Abstract
Neisseria gonorrhoeae has developed resistance to all previous first-line antimicrobial therapies recommended by the United States’ Centers for Disease Control and Prevention over the past 75 years. Now the cephalosporins, the last available antibiotic class that is sufficiently effective, economical, and feasibly delivered in the outpatient setting are also threatened by evolving resistance. Screening for asymptomatic gonorrhea in women and men who have sex with men, treating with a dual antibiotic regimen, ensuring effective partner therapy and remaining vigilant for treatment failures constitute critical activities for clinicians in responding to evolving antimicrobial resistance. In this article, we will review the epidemiology, antimicrobial resistance history, current screening and treatment guidelines, and future treatment options for gonorrhea.
Keywords: Neisseria gonorrhoeae, sexually transmitted disease, antimicrobial resistance
INTRODUCTION
Gonorrhea is one of the most common curable sexually transmitted diseases (STDs), affecting more than 106 million individuals throughout the globe1 and an estimated 700,000 individuals in the United States each year.2 Gonorrhea (GC) disproportionately affects women, men who have sex with men (MSM) and racial/ethnic minorities. In women, untreated Neisseria gonorrhoeae infection can lead to major morbidities including pelvic inflammatory disease, ectopic pregnancy, tubal factor infertility and congenital blindness in offspring.3 In all persons, gonorrhea increases the risk of HIV transmission and acquisition.4 Although once one of public health’s great success stories, gonorrhea is once again a major public health threat with the emergence of multidrug resistance..5
Epidemiology
Gonorrhea rates in the U.S. declined almost 80% from the mid-1970s to the late-1990s, after the introduction of a national gonorrhea control program. With those declines, rates of PID and ectopic pregnancy plummeted.3 However, in the United States since 2002, women have consistently had higher rates of GC than men. Gonorrhea rates are highest among young women aged 15 – 24 years, with 108.9 cases for every 100,000 women in 2011.6 Racial/ethnic disparities in gonorrhea incidence are profound, with rates in African Americans 17-fold rates in whites, and rates in American Indians and Hispanics 4.6-fold and 2.1-fold those in whites, respectively. Gonorrhea incidence also varies substantially by geography, with the highest rates in the U.S. found in southeastern states. Among a network of 12 sentinel STD surveillance sites nationwide, the distribution of gonococcal infections by gender and sexual orientation was 21.6% MSM, 31% heterosexual men and 47.4% women, although the distribution varied widely by geographic region. For example, in San Francisco <10% of diagnosed gonococcal infections occurred in women, whereas in Alabama, Connecticut and Virginia over 60% of infections were in women.6
Worldwide, gonorrhea rates are increasing. The World Health Organization (WHO) estimates that GC cases increased 21% between 2005 and 2008, from 87 million to 106 million annual cases. Although the yearly incidence of GC is higher in men than women worldwide, women bear a larger burden of prevalent infections1 due, in part, to the primarily asymptomatic nature of infections in women. The WHO regions of the Western Pacific (China, Japan, the Philippines, Malaysia, Vietnam, Australia etc), Southeast Asia (India, Korea, Thailand, Bangladesh etc) and Africa have the highest GC rates in the world.1
Antimicrobial Surveillance
The primary source for surveillance of antimicrobial resistance in GC in the U.S. is the Gonococcal Isolate Surveillance Project (GISP), a Centers for Disease Control and Prevention (CDC)-funded collaboration of 28 sentinel clinic sites and 5 regional laboratories. GISP was started in 1986 to provide an evidence base for the selection of gonococcal treatment.7 Although GISP tests only urethral isolates from men diagnosed in STD clinics, the CDC STD Surveillance Network (SSuN) now has pilot programs for enhanced surveillance of extragenital GC isolates from MSM and isolates from patients with possible treatment failures (SSuN cooperative agreement info: CDC-RFA-PS08-865).
The WHO’s Gonococcal Antimicrobial Surveillance Programme (GASP) was introduced in 1992 to monitor antimicrobial resistance in N. gonorrhoeae in the Western Pacific Region. GASP was expanded in 2007 – 2008 with the addition of the South East Asia Region.8 In Europe, antimicrobial surveillance is performed by Euro-GASP which was created in 2004 as part of the European Surveillance of Sexually Transmitted Infections and continues today through the European Centre for Disease Prevention and Control.9
EVOLUTION OF ANTIMICROBIAL RESISTANCE IN NEISSERIA GONORRHOEAE
N. gonorrhoeae is adept at acquiring antimicrobial resistance, and the effort to stay ahead of gonococcal evolution has defined its treatment since the inception of antibiotics. The WHO recommends removing an antibiotic from first-line therapy recommendations for treatment of an STD when > 5% of isolates in a community are resistant to the antibioitc.10 Although there are no empiric data to support a particular threshold, the 5% mark has been influential in the formation of STD treatment guidelines. Sulfonamides, developed in the 1930s, were one of the first widely used classes of antibiotics, but had a relatively short life as gonococcal therapy because resistance emerged within 10 years of their introduction. By the mid-1940s, penicillin became the mainstay of gonococcal treatment. Penicillin was a remarkably resilient therapy, but over the course of four decades the minimum inhibitory concentration (MIC) of penicillin in N. gonorrhoeae gradually rose along with the recommended dose of penicillin for gonorrhea treatment. By the late 1980s, penicillin ceased to be an adequate treatment. Nearly simultaneously, resistance to tetracyclines, an alternate therapy, also emerged.10 Fortunately, at that time, alternative drug classes were available.
Third-generation cephalosporins and quinolones became the recommended therapies in the late 1980s. However, resistance to quinolones emerged rapidly, originally in East and Southeast Asia. By the mid-1990s quinolone-resistant gonorrhea was detected in North America, initially in Hawaii, and then on the West coast of the U.S.10 By 2006, 39% of GC isolates from MSM in the U.S. were quinolone-resistant, and, quinolones were removed from the CDC gonorrhea treatment guidelines.11 Since 2006, CDC has recommended only one class of antimicrobials as a first-line therapy for gonorrhea: the cephalosporins. Following the historical pattern witnessed with quinolone resistance, the gonococcus is now developing resistance to cephalosporins as well.
