Abstract
Hematopoietic growth factors (HGF) are recommended therapy for high dose radiation exposure, but unfavorable administration schedules requiring early and repeat dosing limit the logistical ease with which they can be used. In this report, utilizing our previously described murine model of H-ARS, survival efficacy and effect on hematopoietic recovery of unique PEGylated (PEG) HGF developed by Bolder Biotechnology (BBT) were investigated. The PEGylated-HGF possess longer half-lives and more potent hematopoietic properties than corresponding non-PEGylated-HGFs. C57BL/6 mice underwent single dose lethal irradiation (7.76–8.72 Gy, 137Cs, 0.62–1.02 Gy min−1) and were treated with various dosing regimens of 0.1, 0.3 and 1.0 mg kg−1 of analogs of humanPEG-G-CSF, murinePEG-GM-CSF, or humanPEG-IL-11. Mice were administered one of the HGF analogs at 24–28hr post irradiation, and, in some studies, additional doses given every other day (beginning with the 24–28hr dose) for a total of 3 or 9 doses. 30d survival was significantly increased with only one dose of 0.3mg kg−1 of PEG-G-CSF and PEG-IL-11, or three doses of 0.3mg kg−1 of PEG-GM-CSF (p≤0.006). Enhanced survival correlated with consistently and significantly enhanced WBC, NE, RBC, and PLT recovery for PEG-G- and PEG-GM-CSF, and enhanced RBC and PLT recovery for PEG-IL-11 (p≤0.05). Longer administration schedules or higher doses did not provide a significant additional survival benefit over the shorter, lower dose, schedules. These data demonstrate the efficacy of BBT’s PEG-HGF to provide significantly increased survival with fewer injections and lower drug doses, which may have significant economic and logistical value in the aftermath of a radiation event.
INTRODUCTION
The increasing presence worldwide of radioactive material for therapeutic, energy, or weapon applications underscores the need for medical preparedness for effective treatment in the event of accidental or intentional radiation exposure. However, there are currently no medical countermeasures (MCM) approved to treat severely irradiated individuals, and physicians would likely rely on medications used in chemo- and radiotherapy-induced myelosuppression, such as hematopoietic cytokines, in addition to supportive care.
Supportive care such as antibiotics and fluids can significantly increase the lethal radiation dose for 50% of humans at 60 days post-exposure (LD50/60) from 3.5–4.5 Gy to ~6–7 Gy (Lushbaugh 1969, Vriesendorp and Van Bekkum 1984, Dainiak 2002, Anno et al. 2003). As such, supportive care will be an important factor in treating irradiated individuals. Doses in the range of 2–10 Gy affect primarily the rapidly dividing bone marrow hematopoietic cells, resulting in the hematopoietic (H) syndrome of the acute irradiation syndrome (H-ARS). H-ARS is characterized by a decrease in all classes of white blood cells (WBC), erythrocytes, and platelets, resulting in life-threatening neutropenia and thrombocytopenia, and possible death due to infection and/or bleeding. Stimulation of the hematopoietic system with hematopoietic growth factors (HGF) to enhance production of neutrophils and platelets remains one of the key mitigation strategies for H-ARS.
Hematopoietic growth factors, such as granulocyte-colony stimulating factor (G-CSF), are commonly used to accelerate recovery from chemotherapy-induced neutropenia. G-CSF has been shown to improve survival of animals exposed to otherwise lethal radiation when administered shortly after exposure (Schuening et al. 1989). MacVittie et al. (MacVittie et al. 2005) reported that 17% of dogs exposed to 4 Gy and treated with vehicle survived 30 days, whereas 100% of dogs receiving daily subcutaneous (sc) injections of 0.01mg kg−1 G-CSF for 23 days survived for 30 days. Studies in the authors’ lab (Plett et al. 2012) have shown that daily administration of 0.125mg kg−1 G-CSF beginning 24h post-irradiation and continuing until day 16 resulted in 65% survival of treated mice vs 30% survival in controls. Improved survival correlated with accelerated neutrophil (NE) recovery in G-CSF-treated animals (Plett et al. 2012).
Single administration of G-CSF or murine GM-CSF did not provide a survival benefit when administered to lethally-irradiated mice (Neta 1988, Neta et al. 1988), unless given within 2 hours of radiation (Sureda et al. 1993). Hematopoietic growth factors used to treat hematopoietic complications of chemotherapy, such as G-CSF, GM-CSF, and IL-11, are typically administered by daily subcutaneous (sc) injections for 16–23 days, limiting the attractiveness of these factors for the treatment of H-ARS in a mass casualty radiation event. In the aftermath of such an emergency, patient numbers will be high and medical facilities inundated, making daily treatment schedules challenging. Modification of protein therapeutics with poly(ethylene glycol) (PEG) results in increased size, reduced renal clearance, and prolongation of half-life (Abuchowski et al. 1984, Knauf et al. 1988, Molineux et al. 1999, Bailon et al. 2001), thereby reducing the need for daily dosing. One such PEGylated molecule, Neulasta (PEGylated human G-CSF, Amgen, Inc.), has demonstrated efficacy to enhance neutrophil recovery in the treatment of drug- and radiation-induced neutropenia in animals and humans with only one or two administrations (MacVittie et al. 1990, Patchen et al. 1992, Welte et al. 1996, Bishop et al. 2000).
The authors have developed several novel PEGylated HGF with extended half-life and enhanced hematopoietic efficacy compared to current therapeutics, and are evaluating these PEG-HGF for survival efficacy in their murine model of H-ARS. Of these, human PEG-G-CSF analog BBT-015 [Bolder Biotechnology, Inc (BBT)] possesses a longer half-life and is capable of inducing greater and longer lasting increases in neutrophil numbers than G-CSF or Neulasta (manuscript in preparation). Two other BBT PEG-HGFs, murine (mu) PEG-GM-CSF analog BBT-007 and PEG-IL-11 analog BBT-059, have been shown in rodents to possess longer half-lives and induce longer-lasting increases in hematopoietic cells than their non-PEGylated counterparts (manuscript in preparation). The PEGylated granulopoietic HGFs, PEG-G-CSF and PEG-GM-CSF, through their enhancement of neutrophil recovery, offer defense against infection and are thus warranted for evaluation in H-ARS. The PEG-IL-11 analog BBT-059 may likely speed platelet (PLT) recovery, and may have a role in prevention of hemorrhage in HARS. Combinations of these PEG-HGFs, with their less demanding administration schedules, offer a multi-pronged approach to combating the life-threatening maladies of H-ARS and may be useful in the design of combination therapies that could further enhance survival from high dose radiation.
Efficacy studies for these new MCM will require the use of relevant, applicable and practical animal models adhering to the Food and Drug Administration’s (FDA) Animal Rule (AR). The author’s murine model of H-ARS in C57BL/6 mice has been extensively defined (Plett et al. 2012) and can be conducted under Good Laboratory Practices (GLP) when needed, as recommended by the FDA’s AR for GLP-compliant survival studies (21 CFR Part 58). In this study the authors’ model of H-ARS was utilized to demonstrate the benefit of 1, 3, or 9 doses of PEG-G-CSF, PEG-GM-CSF, or PEG-IL-11 on survival and hematopoietic cell recovery in lethally irradiated C57BL/6 mice.
METHODS
Mice
Specific pathogen free C57BL/6 mice (50/50 male/female; Jackson Laboratory, Bar Harbor, Maine) were received at 10 weeks of age, an age analogous to a “young adult” human. All studies are performed on mice of the same age to avoid age-related changes in radiosensitivity (Grahn and Hamilton 1957, Grahn 1958, Yuhas and Storer 1967, Casarett 1968). Weights ranged from 16.0–21.6gm (females) and 19.6–28.2gm (males). Mice were identified by ear punch and/or tail marks. All studies were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Husbandry
Up to 5 mice per cage were housed in microisolator cages on sterilized, certified direct contact bedding (Alpha Dri) and provided certified commercial extruded lab rodent chow (Harlan 2018SXC) ad libitum in cage hoppers and acidified water (pH 2.0–3.0) in sipper tube bottles. Autoclaved acidified water was provided on days 4–30 post-total body irradiation (TBI) in sipper tubes and wet feed. Animal rooms on a 12-hour light/dark cycle were maintained at 21±3°C with 30–80% relative humidity and at least 10 air changes per hour of 100% conditioned fresh air.
