Abstract
Objectives
To study the impact of continued LGG feeding on rotavirus gastroenteritis in the gnotobiotic pig (Gn) model of virulent human rotavirus (HRV) infection.
Methods
Gn pigs were assigned to treatment groups: (1) mock control, (2) LGG only, (3) HRV only or (4) LGG plus HRV. Nine days before HRV inoculation (3 days of age), pigs were fed LGG with a daily dose increase of 10-fold from 103 until 1012 colony forming units (CFU). The 1012 CFU/dose of LGG feeding continued until post-HRV-inoculation day (PID) 6. Clinical sign (diarrhea), rotavirus fecal shedding, histopathology of the ileum, adherent junction and tight junction protein expression in the ileal epithelial cells, mucin production in the large and small intestinal contents, and serum cytokine responses from PID 2 to PID 6 were examined and compared among the treatment groups.
Results
Clinically, the percentage of pigs developing diarrhea, the mean duration of diarrhea, and the mean cumulative fecal scores were lower in the LGG fed pigs compared to the non-fed pigs after HRV inoculation. LGG partially protected ileal epithelium against HRV-induced compensatory increases of the adherent junction protein α-catenin and β-catenin, tight junction protein occludin, claudin-3 and claudlin-4, and leak protein claudin-2. LGG promoted mucin production as the mucin levels in the large intestinal contents of the LGG+HRV pigs were significantly higher than the HRV only pigs on PID 2. Additionally, LGG maintained the anti-inflammatory cytokine TGF-β level in serum after HRV infection.
Conclusions
LGG is moderately effective for ameliorating rotavirus diarrhea by partially preventing injuries to the epithelium.
Keywords: Rotavirus; probiotic; gastroenteritis, intestinal epithelial barrier; gnotobiotic pigs
INTRODUCTION
Human rotavirus (HRV) is the most important cause of severe dehydrating diarrhea in infants and young children worldwide. Lactobacillus rhamnosus GG strain (LGG) is the most studied probiotic strain for ameliorating HRV diarrhea in children (1–3). However, the mechanism behind this beneficial effect is not well understood. It is unknown whether LGG functions in vivo to preserve intestinal epithelial barrier functions or to facilitate epithelium repair after injury caused by rotavirus infection, as has been implicated in our previous in vitro studies of epithelium and LGG interactions in cell cultures (4).
Intestinal epithelial cells are linked to each other via a series of intercellular junctions, such as tight junctions (TJ) and subjacent adherens junctions (AJ). TJ strictly seal in the intercellular space and prevent the entrance of microorganisms or unwanted substances from the luminal compartment, thus determining intestinal epithelial barrier properties (5, 6). AJ form complexes on the lateral membranes that occur at points of cell-cell contact. TJ are composed by a complex of proteins, including the zonula occludens (ZO), occludin and claudin family members. Among the claudins, claudin-1, -3 and -4 are sealing claudins whereas claudin-2 is a permeability-mediating claudin (leak protein) (7). Another important protein in intestinal epithelia is villin, a cytoskeletal protein of microvilli of epithelial cell brush borders, found principally in absorptive cells of the intestine (8). During rotavirus infections, disruption of the barrier function in intestinal epithelial cell cultures in vitro (9, 10) and development of villous atrophy in vivo (11) have been observed. LGG ameliorates tight junction disruption and partially prevents morphological changes caused by Enterohemorrhagic Escherichia coli (EHEC) O157:H7 in cell cultures (12) and induces intestinal barrier function maturation by promoting claudin-3 expression in a mouse model (13). Hence, in this study we tested our hypothesis that LGG prevents disruption of barrier function and reduce morphological changes of intestinal epithelia during the acute phase of HRV infection in gnotobiotic (Gn) pigs through mechanisms involving modulation of AJ and TJ protein expression, and mucin and cytokine production.
The gastrointestinal mucus layer formed by high molecular mass oligomeric mucin is one of the most important intestinal epithelial barrier properties (6). LGG was shown to increase mucin gene expression and mucin secretion in cell cultures (14–17). It has been reported that intestinal mucins from mice, rats and humans inhibit rotavirus replication in cell cultures (18, 19). Our previous study demonstrated that rotavirus infection stimulated MUC3 mucin secretion in porcine jejunum epithelial cells (IPEC-J2 cell line) (4). However such beneficial effect has not been confirmed in vivo. Hence, the effect of LGG on secreted MUC3 mucin production in the intestine of rotavirus infected Gn pigs was examined in the present study.
Gnotobiotic pigs are an excellent model system for the current study because (1) pigs and humans share great similarities in intestinal epithelial structure, physiology and immune responses; (2) After oral inoculation with the virulent HRV Wa strain, Gn pigs develop signs of disease (diarrhea) and intestinal pathological changes that closely mimic the rotavirus gastroenteritis in human infants; and (3) Gn status excludes the influence of maternal antibodies and commensal microflora. The effect observed in the study is thus totally attributable to the rotavirus or the single probiotic strain LGG. In this study, we demonstrated LGG intestinal colonization in Gn pigs and the protective effects of LGG on HRV infection and diarrhea. We determined the effects of LGG on intestinal epithelial morphology, AJ and TJ protein expression, mucin production in the intestinal contents, and cytokine responses in the serum of the Gn pigs infected with HRV during the acute phase of infection.
MATERIALS AND METHODS
Virus
The Wa strain (G1P1A[8]) virulent HRV (from Dr. Linda Saif, The Ohio State University, USA) was passaged in Gn pigs and the pooled intestinal contents of pigs were used for inoculation at a dose of ~105 fluorescence forming units (FFU). The 50% infectious dose (ID50) of the HRV in neonatal Gn pigs was determined as approximately 1 FFU (20). The virus titer was determined by using cell culture immunofluorescence (CCIF) and was expressed as FFU/ml as described previously (4).
Probiotic bacteria strain and detection of LGG shedding
Lactobacillus rhamnosus GG (ATCC# 53103) were propagated in Lactobacilli MRS broth (Weber Scientific, USA) and the bacteria counts were titrated and were expressed as colony forming units (CFU) per ml as described previously (21). Prior to feeding, the bacteria stored at −80°C were thawed and washed 2 times with 0.1% peptone water by centrifuging at 2000 rpm/min for 10 min at 4°C and were diluted to specified CFU/ml. LGG enumeration from daily rectal swab samples was carried out as previously described (21). The number of CFU on the plates with 20–200 colonies were counted and recorded.
