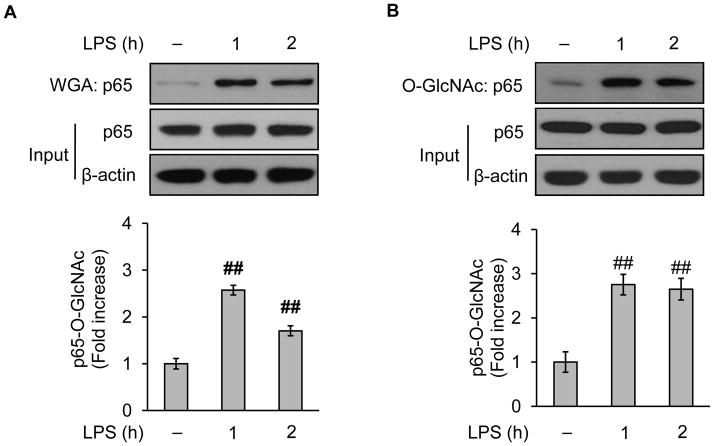
3.3. LPS enhances O-GlcNAc modification of NF-κB/p65
Increasing evidence support the notion that post-translational modulation of NF-κB is crucial in promoting inflammatory gene expression. Recent studies demonstrate that O-GlcNAc modification of NF-κB alters downstream gene expression. O-GlcNAc modification or O-GlcNAcylation is an essential cycling modification consisting of a single O-linked N-Acetylglucosamine sugar attached to the serine or threonine residues of nuclear and cytoplasmic proteins (Hart et al., 2007). We wondered whether LPS affected O-GlcNAc modification of NF-κB in cultured vascular endothelial cells (BAEC). To detect O-GlcNAcylation, we followed the WGA Kit protocol (Zachara et al., 2011) to enrich the O-GlcNAcylated proteins which were then probed with an NF-κB antibody recognizing specifically one of the subunits p65. The Western blotting showed that administration of LPS increased the staining for O-GlcNAcylated NF-κB/p65 (Fig. 2A), without changing total levels of NF-κB/p65 protein in these endothelial cells (Fig. 2A). To confirm results obtained from the WGA approach, we used an anti-O-GlcNAc antibody (CTD110.6) to harvest O-GlcNAc-modified proteins. As presented, a dramatic increase of NF-κB/p65 staining was found in O-GlcNAc coimmunoprecipitates prepared from LPS-treated cells (Fig. 2B), similar to those with the WGA approach (Fig. 2A). Together, LPS increased the O-GlcNAcylation of NF-κB/p65 in endothelial cells. In rest of the study, the WGA approach was adopted to enrich O-GlcNAc-modified proteins.
Fig. 2.
LPS augments O-GlcNAc modification of NF-κB/p65 in vascular endothelial cells. BAEC were pretreated with (100 ng/ml) for up to 2h and the collected cells were subjected to Western blotting after the enrichment of O-GlcNAc modified protein either by (A) WGA protocol or (B) anti-O-GlcNAc antibody (CD110.6). The whole cell lysates were used to assess the protein input as a loading control. Data are expressed as means ± SEM (n=3). ##P<0.01 compared to the vehicle-treated control group.
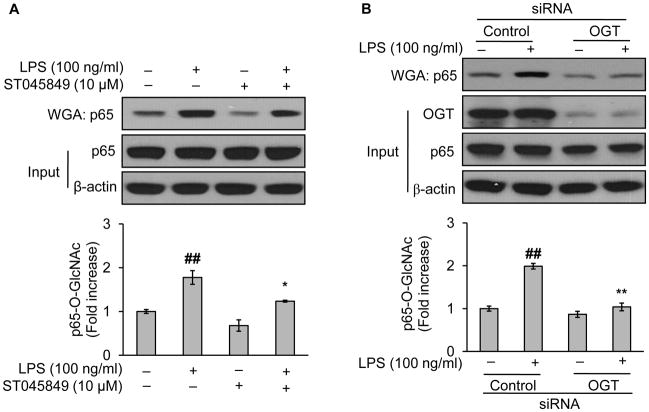
3.4. OGT mediates LPS-increased O-GlcNAc modification of NF-κB/p65
O-GlcNAc transferase (OGT) is a major dynamic enzyme that directly controls O-GlcNAc modification. Specifically, OGT catalyzes O-GlcNAcylation. Increasing evidence support a crucial role of OGT in the regulation of intracellular protein O-GlcNAcylation. We wondered whether OGT was required for the LPS-mediated NF-κB O-GlcNAcylation. To test this, we took pharmacological loss-of-function approach with ST045849, a potent OGT inhibitor (Lima et al., 2011). BAEC were pretreated either with ST045849 or vehicle (culture medium) before LPS challenge. As depicted, LPS reproducibly increased the levels of NF-κB/p65 O-GlcNAcylation in vehicle-treated cells, which was significantly attenuated in ST045849-treated cells (Fig. 3A), although levels of total p65 protein remained unchanged (Fig. 3A). To exclude potential off-target effects of OGT inhibitor, we took genetic loss-of-function approach with siRNA treatment in EA.hy926. As anticipated, OGT-siRNA treatment significantly reduced the protein levels of OGT compared with control-siRNA treatment (Fig. 3B). Incubation of LPS with control siRNA-treated cells increased the O-GlcNAcylation of NF-κB/p65 (Fig. 3A), confirming the effects of LPS in non-siRNA treated cells (Fig. 2 and Fig. 3A). However, the otherwise increased levels of NF-κB/p65 O-GlcNAcylation were largely reduced in OGT-siRNA treated cells (Fig. 3B). These data suggested that OGT mediated the O-GlcNAc modification of NF-κB/p65 induced by LPS.
Fig. 3.
OGT mediates LPS-induced O-GlcNAcylation of NF-κB/p65. Western blotting of indicated proteins of either the whole cell lysates (used to assess the protein input as a loading control) or WGA-enriched O-GlcNAc modified proteins, isolated respectively from endothelial cells being treated (A) either with vehicle (culture medium) or ST045849 (10 μM), a potent OGT inhibitor, for 1 h followed by LPS (100 ng/ml) challenge for 1 h (in BAEC) and (B) either with control or OGT siRNA for 48h followed by LPS (100 ng/ml) challenge for 1 h (in EA.hy926). Data are expressed as means ± SEM (n=3). ##P<0.01 compared to the vehicle-treated control group; *P<0.05, **P<0.01 compared to the LPS-only group or the group of LPS-only with control siRNA treatment.