Irradiation and dosimetry
In the morning of study day 0, mice were placed in single chambers of a Plexiglas irradiation apparatus and exposed to a single uniform total body dose between 7.76 to 8.72 Gy of gamma radiation from a 137Cs radiation source [GammaCell 40; Nordion International, Kanata, Ontario, Canada (0.63–0.68 Gy min−1), or a Mark 1 Irradiator, JL Shepherd, San Fernando, California (1.01–1.02 Gy min−1)]. Each cohort of mice irradiated together was roughly divided among all treatment groups to ensure that each group received the same irradiation exposure conditions. Dosimetry was performed as previously described (Plett et al. 2012). Annual calibration of the radiation dose-lethality relationship in the authors’ laboratory resulted in slightly different LDXX/30 doses over the three year time period that the studies were conducted. Individual survival studies were conducted using two radiation doses and results from each were combined for survival and CBC analyses as described in the statistics section below. Specific irradiation doses and estimated LDXX/30s used to test the PEGylated compounds were: 1) PEG-G-CSF analog BBT-015, 9 dose study: 7.76 (LD50/30) & 7.96 Gy (LD70/30); 1 dose study: 7.86 (LD60/30) & 8.10 Gy (LD80/30), 2) PEG-GM-CSF analog BBT-007, 9 dose study: 7.76 (LD50/30) & 7.96 Gy (LD70/30); 3 dose study: 7.92 (LD50/30) & 8.06 Gy (LD70/30), 3) PEG-IL-11 analog BBT-059, 3 dose study: 7.92 & 8.06 Gy; 1 dose study 8.53 (LD50/30) & 8.72 Gy (LD70/30).
Health status monitoring
Irradiated mice were observed for morbidity and mortality twice daily by trained laboratory personnel and scored on a scale of zero to three for signs meeting the criteria for early euthanasia based on three parameters: the severity of hunched posture, squinted/closed eyes, and decreased activity, as previously described (Plett et al. 2012).
Complete blood counts (CBC)
Mice were tail-bled and CBC with differential were performed at baseline and on various days throughout the study. Two randomly selected mice per group in different cages were bled on d-3 to d-1 for a total of 12–14 baseline CBCs. Six to 9 randomly selected mice per group were bled on days 10, 20, 25, and 30 (or d37 for the 1 dose PEG-IL11 study) post-TBI. Time points for CBC were selected baased on previous studies in mice and NHP (Plett et al. 2012, Farese et al. 2013) that show hematopoietic growth factor mitigators do not effect the rate of the initial loss of blood cells nor the nadir, but only the recovery phase. When possible, the same number of males and females in each group and in separate cages were selected for bleeding and each mouse was bled a maximum of two times during the study at least 14 days apart. CBC were also analyzed from 4–6 non-irradiated, age-matched mice at each time point along with the study mice as an internal control. CBC were performed using a validated HEMAVET® 950FS Hematology System (Drew Scientific, Waterbury, CT) at least 10 mins after collection but within 24hrs.
Radiomitigator manufacture
BBT-015 (PEG-G-CSF)
The long-acting human G-CSF analog, PEG-A141C (BBT-015), was created using site-specific PEGylation (Rosendahl et al. 2005). The protein is homogeneously modified with a single branched, large 40 kDa maleimide-PEG at a unique, non-essential position in the protein (amino acid 141 changed from alanine to cysteine). In vitro bioactivity (as measured by EC50s) is within 2-fold of that of G-CSF. BBT-015 has a 10-fold longer circulating half-life than G-CSF following subcutaneous (sc) administration to rats and stimulates greater and longer lasting increases in circulating NE and white blood cells (WBC) than does G-CSF or Amgen’s 20 kDA-PEG-G-CSF product (Neulasta) in rats. BBT-015 results in faster NE recovery than G-CSF or Neulasta in cyclophosphamide (CPA)-treated rats (manuscript in preparation). Human G-CSF is biologically active in mice rats, dogs and non-human primates (NHP) (MacVittie et al. 2005, Drouet et al. 2008).
BBT-007 (PEG-GM-CSF)
The long-acting murine GM-CSF analog PEG-T3C (BBT-007) was created using site-specific PEGylation technology and is the murine homolog of the human GM-CSF (A3C) analog described by Doherty et al. (Doherty et al. 2005). The protein is modified with a single branched 40kDa-maleimide-PEG at amino acid position 3 (threonine changed to cysteine) of the protein chain. The murine GM-CSF protein is used for mouse ARS studies because human GM-CSF is biologically active in dogs and NHP (Mayer et al. 1987, Schuening et al. 1989, Mayer et al. 1990), but not active in rodents. PEG-T3C’s in vitro bioactivity is comparable to that of murine GM-CSF. BBT-007 has a 10-fold longer circulating half-life than muGM-CSF following sc administration to rats, and stimulates greater and longer lasting increases in circulating NE and WBC than does muGM-CSF. BBT-007 administration causes significantly faster NE recovery than muGM-CSF in CPA-treated rats (manuscript in preparation).
BBT-059 (PEG-IL-11)
The long-acting human PEG-IL-11 analog PEG-*179C (BBT-059) was also created using site-specific PEGylation technology. It is modified with a single branched 40 kDa-PEG at a cysteine residue that was added to the C-terminus of the protein (Lee et al. 2012). BBT-059 has an 8- to 10-fold longer circulating half-life than IL-11 following sc or intravenous (iv) administration to rats and a single sc or iv administration stimulates a 30–60% increase in PLT that lasts for over a week in rats. A comparable single injection of IL-11 has no effect (manuscript in preparation). IL-11 requires multiple daily sc injections to stimulate an increase in circulating platelet levels in rats. Human IL-11 is biologically active in mice, rats, dogs and NHP (Yang and Yang 1994, Goldman 1995, Lee et al. 2012).
Radiomitigator administration
The radiomitigators were administered sc post-TBI as described below. Control groups were similarly injected but with vehicle specified below. The first dose was administered no earlier than 24h post-TBI since it would be difficult to deliver medical care to irradiated personnel in a mass causality event earlier than 24hr post-exposure.
BBT-015 (PEG-G-CSF)
Two different administration schedules were tested, 1) 0.1 or 0.3 mg kg−1, one dose at 24+4 hours after TBI, then every other day from d1 to d17 for a total of 9 doses, or 2) 0.3 mg kg−1 or 1.0 mg kg−1, one dose at 24+4 hours after TBI. Vehicle: 10 mM sodium acetate, pH 4.5, 140 mM NaCl solution.
BBT-007 (PEG-GM-CSF)
Two different administration schedules were tested, 1) 0.1 or 0.3 mg kg−1, one dose at 24+4 hours after TBI, then every other day from d1 to d17 for a total of 9 doses, or 2) 0.3 mg kg−1 or 1.0 mg kg−1, one dose at 24+4 hours after TBI, then every other day from d1 to d5 for a total of 3 doses. Vehicle: 10 mM sodium acetate, pH 4.8, 140 mM NaCl solution.
BBT-059 (PEG-IL-11)
Two different administration schedules were tested, 1) 0.3 or 1.0 mg kg−1, one dose at 24+4 hours after TBI, then every other day from d1 to d5 for a total of 3 doses or 2) 0.3 mg kg−1 or 1.0 mg kg−1, one dose at 24+4 hours after TBI. Vehicle: 20 mM Tris, pH 7.5, 200 mM NaCl, 10% glycerol, 0.05% Tween-20 solution.
Study Design, Sample Size, & Statistical analyses
Each cage was randomized to a radiation exposure dose and individual mice were randomized to treatment groups by a study statistician. Studies testing MCM as mitigators were powered to detect a 30% reduction in mortality (i.e. 70% to 40%, 90% to 60%) with 80% power using a two-tailed 5% significance level. The primary outcome, 30-day survival, was examined using logistic regression, which also included sex, radiation dose and interactions of treatment with dose and gender to examine differential effects. Since the randomization was by mouse, “cage effects”, if present, did not bias the results but the model was adjusted using a Generalized Estimating Equations (GEE) method. Secondary analysis included time to death (overall survival), and was examined using a Cox proportional hazards regression model, which was analogous to the logistic regression and included treatment, gender and radiation dose. Mean survival Time (MST) of decedent mice was performed using analysis of variance including radiation dose as a factor. Unpaired Student t-tests were also utilized to compare differences between groups.