Contemporary Cephalosporin Resistance
To date, there is no universal laboratory definition of GC resistance to cephalosporins (Table 1). Minimum inhibitory concentration (MIC) breakpoints for decreased susceptibility differ for cefixime, an oral third-generation cephalosporin, and ceftriaxone, a parenteral third-generation cephalosporin. Similarly, terminology related to antimicrobial resistance varies. For instance, the U.S. Clinical and Laboratory Standards Institute (CLSI) defines decreased susceptibility for both cefixime and ceftriaxone as an MIC of ≥ 0.5 μg/mL,12 while the CDC defines an “alert value” for cefixime as an MIC ≥ 0.25 μg/mL and an “alert value” for ceftriaxone as an MIC ≥0.125 μg/mL. 12
Table 1.
Comparative Definitions of Minimum Inhibitory Concentration (MIC) Breakpoints for Decreased Susceptibility to Selected Antibiotics and MICs of Isolates Reported as “Superbugs”.
| Cefixime MIC | Ceftriaxone MIC | Azithromycin MIC | |
|---|---|---|---|
| CLSI *– Decreased Susceptibility | ≥ 0.5 μg/mL | ≥ 0.5 μg/mL | NA |
| CDC§ Alert Value | ≥ 0.25 μg/mL | ≥ 0.125 μg/mL | ≥ 2 μg/mL‡ |
| EUCAST† – decreased susceptibility | ≥ 0.25 μg/mL | ≥ 0.25 μg/mL | ≥ 1 μg/mL |
| WHOβ – decreased susceptibility | ≥ 0.25 μg/mL | ≥ 0.125 μg/mL | NA |
| Japanese Isolate H041 | 8 μg/mL | 2 μg/mL^ | 1 μg/mL |
| French Isolate F89 | 4 μg/mL | 1 μg/mL | 1 μg/mL |
CLSI: U.S. Clinical Laboratory Standards Institute;
CDC: U.S. Centers for Disease Control and Prevention;
EUCAST: The European Committee on Antimicrobial Susceptibility Testing;
WHO: World Health Organization;
CLSI does not define azithromycin resistance, but CDC uses 2 μg/mL to indicated decreased susceptibility;
2 mcg/mL by agar dilution method, 4 mcg/mL by e-test method
Decreased cephalosporin susceptibility first emerged among GC strains in Asia. In Japan, between 1999 and 2002, the percentage of N. gonorrhoeae isolates with MIC ≥ 0.5 μg/mL to cefixime increased from zero to 30%.13 Reports from Hong Kong and South Korea also documented increasing cephalosporin resistance, 14, 15 and the WHO GASP for the Western Pacific and South East Asia found at least one country in the region with 56% of its tested isolates exhibiting decreased susceptibility to ceftriaxone,16 suggesting a larger pattern of emerging resistance throughout Asia.
In the U.S., the proportion of GC isolates with CDC-designated cefixime “alert value” MIC increased from 0.1% in 2006 to 1.7% in the first six months of 20115 (see Figure 1). Although the proportion of isolates meeting the CLSI definition for decreased susceptibility to cefixime (≥ 0.5 μg/ml) also rose, these isolates accounted for only 0.1% of all isolates tested in 2006 – 2011. Notably, 77% of the isolates with elevated MICs to cefixime during this time were resistant to tetracycline.12 Between 2006 and 2011 the proportion of tested isolates with alert value MICs for ceftriaxone (≥0.125 μg/mL) rose from 0 to 0.4%.12 Recapitulating the pattern observed in the spread of quinolone-resistant gonorrhea, decreased susceptibility strains are disproportionately found among MSM on the West coast of the U.S.5
Figure 1.
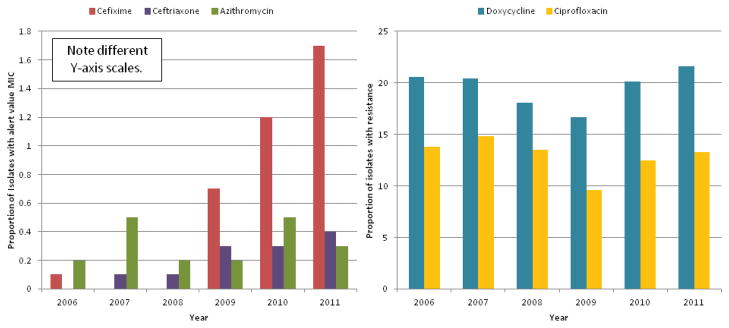
Proportion of Gonococcal Isolates Tested by GISP with CDC ‘Alert Value’ MIC§ to Cefixime*, Ceftriaxone, Azithromycin and with Resistance† to Doxycycline and Ciprofloxacin: 2006 - August 2011.
§CDC defines alert value MIC to Cefixime as ≥0.25 μg/mL, Ceftriaxone ≥0.125 μg/mL, and Azithromycin ≥2.0 μg/mL. †CDC defines doxycycline resistance as MIC ≥2.0 μg/mL and ciprofloxacin resistance as ≥1.0 μg/mL. *Cefixime MIC were not tested in 2007 and 2008. Data compiled from CDC STD Surveillance Reports 2006 – 2011.
Using the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria for decreased susceptibility to cefixime, 9% of isolates tested in Europe in 2010 exhibited decreased susceptibility to cefixime (MIC ≥ 0.25μg/mL). Many countries in Europe, including Germany, Spain, Italy, Greece, Norway, Sweden, and Slovakia report that > 5% of all 2010 – 2012 isolates exhibited decreased susceptibility to cefixime.17 In Austria, Denmark and Slovenia, >20% of isolates had reduced susceptibility to cefixime. However, in 2010, the European CDC did not find any isolates with decreased susceptibility to ceftriaxone.18 In contrast to North American epidemiology, the majority of isolates with decreased susceptibility to cefixime in Europe occur in heterosexual men.