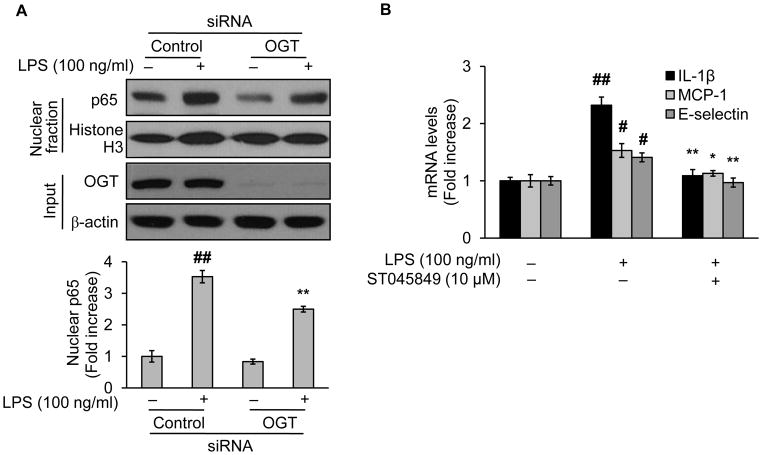
3.5. NF-κB/p65 O-GlcNAcylation is associated with LPS-induced NF-κB nuclear entry
We next determined whether O-GlcNAc modification of NF-κB/p65 resulted in NF-κB nuclear entry, a hallmark as well as an initiate step in LPS-activated NF-κB signaling pathway. To do this, we performed the same experiments in Fig. 3 but isolated the nucleus fraction with a subcellular fractionation kit. Challenge with LPS augmented the NF-κB/p65 staining in nucleus fraction (Fig. 4A), consistent with previous results (Fig. 1A), indicating enhanced nuclear entry of NF-κB/p65. However, this entry was reduced in OGT-knockdown cells (Fig. 4A). Clearly, these changes were associated with those of the NF-κB/p65 O-GlcNAcylation (Fig. 3). We further assessed the functional outcome of NF-κB/p65 O-GlcNAcylation by evaluating NF-κB dependent inflammatory gene expression. As shown, LPS treatment elevated mRNA levels of IL-1β, MCP-1, and E-selectin in vehicle but not in OGT inhibitor-treated cells (Fig. 4B).
Fig. 4.
OGT mediates LPS-induced NF-κB/p65 nucleus entry and activation. (A) Western blotting of the nucleus fraction proteins from EA.hy926 treated either with control or OGT siRNA for 48h followed by LPS (100 ng/ml) challenge for 1 h. (B) mRNA levels of inflammatory cytokines of EA.hy926 either treated with vehicle (culture medium) or ST045849 (10 μM), a potent OGT inhibitor, for 1 h, followed by incubation with LPS (100 ng/ml) for 4 h. The mRNA levels IL-1β, MCP-1, and E-selectin were determined by using RT-PCR as described in detail in Materials and Methods (Inflammatory cytokine gene expression analysis). Data are expressed as means ± SEM (n=3). #P<0.05, ##P<0.01 compared to the vehicle-treated control group; *P<0.05, **P<0.01 compared to the LPS-only group or the group of LPS-only with control siRNA treatment.
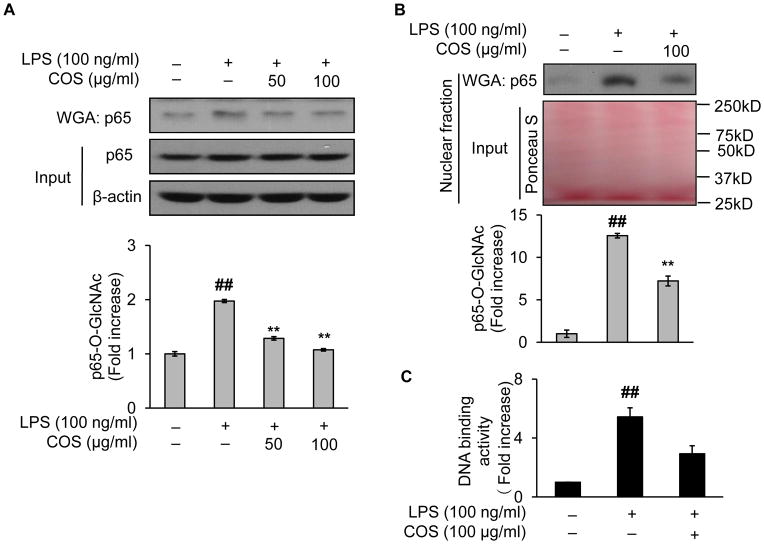
3.6. COS abolish LPS-induced NF-κB/p65 O-GlcNAcylation in endothelial cells
We next decided whether COS would prevent NF-κB/p65 from LPS-induced O-GlcNAcylation. In this regard, we incubated 100 μg/ml of COS for 1 h before endothelial cells were challenged with LPS. As shown, LPS reproducibly induced NF-κB/p65 O-GlcNAcylation compared with the controls (Fig. 5A). Such an effect was abolished when the cells were pretreated with COS (Fig. 5A). Apparently, the effects of COS on levels of NF-κB/p65 O-GlcNAcylation were closely related to COS-mediated downregulation of NF-κB/p65 nucleus translocation (Fig. 1B/C) and mRNA levels of the inflammatory cytokine gene expressions (Fig. 1A) stimulated by LPS. To gain further mechanistic insights, we assessed the levels of NF-κB/p65 O-GlcNAcylation in the nucleus. This was achieved by Western blotting of O-GlcNAc-modified proteins enriched by WGA from nuclear extracts of the cells. As it turned out, NF-κB/p65 O-GlcNAcylation in the nucleus was significantly augmented by LPS. In contrast, when COS was present, the induced O-GlcNAc modification of NF-κB/p65 was greatly attenuated (Fig. 5B). We next measured DNA binding activity of NF-κB with an NF-κB p50/p65 transcription factor assay kit. It was achieved by quantification of the binding of NF-κB (from the enriched nuclear extracts as above) to the DNA containing NF-κB response elements (immobilized on a 96-well plate from the kit). As anticipated, LPS enhanced DNA binding activities of NF-κB (Fig. 5C); however, the presence of COS significantly reduced such activities (Fig. 5C). These data suggested that O-GlcNAc modification of NF-κB may underlie the effect of COS.