CBC data were analyzed using a t-test to compare groups at each time point, and then examined to see if a transformation was necessary to satisfy the assumptions of the t-test before the analysis was implemented. In addition, data for multiple time points were examined together in a two way analysis of variance model with time and treatment as factors. If no treatment by time interaction exists, this analysis has more power than the t-tests. Five mice per group yielded 80% power to detect a 2 standard deviation difference using a two-tailed 5% significance level.
Studies were powered to for the test of the main effect across both irradiation dose groups in the statistical model adjusted for dose. The statistical analysis included tests for interactions between the radiation dose and treatment for survival, MST, or CBC. If a significant interaction (indicating a differential treatment effect for the two radiation doses) was detected, the radiation dose groups would have been analyzed separately. The interactions between radiation dose and treatment for survival, MST or CBC were not observed in any these studies and therefore the results for overall tests are presented.
RESULTS
Survival of irradiated mice after BBT-015 (PEG-G-CSF) administration
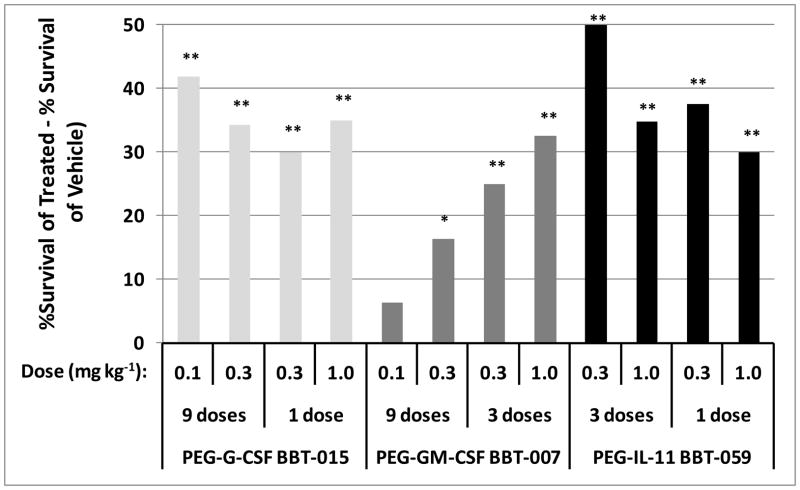
The primary endpoint in survival studies utilizing the mouse H-ARS model is to assess survival efficacy at day 30 of the candidate MCM. Fig. 1 shows a summary of all the survival studies conducted to date using BBT PEG-HGF molecules. For ease of presentation, day 30 survival in each study is presented as the difference in survival of the PEG-HGF treated group to that of the vehicle group (i.e., %survival of treated - % survival of vehicle). Results show that 9 doses of PEG-G-CSF (0.1mg kg−1 or 0.3mg kg−1 every other day from d1 to d17 for 9 doses) significantly enhanced survival by d30 compared to vehicle-treated mice (30d survival = 70%, 63%, and 28%, respectively, p<0.001, Fig. 1). In the next study, one dose of PEG-G-CSF at 0.3mg kg−1 or 1.0mg kg−1 administered 24+4 hours post-irradiation also significantly increased 30 day survival compared to controls (30d survival = 95%, 100%, and 65%, respectively, p≤0.001, Fig. 1), illustrating similar survival efficacy of 1 dose of PEG-G-CSF as 9 doses. In addition, 0.3mg kg−1 appears to provide similar survival efficacy as that of a 3.3-fold higher dose (1.0mg kg−1), suggesting a near maximal response at the lower dosage.
Fig. 1.
Survival of irradiated mice after PEG-HGF administration. Mice were exposed to radiation doses from 7.76 to 8.72 Gy and injected subcutaneously with either 0.1 mg kg−1 day−1, 0.3 mg kg−1 day−1, or 1.0 mg kg−1 day−1 PEG-G-CSF (BBT-015), PEG-GM-CSF (BBT-007), or PEG-IL-11 (BBT-059) 24+4 hours (1 dose), every other day from days 1 to 5 (3 doses), or every other day from day 1 to 17 (9 doses) post irradiation. Control mice were similarly injected with vehicle. Bars in Fig. 1 shows the percentage of PEG-HGF-treated mice that survived above vehicle-treated mice (% survival of treated mice minus % survival of control mice). Thirty day survival was significantly increased for all PEG-HGF-treated mice compared to vehicle treated mice, except for 0.1 mg/kg PEG-GM-CSF-treated mice (*p≤0.05, **p≤0.006, n=39–40 mice per group in each study).
The MST of PEG-G-CSF-treated mice was significantly decreased by 4–7 days compared to the MST of vehicle-treated mice (Table 1, p=0.04). The shortened MST in the PEG-G-CSF-treated mice reflects the finding that onset of death in each group occurs at approximately the same time (day 8 in this study), but deaths in vehicle-treated mice continued to day 25, while the last death in the PEG-G-CSF group was on day 15. Since MST is calculated only from decedent mice, MST of groups where mice continue to die until late time points will be increased.
Table 1.
Mean Survival Time (MST) of decedent mice
| Growth factor | Dose (mg kg-1 day-1) | MSTa (Days±SD) | # of Decedent miceb | Total Miceb |
|---|---|---|---|---|
| 0.1 | 11.3 ± 0.4 | 12 | 40 | |
| PEG-G-CSF BBT-015 | 0.3 | 14.0 ± 4.1 | 17 | 80 |
| 1.0 | NDc | 0 | 40 | |
| Vehicle | 18.5 ± 3.5d | 42 | 79 | |
| PEG-GM-CSF BBT-007 | 0.1 | 18.6 ± 1.6 | 8 | 40 |
| 0.3 | 18.6 ± 2.8 | 8 | 80 | |
| 1.0 | 18.0 | 1 | 40 | |
| Vehicle | 19.7 ± 3.1 | 24 | 78 | |
| PEG-IL-11 BBT-059 | 0.3 | 16.1 ± 4.1 | 6 | 120 |
| 1.0 | 13.1 ± 2.7 | 10 | 79 | |
| Vehicle | 18.9 ± 2.4e | 62 | 120 |
Average of both studies for each growth factor at this dose: 9 and 1 dose for PEG-G-CSF (BBT-015); 9 and 3 dose for PEG-GM-CSF (BBT-007); 3 and 1 dose for PEG-IL-11 (BBT-059).
Numbers reflect the sum from both studies for each growth factor at this dose as described in a above.
ND: No deaths in this group.
Significantly increased, when compared to all PEG-G-CSF-treated groups (p=0.04).
Significantly increased, when compared to all PEG-IL-11-treated groups (p=0.03).
“Overall Survival” is another parameter that reflects the amount of time that mice in a given group are alive, but unlike MST, Overall Survival is calculated using both live and decedent mice. In the current study, Overall Survival of PEG-G-CSF-treated mice was significantly increased compared to vehicle controls (p<0.02), illustrating the overall survival benefit of PEG-G-CSF.
Variability in expected and actual survival
The variability of expected from actual survival in our studies was 20.1±12.8% across all studies ranging from 20% greater lethality to 44% less lethality than expected. This result was somewhat expected, given the steep dose response curves of inbred mice (Grahn and Hamilton 1957), compared to other species (Vriesendorp and Van Bekkum 1984). Our previously published results in C57BL/6 mice (Plett et al. 2012) confirmed the steep dose response curve compared to NHP (Farese et al. 2012). Given the difficulty pinpointing the LDXX/30 in light of the small radiation dose range between LD10/30 and LD90/30, the authors’ lab attempted to control all possible variables including room humidity and temperature, cage location on the rack, light/dark cycles, caging, bedding, feed, vendor and barrier selection. In addition, the radiation dose/lethality response curve was performed approximately annually to adjust for any variation in the radiation response of the C57BL/6 mice used in our experiments, and doses adjusted accordingly in subsequent studies.