Gnotobiotic pig treatment groups
Near-term pigs were derived by surgery from pregnant sows (Large White cross bred) and maintained in Gn isolator units as described (22). All pigs were confirmed as germ-free and seronegative for rotavirus antibodies prior to LGG and HRV exposure. Pigs (both males and females) were randomly assigned to four treatment groups as follows: (1) mock control (Mock), (2) LGG only (LGG), (3) HRV only (HRV) or (4) LGG colonization plus HRV infection (LGG+HRV). From 3 days of age (post-LGG feeding day [PFD] 0 = postpartum day [PPD] 3), pigs in LGG fed groups were orally dosed with LGG starting from 103 CFU in 3ml of 0.1% peptone water with a daily LGG dose increase by 10-fold until 1012 CFU (Fig. 1). From PID 0 to PID 6 (during HRV infection), pigs were fed 1 × 1012 CFU LGG daily. Pigs in non-LGG fed groups were given an equal volume of 0.1% peptone water. At PFD 9 (post-HRV inoculation day [PID] 0), pigs in HRV inoculated groups were orally inoculated with 105 FFU of virulent Wa HRV in 5 ml of diluent (Modified Eagle Medium with 1% non-essential amino acid, 1% antibiotic mixture [penicillin 10,000 I.U. /ml + streptomycin 10,000 MCG/ml + amphotericin 25MCG/ml]), and pigs in non-HRV inoculated groups were orally inoculated with an equal volume of the diluent. Pigs were given 8ml of 100 mM sodium bicarbonate 20 min before HRV inoculation to reduce gastric acidity. All inoculums were given orally using a needleless syringe as previously described (23).
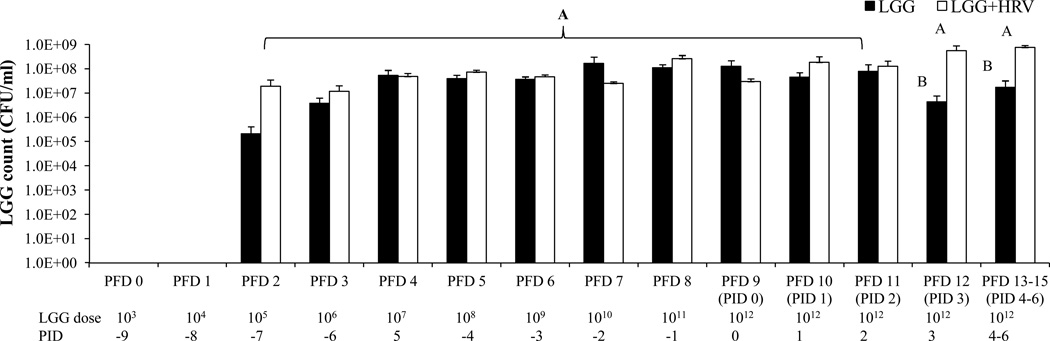
Fig. 1. LGG dosing schedule and counts in fecal samples of Gn pigs.
Nine days before HRV inoculation (3 days of age), pigs were fed LGG with a daily dose increase of 10-fold from 103 until 1012 CFU/dose. LGG counts in rectal swab samples were measured by plating on MRS selective medium from post feeding day (PFD) 0 to PFD 15. Error bars indicate the standard error of the mean. Different letters (A, B) indicate significant difference between LGG+HRV and LGG-only pigs (Kruskal Wallis Test, p<0.05, n=4–6) whereas shared letters indicate no significant difference.
Sample collection
From PFD 0, pigs were examined daily for clinical signs, including % with diarrhea, duration of diarrhea, and diarrhea scores as previously described (24). Fecal swabs were collected daily for detection of LGG and/or HRV shedding. Serum samples were collected at PFD 0, PFD 6 and PFD 10 (PID 0), PFD11 (PID 2) and at euthanasia days for detection of cytokine responses or viremia. Two to three pigs from each group between PID 1–3 and one pig from each group between PID 4–6 on each day were euthanized for sample collection. After euthanasia on PID 2, ileum was collected and fixed for hematoxylin-eosin (H&E) and immunofluorescence staining. The ileal epithelial cells were collected for detection of TJ and AJ protein and villin expressions. Small intestinal contents (SIC) and large intestinal contents (LIC) were collected for detection of rotavirus titers, cytokines response and mucin production. All animal experimental procedures were conducted in accordance with protocols reviewed and approved by the Institutional Animal Care and Use Committee of Virginia Polytechnic Institute and State University.
Detection of virus shedding and viremia by CCIF and ELISA
Post-HRV inoculation, infectious virus titer and virus antigen in rectal swab samples, SIC and LIC were analyzed by CCIF and ELISA, respectively as previously described (4). Rotavirus antigen in serum was measured by ELISA as the indication of viremia (25). The swab, SIC, LIC and serum samples from mock control pigs were used as negative controls.
Histopathology
Segments of ileum (~5mm×10mm) from Gn pigs were fixed in 4% paraformaldehyde and embedded in wax. Tissue sections were cut at 4mm thickness, mounted on slides pre-coated with poly-L-lysine, deparaffinizated with Hemo-De (Meridian Bioscience, Ohio, USA) and rehydrated with graded ethanol. The slides were then stained with H&E and observed under a microscope (Nikon E600, Japan). Semi-quantitative assessment of vacuolated enterocytes was performed as follows. H&E stained transverse sections of the ileum were examined in blinded fashion. The extent of vacuolated enterocytes was determined for animals from the mock (n=1), LGG only (n=1), HRV (n=2) only and HRV+LGG (n=2) groups using the following incidence scale: 1- rare; 2- moderate, and 3- extensive.
Quantification of TJ and AJ proteins by Western blot
The ileal and rectal epithelial cells were collected from the Gn pigs at necropsy and lysed in lysis buffer as previously described (26). Immunoblots were performed with primary antibodies mouse monoclonal antibodies again α-catenin, β-catenin and occludin (Zymed Laboratories Inc., USA), rabbit polyclonal antibodies against claudin-1(Zymed Laboratories Inc., USA) and claudin-3 (Transduction Laboratories, USA), and mouse monoclonal antibodies against claudin-4 (Transduction Laboratories, USA), claudin-2 (Life Technologies, USA) and β-actin (Sigma, USA).
Immunofluorescence staining of AJ and TJ proteins
Slides with ileum sections were prepared as described above for histopathology study and incubated with mouse monoclonal antibodies against α-catenin, β-catenin, occludin or claudin-4, or rabbit polyclonal antibodies against claudin-1 or claudin-3 (Invitrogen, USA) overnight at 4°C, flowing incubation with FITC-conjugated goat anti-mouse IgG1 (1:100 dilution; Santa Cruz Biotechnology, USA) or Alexa Fluor 594 goat anti-rabbit IgG (H+L) (Invitrogen, USA) for 1 h at room temperature. The species specificities of the antibodies are defined by the manufacturers for recognizing mouse/rat/human/canine proteins. Antibodies specifically against porcine TJ and AJ proteins are not commercially available. Mouse IgG1 isotype control antibody (eBiocience, USA) or omitting first antibodies was used in negative controls. The slides were observed under a confocal microscope (Nikon, Eclipse TE2000-E, Japan).
Detection of mucin production in SIC and LIC, and cytokine concentrations in serum, SIC and LIC by ELISA
MUC3 mucin concentration in SIC and LIC was measured by a direct-binding ELISA as previously described (4). Cytokine/chemokine levels in serum, SIC and LIC were measured by sandwich ELISA as previously described (27). Porcine IL-6, IL-8 (R&D system, USA), TNF-α, IFN-γ and TGF-β (Invitrogen, USA) ELISAs were performed according to the manufacturer’s instructions. The detection levels for IL-6, IL-8, IFN-γ and TGF-β were 8.0 pg/ml and for TNF-α was 3.0 pg/ml.