Fig. 5.
COS abolishes LPS-induced NF-κB/p65 O-GlcNAcylation in endothelial cells. BAEC were pretreated with COS (up to 100 μg/ml) for 1 h and then exposed to LPS (100 ng/ml) for 1 h. Western blotting was performed with O-GlcNAc-modified proteins enriched by WGA either from (A) whole cell lysates (the protein loading or input was assessed by Western blotting on whole cell lysates) or (B) nuclear fraction isolated with NE-PER® Nuclear and Cytoplasmic Extraction Reagents (the protein loading or input was assessed by Ponceau S staining of the blot). (C) DNA binding activity was measured by following the instruction of the NF-kB p50/p65 Transcription Factor Assay Kit provided by the manufacture (Abcam). It was achieved by quantification of the binding of NF-κB (from WGA-enriched nuclear extracts) to the DNA containing NF-κB response elements (immobilized on a 96-well plate from the kit). Data are expressed as means ± SEM (n=3). ##P<0.01 compared to the vehicle-treated control group; **P<0.01 compared to the LPS-only group.
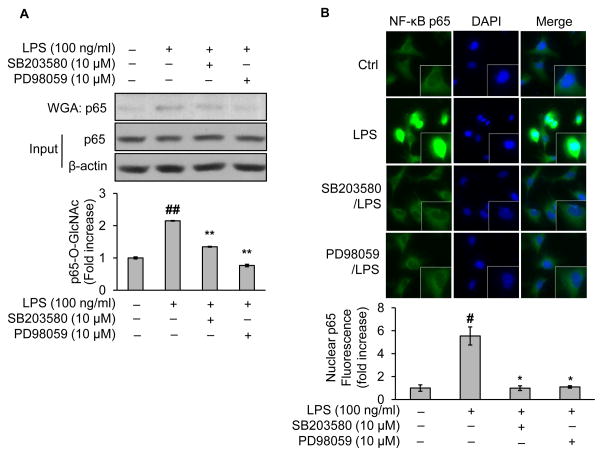
3.7. Blockade of MAP kinase inhibits NF-κB/p65 O-GlcNAcylation and nucleus translocation
MAPK mediated COS-exerted anti-inflammatory effects both in immune cells and endothelia cells treated with LPS. We wondered whether MAPK mediated O-GlcNAc modification of NF-κB/p65 induced by LPS. To this end, endothelial cells were pretreated with SB203580, a specific MAPK inhibitor. Compared with vehicle-treated controls, LPS treatment increased O-GlcNAc modification of NF-κB/p65 (Fig. 6A); however, the enhancement was drastically diminished by MAPK inhibitor (Fig. 6A). Notably, the changes in levels of NF-κB/p65 O-GlcNAcylation were mirrored by those of the NF-κB nucleus translocation under same conditions (Fig. 6B). Similar results were obtained when the MAPK inhibitor was replaced by PD98059, a selective inhibitor of MAPK kinase, which significantly reduced LPS-elicited NF-κB/p65 O-GlcNAcylation (Fig. 6A) and nuclear entry (Fig. 6B). In sum, these data implicated that MAPK modulated NF-κB/p65 O-GlcNAcylation and the consequent nucleus translocation.
Fig. 6.
Blockade of p38 MAPK pathway inhibits O-GlcNAcylation and activation of NF-κB/p65 in LPS-treated endothelial cells. BAEC were pretreated either with SB203580 (10 μM, a specific inhibitor of p38 MAPK) or PD98059 (10 μM, a selective inhibitor of MAPK kinase or MEK) for 1 h followed by LPS (100 ng/ml) incubation for 1 h. The treated cells were subjected to (A) Western blotting of proteins either from WGA-enriched O-GlcNAc modified proteins or whole cell lysates (to assess protein input as a loading control); (B) immunofluorescent staining of NF-κB or DAPI with a kit including ProLong® Gold and SlowFade® Gold Antifade. The secondary antibody was conjugated with a fluorescent green dye (Alexa Fluor 488). Nuclear p65 fluorescence was quantified. All images shown are representative of three independent experiments. Data are expressed as means ± SEM (n=3). #P<0.05, ##P<0.01 compared to the vehicle-treated control group; *P<0.05, **P<0.01 compared to the LPS-only group.
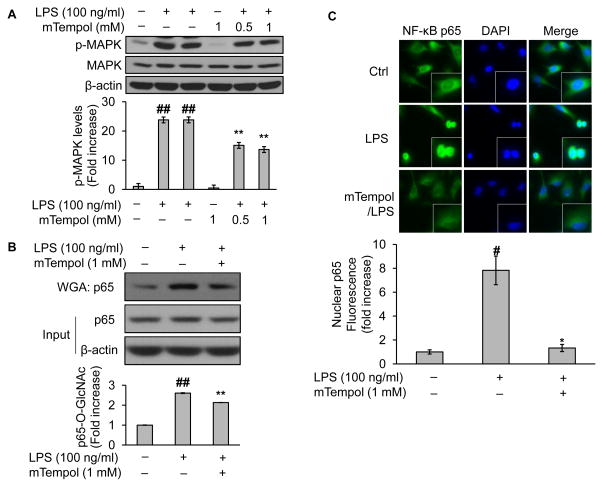
3.8. Superoxide scavenger blocks NF-κB/p65 O-GlcNAcylation and nucleus translocation
LPS is well known for its capacity to induce superoxide in endothelial cells (Al Ghouleh & Magder, 2008), whereas superoxide has been shown to activate MAPK (Chan et al., 2005). We wondered whether superoxide contributed to LPS-induced O-GlcNAc modification of NF-κB/p65. To test this, we pretreated endothelial cells with mTempol (1mM for 1h), a mitochondria-targeted antioxidant with superoxide scavenging properties (Dikalova et al., 2010), before LPS challenge. As predicted, LPS activated MAPK as shown by increased expression of phosphorylated MAPK (Fig. 7A), which was significantly less activated in the presence of mTempol (Fig. 7A). Repeatedly, LPS treatment increased O-GlcNAc modification of NF-κB/p65 (Fig. 7B); such an elevation was reduced when mTempol was pre-incubated (Fig. 7B), in a similar trend to the changes of MAPK phosphorylation (Fig. 7A). Importantly, preincubation with mTempol significantly reduced LPS-mediated NF-κB nucleus translocation (Fig. 7C), further supporting a potential cause-and-effect relationship between NF-κB modification and nuclear entry which was modulated by superoxide.