CBC profiles of irradiated mice after BBT-015 (PEG-G-CSF) administration
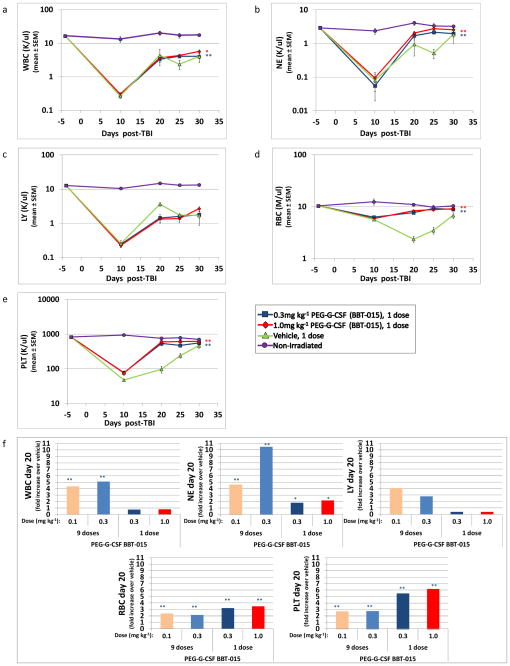
CBC values of the irradiated and treated mice were assessed on day 10 (during nadir, except for RBC, which continued to decrease until d20) shown here and in previous studies (Plett et al. 2012), and days 20, 25, and 30 post-TBI to determine whether increased survival correlated with enhanced hematopoietic cell recovery. While the nadir of all hematopoietic cells at d10 post-TBI was similar for both PEG-G-CSF-treated and untreated mice, recovery of NE, WBC, red blood cells (RBC) and PLT was significantly accelerated in mice treated with one dose of PEG-G-CSF, compared to vehicle control (Fig. 2a–e, p≤0.05). These differences were particularly evident for most CBC parameters at d20 post-TBI, and are highlighted in Fig. 2f, which shows the fold increase of WBC, NE, LY, RBC, and PLT levels in PEG-G-CSF-treated over vehicle-treated mice (p≤0.05). LY recovery was not significantly different in treated and untreated mice in the 9 or 1 dose experiments (Fig. 2c and 2f). Similar CBC results were obtained in the one and nine dose (data not shown), PEG-G-CSF studies. These results suggest a predominant effect of PEG-G-CSF on cells of the myeloid, erythroid and megakaryocytic lineage.
Fig. 2.
Hematopoietic cell recovery in irradiated mice after PEG-G-CSF (BBT-015) administration. C57BL/6 mice were irradiated with 7.86 or 8.10 Gy and treated with 0.3mg kg−1 or 1.0mg kg−1 given once 24+4 hours post irradiation (panels a-e). Mice were assessed for peripheral blood cells using a validated HEMAVET® 950FS Hematology System pre-irradiation and post-irradiation, at time points indicated in the graphs showing mean±SEM of WBC (panel a), NE (panel b), LY (panel c), RBC (panel d) and PLT (panel e). CBC profiles show a significant increase in overall recovery of WBC, NE, RBC, and PLT in PEG-G-CSF-treated mice when compared to vehicle-treated mice (**p≤0.006, n=12–13 mice in baseline time point, n=6–8 mice per post-irradiation time point, n=4–6 mice in non-irradiated group, red asterisks represent significant differences for 0.3mg kg−1 dose and blue asterisks for 1.0mg kg−1 dose). Panel f shows the fold increase of all CBC parameters in PEG-G-CSF-treated over vehicle-treated mice on d20 for both the 9 dose and 1 dose studies. Significant increases over vehicle control were calculated from the raw CBC data and are indicated above the bar. (*p≤0.05, **p≤0.006, n=8, for panel f, 1 equals no change).
Survival of irradiated mice after BBT-007 (PEG-GM-CSF) administration
As observed with PEG-G-CSF, PEG-GM-CSF-treated mice receiving 0.3mg kg−1 every other day from day one to d17, for a total of 9 doses, also exhibited significantly increased survival (90%) at d30 post lethal irradiation compared with vehicle-treated controls (74%, p≤0.05, Fig. 1). While 80% of mice treated with 9 doses of 0.1mg kg−1 survived (6% more compared to vehicle controls), this did not reach statistical significance (Fig. 1). A further study showed that just three doses of PEG GM-CSF (0.3mg kg−1 or 1.0mg kg−1) given 24+4 hours, d3, and d5 post irradiation resulted in significantly increased survival of 25–33% more mice than vehicle-treated mice (90%, 98%, and 65%, respectively, p≤0.006, Fig. 1).
Onset and last day of death was similar between the 2 groups (12–22 days and 7–26 days for PEG-GM-CSF and vehicle-treated mice, respectively), resulting in similar MST between PEG-GM-CSF-treated and vehicle groups (Table 1). Overall survival, on the other hand, was significantly increased in PEG-GM-CSF mice (p<0.04), illustrating the significantly lower lethality in mice treated with PEG-GM-CSF.
CBC profiles of irradiated mice after BBT-007 (PEG-GM-CSF) administration
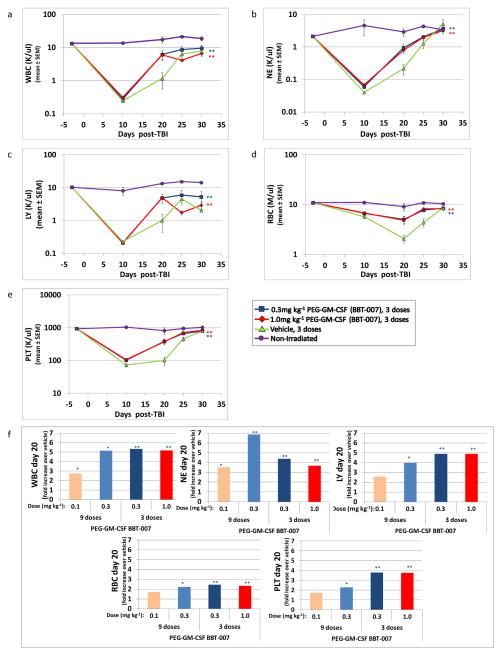
The significantly increased 30 day survival of PEG-GM-CSF-treated mice (3 doses of 0.3mg kg−1 or 1.0mg kg−1) was correlated with significantly increased kinetics of recovery of WBC, NE, LY, RBC, and PLT compared to controls (p≤0.006, Fig. 3a–e). Most dramatic differences in recovery of these five CBC parameters occurred on d20 post TBI, and are plotted as fold increase over controls in Fig. 3f (p≤0.05). As seen with PEG-G-CSF, the nadir of hematopoietic cells (except RBC) at d10 post-irradiation was similar for both PEG-GM-CSF treated and untreated mice, indicating that treatment with PEG-GM-CSF did not prevent the loss of hematopoietic cells early post-TBI but was effective in promoting faster recovery. The decline in the RBCs until d20 post-TBI was significantly more pronounced in the vehicle-treated compared to the PEG-GM-CSF-treated groups. Similar CBC results were observed in the three and nine dose (data not shown) PEG-GM-CSF studies. These results document the efficacy of PEG-GM-CSF to enhance recovery of all hematopoietic lineages in this murine model of HARS.
Fig. 3.
Hematopoietic cell recovery in irradiated mice after PEG-GM-CSF (BBT-007) administration. C57BL/6 mice were irradiated with 7.92 or 8.06 Gy and treated with 0.3mg kg−1 or 1.0mg kg−1 given on days 1, 3, and 5 post irradiation (panels a-e). Mice were assessed for peripheral blood cells using a validated HEMAVET® 950FS Hematology System pre-irradiation and post-irradiation, at time points indicated in the graph showing mean±SEM of WBC (panel a), NE (panel b), LY (panel c), RBC (panel d) and PLT (panel e). CBC profiles show a significant increase in overall recovery of WBC, NE, LY, RBC, PLT in PEG-GM-CSF-treated mice when compared to vehicle-treated mice (*p≤0.05, **p≤0.006, n=12 mice in baseline time point, n=7–8 mice per post-irradiation time point, n=4 mice in non-irradiated group; red asterisks represent significant differences for 0.3mg kg−1 dose and blue asterisks for 1.0mg kg−1 dose). Panel f shows fold increase of all CBC parameters in PEG-GM-CSF-treated over vehicle-treated mice for both the 9 dose and 3 dose studies on day 20. Significant increases over vehicle control were calculated from the raw CBC data and are indicated above the bars (*p≤0.05, **p≤0.006, n=8 for panel f, 1 equals no change).