Cephalosporin Treatment Failures
The clinical correlates of cephalosporin MIC values in GC are not known, but treatment failures to both cefixime and ceftriaxone in persons infected with reduced susceptibility isolates have been reported. The first reported treatment failures to oral cephalosporins occurred in Japan in the early 2000s in patients treated with multiple doses of 200 mg cefixime against isolates exhibiting MICs ranging from 0.125 μg/mL to 1 μg/mL.19, 20 The Japanese authorities responded quickly and transitioned to ceftriaxone as first-line treatment for gonorrhea in 2006.21 In 2010, the first two cases of cefixime treatment failure in Europe were reported in heterosexual men in Norway, both of whom failed treatment with 400 mg of cefixime, but were cured with 500mg of intramuscular ceftriaxone.22 Cefixime treatment failures were then documented in England,23 Austria,24 France,25 and Canada.26 In contrast to previous reports, the GC strains isolated from the Canadian series of 9 patients with treatment failures all had cefixime MICs of 0.12 μg/mL or lower26 – at least one dilution below the CLSI and EUCAST designated elevated MIC level.
Ceftriaxone treatment failures were heralded by a 2011 case report of pharyngeal gonorrhea in a female sex worker in Kyoto, Japan. The infecting strain, named H041, had a ceftriaxone MIC of 2 μg/mL, and was called the ‘superbug’ because it also has high level resistance to cefixime, penicillin and levofloxacin.27 Subsequently, ceftriaxone treatment failures were reported from Sweden,28 Slovenia,29 France25 and Spain.30 The French isolate, F89, is considered the second strain of the gonococcal ‘superbug’, due to resistance to cefixime, ceftriaxone, ciprofloxacin, azithromycin, tetracycline and penicillin.25 Although cases of ceftriaxone treatment failure have been rare to date, they are particularly concerning because they indicate the possibility of widespread, high-level cephalosporin resistance, leading to speculation about the possibility of an era of untreatable gonorrhea.
Molecular mechanisms of resistance
N. gonorrhoeae has developed resistance to antimicrobials over time primarily due to its ability to scavenge DNA of other Neisseria species and incorporate exogenous DNA into its own genome (transformation). The gonococcus also develops resistance through acquisition of plasmids from other bacteria (conjugation), and spontaneous point mutations in response to antibiotic pressure.31
Three main resistance mutations result in decreased susceptibility to beta-lactams: penA, mtrR and penB. penA encodes a change in penicillin binding protein 2 (PBP2), the primary site for beta-lactams’ mechanism of action. mtrR causes an efflux pump to be over-expressed, which leads to increased efflux of antibiotics, particularly ceftriaxone, and can also stimulate penB resistance mutation. penB alters the outermembrane porin (porB1b) to prevent cephalosporins from entering the cell. One potential explanation for the greater prevalence of cefixime resistance than ceftriaxone resistance among GC is that although nearly all cefixime resistance is due to the mosaic penA allele, ceftriaxone requires penA, mtrR and penB mutations to result in clinically relevant resistance.21, 31, 32
SCREENING AND DIAGNOSIS
Gonorrhea can infect multiple mucosal surfaces including urethral, cervicovaginal, oropharyngeal, rectal and conjunctival sites. In men, urethral GC is almost always symptomatic. In contrast, at least 80% of women infected with GC will not exhibit symptoms.3 For this reason, screening of asymptomatic women and partner treatment of heterosexual men with urethral infection are the cornerstones of gonorrhea control in women. In MSM, although 25–30% of urethral cases occur with concurrent pharyngeal infection,33–36 the majority of all gonococcal infections in MSM are isolated to extra-genital sites, the pharynx and/or rectum.37 Extra-genital infections are overwhelmingly asymptomatic38 and under-diagnosed. The persistence of asymptomatic extra-genital gonococcal infections in a community provides an important reservoir for ongoing transmission
Screening Recommendations
The CDC recommends that all sexually active women under 25 years of age undergo annual screening for gonorrhea, and all pregnant women be screened in the first trimester with either a urine, vaginal or endocervical NAAT. (Box 1) Routine screening is also recommended for women over 25 years of age who are at high risk of infection (multiple partners, previously history of STD, commercial sex work, or are part of a population with high prevalence of disease).39 CDC guidelines recommend gonorrhea screening for all sexually active MSM annually with urine NAAT if they report insertive anal or oral intercourse, at the rectum if they report receptive anal intercourse, and at the pharynx if they report performing oral sex. Men who are at high risk of STDs, defined in the CDC guidelines as having multiple or anonymous partners, methamphetamine or amyl nitrate use, or bacterial STD in the previous year, should be screened every 3–6 months.39
Box 1. Screening Recommendations.
All women under 25 years of age should be screened annually with urine or vaginal NAAT.
Women over 25 years of age at high risk for gonorrhea (commercial sex work, multiple partners, a population with high prevalence of disease etc) should also routinely screen.
All pregnant women should be screened during their first trimester.
All men who have sex with men (MSM) should be screened at least annually at all sites exposed in the last 12 months with NAAT (urethra, rectum, pharynx).
MSM at high risk of infection (> 10 partners per year, bacterial STD in the prior 12 months, use amphetamines or amyl nitrates, unprotect anal intercourse) should screen as frequently as every 3 months.
Screening of the pharynx and rectum is of utmost importance in MSM. Between 36% and 85% of GC infections in MSM are missed by urethral testing alone.37, 38 The data on screening for women for extra-genital infection is mixed. Studies conducted in the 1970s and 1980s found that among women with genital tract infections with N. gonorrhoeae, approximately 10–25% had concurrent pharyngeal gonorrhea,33–36, 40–42 and nearly 40% had concurrent rectal infection.42 However, isolated extra-genital infections (without concurrent cervical infection) in women were not common in those studies, and the U.S. CDC does not recommend extra-genital screening for women.42–45 However, recent studies showing higher rates of extra-genital co-infection have stimulated interest in revisiting extragenital screening recommendations for women.43,46
Diagnostic Technology
The advent of nucleic acid amplification tests (NAATs), which detect DNA or RNA, has changed the face of GC screening dramatically. Previous to the widespread use of NAATs, GC was diagnosed either in the clinic with a gram stain of infected fluid or with culture. Culture is insensitive (50–70% sensitivity),3 but highly specific for the detection of GC. NAATs, on the other hand, are both highly sensitive and specific and can be used to screen genital sites, urine or other mucosal areas such as the throat and rectum. Because NAATs can detect nonviable genetic material, transportation and storage of specimens is less cumbersome than with culture, allowing for specimen collection at non-clinical venues. Although the FDA has yet to approve the use of NAATs on extra-genital sites, many studies that have examined the performance of NAATs on pharyngeal and rectal specimens have validated their use,47–49 and the U.S. CDC now recommends the use of NAAT for extra-genital screening.39
While the uptake of NAAT for GC testing is a positive step for gonorrhea control, the major drawback to this technology is the loss of widespread antimicrobial susceptibility testing. Assays designed to detect molecular markers of cephalosporin resistance in GC are under study, but are not commercially available or in widespread use.50 Culture and antimicrobial susceptibility testing is not widely available outside of sites that participate in GISP and GASP, but clinicians who suspect a possible treatment failure should contact local public health authorities for assistance arranging for culture-based testing.