Statistical analysis
LGG counts, virus titers, ELISA OD values, and cytokine concentrations were compared among treatment groups using the non-parametric Kruskal-Wallis rank sum test. Proportions of virus shedding and diarrhea among treatment groups were compared using Fisher’s exact test. Mean duration of virus shedding and diarrhea and mean cumulative fecal consistence scores among the treatment groups were compared using one-way analysis of variance (ANOVA)-general linear model, followed by Duncan’s multiple range tests. Statistical significance was assessed at p<0.05. All statistical analysis was performed using SAS program 9.2 (SAS Institute, INC, USA).
RESULTS
LGG reduced HRV diarrhea and virus shedding
The clinical signs and virus shedding in pigs are summarized in Table 1. LGG feeding alone did not cause diarrhea or any other side effects in Gn pigs. After HRV inoculation, 100% HRV pigs and 75% of the LGG+HRV pigs, but none of the LGG and Mock pigs, developed diarrhea. All HRV and LGG+HRV pigs developed fecal virus shedding and viremia. The LGG+HRV pigs had a significantly delayed (4.0 vs 2.3 days) onset of diarrhea and a delayed onset of fecal virus shedding compared to the HRV pigs. Additionally, the mean durations of diarrhea, fecal virus shedding and viremia were shorter, mean cumulative score of diarrhea, peak virus titer, and the percentage of pigs with diarrhea were all lower in the LGG+HRV pigs than the HRV pigs, although these differences were not statistically significant between the LGG+HRV and the HRV pigs. Taken together, LGG demonstrated a clear but rather moderate protective effect on HRV induced diarrhea and virus replication in Gn pigs.
Table 1.
Clinical signs, virus shedding and viremia in Gn pigs after HRV inoculation
| Clinical signs | Fecal virus shedding (by CCIF and/or ELISA) | Viremia (by ELISA) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | n | % with diarrheaa * |
Mean days to onset** |
Mean duration days** |
Mean cumulative scoreb ** |
% shed virus* |
Mean days to onset** |
Mean duration days** |
Mean peak titer (FFU/ml)c,** |
% with viremia* |
Mean days to onset** |
Mean duration days** |
| Mock | 4 | 0B | na | 0B(0)d | 1.5B(0.4) | 0B | na | 0B(0) | 0B(0) | 0B | na | 0B(0) |
| LGG | 4 | 0B | nae | 0B(0) | 2.5B(0.4) | 0B | na | 0B(0) | 0B(0) | 0B | na | 0B(0) |
| HRV | 4 | 100A | 2.3B(0.3) | 3.3A(0.5) | 8.9A(1.6) | 100A | 1.3A(0.3) | 4.3A(0.5) | 7.8×102 A | 100A | 2A(0) | 3.3A(0.6) |
| LGG+HRV | 4 | 75A | 4.0A(0) | 2.0A(0.6) | 8.3A(1.6) | 100A | 1.8A(0.5) | 3.8A(0.6) | 6.0×102 A | 100A | 2A(0) | 2.8A(0.3) |
Pigs with daily fecal scores of ≥2 were considered diarrheic. Fecal consistency was scored as follows: 0, normal; 1, pasty; 2, semiliquid; and 3, liquid.
Mean cumulative score calculation included all the pigs in each group.
FFU, fluorescent focus forming units.
Standard error of the mean.
na, not applicable.
Proportions in the same column with different superscript capital letters (A,B) indicate significant different among different groups (Fisher’s exact test, p≤0.05), while shared letters indicate no significant difference.
Mean in the same column with different superscript capital letters (A,B) indicated significant different among different groups (Kruskal-Wallis Test, p≤0.05), while shared letters indicate no significant difference.
LGG growth was increased after HRV infection
LGG was detectable in rectal swab samples from all LGG only and LGG+HRV from PFD 2 (Fig. 1). LGG counts in LGG only pigs during the entire feeding period and in LGG+HRV pigs before HRV inoculation were maintained at the levels of 107–108 CFU/ml, even though the amount of LGG fed increased daily until reaching 1012 CFU. Thus, intestinal LGG load was kept in certain range and did not increase with the increasing LGG intake as the bacterial load in the intestine is regulated by the host immunity (28). However, after HRV inoculation, the counts in the LGG+HRV group increased to 109 CFU/ml and was significantly higher than the LGG only pigs from PID 3–6, thus HRV infection significantly increased LGG growth in the gut of Gn pigs.
The bacterial colonies on MRS agar plates from SIC, LIC and rectal swab samples had identical morphology as the colonies from the original LGG inoculums. The serially diluted SIC, LIC and rectal swab samples were also planted on regular blood agar plates as well as in thioglycollate broth and cultured at 37°C for 24–48 h. No bacterial colonies were found from any pig of non-LGG fed groups and the bacterial colony on the blood agar or in thioglycollate broth from the pigs of LGG fed groups was identified as LGG, suggesting that no extraneous bacterial contamination occurred.