Survival of irradiated mice after BBT-059 (PEG-IL-11) administration
IL-11 is a cytokine biologically active in a variety of non-hematopoietic and hematopoietic cells, particularly megakaryocytes and platelets, and is currently used for treatment of thrombocytopenia in cancer patients (Kaye 1998, Schwertschlag et al. 1999, Reynolds 2000). In the current studies, 3 doses of 0.3mg kg−1 or 1.0mg kg−1 PEG-IL-11 on days 1, 3, and 5 were effective in significantly increasing 30 day survival compared to controls (98%, 92%, and 48%, respectively, p≤0.006, Fig. 1). As observed with PEG-G-CSF, administration of just one dose of PEG-IL-11 (0.3mg kg−1 or 1.0mg kg−1 at 24+4 hours post irradiation) also significantly increased 30 day survival compared to vehicle controls (90%, 83%, and 53%, respectively, p≤0.006, Fig. 1). The lower survival of mice treated with 1.0mg kg−1 PEG-IL-11 prompted the authors to consider that this dose might be somewhat toxic compared to 0.3mg kg−1, so total moribund scores over 30 days were compared, but no differences were noted (data not shown).
Similar to studies with PEG-G-CSF, the MST of PEG-IL-11-treated mice was decreased compared to vehicle-treated mice, albeit by fewer days. Whereas MST of PEG-G-CSF mice was ~6 days less than controls, MST of PEG-IL-11-treated mice was 3–4 days less than controls (Table 1, p=0.03 for the 1.0mg kg−1 group). The shortened MST in PEG-IL-11-treated mice, similar to that of PEG-G-CSF-treated mice, resulted from the fact that vehicle-treated mice continued to die to day 25, while the last death in the PEG-IL-11 group was day 18.
Overall Survival was significantly increased in the PEG-IL-11 studies (p<0.001), again reflecting the overall survival benefit of PEG-IL-11 as a radiomitigator in H-ARS.
CBC profiles of irradiated mice after BBT-059 (PEG-IL-11) administration
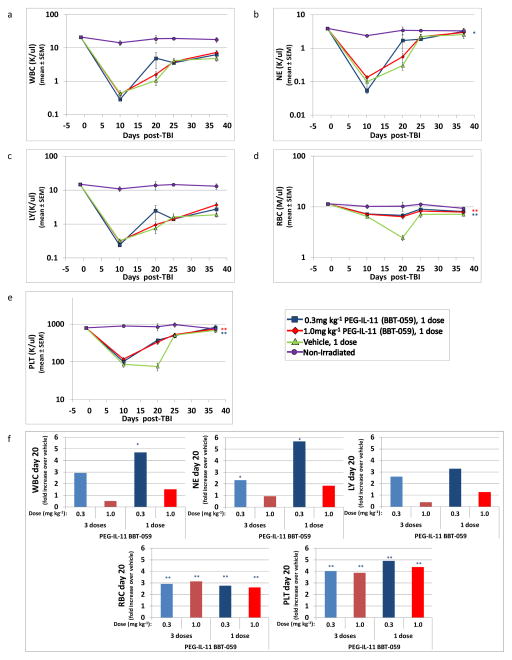
As anticipated from its known effects on hematopoiesis, the primary effect of PEG-IL-11 on hematopoietic cell recovery was on platelets, as previously reported in mice (Du et al. 1993, Neben et al. 1993) and in NHP (Schlerman et al. 1996), although RBC recovery was also significantly increased in PEG-IL-11-treated mice (Figs. 4d and 4e, 1 dose of 0.3mg kg−1 or 1.0mg kg−1 24+4 hours post-irradiation, **p≤0.006). Enhanced neutrophil recovery was only evident in mice treated with 0.3mg kg−1 PEG-IL-11 (Fig. 4b; *p≤0.05). Similar to recovery of CBC parameters with PEG-G-CSF and PEG-GM-CSF, the most dramatic changes in CBC parameters between PEG-IL-11 and vehicle-treated mice occurred on day 20, and are illustrated as fold change on fig. 4f (p≤0.05). Similar CBC results were observed in the three (data not shown) and one dose PEG-IL-11-CSF studies. Also similar to PEG-G-CSF and PEG-GM-CSF, PEG-IL-11 had no effect on the nadir of WBC, NE, and LY, while the decline in the RBCs and PLT up to d20 post-TBI was significantly greater in the vehicle-treated compared to the PEG-GM-CSF-treated groups.
Fig. 4.
Hematopoietic cell recovery in irradiated mice after PEG-IL-11 (BBT-059) administration. C57BL/6 mice were irradiated with 8.53 or 8.72 Gy and treated with 0.3mg kg−1 or 1.0mg kg−1 given once 24+4 hours post irradiation (panels a-e). Mice were assessed for peripheral blood cells using a validated HEMAVET® 950FS Hematology System pre-irradiation and post-irradiation, at time points indicated in the graph showing mean±SEM of WBC (panel a), NE (panel b), LY (panel c), RBC (panel d) and PLT (panel e). CBC profiles show a significant increase in overall recovery of NE, RBC and PLT in PEG-IL-11-treated mice, when compared to vehicle-treated mice (*p≤0.05, **p≤0.006, n=12–14 mice in baseline time point, n=7–9 mice/post-irradiation time point, n=4 mice in non-irradiated group; red asterisks represent significant differences for 0.3mg kg−1 dose and blue asterisks for 1.0mg kg−1 dose). Panel f shows the fold increase of all CBC parameters in PEG-IL-11-treated over vehicle-treated mice on d20 for both the 3 dose and 1 dose studies. Significant increases over vehicle control were calculated from the raw CBC data and are indicated above the bars (*p≤0.05, **p≤0.006, n=7–9 mice for panel f, 1 equals no change).
DISCUSSION
These results demonstrate the ability of Bolder Biotechnology’s PEGylated G-CSF, PEGylated GM-CSF, and PEGylated IL-11 to significantly enhance survival up to 100% in lethally-irradiated mice treated with only 1 dose (PEG-G-CSF and PEG-IL-11) or 3 doses (PEG-GM-CSF) by 24–28hr post-exposure. Survival enhancement seen with 1 to 3 doses of these PEGylated cytokines is similar to that obtained with 16–23 daily doses of non-PEGylated G-CSF (MacVittie et al. 2005, Plett et al. 2012) Few studies have demonstrated significant survival efficacy after just 1–2 injections of non-PEGylated HGF, but only when administered within hours of radiation exposure and only if delivered in combination with other growth factors (Van der Meeren et al. 2002, Drouet et al. 2004, Van der Meeren et al. 2004, Herodin et al. 2007). Such administration protocols are challenging logistically, practically, and from a regulatory viewpoint since multiple MCM would likely be represented by different companies. Single administration of MCM with a broad safety window are most desirable for use in a radiation event.
The initial dosing regimen of 9 every other day sc injections (days 1–17) of PEG G-CSF BBT-015 or PEG-muGM-CSF BBT-007 was chosen based partly on the authors’ (Plett et al. 2012) and other’s previous studies (Doherty et al. 2005, Rosendahl et al. 2005), showing that daily dosing of G-CSF from day 1–16 post-TBI significantly improved survival in the mouse HARS model. Dosing until day 16 or 17 ensures that mice receive several doses of the drug past the neutrophil nadir, which occurs around day 10 in the mouse H-ARS model. The authors’ used an every other day dosing regimen rather than a daily dosing regimen for BBT-015 and muBBT-007 because of the longer in vivo half-lives of the proteins relative to G-CSF and muGM-CSF [(Rosendahl et al. 2005) and unpublished results]. G-CSF and GM-CSF typically are administered to cancer patients with chemotherapy-induced neutropenia for several days after the neutrophil nadir. The second BBT-015 study used a single injection of BBT-015 administered 24h post-TBI based on the authors’ unpublished studies showing that a single administration of BBT-015 was sufficient to accelerate neutrophil recovery in a rat chemotherapy-induced neutropenia model. The second muBBT-007 study used 3 every other day injections of muBBT-007 (dosing on days 1, 3 and 5), also based on the authors’ unpublished data showing efficacy to increase neutrophils in a rat chemotherapy-induced neutropenia model for a related PEGylated muGM-CSF analog. A future study will test whether a single dose of muBBT-007 administered 24h post-TBI will improve survival in the mouse HARS model.