Specimen Collection
Because NAATs require minimal technique for specimen collection, the type of specimen collection is more flexible than with culture. Since the introduction of NAATs, vaginal swabs have become the preferred specimen for gonococcal screening in women due to their superior sensitivity, although urine and cervical specimens are also highly sensitive.51 In a study comparing first-void urine, self-collected vaginal specimens and clinician-collected endocervical specimens, vaginal swabs yielded higher rates of GC detection than the other two methods.52
Another promising use of NAATs is for patient-collected specimens. Self-obtained testing facilitates screening in non-clinical settings, such as in the home or at outreach sites. Programs that offer home-based self-collected vaginal swabs increase testing volume,53 case finding,54 and successfully reach at-risk populations.53–56 Using the Aptima Combo 2, self-collected vaginal swabs perform comparably (sensitivity 98.7%, specificity 99.6%) to clinician-collected vaginal swabs (sensitivity, 96.2%, specificity 99.4%).51 Patients find self-collected vaginal swabs acceptable57 and most women prefer self-collection to a speculum exam or urine collection.55, 56
Self-collection of extra-genital specimens in MSM performs comparatively if not better than clinician-obtained specimens.58–60 In a study of 480 MSM, the concordance between self-collected and clinician-collected pharyngeal specimens for gonorrhea was 96.6%.58 Concordance between rectal self-testing and clinician-testing was 97.1% in another study.60 Moreover, self-collection of extra-genital specimens is acceptable to MSM, and when given a choice for asymptomatic screening, some MSM may prefer home-based testing.61
TREATMENT
Current Treatment Guidelines
The current U.S. CDC recommended treatment for all uncomplicated gonococcal infections is two-drug combination therapy with ceftriaxone 250mg intramuscularly (IM) and either azithromycin 1g orally once or doxycycline 100mg orally twice daily for 7 days.12, 39 (Box 2) A single oral 400mg dose of cefixime in combination with azithromycin or doxycycline has recently been downgraded to an alternative treatment reserved for uncomplicated urogenital infections.12 Cefixime’s removal from first-line treatment recommendations was based on the increasing proportion of isolates with ‘alert value’ MICs,5 reports of treatment failures,22–25 and its inadequacy in eradicating pharyngeal infections39, 62 which are often undiagnosed.63, 64 Highly effective treatment of pharyngeal gonorrhea is a priority because untreated gonococcal infection in the oropharynx may contribute to sustained community transmission and promote the emergence of antimicrobial resistance due to acquisition of resistance genes from commensal Neisseria species.21, 64
Box 2. Current CDC Treatment Guidelines.
-
For all uncomplicated gonococcal infections:
ceftriaxone 250mg IM
PLUS
azithromycin 1gm orally once OR doxycycline 100mg orally twice daily for seven days
If ceftriaxone is not available, 400mg cefixime orally once plus either azithromycin or doxycycline can be considered an alternative therapy, except for pharyngeal gonorrhea.
Penicillin or cephalosporin allergic patients may be treated with 2 g azithromycin orally once.
Persons treated with a non-ceftriaxone containing regimen should return for a test-of-cure 7–10 days following therapy.
All persons with gonococcal infection should return for rescreening 3 months following treatment.
The decision to recommend dual therapy was largely based on expert opinion, experience with other multi-drug resistant organisms where two or more drug regimens more effectively eradicate infection than single drug regimens, and the theory that using two drugs may diminish the risk of inducing or selecting for resistance.10, 65 In practice, dual therapy for gonorrhea has been given for years as empiric co-treatment of chlamydial infections. Limited observational data support the rationale to move to dual therapy. Two retrospective studies suggest that treatment with cefixime and azithromycin is comparable to ceftriaxone, and superior to oral cephalosporin therapy alone or in combination with doxycycline for pharyngeal gonorrhea.66,67
Some nations have elected to increase the recommended dose of their chosen cephalosporin for gonorrhea treatment in an effort to combat emerging resistance. STD treatment guidelines in the United Kingdom recommend ceftriaxone 500mg IM plus azithromycin 1 g.68 Chinese and Japanese guidelines recommend 1 g of ceftriaxone21 and Canadian guidelines recommend cefixime 800mg once for most uncomplicated gonococcal infections, but prefer ceftriaxone 250mg for MSM and pharyngeal infections.69
Potential future treatment options
At present, there is only one novel drug approaching clinical trial stage for treatment of cephalosporin-resistant gonorrhea. Solithromycin is an oral fluoroketolide, a form of a macrolide, that functions by binding to three binding sites on the bacterial ribosome. In vitro studies have found that solithromycin has good activity against cephalosporin-resistant gonorrhea, and against some isolates with low-level azithromycin resistance.21, 70 Further, it has in vitro activity against Chlamydia trachomatis and Mycoplasma genitalium,70, 71 broadening its potential applicability in STD treatment.
Other options for the treatment of resistant gonorrhea include increasing the dose and duration of cephalosporins, reviving the use of older antibiotics, creating new combinations of antibiotics, and changing the tradition of STD treatment from empiric treatment to antimicrobial susceptibility-guided therapy for those with asymptomatic infections. Unemo and colleagues reported that, among GC isolates with “resistant” level MICs to ceftriaxone (≥0.5 μg/mL), the ertapenem MICs were much lower suggesting that ertapenem could function as a back-up regimen for ceftriaxone-resistant gonorrhea.72 Spectinomycin, not currently available in the U.S., remains an option for uncomplicated anogenital gonococcal infection.10 However, spectinomycin is not efficacious at eradicating GC from the pharynx and resistance can emerge rapidly due to a single point mutation.21 Gentamicin has been used successfully as a single agent in practice in Malawi for years without the emergence of resistance,21, 73 but a meta-analysis found that gentamicin did not reach the pre-specified population level of effectiveness (>95%) to warrant recommending its use for gonorrhea treatment.74 Gemifloxacin is a fluoroquinolone with persevered in vitro activity against gonorrhea with ciprofloxacin resistance. The results of a clinical trial evaluating combination therapy with gemifloxacin (320 mg orally) plus azithromycin (2 g orally) compared to azithromycin (2g orally) plus gentamicin (240mg IM) demonstrated high microbiological cure rates with both regimens (99.5% and 98.5%, respectively).