HRV infection induced villous atrophy and inflammatory cell infiltration in ileum of Gn pigs from PID 2 with or without LGG feeding
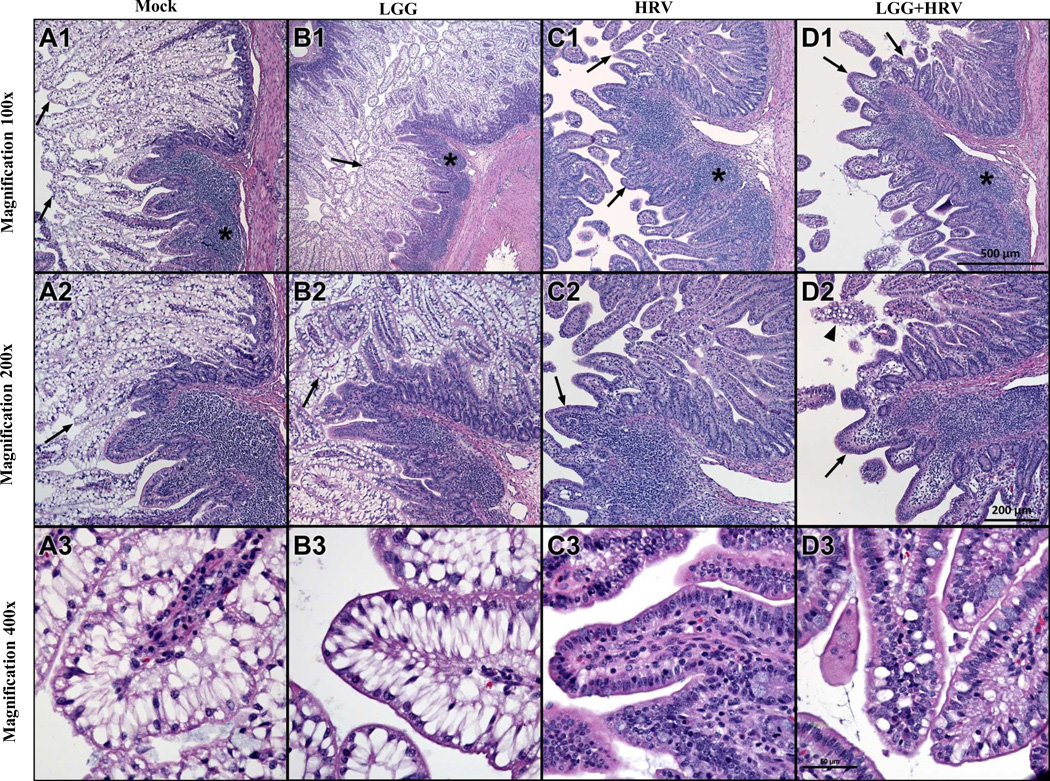
The ileal histopathology from Mock, LGG only, HRV only and LGG+HRV pigs was observed from PID 1 to PID 6. Series of photomicrographs of mucosa of ileum from the pigs on PID 2 were presented at Fig. 2. In the mock control pigs and LGG only pigs, the villi of ileal epithelium are long and covered with a layer of vacuolated enterocytes (Fig 2. A1–3, B1–3; scored as 3-extensive), which extend from the tips of the villi to a level at or just above the innermost region of the glands of Lieberkuhn (considering the intestinal lumen as innermost and the serosa outermost). The vacuoles are unstained and largely fill the cytoplasm of the cells. Except as follows, the lamina propria has few leukocytes, mainly lymphocytes and eosinophils. Lymphocyte-rich Peyer’s patches are regionally located in the submucosa and often extend into the lamina propria of overlying mucosa. The four pig groups at PID 1 and the mock control and LGG only pigs at PID 3–6 (data not shown) had similar ileal histology to the mock control and LGG only pigs at PID 2. From PID 2 on, the HRV and LGG+HRV pigs had a distinctly different histologic appearance from the mock control and LGG pigs (Fig. 2). The villi have lost the vacuolated enterocytes, which led to shortened and blunted villi (villous atrophy). Peyer’s patches appear more extensive and their extension into overlying lamina propria was much more prominent, suggesting inflammatory cell infiltration. Sloughed enterocytes with pyknotic nuclei are noted in the intestinal lumen. At PID 2, the LGG+HRV pigs had modest preservation of vacuolated enterocytes, with moderate numbers of vacuolated enterocytes present at the tips of some villi (Fig. 2 D3; scored as 2-moderate) compared to the HRV only pigs with very few vacuolated enterocytes (Fig. 2 C3; scored as 1-rare).
Fig. 2. Histopathology of ileum of Gn pigs on PID 2.
Series of photomicrographs of mucosa of ileum from a mock inoculated control (A), LGG alone (B), HRV alone (C) and LGG+HRV (D) exposed Gn pigs euthanized on PID 2 were acquired from H& stained cross-sections. The sections include a region containing Peyer's patch (* A1, B1, C1, D1) and adjacent mucosa. Note the prominence of pale-stained vacuolated enterocytes lining long, thin villi in the mock exposed control and LGG-exposed animals (arrows A1–2, B1–2 and shown at higher magnification A3, B3). In the two HRV infected groups the vacuolated cells are largely lost, and villi are shortened, blunted and have increased lymphocytes in the lamina propria (arrows in C1–2, D1–2). There is modest preservation of vacuolated enterocytes in LGG+HRV pigs (arrowhead in D2 and higher magnification image in D3) compared to HRV alone pigs (C2–3). Scale bar for each row of images is seen in D1–3.
LGG decreased the levels of AJ, TJ and leak proteins in ileum after HRV infection
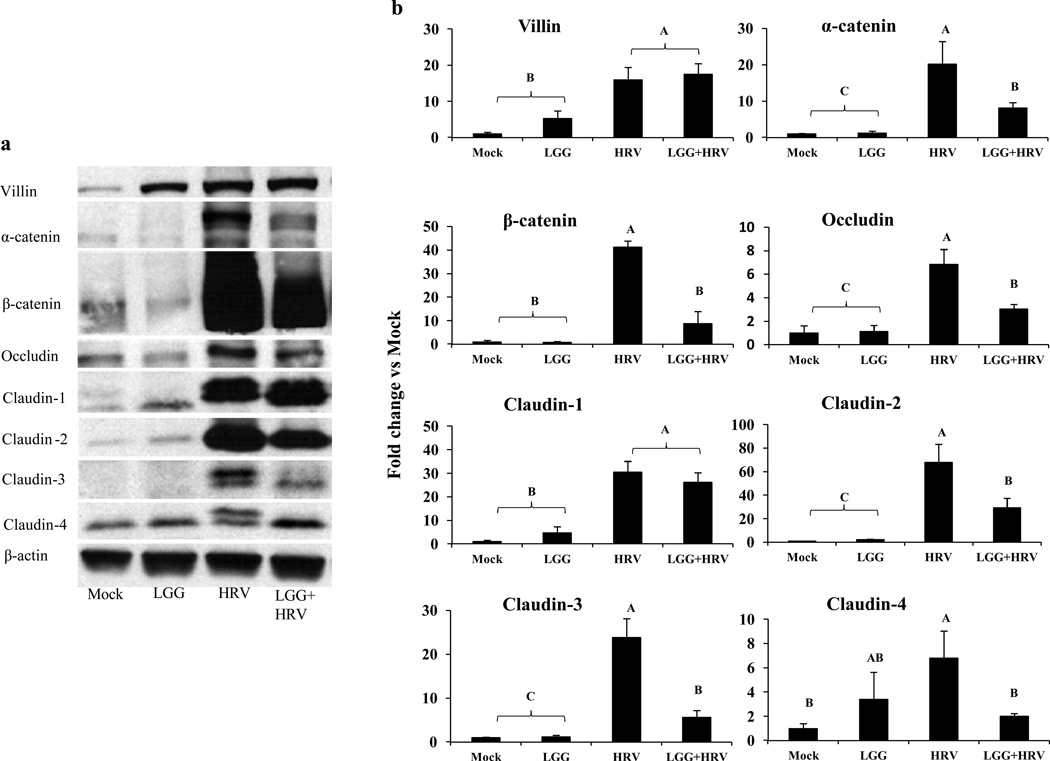
Expression of villin, AJ proteins (α-catenin and β-catenin), TJ proteins (occludin, claudin-1, claudin-3, and claudin-4) and the leak protein claudin-2 in the ileal epithelium cells were investigated by using Western blotting analysis. At PID 2, except for claudin-4 in LGG only pigs, the amount of all these proteins in the non-HRV inoculated pigs (Mock and LGG pigs) were significantly lower than those of the HRV inoculated pigs (HRV and LGG+HRV pigs) (Fig. 3a and 3b). Thus HRV infection significantly increased the villin, TJ, and AJ protein expression in the ileum of Gn pigs. More importantly, except for villin and claudin-1, expression levels of AJ proteins (α-catenin and β-catenin), TJ proteins (occludin, claudin-3, and claudin-4) and the leak protein claudin-2 in the ileum of LGG+HRV pigs were significantly lower than HRV-only pigs (Fig. 3a and 3b). However, the levels of α-catenin, occludin, claudin-1, claudin-2, and claudin-3 in the LGG+HRV pigs were significantly higher compared to the Mock and LGG only pigs (Fig. 3a and 3b); therefore, there was only a partial protective effect of LGG on the HRV-induced interruption of the tight junctions of the ileum.