Fig. 7.
Scavenging superoxide obliterates the O-GlcNAc modification and nucleus translocation of NF-κB/p65 in LPS-challenged endothelial cells. BAEC were pretreated either with vehicle (culture medium) or mTempol (up to 1mM, a mitochondria-targeted antioxidant with superoxide scavenging properties) for up to 1 h followed by incubation with LPS (100 ng/ml) for 1 h. The treated cells were subjected to (A) assessment of the role of superoxide scavenger on LPS-induced MAPK activation by Western blot; (B) Western blotting of proteins either from WGA-enriched O-GlcNAc modified proteins or whole cell lysates (to assess protein input as a loading control); (B) immunofluorescent staining of NF-κB or DAPI with a kit including ProLong® Gold and SlowFade® Gold Antifade. The secondary antibody was conjugated with a fluorescent green dye (Alexa Fluor 488). Nuclear p65 fluorescence was quantified. All images shown are representative of three independent experiments. Data are expressed as means ± SEM (n=3). #P<0.05, ##P<0.01 compared to the vehicle-treated control group; *P<0.05, **P<0.01 compared to the LPS-only group.
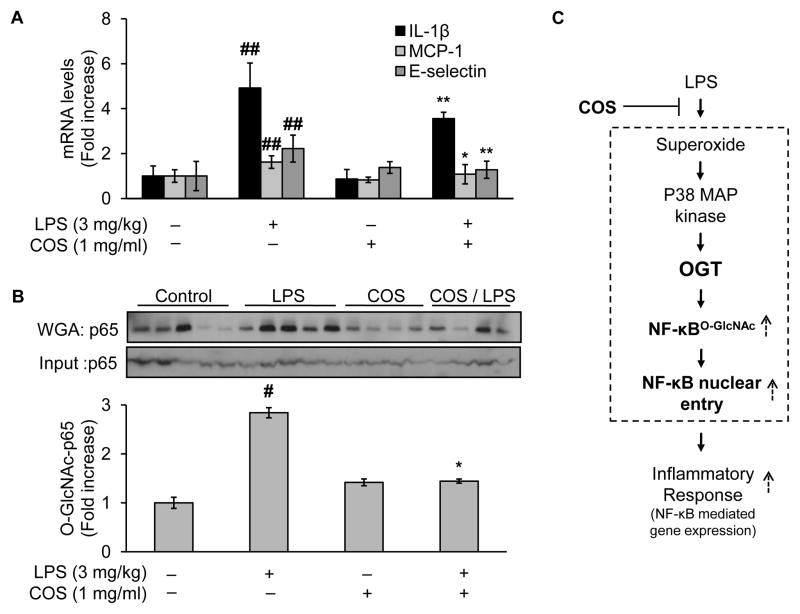
3.9. Administration of COS inhibits vascular inflammatory response in LPS-treated mice
To extend our findings to physiologically relevant setting, we sought to test whole animal in vivo. In this regard, we selected a LPS mouse model, which has long been used to evaluate acute pro-inflammatory response to LPS either in immune cells (Xu et al., 2003) or beyond (Goldblum et al., 1994; Kisseleva et al., 2006). We chose to test vascular tissues in which aberrant inflammatory response has been attributed to the pathogenesis of atherosclerotic cardiovascular diseases (Packard & Libby, 2008). Indeed, mice received LPS injection (3mg/kg, i.p.) upregulated the gene expression of NF-κB-dependent inflammatory cytokines, as evidenced by the mRNA levels of these genes in vasculature-rich tissues (lung, which met the minimum requirement of weight for assessment of tissue mRNA levels) isolated from the treated mice 24h after LPS injection (Fig. 8A). However, such an upregulation was absent in mice receiving COS administration for 2 weeks before LPS injection (COS: 1mg/ml in normal drinking water, for 2 weeks) (Fig. 8A). Of note, administration of COS alone for the same duration (COS: 1mg/ml in normal drinking water, for 2 weeks) did not invoke the inflammatory response (Fig. 8A), similar to the effects of vehicle-treated controls (normal drinking water without COS) (Fig. 8A).
Fig. 8.
Administration of COS inhibits vascular inflammatory response in LPS-treated mice, likely through reduction of LPS-induced NF-κB O-GlcNAcylation. Male C57BL/6J mice (10 weeks of age, 20–30g) were randomly assigned into two major groups: the COS and the non-COS treatment groups (COS-group: 1mg/ml in drinking water, 2 weeks; non-COS group: normal drinking water). Each major group was further divided into two subgroups treated either with LPS or vehicle (LPS from E.coli: 3 mg/kg, i.p.; vehicle: PBS). All mice were euthanized 24h after the acute injection of either LPS/vehicle for tissues collection. Aortic tissues were then subjected to evaluation of (A) mRNA levels of inflammatory cytokines including IL-1β, MCP-1, and E-selectin; and (B) Western blotting of proteins either from WGA-enriched O-GlcNAc modified proteins or whole cell lysates (to assess protein input as a loading control). (C) Proposed mechanism by which COS exerts its anti-inflammatory effects induced by LPS: COS counteracts LPS-elicited vascular endothelial inflammatory response through a mechanism involving OGT-mediated O-GlcNAcylation, nucleus translocation, and activation of NF-κB/p65, both in cultured endothelial cells and experimental mouse model. Text in bold highlighted the new findings in the present study. Data are expressed as means ± SEM. #P<0.05, ##P<0.01 compared to the vehicle-treated control group; *P<0.05, **P<0.01 compared to the LPS-only group.
3.10. LPS enhances aortic NF-κB/p65 O-GlcNAcylation, which is prevented in COS-treated mice
We further evaluated the levels of O-GlcNAc modification of NF-κB/p65 in aortic tissues. In line with the results obtained from cultured cells, acute administration of LPS (3mg/kg, i.p.) induced a 2 fold increase of O-GlcNAcylation levels of NF-κB/p65 (Fig. 8B). However, pretreatment with COS for 2 weeks (3mg/kg in drinking water) markedly reduced the levels of NF-κB/p65 O-GlcNAcylation in LPS-treated mice (Fig. 8B). Importantly, the changes in levels of NF-κB/p65 O-GlcNAcylation were closely associated with the activation of NF-κB pathway, evidenced by the alteration in NF-κB-dependent inflammatory gene expression in aortic tissues in response to administration of LPS and/or COS (Fig. 8A). In all experiment groups, the expression levels of total NF-κB/p65 protein remained similar (Fig. 8B). Taken together, LPS increased O-GlcNAcylation of NF-κB/p65 and enhanced inflammatory cytokine gene expression, which could be prevented by administration of COS in mouse model in vivo.