Test-of-Cure and Rescreening
Test-of-cure, a repeat test after GC treatment, is intended to detect treatment failures and is typically done 1 – 4 weeks following treatment. In contrast, rescreening is intended to detect reinfection after successful treatment and is recommended 3 months following treatment for all persons diagnosed with gonorrhea due to the high risk of reinfection.75 In practice, less than half of persons treated for gonorrhea complete rescreening. Prior to the revised gonorrhea treatment guidelines in late 2012, tests-of-cure were reserved for pregnant women, persons who did not complete recommended therapy, and persons with persistent symptoms. Current guidelines recommend test-of-cure for all persons with gonorrhea 7–10 days after treatment if they are not treated with a ceftriaxone-containing regimen.12 Although culture is preferable in order to distinguishing active infection from residual DNA of a successfully treated infection, CDC guidelines consider a positive NAAT 7 days post-treatment to represent a clinical failure.
GISP represents another mechanism to monitor for possible treatment failures. GISP laboratories notify ordering providers and health departments when an isolate with an elevated MIC to cefixime is detected. In such cases, the clinicians and health department should coordinate efforts to contact the patient for retesting at all exposed sites, treatment with first-line therapy, and partner services to ensure that all partners are tested and treated with a regimen containing ceftriaxone. 7, 76
Partner Treatment
Testing and treatment of sexual contacts is a central aspect of gonorrhea control. In all U.S. states, clinicians are legally required to report cases of gonorrhea, and all sexual partners from the past 60 days should receive screening and empiric treatment. Expedited partner therapy (EPT), whereby patients deliver appropriate treatment directly to their sexual partners, increases partner treatment completion and reduces the likelihood of patients testing GC positive at follow-up. 77 Legal regulations regarding EPT vary by state, and EPT is not generally recommended for MSM due to high rates of undiagnosed HIV and STD co-infections in partners of men with gonorrhea. The CDC Cephalosporin-Resistant Neisseria Gonorrhoeae Public Health Response Plan76 recommends that persons exposed to GC be informed that dual therapy with ceftriaxone is the most effective treatment and be advised to seek clinical evaluation. However, EPT with cefixime and azithromycin is still an option encouraged for partner treatment of heterosexuals with gonorrhea in many states.12, 76 The potential for poorer treatment efficacy of decreased susceptibility GC isolates with a fully oral EPT regimen in sex partners of persons with gonorrhea must be considered in the context of higher treatment completion with EPT. Not all contacts to gonorrhea have gonococcal infection, and reduced susceptibility to cefixime in GC isolates from heterosexual men remains very rare.7 Thus, at least at present, the public health benefits of EPT likely outweigh the risk of EPT-associated treatment failure.
A gonorrhea vaccine?
The ultimate solution for infectious disease public health prevention is a vaccine. Unfortunately efforts to develop a vaccine against gonorrhea have been met with many challenges. The primary challenge is the lack of sustained, systemic host immunity to GC. The immune response to gonococcal infections primarily occurs locally at the mucosal level. This local immunity is not sustained; hence individuals may be reinfected multiple times with the same strain. At the level of the organism, the gonococcal surface exhibits a variety of antigens, which can change over time, making identification of an antibody target difficult. Despite these impediments investigators have made successful early steps toward a gonococcal vaccine.21, 78
SUMMARY AND CONCLUSION
Gonorrhea remains an important communicable disease throughout the globe, and the gonococcus’ continual evolution to evade antimicrobials combined with a current lack of novel antimicrobial therapies makes cephalosporin-resistant gonorrhea a major public health threat. Increased screening of persons at risk for gonorrhea, appropriate and timely treatment for infected persons and their partners, active surveillance for antimicrobial resistance, and mobilization of public health resources in response to suspected cases of treatment failure are crucial for control of cephalosporin-resistant gonorrhea.
KEY POINTS.
Gonorrhea is the second most common bacterial STD worldwide. Untreated infection can lead to infertility in women and increase the risk of HIV transmission and acquisition.
Cephalosporin-resistant Neisseria gonorrhoeae is now a major public health threat as strains demonstrating decreased susceptibility to oral cephalosporins become more common.
Although a few ceftriaxone treatment failures have been reported worldwide, intramuscular ceftriaxone combined with oral azithromycin remains a highly effective treatment for gonorrhea.
Screening for asymptomatic infections in at-risk women and men who have sex with men is central to gonorrhea control
Acknowledgments
DISCLOSURES
Funding sources:
Dr. Barbee: National Institutes of Health Sexually Transmitted Diseases Training Grant [T32 67-4198], National Institutes of Allergy and Infectious Diseases Division of Microbiology and Infectious Diseases Contract # HHSN272200800026C and the Seattle Sexually Transmitted Diseases Prevention Training Center.
Dr. Dombrowski: National Institutes of Mental Health (K23MH090923) and the University of Washington Center for AIDS Research (CFAR), an NIH funded program (P30 AI027757) which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, and NIA.