Fig. 3. Western blot detection of TJ and AJ proteins in ileum of Gn pigs on PID 2.
Villin protein, AJ proteins (α-catenin and β-catenin), TJ proteins (occludin, claudin-1, claudin-3 and claudin-4), and leak protein claudin-2 of intestinal epithelial cells from ileum were detected by Western blot. The β-actin is a housekeeping protein. Fig. 3a, the representative results of each protein from the 4 treatment groups. Fig. 3b, the mean fold changes in different treatment groups over the mean level of each protein in the mock control group. Different letters (A, B, C) indicate significant difference among groups (Kruskal Wallis Test, p<0.05, n=3–5) whereas shared letters indicate no significant difference.
It is noticeable that the villin protein level was the lowest in the mock Mock pigs, followed by the LGG only pigs at PID 2 (Fig. 3). The HRV and LGG+HRV pigs had significantly higher amount of villin protein in ileum than the Mock and LGG only pigs (Fig. 3b).
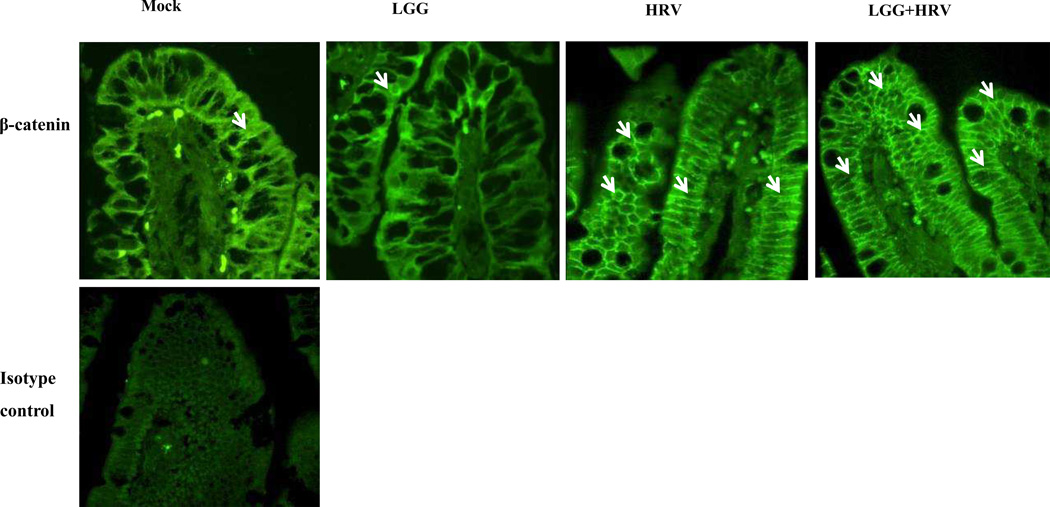
At PID 2, the locations of β-catenin in the ileal enterocytes was visualized by immunostaining and shown in Fig.4. Corresponding to the distinctly different histologic appearance between HRV infected pigs and non-HRV infected pigs (Fig. 2), the display of β-catenin in HRV infected pigs (HRV and LGG+HRV) was substantially stronger and more dense than the non-HRV infected pigs (Mock and LGG) (Fig. 4).
Fig. 4. Visualizing AJ protein β-catenin in ileum of Gn pigs.
β-catenin on the epithelial cells of ileum collected from Gn pigs in the 4 treatment groups was stained with primary anti β-catenin monoclonal antibodies and FITC conjugated secondary antibody. The images were captured by using a confocal microscope at the magnification of 400×. White arrows indicate β-catenin at some locations. There were no differences among all the time points within each group, therefore representative ones on PID 2 are shown.
We also determined the expression of villi protein as well as the expression and location of TJ, AJ and leaky proteins from the four pig groups at PID 3–6 (data not shown); there were similar trends among different pig groups as at PID 2.
LGG enhanced MUC3 mucin secretion in HRV infected Gn pigs at early stage
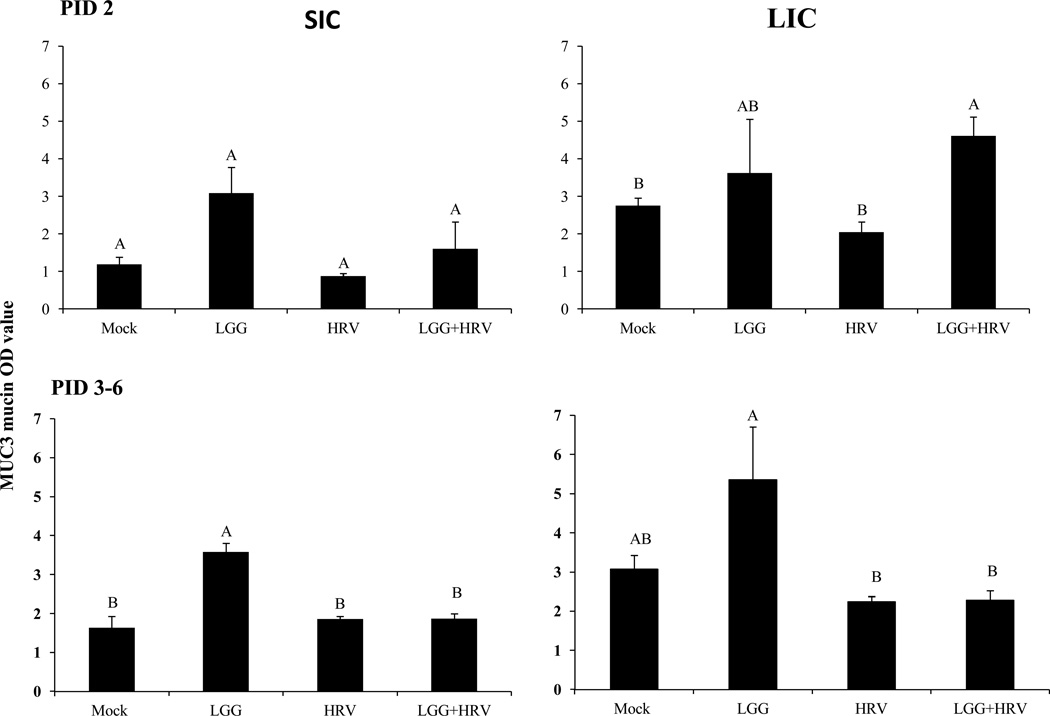
The LGG only pigs had higher or significantly higher levels of MUC3 mucin in SIC and LIC than the HRV-only pigs and mock controls at PID 2 and PID 3–6 (Fig. 5).The mucin levels in the LIC of the LGG+HRV pigs were significantly higher than the HRV only pigs and mock control pigs at PID 2.