Van der Meeren et al. (2002) reported that daily dosing of IL-11 for 5 days beginning 2 h post-TBI significantly improved survival of lethally irradiated mice. The first mouse H-ARS study with PEG IL-11 BBT-059 mimicked this study by dosing the drug for 5 days post-TBI. An every other day dosing regimen rather than a daily dosing regimen was used for BBT-059 because of the longer in vivo half-life of BBT-059 compared to IL-11 (unpublished results). The second BBT-059 ARS study compared 3 every other day doses (dosing on days 1, 3 and 5) with a single dose administered on day 1. A single dose of BBT-059 was tested based on the authors’ unpublished data showing that a single injection of PEG IL-11 BBT-059, but not IL-11, stimulated an increase in circulating platelets that lasted for 7–10 days in rats.
The potential of combination therapy with different MCM to target H-ARS from several mechanistic angles will likely lead to strategies with improved efficacy compared to single administration strategies. In this regard, combination therapy with Bolder Biotechnology’s PEGylated MCM remains an exciting possibility from both a scientific and therapeutic view, yet to be explored.
The similarly enhanced survival seen in mice treated with the 0.3mg kg−1 and 1.0mg kg−1 doses of the three growth factors suggests that the lower 0.3mg kg−1 dose may approach maximal effectiveness. Only IL-11 showed a slight decrease in survival at the higher dose, suggesting possible detrimental effects of the higher dose compared to the lower dose. The authors did not observe higher morbidity in the 1.0mg kg−1 group compared with the 0.3mg kg−1 group, but morbidity is an indirect way of assessing toxicity. It would be useful to include more sensitive toxicity assessments in future studies of the 1.0mg kg−1 PEG-IL-11 dose. While these studies were not powered sufficiently to detect small survival differences between HGF-treatment groups, in the case of PEG-GM-CSF, the higher dose seemed to provide a moderately greater survival benefit than the lower doses studied. On the other hand, being able to utilize a lower dose may prove beneficial when combination therapies of these three growth factors are explored, and to prevent potential side effects seen with G-CSF, GM-CSF and IL-11 drugs, including bone pain, various types of edema, splenic rupture (in non myeloablated patients), or capillary leaking (Wadhwa and Thorpe 2008). Neutralizing antibody formation by the host can be a concern in patients with intact immune systems, but is likely not an issue in the lethally-irradiated population. Regardless, dosing for longer periods of time could increase the chances of blocking antibodies developing, which would result in inactivation of the therapeutic agent. Repeated dosing also requires repeated handling of subjects, which, at least in the mouse H-ARS model, has detrimental effects on survival (Plett et al. 2012)
An additional problem in a mass casualty event is the lack of reliable dosimetry. Current biodosimetric methods are time-consuming and imprecise, and may not provide accurate biological dose-estimations within a logistically- or medically-feasible time frame. This becomes important, as the biodosimetry would direct the type of therapy that would be appropriate. With respect to cytokine therapy, it may be safer to administer a single dose of a PEG-HGF, shown here to be effective in mice, to individuals where the radiation exposure level was unknown or even zero, rather than multiple daily doses which could result in side effects such splenic rupture (Masood et al. 2008). Treatment of healthy individuals with a PEGylated HGF has its own potential side-effect issues, as it is difficult to “undo” the long half-life imparted by the PEG moiety. Although PEGylated cytokines have increased half-lives, single administrations at lower doses would be less likely to induce side effects, while being sufficient to effectively treat H-ARS symptoms. Single dose administration would also allow quicker discharge of patients not requiring hospitalization or monitoring, but where treatment was prescribed.
The enhanced survival observed in these studies with each of the three PEG-HGF was strongly correlated with significantly faster recovery of CBC parameters. In addition to its expected stimulation of neutrophil recovery (Demetri 1992), PEG-G-CSF also significantly increased recovery of WBC, RBC, and platelets, similar to previous reports (Shimoda et al. 1993, Franzke et al. 2003). The primary targets of GM-CSF are granulocytes and monocytes, yet PEG-GM-CSF significantly increased recovery of not only neutrophils, but also all other hematopoietic cell types examined. PEG-GM-CSF was the only HGF in the current study to significantly enhance recovery of lymphocytes, illustrating its broad spectrum of hematopoietic activity. While PEG-IL-11 predictably increased recovery of its primary target, platelets, it also enhanced recovery of RBC and in some studies, neutrophils as well. These data are in agreement with others that documented stimulatory effects of IL-11 on erythropoiesis, lymphopoiesis and myelopoiesis (Wadhwa and Thorpe 2008).
The authors previously reported (Plett et al. 2012) that stress effects from frequent blood sampling for CBC (once every 5 days) and/or handling, significantly increased mortality of lethally irradiated mice. Therefore, in the current studies, each mouse was bled a maximum of two times during the study and each bleed was 14 days apart. It is not possible to know in the current design whether survival was negatively impacted by even the two bleeds, but since the control groups were bled on the same schedule, all groups should have been similarly affected. Given the greater survival in treated groups, there does not appear to be a detrimental effect on survival.
Despite the belief that the primary cause of death from H-ARS is infection and hemorrhage due to neutropenia and thrombocytopenia, the general thought when considering mechanisms for HGF-mediated survival from H-ARS is that of increased recovery of neutrophils. In this regard, the authors’ data with PEG-IL-11 is of particular interest since these data showed strong correlation of survival with significantly enhanced recovery of primarily platelets and RBC, with a variable response of neutrophils. These data suggest that reversal of thrombocytopenia, and possibly anemia, with little or moderate recovery of neutropenia may be sufficient to significantly increase survival from H-ARS.
These findings again highlight the potential benefit of a multi-pronged approach to HARS mitigation with combination therapy. Enhancing the recovery of different hematopoietic cell lineages through the use of specific HGF targeting different cell populations will undoubtedly result in enhanced survival from H-ARS and likely push the LD50 to much higher levels. Whether these PEG-HGF function by directly stimulating the few committed hematopoietic stem and/or progenitors remaining after lethal radiation exposure, or rather by indirect effects on the hematopoietic stem cell niche or other cells, remains to be determined. Elucidation of these direct or indirect effects will need to be addressed in future studies for complete understanding of how these MCMs are acting to increase survival and accelerate recovery of multiple hematopoietic cell lineages. Future studies will also examine the possible complementarity of these cytokines for increasing the recovery of all hematopoietic lineages.
Another parameter assessed in these studies is the mean survival time (MST) of decedents, or time to death. MST is commonly viewed as the “window of opportunity”, where MCM are working to effect survival. Our studies show that the MST of both PEG-G-CSF and PEG-IL-11 were significantly shorter compared to vehicle control. This finding reflects the fact that while onset of death in both groups commences within the first two weeks after irradiation, mice in the control group continue to die throughout the 30day time period, while PEG-HGF-treated mice rarely expire beyond 2 weeks post-exposure.
The authors have shown that repopulation in the bone marrow begins within one week post-TBI (Plett et al. 2012), which precedes recovery of peripheral blood cells. One mechanism whereby PEG-HGF may exert their radiation-mitigating effects is by enhancing survival of HSC or HPC in the marrow early after radiation exposure, resulting in increased numbers of these important cells and more rapid expansion of blood cells during the recovery phase. If MCM are administered too late post-exposure or not at all, HSC and HPC likely undergo radiation-induced apoptosis due to direct effects of radiation, and/or the noxious post-radiation microenvironment and high levels of circulating free radicals. Loss of HSC and HPC results in a reduction of the available progenitor pool for blood cell production, thus increasing the chances of opportunistic infection, hemorrhage, and death. PEG-HGF also may exert their radiation-mitigating effects by stimulating expansion of lineage-specific progeny of surviving HSC/HPC to accelerate recovery of the blood cell pool. Continued presence of HGF post-TBI (through repeated injections of non-PEG-HGF or via prolonged half-life of PEG-HGF) could potentially provide a dual benefit by both improving survival of HSC and HPC and by stimulating expansion of lineage-specific progeny of the rescued HSC/HPC. The observation that different PEG-HGFs affect recovery of different hematopoietic cell lineages to different extents suggests that the proteins act on distinct classes of HSC and HPC populations. This finding suggests that combination therapy with PEG-HGFs targeting different cell populations could have additive or synergistic effects on survival and hematopoietic recovery cell following radiation exposure. Future studies will test this possibility.