Footnotes
Conflicts of Interest: Dr. Barbee: none
Dr. Dombrowski: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Global Incidence and Prevalence of Selected Curable Sexually Transmitted Infections - 2008. World Health Organization; 2012. [Google Scholar]
- 2.CDC. Sexually Transmitted Disease Surveillance, 2010. Atlanta, GA: US Department of Health and Human Services; 2011. [Google Scholar]
- 3.Gerald L, Mandell JEB, Dolin Raphael, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 7. Elsevier; 2009. [Google Scholar]
- 4.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sexually transmitted infections. 1999;75(1):3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolan GA, Sparling PF, Wasserheit JN. The emerging threat of untreatable gonococcal infection. The New England journal of medicine. 2012;366(6):485–7. doi: 10.1056/NEJMp1112456. [DOI] [PubMed] [Google Scholar]
- 6.CDC. Sexually Transmitted Disease Surveillance, 2011. Atlanta, GA: US Department of Health and Human Services; 2012. [Google Scholar]
- 7.Kirkcaldy RD, Zaidi A, Hook EW, et al. Neisseria gonorrhoeae Antimicrobial Resistance Among Men Who Have Sex With Men and Men Who Have Sex Exclusively With Women: The Gonococcal Isolate Surveillance Project, 2005–2010. Annals of internal medicine. 2013;158(5 Pt 1):321–8. doi: 10.7326/0003-4819-158-5-201303050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tapsall JW, Limnios EA, Abu Bakar HM, et al. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific and South East Asian regions, 2007–2008. Communicable diseases intelligence. 2010;34(1):1–7. [PubMed] [Google Scholar]
- 9.Cole MJ, Unemo M, Hoffmann S, Chisholm SA, Ison CA, van de Laar MJ. The European gonococcal antimicrobial surveillance programme, 2009. Euro Surveill. 2011;16(42) [PubMed] [Google Scholar]
- 10.Workowski KA, Berman SM, Douglas JM., Jr Emerging antimicrobial resistance in Neisseria gonorrhoeae: urgent need to strengthen prevention strategies. Annals of internal medicine. 2008;148(8):606–13. doi: 10.7326/0003-4819-148-8-200804150-00005. [DOI] [PubMed] [Google Scholar]
- 11.Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1–94. [PubMed] [Google Scholar]
- 12.Update to CDC’s Sexually Transmitted Diseases Treatment Guidelines, 2010: Oral Cephalosporins No Longer a Recommended Treatment for Gonococcal Infections. Mmwr. 2012;61(31):590– 4. [PubMed] [Google Scholar]
- 13.Ito M, Yasuda M, Yokoi S, et al. Remarkable increase in central Japan in 2001–2002 of Neisseria gonorrhoeae isolates with decreased susceptibility to penicillin, tetracycline, oral cephalosporins, and fluoroquinolones. Antimicrobial agents and chemotherapy. 2004;48(8):3185–7. doi: 10.1128/AAC.48.8.3185-3187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo JY, Ho KM, Leung AO, et al. Ceftibuten resistance and treatment failure of Neisseria gonorrhoeae infection. Antimicrobial agents and chemotherapy. 2008;52(10):3564–7. doi: 10.1128/AAC.00198-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H, Hong SG, Soe Y, et al. Trends in antimicrobial resistance of Neisseria gonorrhoeae isolated from Korean patients from 2000 to 2006. Sex Transm Dis. 2011;38(11):1082–6. doi: 10.1097/OLQ.0b013e31822e60a4. [DOI] [PubMed] [Google Scholar]
- 16.Lahra MM. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific and South East Asian Regions, 2010. Communicable diseases intelligence. 2012;36(1):95–100. [PubMed] [Google Scholar]
- 17.Van de Laar M, Spiteri G. Increasing trends of gonorrhoea and syphilis and the threat of drug-resistant gonorrhoea in Europe. Euro Surveill. 2012;17(29) [PubMed] [Google Scholar]
- 18.European Centre for Disease Prevention and Control. Gonococcal antimicrobial susceptibility surveillance in Europe – 2010. Stockholm: ECDC; 2012. [Google Scholar]
- 19.Yokoi S, Deguchi T, Ozawa T, et al. Threat to cefixime treatment for gonorrhea. Emerging infectious diseases. 2007;13(8):1275–7. doi: 10.3201/eid1308.060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deguchi T, Yasuda M, Yokoi S, et al. Treatment of uncomplicated gonococcal urethritis by double-dosing of 200 mg cefixime at a 6-h interval. J Infect Chemother. 2003;9(1):35–9. doi: 10.1007/s10156-002-0204-8. [DOI] [PubMed] [Google Scholar]
- 21.Unemo M, Nicholas RA. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future microbiology. 2012;7:1401–22. doi: 10.2217/fmb.12.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unemo M, Golparian D, Syversen G, Vestrheim DF, Moi H. Two cases of verified clinical failures using internationally recommended first-line cefixime for gonorrhoea treatment, Norway, 2010. Euro Surveill. 2010;15(47) doi: 10.2807/ese.15.47.19721-en. [DOI] [PubMed] [Google Scholar]
- 23.Ison CA, Hussey J, Sankar KN, Evans J, Alexander S. Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Euro Surveill. 2011;16(14) [PubMed] [Google Scholar]
- 24.Unemo M, Golparian D, Stary A, Eigentler A. First Neisseria gonorrhoeae strain with resistance to cefixime causing gonorrhoea treatment failure in Austria, 2011. Euro Surveill. 2011;16(43) [PubMed] [Google Scholar]
- 25.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. High-level cefiximeand ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrobial agents and chemotherapy. 2012;56(3):1273–80. doi: 10.1128/AAC.05760-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen VG, Mitterni L, Seah C, et al. Neisseria gonorrhoeae treatment failure and susceptibility to cefixime in Toronto, Canada. JAMA. 2013;309(2):163– 70. doi: 10.1001/jama.2012.176575. [DOI] [PubMed] [Google Scholar]
- 27.Ohnishi M, Golparian D, Shimuta K, et al. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrobial agents and chemotherapy. 2011;55(7):3538–45. doi: 10.1128/AAC.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unemo M, Golparian D, Hestner A. Ceftriaxone treatment failure of pharyngeal gonorrhoea verified by international recommendations, Sweden, July 2010. Euro Surveill. 2011;16(6) [PubMed] [Google Scholar]