Fig. 5. MUC3 mucin concentrations in SIC and LIC of Gn pigs.
The MUC3 mucin concentrations in SIC and LIC on PID 2–6 were measured by a direct-binding ELISA and the results are shown as the mean OD value. Data from PID 3–6 were combined within each group. Error bars indicate the standard error of the mean. Different letters (A, B) indicate significant difference among groups (Kruskal Wallis Test, p<0.05, n=4) whereas shared letters indicate no significant difference.
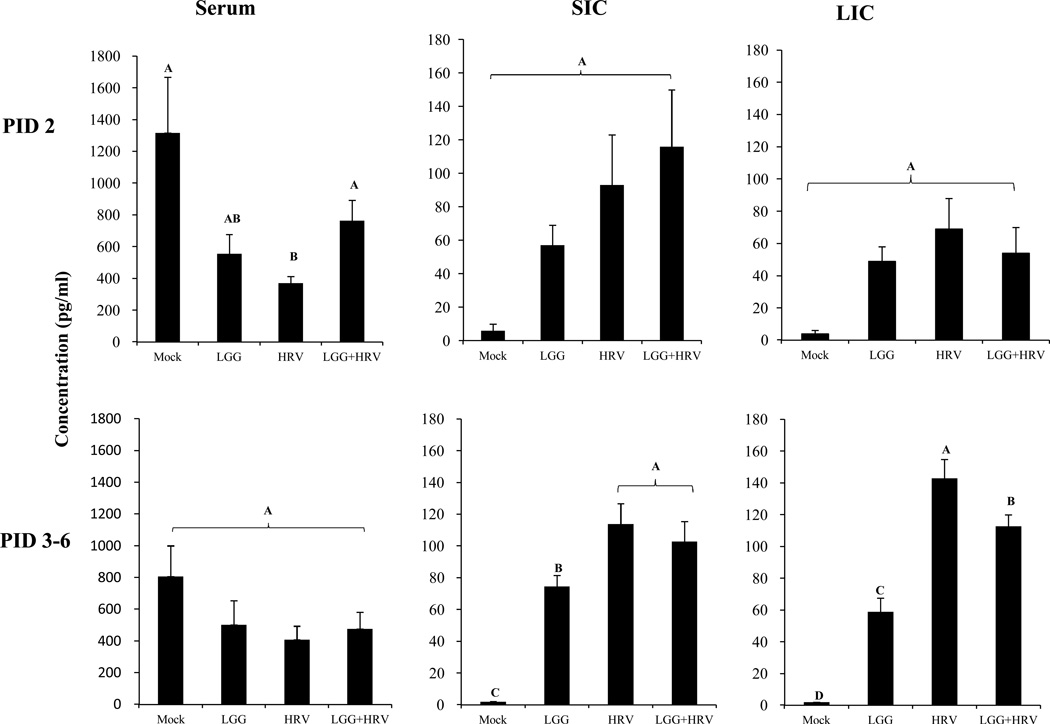
LGG increased TGF-β levels in serum of HRV infected Gn pigs at early stage
Mean TGF-β concentrations in serum, SIC and LIC at PID 2 and PID 3–6 are depicted in Fig. 6. HRV infection alone significantly decreased the serum TGF-β concentrations compared to the Mock pigs at PID 2. The serum TGF-β concentrations in the LGG+HRV pigs were significantly higher than the HRV only pigs and statistically similar to the mock controls (Fig. 6). Thus, LGG prevented the significant decline of the serum TGF-β level after HRV infection at PID 2. LGG did not have a significant influence on IL-6, IL-8, TNF-α, and IFN-γ in serum (data not shown).
Fig. 6. Mean TGF-β concentrations in serum, SIC and LIC of Gn pigs.
The cytokine concentrations were measured by ELISA on PID 2–6. The data from PID 3–6 were combined within each group. Error bars indicate the standard error of the mean. Different letters (A, B, C, D) indicate significant difference among groups (Kruskal Wallis Test, p<0.05, n=4), whereas shared letters indicate no significant difference.
At PID 3–6, the TGF-β concentrations in SIC and LIC of the HRV only and LGG+HRV pigs were significantly higher than the LGG only and mock control pigs; the LGG only pigs were significantly higher than the Mock pigs, thus HRV infection, LGG colonization, or LGG plus HRV induced significantly increased TGF-β production in the intestine at PID 3–6. LGG significantly reduced TGF-β level in LIC of the LGG+HRV pigs compared to HRV-only pigs at PID 3–6, suggesting an attenuating effect of LGG on HRV-induced intestinal TGF-β response (Fig. 6). Concentrations of IL-6, IL-8, TNF-α, and IFN-γ in SIC and LIC did not differ significantly between HRV only and LGG+HRV pigs (data not shown).
DISCUSSION
LGG colonization had a clear and moderate protective effect on HRV-induced diarrhea and virus replication in Gn pigs. The moderate effectiveness of LGG in HRV-infected Gn pigs closely mimics the results of human clinical trials (1–3, 29–33), further confirming the usefulness of the Gn pig model of HRV infection and diarrhea in the studies of probiotic-host-virus interactions.
HRV infection significantly increased the LGG growth. This is consistent with our previous observation that rotavirus infection significantly increased the growth of lactobacilli (L. acidophilus and L. reuteri) in Gn pigs (21). One of possible contributing factor to this observation is the change of the cytokine profile (i.e., increased IFN-γ and IL-12; decreased TGF-β levels) after rotavirus infection (34), which down regulates LGG-specific IgA antibody production. Intestinal IgA antibodies against gut commensals play an important role in controlling the bacteria load in the gut (35).
TGF-β is also an improtant anti-inflammatory cytokines and promotes enhancement of intestinal epithelail TJ barrier function and protects against TJ protein disruption (36, 37). LGG significantly attenuated the decrease of TGF-β level in serum of LGG+HRV pigs compared with the HRV-only pigs, which may contribute to the preservation and restoration of the gut homeostasis after HRV infection.
Intestinal epithelium is a highly organized system built up by a population of proliferative cells that are rapidly renewed and migrate along the crypt-villus axis, differentiating into functional mature cells (38). In germ-free pigs (Mock pigs) or Gn pigs monoassociated with LGG (LGG alone), vacuolated enterocytes in the villi of ileal epithelium are abundant; this observation has also been reported by others (39). LGG feeding alone did not have a significant effect on the morphology of ileal epithelium of Gn pigs over the time period (PID 1–6). It is possible that the effects of LGG colonization on the ileal epithelium morphology have occurred soon after LGG initial feeding and prior to 10 days of age (PID 1). LGG mono-association in Gn rats significantly increased the total number of crypt cells and the depth of the crypt in ileum at 3 days after initial LGG feeding and there were no further changes between 3 and 21 days (40). The germ-free pigs had weak villin protein expression likely due to the lack of gut microbial stimulation. However, with the LGG colonization and/or HRV infection, the villin protein expression level increased. Villin is a marker of both enterocyte differentiation and epithelial cell polarity on microvilli (8). Thus, either LGG colonization or HRV infection can stimulate the differentiation and epithelial cell polarization of microvilli, a reparative response to HRV-induced injury.