Increasing the hematopoietic cell recovery in addition to antibiotic supportive care would likely decrease the effect of bacterial infection either by translocation from radiation-induced intestinal damage or from exterior sources and possible wounds inflicted by the mass casualty event. Adding these cytokines to the care of severely irradiated individuals should be able to not only increase survival, but also decrease morbidity and potentially reduce the need for prolonged hospitalization. In addition, it is hoped that HGF therapy for H-ARS may also result in long-term benefits by reducing some of the deleterious effects of residual bone marrow damage (RBMD) to the hematopoietic system, previously described in survivors of this murine H-ARS model (Chua et al. 2012). Future studies will examine the benefit of these PEG-HGF MCM on RBMD.
One aspect of this model that required continual modification, as noted by the varied radiation doses utilized in the studies presented here, was the changing level of lethality in the C57BL/6 mice with specific radiation doses. This problem was addressed by conducting dose response curve experiments approximately annually to adjust the doses that would result in the desired lethality, further refining our mouse model of H-ARS.
CONCLUSIONS
These results demonstrate that an animal H-ARS model such as the one developed in C57BL/6 mice (Plett et al 2012) can be implemented successfully to assess MCM effectiveness for enhancing survival, and also for assessing possible MCM mechanism of action on hematopoietic cells. We have shown that the three PEGylated HGFs assessed in these studies, PEG-G-CSF, PEG-GM-CSF, and PEG-IL-11 all significantly increased survival with only one dose of 0.3mg kg−1 for PEG-G-CSF and PEG-IL-11, or as few as three doses of 0.3mg kg−1 for PEG-GM-CSF. All three cytokines showed significantly enhanced recovery of RBC and PLT, with the granulopoietic cytokines enhancing recovery of WBC and NE as well. These results validate the C57BL/6 mouse H-ARS model as a useful screening tool for assessing efficacy of potential MCM, and bringing to FDA approval these and other potential radiation MCMs.
Acknowledgments
Funding: This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Grant No. 1R43 AI084301, 1R43 AI084288, and 1R43 AI088928. We acknowledge the valuable discussion, insight, and constructive critique of our UMB and NIAID colleagues, Tom MacVittie, Ann Farese, David Cassatt, and Bert Maidment who were instrumental in our model development. The publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, or Department of Health and Human Services.
References
- Abuchowski A, Kazo GM, Verhoest CR, Jr, Van Es T, Kafkewitz D, Nucci ML, Viau AT, Davis FF. Cancer therapy with chemically modified enzymes. I. Antitumor properties of polyethylene glycol-asparaginase conjugates. Cancer Biochem Biophys. 1984;7:175–86. [PubMed] [Google Scholar]
- Anno GH, Young RW, Bloom RM, Mercier JR. Dose response relationships for acute ionizing-radiation lethality. Health Phys. 2003;84:565–75. doi: 10.1097/00004032-200305000-00001. [DOI] [PubMed] [Google Scholar]
- Bailon P, Palleroni A, Schaffer CA, Spence CL, Fung WJ, Porter JE, Ehrlich GK, Pan W, Xu ZX, Modi MW, Farid A, Berthold W, Graves M. Rational design of a potent, long-lasting form of interferon: A 40 kda branched polyethylene glycol-conjugated interferon alpha-2a for the treatment of hepatitis c. Bioconjug Chem. 2001;12:195–202. doi: 10.1021/bc000082g. [DOI] [PubMed] [Google Scholar]
- Bishop MR, Tarantolo SR, Geller RB, Lynch JC, Bierman PJ, Pavletic ZS, Vose JM, Kruse S, Dix SP, Morris ME, Armitage JO, Kessinger A. A randomized, double-blind trial of filgrastim (granulocyte colony-stimulating factor) versus placebo following allogeneic blood stem cell transplantation. Blood. 2000;96:80–5. [PubMed] [Google Scholar]
- Casarett A. Radiation biology. Englewood. New Jersey: Prentice-Hall Inc; 1968. [Google Scholar]
- Chua HL, Plett PA, Sampson CH, Joshi M, Tabbey R, Katz BP, MacVittie TJ, Orschell CM. Long-term hematopoietic stem cell damage in a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2012;103:356–66. doi: 10.1097/HP.0b013e3182666d6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30:513. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- Demetri GD. Hematopoietic growth factors: Current knowledge, future prospects. Current Problems in Cancer. 1992;16:177–259. [PubMed] [Google Scholar]
- Doherty DH, Rosendahl MS, Smith DJ, Hughes JM, Chlipala EA, Cox GN. Site-specific pegylation of engineered cysteine analogues of recombinant human granulocyte-macrophage colony-stimulating factor. Bioconjug Chem. 2005;16:1291–8. doi: 10.1021/bc050172r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouet M, Delaunay C, Grenier N, Garrigou P, Mayol JF, Herodin F. Cytokines in combination to treat radiation-induced myelosuppresssion: Evaluation of scf + glycosylated epo + pegylated g-csf as an emergency treatment in highly irradiated monkeys. Haematologica. 2008;93:465–6. doi: 10.3324/haematol.12183. [DOI] [PubMed] [Google Scholar]
- Drouet M, Mourcin F, Grenier N, Leroux V, Denis J, Mayol JF, Thullier P, Lataillade JJ, Herodin F. Single administration of stem cell factor, flt-3 ligand, megakaryocyte growth and development factor, and interleukin-3 in combination soon after irradiation prevents nonhuman primates from myelosuppression: Long-term follow-up of hematopoiesis. Blood. 2004;103:878–85. doi: 10.1182/blood-2003-05-1400. [DOI] [PubMed] [Google Scholar]
- Du XX, Neben T, Goldman S, Williams DA. Effects of recombinant human interleukin-11 on hematopoietic reconstitution in transplant mice: Acceleration of recovery of peripheral blood neutrophils and platelets. Blood. 1993;81:27–34. [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Katz BP, Smith CP, Gibbs A, Cohen DM, MacVittie TJ. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiation Research. 2013;179:89–100. doi: 10.1667/RR3049.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Katz BP, Smith CP, Jackson W, 3rd, Cohen DM, MacVittie TJ. A nonhuman primate model of the hematopoietic acute radiation syndrome plus medical management. Health Phys. 2012;103:367–82. doi: 10.1097/HP.0b013e31825f75a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzke A, Piao W, Lauber J, Gatzlaff P, Konecke C, Hansen W, Schmitt-Thomsen A, Hertenstein B, Buer J, Ganser A. G-csf as immune regulator in t cells expressing the g-csf receptor: Implications for transplantation and autoimmune diseases. Blood. 2003;102:734–9. doi: 10.1182/blood-2002-04-1200. [DOI] [PubMed] [Google Scholar]
- Goldman SJ. Preclinical biology of interleukin 11: A multifunctional hematopoietic cytokine with potent thrombopoietic activity. Stem Cells. 1995;13:462–71. doi: 10.1002/stem.5530130503. [DOI] [PubMed] [Google Scholar]
- Grahn D. Acute radiation response of mice from a cross between radiosensitive and radioresistant strains. Genetics. 1958;43:835–843. doi: 10.1093/genetics/43.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn D, Hamilton K. Genetic variation in the acute lethal response of four inbred mouse strains to whole body x-irradiation. Genetics. 1957;42:189–198. doi: 10.1093/genetics/42.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herodin F, Grenier N, Drouet M. Revisiting therapeutic strategies in radiation casualties. Exp Hematol. 2007;35:28–33. doi: 10.1016/j.exphem.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Kaye JA. Fda licensure of neumega to prevent severe chemotherapy-induced thrombocytopenia. Stem Cells. 1998;16(Suppl 2):207–23. doi: 10.1002/stem.5530160724. [DOI] [PubMed] [Google Scholar]