- 29.Unemo M, Golparian D, Potocnik M, Jeverica S. Treatment failure of pharyngeal gonorrhoea with internationally recommended first-line ceftriaxone verified in Slovenia, September 2011. Euro Surveill. 2012;17(25) [PubMed] [Google Scholar]
- 30.Carnicer-Pont D, Smithson A, Fina-Homar E, Bastida MT. First cases of Neisseria gonorrhoeae resistant to ceftriaxone in Catalonia, Spain, May 2011. Enfermedades infecciosas y microbiologia clinica. 2012;30(4):218–9. doi: 10.1016/j.eimc.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Unemo M, Shafer WM. Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future. Annals of the New York Academy of Sciences. 2011;1230:E19–28. doi: 10.1111/j.1749-6632.2011.06215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindberg R, Fredlund H, Nicholas R, Unemo M. Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: association with genetic polymorphisms in penA, mtrR, porB1b, and ponA. Antimicrobial agents and chemotherapy. 2007;51(6):2117–22. doi: 10.1128/AAC.01604-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiesner PJ, Tronca E, Bonin P, Pedersen AH, Holmes KK. Clinical spectrum of pharyngeal gonococcal infection. The New England journal of medicine. 1973;288(4):181–5. doi: 10.1056/NEJM197301252880404. [DOI] [PubMed] [Google Scholar]
- 34.Bro-Jorgensen A, Jensen T. Gonococcal pharyngeal infections. Report of 110 cases. The British journal of venereal diseases. 1973;49(6):491–9. doi: 10.1136/sti.49.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tice AW, Jr, Rodriguez VL. Pharyngeal gonorrhea. Jama. 1981;246(23):2717–9. [PubMed] [Google Scholar]
- 36.Kinghorn G. Pharyngeal gonorrhoea: a silent cause for concern. Sexually transmitted infections. 2010;86(6):413–4. doi: 10.1136/sti.2010.043349. [DOI] [PubMed] [Google Scholar]
- 37.Marcus JL, Bernstein KT, Kohn RP, Liska S, Philip SS. Infections missed by urethral-only screening for chlamydia or gonorrhea detection among men who have sex with men. Sex Transm Dis. 2011;38(10):922–4. doi: 10.1097/OLQ.0b013e31822a2b2e. [DOI] [PubMed] [Google Scholar]
- 38.Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis. 2005;41(1):67–74. doi: 10.1086/430704. [DOI] [PubMed] [Google Scholar]
- 39.Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110. [PubMed] [Google Scholar]
- 40.Sulaiman MZ, Bates CM, Bittiner JB, Dixon CA, Slack RC. Response of pharyngeal gonorrhoea to single dose penicillin treatment. Genitourinary medicine. 1987;63(2):92–4. doi: 10.1136/sti.63.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed-Jushuf IH, Bradley MG, Rao PM. Oropharyngeal carriage of Neisseria gonorrhoeae and its response to treatment in patients with anogenital infection. Genitourinary medicine. 1988;64(1):64–5. doi: 10.1136/sti.64.1.64-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Handsfield HH, Knapp JS, Diehr PK, Holmes KK. Correlation of auxotype and penicillin susceptibility of Neisseria gonorrhoeae with sexual preference and clinical manifestations of gonorrhea. Sex Transm Dis. 1980;7(1):1–5. doi: 10.1097/00007435-198001000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Giannini CM, Kim HK, Mortensen J, Mortensen J, Marsolo K, Huppert J. Culture of non-genital sites increases the detection of gonorrhea in women. Journal of pediatric and adolescent gynecology. 2010;23(4):246–52. doi: 10.1016/j.jpag.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Kinghorn GR, Rashid S. Prevalence of rectal and pharyngeal infection in women with gonorrhoea in Sheffield. The British journal of venereal diseases. 1979;55(6):408–10. doi: 10.1136/sti.55.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raychaudhuri M, Birley HD. Audit of routine rectal swabs for gonorrhoea culture in women. International journal of STD & AIDS. 2010;21(2):143–4. doi: 10.1258/ijsa.2009.009076. [DOI] [PubMed] [Google Scholar]
- 46.Javanbakht M, Gorbach P, Stirland A, Chien M, Kerndt P, Guerry S. Prevalence and correlates of rectal Chlamydia and gonorrhea among female clients at sexually transmitted disease clinics. Sex Transm Dis. 2012;39(12):917–22. doi: 10.1097/OLQ.0b013e31826ae9a2. [DOI] [PubMed] [Google Scholar]
- 47.Schachter J, Moncada J, Liska S, Shayevich C, Klausner JD. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis. 2008;35(7):637–42. doi: 10.1097/OLQ.0b013e31817bdd7e. [DOI] [PubMed] [Google Scholar]
- 48.Bachmann LH, Johnson RE, Cheng H, et al. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. Journal of clinical microbiology. 2010;48(5):1827–32. doi: 10.1128/JCM.02398-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bachmann LH, Johnson RE, Cheng H, Markowitz LE, Papp JR, Hook EW., 3rd Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae oropharyngeal infections. Journal of clinical microbiology. 2009;47(4):902–7. doi: 10.1128/JCM.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandori M, Barry PM, Wu A, et al. Mosaic penicillin-binding protein 2 in Neisseria gonorrhoeae isolates collected in 2008 in San Francisco, California. Antimicrobial agents and chemotherapy. 2009;53(9):4032–4. doi: 10.1128/AAC.00406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis. 2005;32(12):725–8. doi: 10.1097/01.olq.0000190092.59482.96. [DOI] [PubMed] [Google Scholar]
- 52.Shafer MA, Moncada J, Boyer CB, Betsinger K, Flinn SD, Schachter J. Comparing first-void urine specimens, self-collected vaginal swabs, and endocervical specimens to detect Chlamydia trachomatis and Neisseria gonorrhoeae by a nucleic acid amplification test. Journal of clinical microbiology. 2003;41(9):4395–9. doi: 10.1128/JCM.41.9.4395-4399.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert review of anti-infective therapy. 2011;9(2):183–94. doi: 10.1586/eri.10.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rotblatt H, Montoya JA, Plant A, Guerry S, Kerndt PR. There’s No Place Like Home: First-Year Use of the “I Know” Home Testing Program for Chlamydia and Gonorrhea. American journal of public health. 2013 doi: 10.2105/AJPH.2012.301010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graseck AS, Secura GM, Allsworth JE, Madden T, Peipert JF. Home screening compared with clinic-based screening for sexually transmitted infections. Obstetrics and gynecology. 2010;115(4):745–52. doi: 10.1097/AOG.0b013e3181d4450d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chernesky MA, Hook EW, 3rd, Martin DH, et al. Women find it easy and prefer to collect their own vaginal swabs to diagnose Chlamydia trachomatis or Neisseria gonorrhoeae infections. Sex Transm Dis. 2005;32(12):729–33. doi: 10.1097/01.olq.0000190057.61633.8d. [DOI] [PubMed] [Google Scholar]