4. Discussion
The present study provided evidence to support the hypothesis that COS exert an anti-inflammatory effect through posttranslational modulation of NF-κB, the transcriptional factor key to gene expression of major inflammatory cytokines. Mechanistically, LPS induced OGT-mediated NF-κB O-GlcNAcylation in cultured cells, which was closely associated with enhanced NF-κB nucleus translocation and the resultant upregulation of inflammatory gene expression. Importantly, this connection was recapitulated in LPS mouse model in vivo. Given the indispensible role of OGT in this mechanism, this study identified a potentially new mechanism by which COS confer a resistance to inflammation in vascular endothelial cells (Fig. 8C).
An important finding in this study was the demonstration both in the cell and mouse model that LPS-induced O-GlcNAc modification NF-κB/p65 was linked to NF-κB pathway activation in vascular endothelial cells. NF-κB serves as a critical regulator of cytokine production, cell activation, and proliferation both in immune cells (Li & Verma, 2002; Vallabhapurapu & Karin, 2009) and vascular endothelial cells (Pober & Sessa, 2007). NF-κB activation represents the major mechanisms by which LPS upregulates NF-κB-dependent inflammatory gene expressions (Dumitru et al., 2000; Natoli, 2004, 2012). Activation of NF-κB requires posttranslational modifications, such as phosphorylation and acetylation. NF-κB O-GlcNAcylation-induced activation of NF-κB-dependent genes has been found in T lymphocytes (Golks et al., 2007), high glucose-treated vascular smooth muscle cells (Yang et al., 2008) and Mesangial cells (James et al., 2002), and recently in HEK cells (Allison et al., 2012). Our study extends the findings to vascular endothelial cells both in the cell and mouse models that LPS-induced O-GlcNAc modification of NF-κB/p65 is associated with activation of NF-κB pathway, likely through a similar mechanism involving disruption of the interaction with IκB, although direct involvement in nuclear translocation of NF-κB is also possible (Ozcan et al., 2010). The finding has also provided a testable mechanism by which COS affords the anti-inflammatory function.
Another interesting finding in the present study was the involvement of OGT in LPS-induced O-GlcNAc modification (Fig. 3), nuclear entry (Fig. 4A), and activation nuclear entry (Fig. 4B) of NF-κB. This finding is in line with a recent report that LPS enhances OGT-mediated O-GlcNAcylation by activating OGT (Ryu & Do, 2011). Furthermore, increased O-GlcNAcylation has been shown contributable to insulin resistance and hence the development of diabetes (McClain & Crook, 1996), elevated vascular contractility in response to endothelin-1 (Lima et al., 2010), and DOCA-salt hypertension (Lima et al., 2009). However, acute incubation ex vivo with rat aortas of O-GlcNAc-increasing agents such as glucosamine prevented inflammation-induced vascular dysfunction (Hilgers et al., 2012). Similarly, increased O-GlcNAc levels have been shown to reverse NF-κB activation in macrophage (Zou et al., 2009) and inhibit inflammatory responses to acute arterial injury (Xing et al., 2008). The discrepancies and even contradictions in these and perhaps other similar studies might attribute to a major difference in modes of OGT activation, durations of GlcNAc modulation, and even the cell types and animal models adopted. However, the dynamics of O-GlcNAc modification are complex (Hart & Copeland, 2010; Hart et al., 2007; Wells et al., 2001) and so is OGT-mediated O-GlcNAc modification of transcriptional factors (Ozcan et al., 2010). It needs further studies to define exactly how OGT regulates NF-κB-mediated inflammatory response.
The most important finding in the current study was the identification of the mechanism by which COS abolished LPS-induced activation of NF-κB pathway. Specifically, administration of COS both in the cell and mouse models lowered the levels of NF-κB/p65 O-GlcNAcylation induced by LPS. The downregulation in NF-κB O-GlcNAcylation may contribute to the reduction in NF-κB/p65 nucleus translocation and activation of NF-κB pathway, because treatment with COS diminished LPS-elicited inflammatory response, evidenced by reduced gene expression of inflammatory cytokines both in cultured cells and mice in vivo. It is yet unknown exactly how COS regulate NF-κB/p65 O-GlcNAcylation; however, it is known that COS inhibit LPS-mediated inflammatory response (Liu et al., 2010b) and hydrogen peroxide-induced apoptosis (Liu et al., 2010a) through suppression of MAPK, an important kinase known to be activated by superoxide (Chan et al., 2005). Interestingly, inhibition of either MAPK (or MAPK kinase) (Fig. 6) or superoxide (Fig. 7) abrogated LPS-induced O-GlcNAc modification of NF-κB and nuclear translocation. Given the requirement of OGT in LPS-induced NF-κB O-GlcNAcylation (Fig. 3) and the resultant activation of NF-κB pathway (Fig. 4B, Fig. 5B and 5C), it is likely that COS interrupt NF-κB/p65 O-GlcNAcylation by blocking the impact of either MAPK or superoxide on OGT. Such an impact has been demonstrated: the activation of MAPK increased OGT-mediated O-GlcNAc modification in TGF-β-treated retinal pigment epithelial cells (Chung et al., 2011), although modulation of OGT could affect MAPK in high glucose-treated renal cells (Goldberg et al., 2011) and in mouse embryonic neural precursor cells (Yanagisawa & Yu, 2009). These results implicate that COS-suppressed O-GlcNAc modification of NF-κB is an important integrated component of the mechanism by which COS inhibit NF-κB pathway. It warrants further investigation on how COS modulate the complex crosstalk among MAPK, superoxide, and OGT to exert its anti-inflammatory function. It is worth noting that as a logical extension to the published studies, the present paper focused only on intracellular effects of COS; however, it does not exclude the extracellular impacts from COS as found in cultured immune cells (Qiao et al., 2010). It also merits further investigation on the role of COS in not only the prevention but also the treatment of inflammation.