Rotavirus infection caused the near total loss of the vacuolated enterocytes, as the infected cells die quickly and exfoliate into the intestinal lumen during villous atrophy (11). Modest preservation of vacuolated enterocytes was observed in LGG+HRV pigs compared to HRV-only pigs, which is associated with the moderately reduced clinical signs in these pigs. Damage to enterocyte membranes and TJ proteins, coupled with alterations in mucous secretion and immune cell activity, can increase the permeability of the intestinal barrier (41). However, the intestinal epithelium is programmed to rapidly heal any damage or injury and reseal the intestinal barrier after injury (42). The regeneration process of epithelium started immediately following the injury caused by HRV replication. By PID 2, the shortened villi with compact enterocytes and increased levels of villin, AJ and TJ proteins were evident in all the HRV inoculated Gn pigs. Study of methotrexate (MTX)-induced intestinal injury in rats reported the similar observation that β-catenin protein level increased in the treated rats compared to the controls at 72 hours after MTX-induced damage (43). More importantly, the decreased AJ and TJ protein expression in the ileum of LGG+HRV pigs compared to HRV alone pigs indicates that LGG attenuated the HRV-associated ileal injury and the associated compensatory/regenerative process after viral infection in the Gn pigs.
Consistent with previous findings that LGG stimulates the increase of MUC3 mucin mRNA expression and production, in our current study LGG increased MUC3 mucin concentrations in the SIC (slightly) and LIC (significantly) of the HRV-infected pigs at PID 2, suggesting that the increased mucin concentration may have played a role in the protective effect of LGG on rotavirus infection and diarrhea.
In summary, LGG slightly reduced the injury of ileum, modulated epithelial cell functions by attenuating the compensatory increases of AJ and TJ protein, increased villin expression and intestinal MUC3 mucin production, and maintained normal TGF-β level in serum after HRV infection. These functions of LGG could have contributed to the mechanisms behind the moderate protective effect of LGG on HRV induced diarrhea in neonatal Gn pigs.
We recognize the limitation of current studies. The small number of pigs from each group prevented us from observing statistically significant differences in most clinical signs. For the same reason, we have to combine the data of mucin and cytokine responses at PID 3–6 for each group. The high variability of cytokine responses makes it difficult to draw conclusions from the data. Despite these weaknesses of the studies, our findings are still significant because this is the first time the direct effects of LGG on the pathogenesis of rotavirus infection were studied in vivo in the well-controlled experimental Gn pig model that is most closely mimicking human infants infected with HRV. In general, the beneficial effect of probiotics as a cure is low: when studied in children with acute infectious diarrhea, the effect of probiotics was mostly described as shortening the duration of diarrhea by ~1 day (45, 46). Our study using the Gn pig model confirms the modest effect of LGG. Therefore, we propose that probiotics as adjuvants for vaccines (47) may have a more substantial impact than when they are used alone as therapeutic agents to treat infectious diseases.
Acknowledgment
We thank Dr. Kevin Pelzer, Dr. Marlice Vonck, Pete Jobst, Andrea Pulliam and Shannon Viers for animal care at Virginia-Maryland Regional College of Veterinary Medicine, Virginia Polytechnic Institute and State University.
Funds: This work was partially supported by a grant from the NCCAM, National Institutes of Health (R01AT004789) and the start-up fund from Virginia Tech to LY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no conflicts of interest.
References
- 1.Guandalini S. Probiotics for children with diarrhea: an update. J Clin Gastroenterol. 2008;42(Suppl 2):S53–S57. doi: 10.1097/MCG.0b013e3181674087. [DOI] [PubMed] [Google Scholar]
- 2.Guandalini S. Probiotics for children: use in diarrhea. J Clin Gastroenterol. 2006;40(3):244–248. doi: 10.1097/00004836-200603000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Szajewska H, Wanke M, Patro B. Meta-analysis: the effects of Lactobacillus rhamnosus GG supplementation for the prevention of healthcare-associated diarrhoea in children. Aliment Pharmacol Ther. 2011;34(9):1079–1087. doi: 10.1111/j.1365-2036.2011.04837.x. [DOI] [PubMed] [Google Scholar]
- 4.Liu F, Li G, Wen K, et al. Porcine small intestinal epithelial cell line (IPEC-J2) of rotavirus infection as a new model for the study of innate immune responses to rotaviruses and probiotics. Viral Immunol. 2010;23(2):135–149. doi: 10.1089/vim.2009.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landau D. Epithelial paracellular proteins in health and disease. Curr Opin Nephrol Hypertens. 2006;15(4):425–429. doi: 10.1097/01.mnh.0000232883.43093.76. [DOI] [PubMed] [Google Scholar]
- 6.Laukoetter MG, Bruewer M, Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol. 2006;22(2):85–89. doi: 10.1097/01.mog.0000203864.48255.4f. [DOI] [PubMed] [Google Scholar]
- 7.Markov AG, Kruglova NM, Fomina YA, et al. Altered expression of tight junction proteins in mammary epithelium after discontinued suckling in mice. Pflugers Arch. 2012;463(2):391–398. doi: 10.1007/s00424-011-1034-2. [DOI] [PubMed] [Google Scholar]
- 8.West AB, Isaac CA, Carboni JM, et al. Localization of villin, a cytoskeletal protein specific to microvilli, in human ileum and colon and in colonic neoplasms. Gastroenterology. 1988;94(2):343–352. doi: 10.1016/0016-5085(88)90421-0. [DOI] [PubMed] [Google Scholar]
- 9.Beau I, Cotte-Laffitte J, Amsellem R, et al. A protein kinase A-dependent mechanism by which rotavirus affects the distribution and mRNA level of the functional tight junction-associated protein, occludin, in human differentiated intestinal Caco-2 cells. J Virol. 2007;81(16):8579–8586. doi: 10.1128/JVI.00263-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nava P, Lopez S, Arias CF, et al. The rotavirus surface protein VP8 modulates the gate and fence function of tight junctions in epithelial cells. J Cell Sci. 2004;117(Pt 23):5509–5519. doi: 10.1242/jcs.01425. [DOI] [PubMed] [Google Scholar]
- 11.Yuan L, Stevenson G, Saif L. Rotavirus and Reovirus. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, editors. Diseases of Swine. Ames, Iowa: Blackwell Publishing; 2006. pp. 435–454. [Google Scholar]