- 57.Wiesenfeld HC, Lowry DL, Heine RP, et al. Self-collection of vaginal swabs for the detection of Chlamydia, gonorrhea, and trichomoniasis: opportunity to encourage sexually transmitted disease testing among adolescents. Sex Transm Dis. 2001;28(6):321–5. doi: 10.1097/00007435-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Freeman AH, Bernstein KT, Kohn RP, Philip S, Rauch LM, Klausner JD. Evaluation of Self-Collected Versus Clinician-Collected Swabs for the Detection of Chlamydia trachomatis and Neisseria gonorrhoeae Pharyngeal Infection Among Men Who Have Sex With Men. Sex Transm Dis. 2011;38(11):1036–9. doi: 10.1097/OLQ.0b013e318227713e. [DOI] [PubMed] [Google Scholar]
- 59.Alexander S, Ison C, Parry J, et al. Self-taken pharyngeal and rectal swabs are appropriate for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in asymptomatic men who have sex with men. Sexually transmitted infections. 2008;84(6):488–92. doi: 10.1136/sti.2008.031443. [DOI] [PubMed] [Google Scholar]
- 60.Sexton ME, Baker JJ, Nakagawa K, et al. How reliable is self-testing for gonorrhea and chlamydia among men who have sex with men? The Journal of family practice. 2013;62(2):70–8. [PubMed] [Google Scholar]
- 61.Wayal S, Llewellyn C, Smith H, Fisher M. Home sampling kits for sexually transmitted infections: preferences and concerns of men who have sex with men. Culture, health & sexuality. 2011;13(3):343–53. doi: 10.1080/13691058.2010.535018. [DOI] [PubMed] [Google Scholar]
- 62.Moran JS. Treating uncomplicated Neisseria gonorrhoeae infections: is the anatomic site of infection important? Sex Transm Dis. 1995;22(1):39–47. doi: 10.1097/00007435-199501000-00007. [DOI] [PubMed] [Google Scholar]
- 63.Moran JS, Levine WC. Drugs of choice for the treatment of uncomplicated gonococcal infections. Clin Infect Dis. 1995;20 (Suppl 1):S47–65. doi: 10.1093/clinids/20.supplement_1.s47. [DOI] [PubMed] [Google Scholar]
- 64.Weinstock H, Workowski KA. Pharyngeal gonorrhea: an important reservoir of infection? Clin Infect Dis. 2009;49(12):1798–800. doi: 10.1086/648428. [DOI] [PubMed] [Google Scholar]
- 65.Whiley DM, Goire N, Lahra MM, et al. The ticking time bomb: escalating antibiotic resistance in Neisseria gonorrhoeae is a public health disaster in waiting. The Journal of antimicrobial chemotherapy. 2012;67(9):2059–61. doi: 10.1093/jac/dks188. [DOI] [PubMed] [Google Scholar]
- 66.Barbee LA, Kerani RP, Dombrowski JC, Soge OO, Golden MR. A Retrospective Comparative Study of Two-Drug Oral and Intramuscular Cephalosporin Treatment Regimens for Pharyngeal Gonorrhea. Clin Infect Dis. 2013 doi: 10.1093/cid/cit084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sathia L, Ellis B, Phillip S, Winston A, Smith A. Pharyngeal gonorrhoea - is dual therapy the way forward? International journal of STD & AIDS. 2007;18(9):647–8. doi: 10.1258/095646207781568556. [DOI] [PubMed] [Google Scholar]
- 68.Bignell C, Fitzgerald M. UK national guideline for the management of gonorrhoea in adults, 2011. International journal of STD & AIDS. 2011;22(10):541–7. doi: 10.1258/ijsa.2011.011267. [DOI] [PubMed] [Google Scholar]
- 69. [Accessed February 4, 2013];Public Health Agency of Canada Important Notice on Gonococcal Infection. 2011 at http://www.phac-aspc.gc.ca/std-mts/sti-its/alert/2011/alert-gonoeng.php.
- 70.Workowski K. Treatment in an Era of Dwindling Treatment Options. Oral presentation at the CDC National STD Prevention Conference 2012; Minneapolis, MN. March 12–15, 2012; 2012. [Google Scholar]
- 71.Roblin PM, Kohlhoff SA, Parker C, Hammerschlag MR. In vitro activity of CEM-101, a new fluoroketolide antibiotic, against Chlamydia trachomatis and Chlamydia (Chlamydophila) pneumoniae. Antimicrobial agents and chemotherapy. 2010;54(3):1358–9. doi: 10.1128/AAC.01343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Unemo M, Golparian D, Limnios A, et al. In vitro activity of ertapenem versus ceftriaxone against Neisseria gonorrhoeae isolates with highly diverse ceftriaxone MIC values and effects of ceftriaxone resistance determinants: ertapenem for treatment of gonorrhea? Antimicrobial agents and chemotherapy. 2012;56(7):3603–9. doi: 10.1128/AAC.00326-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brown LB, Krysiak R, Kamanga G, et al. Neisseria gonorrhoeae antimicrobial susceptibility in Lilongwe, Malawi, 2007. Sex Transm Dis. 2010;37(3):169–72. doi: 10.1097/OLQ.0b013e3181bf575c. [DOI] [PubMed] [Google Scholar]
- 74.Dowell D, Kirkcaldy RD. Effectiveness of gentamicin for gonorrhoea treatment: systematic review and meta-analysis. Sexually transmitted infections. 2012;88(8):589–94. doi: 10.1136/sextrans-2012-050604. [DOI] [PubMed] [Google Scholar]
- 75.Peterman TA, Tian LH, Metcalf CA, et al. High incidence of new sexually transmitted infections in the year following a sexually transmitted infection: a case for rescreening. Annals of internal medicine. 2006;145(8):564–72. doi: 10.7326/0003-4819-145-8-200610170-00005. [DOI] [PubMed] [Google Scholar]
- 76.Cephalosporin-resistant Neisseria Gonorrhoeae Public Health Response Plan. Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 77.Golden MR, Whittington WL, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. The New England journal of medicine. 2005;352(7):676–85. doi: 10.1056/NEJMoa041681. [DOI] [PubMed] [Google Scholar]
- 78.Zhu W, Chen CJ, Thomas CE, Anderson JE, Jerse AE, Sparling PF. Vaccines for gonorrhea: can we rise to the challenge? Frontiers in microbiology. 2011;2:124. doi: 10.3389/fmicb.2011.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]