Taken together, LPS enhances gene expression of the inflammatory cytokines in vascular endothelial cells through a mechanism involving OGT-mediated O-GlcNAcylation and nucleus translocation of NF-κB; mechanistically, these are mediated by MAP kinase and superoxide generation activated by LPS. However, administration of COS, both to cultured endothelial cells and experimental mouse model, attenuates these impacts. We conclude that COS suppress vascular endothelial inflammatory response induced by LPS through a mechanism involving MAP kinase, superoxide, and OGT-mediated O-GlcNAc modification and nucleus translocation of NF-κB. These insights may help develop COS-based pharmacological strategies to regulate inflammation.
Highlights.
We propose a new mechanism by which COS inhibits NF-κB activation induced by LPS
LPS enhances NF-κB O-GlcNAcylation and activates NF-κB pathway
OGT mediates LPS-induced O-GlcNAc modification of NF-κB
COS reduces LPS-induced inflammatory response by decreasing NF-κB O-GlcNAcylation
COS attenuates NF-κB O-GlcNAcylation and inflammatory response in LPS-treated mice
Acknowledgments
This work was supported in part by an NIH Grant from the COBRE Program of the National Center for Research Resources (P20 RR 024215-05) and of the National Institute of General Medical Sciences (9P20GM104934-06) (JX, Project 2). This work was also supported by a National Scientist Development Grant (10SDG2600164) from the American Heart Association (JX), a Junior Faculty Award (1-12-JF-58) from the American Diabetes Association (JX), and a Research Award (HR11-200) from the Oklahoma Center for Advancement of Science and Technology (JX). Q.S. Xu and Y.G. Du were supported by the National Natural Science Foundation of China (No. 31072065 and No. 31100589).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Ghouleh I, Magder S. Nicotinamide adenine dinucleotide phosphate (reduced form) oxidase is important for LPS-induced endothelial cell activation. Shock. 2008;29(5):553–559. doi: 10.1097/SHK.0b013e318157ebc8. [DOI] [PubMed] [Google Scholar]
- Allison DF, Wamsley JJ, Kumar M, Li D, Gray LG, Hart GW, Jones DR, Mayo MW. Modification of RelA by O-linked N-acetylglucosamine links glucose metabolism to NF-kappaB acetylation and transcription. Proc Natl Acad Sci U S A. 2012;109(42):16888–16893. doi: 10.1073/pnas.1208468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitoyannis IS, Nakayama A, Aiba AI. Chitosan and gelatin based edible films: state diagrams, mechanical and permeation properties. Carbohydrate Polymers. 1998;(37):371–382. [Google Scholar]
- Bierhaus A, Chen J, Liliensiek B, Nawroth PP. LPS and cytokine-activated endothelium. Semin Thromb Hemost. 2000;26(5):571–587. doi: 10.1055/s-2000-13214. [DOI] [PubMed] [Google Scholar]
- Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res. 2005;97(8):772–780. doi: 10.1161/01.RES.0000185804.79157.C0. [DOI] [PubMed] [Google Scholar]
- Choi EH, Yang HP, Chun HS. Chitooligosaccharide ameliorates diet-induced obesity in mice and affects adipose gene expression involved in adipogenesis and inflammation. Nutr Res. 2012;32(3):218–228. doi: 10.1016/j.nutres.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Chung EJ, Chun JN, Jung SA, Cho JW, Lee JH. TGF-beta-stimulated aberrant expression of class III beta-tubulin via the ERK signaling pathway in cultured retinal pigment epithelial cells. Biochem Biophys Res Commun. 2011;415(2):367–372. doi: 10.1016/j.bbrc.2011.10.074. [DOI] [PubMed] [Google Scholar]
- Chung MJ, Park JK, Il Park Y. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. International Immunopharmacology. 2012;12(2):453–459. doi: 10.1016/j.intimp.2011.12.027. [DOI] [PubMed] [Google Scholar]
- Da Silva CA, Pochard P, Lee CG, Elias JA. Chitin Particles Are Multifaceted Immune Adjuvants. American Journal of Respiratory and Critical Care Medicine. 2010;182(12):1482–1491. doi: 10.1164/rccm.200912-1877OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107(1):106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du YZ, Wang L, Yuan H, Wei XH, Hu FQ. Preparation and characteristics of linoleic acid-grafted chitosan oligosaccharide micelles as a carrier for doxorubicin. Colloids Surf B Biointerfaces. 2009;69(2):257–263. doi: 10.1016/j.colsurfb.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103(7):1071–1083. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Eijsink VGH, Aam BB, Heggset EB, Norberg AL, Sorlie M, Varum KM. Production of Chitooligosaccharides and Their Potential Applications in Medicine. Marine Drugs. 2010;8(5):1482–1517. doi: 10.3390/md8051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes J, Sereno J, Garrido P, Parada B, Cunha M, Reis F, Pintado M, Santos-Silva A. Inhibition of Bladder Tumor Growth by Chitooligosaccharides in an Experimental Carcinogenesis Model. Marine Drugs. 2012;10(12):2661–2675. doi: 10.3390/md10122661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes JC, Spindola H, de Sousa V, Santos-Silva A, Pintado ME, Malcata FX, Carvalho JE. Anti-inflammatory activity of chitooligosaccharides in vivo. Mar Drugs. 2010;8(6):1763–1768. doi: 10.3390/md8061763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg H, Whiteside C, Fantus IG. O-linked beta-N-acetylglucosamine supports p38 MAPK activation by high glucose in glomerular mesangial cells. Am J Physiol Endocrinol Metab. 2011;301(4):E713–726. doi: 10.1152/ajpendo.00108.2011. [DOI] [PubMed] [Google Scholar]