- Knauf MJ, Bell DP, Hirtzer P, Luo ZP, Young JD, Katre NV. Relationship of effective molecular size to systemic clearance in rats of recombinant interleukin-2 chemically modified with water-soluble polymers. J Biol Chem. 1988;263:15064–70. [PubMed] [Google Scholar]
- Lee HT, Park SW, Kim M, Ham A, Anderson LJ, Brown KM, D’Agati VD, Cox GN. Interleukin-11 protects against renal ischemia and reperfusion injury. Am J Physiol Renal Physiol. 2012;303:F1216–24. doi: 10.1152/ajprenal.00220.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushbaugh C. Reflections on some recent progress in human radiobiology. In: Augenstein L, Mason R, Zelles M, editors. Advances in radiation biology. New York, NY: Academic Press; 1969. p. 277. [Google Scholar]
- MacVittie TJ, Farese AM, Jackson W., 3rd Defining the full therapeutic potential of recombinant growth factors in the post radiation-accident environment: The effect of supportive care plus administration of g-csf. Health Phys. 2005;89:546–55. doi: 10.1097/01.hp.0000173143.69659.5b. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ, Monroy RL, Patchen ML, Souza LM. Therapeutic use of recombinant human g-csf (rhg-csf) in a canine model of sublethal and lethal whole-body irradiation. International Journal of Radiation Biology. 1990;57:723–36. doi: 10.1080/09553009014550891. [DOI] [PubMed] [Google Scholar]
- Masood N, Shaikh AJ, Memon WA, Idress R. Splenic rupture, secondary to g-csf use for chemotherapy induced neutropenia: A case report and review of literature. Cases J. 2008;1:418. doi: 10.1186/1757-1626-1-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer P, Lam C, Obenaus H, Liehl E, Besemer J. Recombinant human gm-csf induces leukocytosis and activates peripheral blood polymorphonuclear neutrophils in nonhuman primates. Blood. 1987;70:206–13. [PubMed] [Google Scholar]
- Mayer P, Werner FJ, Lam C, Besemer J. In vitro and in vivo activity of human recombinant granulocyte-macrophage colony-stimulating factor in dogs. Exp Hematol. 1990;18:1026–33. [PubMed] [Google Scholar]
- Molineux G, Kinstler O, Briddell B, Hartley C, McElroy P, Kerzic P, Sutherland W, Stoney G, Kern B, Fletcher FA, Cohen A, Korach E, Ulich T, McNiece I, Lockbaum P, Miller-Messana MA, Gardner S, Hunt T, Schwab G. A new form of filgrastim with sustained duration in vivo and enhanced ability to mobilize pbpc in both mice and humans. Exp Hematol. 1999;27:1724–34. doi: 10.1016/s0301-472x(99)00112-5. [DOI] [PubMed] [Google Scholar]
- Neben TY, Loebelenz J, Hayes L, McCarthy K, Stoudemire J, Schaub R, Goldman SJ. Recombinant human interleukin-11 stimulates megakaryocytopoiesis and increases peripheral platelets in normal and splenectomized mice. Blood. 1993;81:901–8. [PubMed] [Google Scholar]
- Neta R. Cytokines in radioprotection and therapy of radiation injury. Biotherapy. 1988;1:41–5. doi: 10.1007/BF02170134. [DOI] [PubMed] [Google Scholar]
- Neta R, Oppenheim JJ, Douches SD. Interdependence of the radioprotective effects of human recombinant interleukin 1 alpha, tumor necrosis factor alpha, granulocyte colony-stimulating factor, and murine recombinant granulocyte-macrophage colony-stimulating factor. J Immunol. 1988;140:108–11. [PubMed] [Google Scholar]
- Patchen ML, MacVittie TJ, Souza LM. Postirradiation treatment with granulocyte colony-stimulating factor and preirradiation wr-2721 administration synergize to enhance hemopoietic reconstitution and increase survival. International Journal of Radiation Oncology, Biology, Physics. 1992;22:773–9. doi: 10.1016/0360-3016(92)90522-j. [DOI] [PubMed] [Google Scholar]
- Plett PA, Sampson CH, Chua HL, Joshi M, Booth C, Gough A, Johnson CS, Katz BP, Farese AM, Parker J, MacVittie TJ, Orschell CM. Establishing a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2012;103:343–55. doi: 10.1097/HP.0b013e3182667309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CH. Clinical efficacy of rhil-11. Oncology (Williston Park) 2000;14:32–40. [PubMed] [Google Scholar]
- Rosendahl M, Doherty D, Smith D, Bendele A, Cox G. Site-specific protein pegylation: Application to cysteine analogs of recombinant human granulocyte colony-stimulating factor. BioProcess International. 2005;3:52–62. [PMC free article] [PubMed] [Google Scholar]
- Schlerman FJ, Bree AG, Kaviani MD, Nagle SL, Donnelly LH, Mason LE, Schaub RG, Grupp SA, Goldman SJ. Thrombopoietic activity of recombinant human interleukin 11 (rhuil-11) in normal and myelosuppressed nonhuman primates. Stem Cells. 1996;14:517–32. doi: 10.1002/stem.140517. [DOI] [PubMed] [Google Scholar]
- Schuening FG, Storb R, Goehle S, Graham TC, Appelbaum FR, Hackman R, Souza LM. Effect of recombinant human granulocyte colony-stimulating factor on hematopoiesis of normal dogs and on hematopoietic recovery after otherwise lethal total body irradiation. Blood. 1989;74:1308–13. [PubMed] [Google Scholar]
- Schuening FG, Storb R, Goehle S, Nash R, Graham TC, Appelbaum FR, Hackman R, Sandmaier BM, Urdal DL. Stimulation of canine hematopoiesis by recombinant human granulocyte-macrophage colony-stimulating factor. Exp Hematol. 1989;17:889–94. [PubMed] [Google Scholar]
- Schwertschlag US, Trepicchio WL, Dykstra KH, Keith JC, Turner KJ, Dorner AJ. Hematopoietic, immunomodulatory and epithelial effects of interleukin-11. Leukemia. 1999;13:1307–15. doi: 10.1038/sj.leu.2401514. [DOI] [PubMed] [Google Scholar]
- Shimoda K, Okamura S, Harada N, Kondo S, Okamura T, Niho Y. Identification of a functional receptor for granulocyte colony-stimulating factor on platelets. J Clin Invest. 1993;91:1310–3. doi: 10.1172/JCI116330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureda A, Valls A, Kadar E, Algara M, Ingles-Esteve J, Bigas A, Jaume M, Lacruz M, Tobajas LM, Rutllant M, et al. A single dose of granulocyte colony-stimulating factor modifies radiation-induced death in b6d2f1 mice. Exp Hematol. 1993;21:1605–7. [PubMed] [Google Scholar]
- Van der Meeren A, Mouthon MA, Gaugler MH, Vandamme M, Gourmelon P. Administration of recombinant human il11 after supralethal radiation exposure promotes survival in mice: Interactive effect with thrombopoietin. Radiat Res. 2002;157:642–9. doi: 10.1667/0033-7587(2002)157[0642:aorhia]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Van der Meeren A, Mouthon MA, Vandamme M, Squiban C, Aigueperse J. Combinations of cytokines promote survival of mice and limit acute radiation damage in concert with amelioration of vascular damage. Radiat Res. 2004;161:549–59. doi: 10.1667/rr3164. [DOI] [PubMed] [Google Scholar]
- Vriesendorp H, Van Bekkum D. Susceptibility to total-body irradiation. In: Broerse J, MacVitties T, editors. Response of different species to total body irradiaton. Amsterdam: Martinus Nijhoff; 1984. [Google Scholar]
- Wadhwa M, Thorpe R. Haematopoietic growth factors and their therapeutic use. Thromb Haemost. 2008;99:863–73. [PubMed] [Google Scholar]
- Welte K, Gabrilove J, Bronchud MH, Platzer E, Morstyn G. Filgrastim (r-methug-csf): The first 10 years. Blood. 1996;88:1907–29. [PubMed] [Google Scholar]
- Yang L, Yang YC. Regulation of interleukin (il)-11 gene expression in il-1 induced primate bone marrow stromal cells. J Biol Chem. 1994;269:32732–9. [PubMed] [Google Scholar]
- Yuhas JM, Storer JB. The effect of age on two modes of radiation death and on hematopoietic cell survival in the mouse. Radiat Res. 1967;32:596–605. [PubMed] [Google Scholar]