- 12.Johnson-Henry KC, Donato KA, Shen-Tu G, et al. Lactobacillus rhamnosus strain GG prevents enterohemorrhagic Escherichia coli O157:H7-induced changes in epithelial barrier function. Infect Immun. 2008;76(4):1340–1348. doi: 10.1128/IAI.00778-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel RM, Myers LS, Kurundkar AR, et al. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol. 2012;180(2):626–635. doi: 10.1016/j.ajpath.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mack DR, Ahrne S, Hyde L, et al. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52(6):827–833. doi: 10.1136/gut.52.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mack DR, Michail S, Wei S, et al. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol. 1999;276(4 Pt 1):G941–G950. doi: 10.1152/ajpgi.1999.276.4.G941. [DOI] [PubMed] [Google Scholar]
- 16.Mattar AF, Teitelbaum DH, Drongowski RA, et al. Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr Surg Int. 2002;18(7):586–590. doi: 10.1007/s00383-002-0855-7. [DOI] [PubMed] [Google Scholar]
- 17.Caballero-Franco C, Keller K, De Simone C, et al. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G315–G322. doi: 10.1152/ajpgi.00265.2006. [DOI] [PubMed] [Google Scholar]
- 18.Chen CC, Baylor M, Bass DM. Murine intestinal mucins inhibit rotavirus infection. Gastroenterology. 1993;105(1):84–92. doi: 10.1016/0016-5085(93)90013-3. [DOI] [PubMed] [Google Scholar]
- 19.Yolken RH, Ojeh C, Khatri IA, et al. Intestinal mucins inhibit rotavirus replication in an oligosaccharide-dependent manner. J Infect Dis. 1994;169(5):1002–1006. doi: 10.1093/infdis/169.5.1002. [DOI] [PubMed] [Google Scholar]
- 20.Ward LA, Rosen BI, Yuan L, et al. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77(Pt 7):1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Azevedo MS, Gonzalez AM, et al. Influence of probiotic Lactobacilli colonization on neonatal B cell responses in a gnotobiotic pig model of human rotavirus infection and disease. Vet Immunol Immunopathol. 2008;122(1–2):175–181. doi: 10.1016/j.vetimm.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer RC, Bohl EH, Kohler EM. Procurement and Maintenance of Germ-Free Swine for Microbiological Investigations. Appl Microbiol. 1964;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Azevedo MS, Wen K, et al. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine. 2008;26(29–30):3655–3661. doi: 10.1016/j.vaccine.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan L, Ward LA, Rosen BI, et al. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70(5):3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azevedo MS, Yuan L, Jeong KI, et al. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J Virol. 2005;79(9):5428–5436. doi: 10.1128/JVI.79.9.5428-5436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao D, Luo Y, Markowitz D, et al. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One. 2009;4(11):e7965. doi: 10.1371/journal.pone.0007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azevedo MS, Yuan L, Pouly S, et al. Cytokine responses in gnotobiotic pigs after infection with virulent or attenuated human rotavirus. J Virol. 2006;80(1):372–382. doi: 10.1128/JVI.80.1.372-382.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portal-Celhay C, Bradley ER, Blaser MJ. Control of intestinal bacterial proliferation in regulation of lifespan in Caenorhabditis elegans. BMC Microbiol. 2012;12:49. doi: 10.1186/1471-2180-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guarino A, Canani RB, Spagnuolo MI, et al. Oral bacterial therapy reduces the duration of symptoms and of viral excretion in children with mild diarrhea. J Pediatr Gastroenterol Nutr. 1997;25(5):516–519. doi: 10.1097/00005176-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Isolauri E, Juntunen M, Rautanen T, et al. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics. 1991;88(1):90–97. [PubMed] [Google Scholar]
- 31.Teran CG, Teran-Escalera CN, Villarroel P. Nitazoxanide vs. probiotics for the treatment of acute rotavirus diarrhea in children: a randomized, single-blind, controlled trial in Bolivian children. Int J Infect Dis. 2009;13(4):518–523. doi: 10.1016/j.ijid.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Rosenfeldt V, Michaelsen KF, Jakobsen M, et al. Effect of probiotic Lactobacillus strains in young children hospitalized with acute diarrhea. Pediatr Infect Dis J. 2002;21(5):411–416. doi: 10.1097/00006454-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Guandalini S, Pensabene L, Zikri MA, et al. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr. 2000;30(1):54–60. doi: 10.1097/00005176-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 34.Azevedo MS, Zhang W, Wen K, et al. Lactobacillus acidophilus and Lactobacillus reuteri modulate cytokine responses in gnotobiotic pigs infected with human rotavirus. Benef Microbes. 2012;3(1):33–42. doi: 10.3920/BM2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28(6):740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howe KL, Reardon C, Wang A, et al. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am J Pathol. 2005;167(6):1587–1597. doi: 10.1016/s0002-9440(10)61243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci. 2009;14:2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaeffer B. Mammalian intestinal epithelial cells in primary culture: a mini-review. In Vitro Cell Dev Biol Anim. 2002;38(3):123–134. doi: 10.1290/1071-2690(2002)038<0123:MIECIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 39.Shirkey TW, Siggers RH, Goldade BG, et al. Effects of commensal bacteria on intestinal morphology and expression of proinflammatory cytokines in the gnotobiotic pig. Exp Biol Med (Maywood) 2006;231(8):1333–1345. doi: 10.1177/153537020623100807. [DOI] [PubMed] [Google Scholar]
- 40.Banasaz M, Norin E, Holma R, et al. Increased enterocyte production in gnotobiotic rats mono-associated with Lactobacillus rhamnosus GG. Appl Environ Microbiol. 2002;68(6):3031–3034. doi: 10.1128/AEM.68.6.3031-3034.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.West NP, Pyne DB, Peake JM, et al. Probiotics, immunity and exercise: a review. Exerc Immunol Rev. 2009;15:107–126. [PubMed] [Google Scholar]
- 42.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169(6):1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ben-Lulu S, Pollak Y, Mogilner J, et al. Dietary transforming growth factor-beta 2 (TGF-beta2) supplementation reduces methotrexate-induced intestinal mucosal injury in a rat. PLoS One. 2012;7(9):e45221. doi: 10.1371/journal.pone.0045221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCracken VJ, Chun T, Baldeon ME, et al. TNF-alpha sensitizes HT-29 colonic epithelial cells to intestinal lactobacilli. Exp Biol Med (Maywood) 2002;227(8):665–670. doi: 10.1177/153537020222700817. [DOI] [PubMed] [Google Scholar]
- 45.Van Niel CW, Feudtner C, Garrison MM, et al. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics. 2002;109(4):678–684. doi: 10.1542/peds.109.4.678. [DOI] [PubMed] [Google Scholar]
- 46.Majamaa H, Isolauri E, Saxelin M, et al. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 1995;20(3):333–338. doi: 10.1097/00005176-199504000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Licciardi PV, Tang ML. Vaccine adjuvant properties of probiotic bacteria. Discov Med. 2011;12(67):525–533. [PubMed] [Google Scholar]