- Goldblum SE, Brann TW, Ding X, Pugin J, Tobias PS. Lipopolysaccharide (LPS)-binding protein and soluble CD14 function as accessory molecules for LPS-induced changes in endothelial barrier function, in vitro. J Clin Invest. 1994;93(2):692–702. doi: 10.1172/JCI117022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golks A, Tran TT, Goetschy JF, Guerini D. Requirement for O-linked N-acetylglucosaminyltransferase in lymphocytes activation. EMBO J. 2007;26(20):4368–4379. doi: 10.1038/sj.emboj.7601845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart GW, Copeland RJ. Glycomics hits the big time. Cell. 2010;143(5):672–676. doi: 10.1016/j.cell.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446(7139):1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- Heo KS, Chang E, Le NT, Cushman H, Yeh ET, Fujiwara K, Abe J. De-SUMOylation Enzyme of Sentrin/SUMO-Specific Protease 2 Regulates Disturbed Flow-Induced SUMOylation of ERK5 and p53 that Leads to Endothelial Dysfunction and Atherosclerosis. Circ Res. 2013;112(6):911–923. doi: 10.1161/CIRCRESAHA.111.300179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgers RH, Xing D, Gong K, Chen YF, Chatham JC, Oparil S. Acute O-GlcNAcylation prevents inflammation-induced vascular dysfunction. Am J Physiol Heart Circ Physiol. 2012;303(5):H513–522. doi: 10.1152/ajpheart.01175.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain I, Singh T, Chittenden C. Preparation of chitosan oligomers and characterization: their antifungal activities and decay resistance. Holzforschung. 2012;66(1):119–125. [Google Scholar]
- James LR, Tang D, Ingram A, Ly H, Thai K, Cai L, Scholey JW. Flux through the hexosamine pathway is a determinant of nuclear factor kappaB- dependent promoter activation. Diabetes. 2002;51(4):1146–1156. doi: 10.2337/diabetes.51.4.1146. [DOI] [PubMed] [Google Scholar]
- Jin HY, Song B, Oudit GY, Davidge ST, Yu HM, Jiang YY, Gao PJ, Zhu DL, Ning G, Kassiri Z, Penninger JM, Zhong JC. ACE2 deficiency enhances angiotensin II-mediated aortic profilin-1 expression, inflammation and peroxynitrite production. PLoS One. 2012;7(6):e38502. doi: 10.1371/journal.pone.0038502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadl A, Leitinger N. The role of endothelial cells in the resolution of acute inflammation. Antioxid Redox Signal. 2005;7(11–12):1744–1754. doi: 10.1089/ars.2005.7.1744. [DOI] [PubMed] [Google Scholar]
- Kim JA, Ahn BN, Kong CS, Kim SK. Chitooligomers inhibit UV-A-induced photoaging of skin by regulating TGF-beta/Smad signaling cascade. Carbohydrate Polymers. 2012;88(2):490–495. [Google Scholar]
- Kim SK, Rajapakse N. Enzymatic production and biological activities of chitosan oligosaccharides (COS) Carbohydrate Polymers. 2005;62(4):357–368. [Google Scholar]
- Kisseleva T, Song L, Vorontchikhina M, Feirt N, Kitajewski J, Schindler C. NF-kappaB regulation of endothelial cell function during LPS-induced toxemia and cancer. J Clin Invest. 2006;116(11):2955–2963. doi: 10.1172/JCI27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Lima VV, Giachini FR, Carneiro FS, Carneiro ZN, Saleh MA, Pollock DM, Fortes ZB, Carvalho MH, Ergul A, Webb RC, Tostes RC. O-GlcNAcylation contributes to augmented vascular reactivity induced by endothelin 1. Hypertension. 2010;55(1):180–188. doi: 10.1161/HYPERTENSIONAHA.109.143818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima VV, Giachini FR, Carneiro FS, Carvalho MH, Fortes ZB, Webb RC, Tostes RC. O-GlcNAcylation contributes to the vascular effects of ET-1 via activation of the RhoA/Rho-kinase pathway. Cardiovasc Res. 2011;89(3):614–622. doi: 10.1093/cvr/cvq338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima VV, Giachini FR, Choi H, Carneiro FS, Carneiro ZN, Fortes ZB, Carvalho MH, Webb RC, Tostes RC. Impaired vasodilator activity in deoxycorticosterone acetate-salt hypertension is associated with increased protein O-GlcNAcylation. Hypertension. 2009;53(2):166–174. doi: 10.1161/HYPERTENSIONAHA.108.116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, He JL, Li WM, Yang Z, Wang YX, Bai XF, Yu C, Du YG. Chitosan oligosaccharides protect human umbilical vein endothelial cells from hydrogen peroxide-induced apoptosis. Carbohydrate Polymers. 2010a;80(4):1062–1071. [Google Scholar]
- Liu H, Yu S, Xu W, Xu J. Enhancement of 26S Proteasome Functionality Connects Oxidative Stress and Vascular Endothelial Inflammatory Response in Diabetes Mellitus. Arterioscler Thromb Vasc Biol. 2012a;32(9):2131–2140. doi: 10.1161/ATVBAHA.112.253385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yu S, Zhang H, Xu J. Angiogenesis Impairment in Diabetes: Role of Methylglyoxal-Induced Receptor for Advanced Glycation Endproducts, Autophagy and Vascular Endothelial Growth Factor Receptor 2. PLoS One. 2012b;7(10):e46720. doi: 10.1371/journal.pone.0046720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, Huang P, Ma P, Liu QS, Yu C, Du YG. Chitosan oligosaccharides suppress LPS-induced IL-8 expression in human umbilical vein endothelial cells through blockade of p38 and Akt protein kinases. Acta Pharmacol Sin. 2011;32(4):478–486. doi: 10.1038/aps.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, Li WM, Li XY, Xu QS, Liu QS, Bai XF, Yu C, Du YG. Chitosan oligosaccharides inhibit the expression of interleukin-6 in lipopolysaccharide-induced human umbilical vein endothelial cells through p38 and ERK1/2 protein kinases. Basic Clin Pharmacol Toxicol. 2010b;106(5):362–371. doi: 10.1111/j.1742-7843.2009.00493.x. [DOI] [PubMed] [Google Scholar]
- Ma P, Liu HT, Wei P, Xu QS, Bai XF, Du YG, Yu C. Chitosan oligosaccharides inhibit LPS-induced over-expression of IL-6 and TNF-α in RAW264.7 macrophage cells through blockade of mitogen-activated protein kinase (MAPK) and PI3K/Akt signaling pathways. Carbohydrate Polymers. 2011;84(4):1391–1398. [Google Scholar]
- Malcata FX, Fernandes JC, Tavaria FK, Fonseca SC, Ramos OS, Pintado ME. In Vitro Screening for Antimicrobial Activity of Chitosans and Chitooligosaccharides, Aiming at Potential Uses in Functional Textiles. Journal of Microbiology and Biotechnology. 2010;20(2):311–318. doi: 10.4014/jmb.0904.04038. [DOI] [PubMed] [Google Scholar]
- Marques M, Brown SA, Rodrigues-Pereira P, Natalia M, Cordeiro DS, Morales-Helguera A, Cobrado L, Queiros L, Freitas R, Fernandes J, Correia-Sa I, Rodrigues AG, Amarante J. Animal model of implant capsular contracture: effects of chitosan. Aesthet Surg J. 2011;31(5):540–550. doi: 10.1177/1090820X11411475. [DOI] [PubMed] [Google Scholar]
- McClain DA, Crook ED. Hexosamines and insulin resistance. Diabetes. 1996;45(8):1003–1009. doi: 10.2337/diab.45.8.1003. [DOI] [PubMed] [Google Scholar]
- Meier FM, Frommer KW, Peters MA, Brentano F, Lefevre S, Schroder D, Kyburz D, Steinmeyer J, Rehart S, Gay S, Muller-Ladner U, Neumann E. Visfatin/pre-B-cell colony-enhancing factor (PBEF), a proinflammatory and cell motility-changing factor in rheumatoid arthritis. J Biol Chem. 2012;287(34):28378–28385. doi: 10.1074/jbc.M111.312884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KS, Kim MK, Shon YH. Chemopreventive effect of chitosan oligosaccharide against colon carcinogenesis. J Microbiol Biotechnol. 2007;17(9):1546–1549. [PubMed] [Google Scholar]
- Natoli G. Little things that count in transcriptional regulation. Cell. 2004;118(4):406–408. doi: 10.1016/j.cell.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Natoli G. NF-kappaB and chromatin: ten years on the path from basic mechanisms to candidate drugs. Immunol Rev. 2012;246(1):183–192. doi: 10.1111/j.1600-065X.2012.01103.x. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Nishimura S, Nishi N, Saiki I, Tokura S, Azuma I. Immunological activity of chitin and its derivatives. Vaccine. 1984;2(1):93–99. doi: 10.1016/s0264-410x(98)90039-1. [DOI] [PubMed] [Google Scholar]
- Ozcan S, Andrali SS, Cantrell JE. Modulation of transcription factor function by O-GlcNAc modification. Biochim Biophys Acta. 2010;1799(5–6):353–364. doi: 10.1016/j.bbagrm.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54(1):24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- Pan L, Li Y, Jia L, Qin Y, Qi G, Cheng J, Qi Y, Li H, Du J. Cathepsin S deficiency results in abnormal accumulation of autophagosomes in macrophages and enhances Ang II-induced cardiac inflammation. PLoS One. 2012;7(4):e35315. doi: 10.1371/journal.pone.0035315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangestuti R, Bak SS, Kim SK. Attenuation of pro-inflammatory mediators in LPS-stimulated BV2 microglia by chitooligosaccharides via the MAPK signaling pathway. Int J Biol Macromol. 2011;49(4):599–606. doi: 10.1016/j.ijbiomac.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7(10):803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- Qiao Y, Bai XF, Du YG. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int Immunopharmacol. 2011;11(1):121–127. doi: 10.1016/j.intimp.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Qiao Y, Ruan YY, Xiong CN, Xu QS, Wei P, Ma P, Bai XF, Du YG. Chitosan oligosaccharides suppressant LPS binding to TLR4/MD-2 receptor complex. Carbohydrate Polymers. 2010;82(2):405–411. [Google Scholar]
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu IH, Do SI. Denitrosylation of S-nitrosylated OGT is triggered in LPS-stimulated innate immune response. Biochem Biophys Res Commun. 2011;408(1):52–57. doi: 10.1016/j.bbrc.2011.03.115. [DOI] [PubMed] [Google Scholar]
- Sato C, Shikata K, Hirota D, Sasaki M, Nishishita S, Miyamoto S, Kodera R, Ogawa D, Tone A, Kataoka HU, Wada J, Kajitani N, Makino H. P-selectin glycoprotein ligand-1 deficiency is protective against obesity-related insulin resistance. Diabetes. 2011;60(1):189–199. doi: 10.2337/db09-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavaria FK, Soares JC, Reis IL, Paulo MH, Malcata FX, Pintado ME. Chitosan: antimicrobial action upon staphylococci after impregnation onto cotton fabric. Journal of Applied Microbiology. 2012;112(5):1034–1041. doi: 10.1111/j.1365-2672.2012.05274.x. [DOI] [PubMed] [Google Scholar]
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001;291(5512):2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- Xing D, Feng W, Not LG, Miller AP, Zhang Y, Chen YF, Majid-Hassan E, Chatham JC, Oparil S. Increased protein O-GlcNAc modification inhibits inflammatory and neointimal responses to acute endoluminal arterial injury. Am J Physiol Heart Circ Physiol. 2008;295(1):H335–342. doi: 10.1152/ajpheart.01259.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lucas R, Schuchmann M, Kuhnle S, Meergans T, Barreiros AP, Lohse AW, Otto G, Wendel A. GM-CSF restores innate, but not adaptive, immune responses in glucocorticoid-immunosuppressed human blood in vitro. J Immunol. 2003;171(2):938–947. doi: 10.4049/jimmunol.171.2.938. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Yu RK. O-linked beta-N-acetylglucosaminylation in mouse embryonic neural precursor cells. J Neurosci Res. 2009;87(16):3535–3545. doi: 10.1002/jnr.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WH, Park SY, Nam HW, Kim do H, Kang JG, Kang ES, Kim YS, Lee HC, Kim KS, Cho JW. NFkappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci U S A. 2008;105(45):17345–17350. doi: 10.1073/pnas.0806198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HJ, Moon ME, Park HS, Im SY, Kim YH. Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW 264.7 macrophage cells. Biochem Biophys Res Commun. 2007;358(3):954–959. doi: 10.1016/j.bbrc.2007.05.042. [DOI] [PubMed] [Google Scholar]
- Zachara NE, Vosseller K, Hart GW. Detection and analysis of proteins modified by O-linked N-acetylglucosamine. Curr Protoc Mol Biol. 2011;Chapter 17(Unit 17):16. doi: 10.1002/0471142727.mb1706s95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Yang S, Champattanachai V, Hu S, Chaudry IH, Marchase RB, Chatham JC. Glucosamine improves cardiac function following trauma-hemorrhage by increased protein O-GlcNAcylation and attenuation of NF-{kappa}B signaling. Am J Physiol Heart Circ Physiol. 2009;296(2):H515–523. doi: 10.1152/ajpheart.01025.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]