Abstract
Experimental studies have shown heart rates to decrease from embryo to hatchling stage in turtles, remain steady in skinks, and increase in birds. However, no snake species has been studied in this regard. I recorded heart rate evolution trajectories from embryo to juvenile stage in 78 eggs from two species of European Natricine snakes. Unexpectedly, snakes behaved more like birds than turtles or lizards: heart rates increased after hatching in both N. maura and N. natrix, respectively by 43.92 ± 22.84% and 35.92 ± 24.52%. Heart rate shift was not related to an abrupt elevation of metabolism per se (snakes that increased their heart rates the most sharply grew the least after birth), but rather due to a number of smaller eggs that experienced lower than normal heart rates throughout the incubation and recovered a normal heart rate post-birth. This finding is discussed in the light of hatching synchrony benefits.
Ontogenetic shifts in heart rates from embryo to hatchling stage are known to occur in a variety of amniotic vertebrates, in both ectotherms and endotherms. Such shifts may translate into a drop in heart rate (in turtles1,2,3,4), little or no change (in skinks5,6) or an increase in heart rate (in birds7). These shifts in heart rates may reflect the tremendous physiological changes accompanying hatching, from respiration, locomotion, through to energetic independence of the newly hatched organism8. However, why the direction and intensity of changes varies amongst groups remains an open question.
In birds, Pearson & Tazawa7 suggested that the ontogenetic shift in heart rate may be explained by the development of endothermic metabolism, hence an elevation in metabolic rate. In the emydid turtles Chrysemys picta and Graptemys pseudogeographica kohniiturtles, it was proposed that a drop in heart rate from embryo to hatchling may be adaptive4. That is, while developing embryos may benefit from accelerated developmental rate (earlier hatching typically enhances offspring fitness9,10), young turtles no longer have a guaranteed food supply (i.e. yolk contained within the egg). Young turtles thus benefit from a lower post-hatching metabolic rate presumably in order to avoid fast depletion of resources in the face of uncertain levels of food availability4. Squamate reptiles such as the three-lined skink (Bassiana duperreyi) and the eastern fence lizards (Sceloporus undulatus) seem to show a different pattern, with little or no ontogenetic shift in heart rates from embryo to hatchling stages5,6. Du et al.4 explained this lack of consistency by the fact that annual survival rates average much higher in turtles than in squamates11. As a result, short-lived lizards (i.e. compared to turtles) may adopt “riskier” life-history tactics, expending energy at high rates despite the uncertainty about future food availability12.
Clearly, the current scarcity of experimental data, both within and amongst amniotic taxa, greatly limits our understanding of the physiological processes involved in over-birth shifts in heart rates, their interactions with life history traits (i.e. sex, body size, growth rates), and ultimately the adaptive value of such shifts. Further, to my knowledge, no snake species has previously been studied in this regard, so that no general pattern in heart rates can be yet defined amongst squamates.
The purpose of this study was thus to advance our knowledge in this regard, with special emphasis given to the physiological aspects of heart rate variations from embryo to hatchling. I recorded heart rate evolution trajectories from embryo to juvenile stage in 78 eggs from two species of European natricine snakes (the viperine snake Natrix maura and grass snake Natrix natrix) so as to precisely document the potential interactions amongst species, sex, egg mass, hatchling body size, post-hatching growth rates and resting metabolism.
Results
Trait comparison between sexes, species and interactions
Egg mass
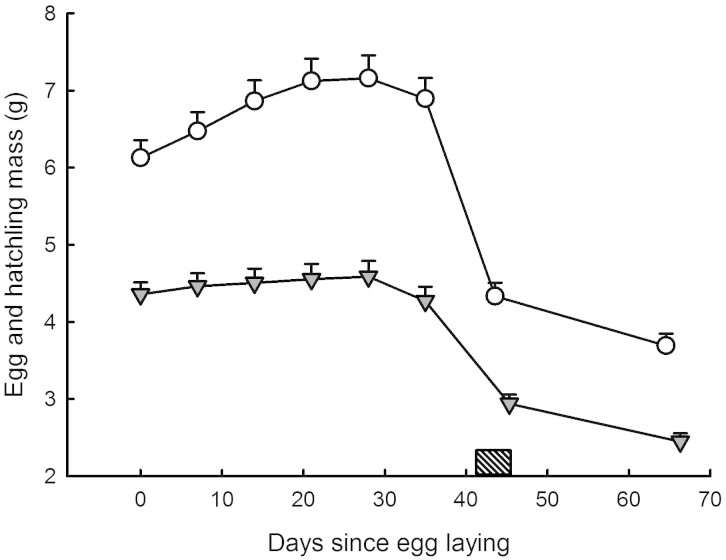
A two factor repeated measures ANOVA with species and sex as factors and the five successive egg masses as repeated measures yielded F4, 284 = 1.80; P = 0.13 (global interaction term; see Table 1). There was no effect of sex on egg mass evolution trajectories. N. natrix eggs were heavier at oviposition, and grew at higher rates than N. maura eggs (14.95 ± 6.76% increase in mass versus 4.10 ± 6.65% respectively; P < 0.001) throughout the incubation period. Maximum egg mass was reached around day 28 of the incubation period in both species (Fig. 1). I calculated a mean egg mass, averaged across the incubation period, for each individual within each species.
Table 1. Statistical results of a repeated measure ANOVA with species and sex as factors and egg mass as repeated variable.
| Effect | SS | Df | MS | F | P |
|---|---|---|---|---|---|
| Species | 2.368 | 1 | 2.368 | 51.94 | 0.0001 |
| Sex | .052 | 1 | 0.052 | 1.13 | 0.291 |
| Species * Sex | .023 | 1 | 0.023 | 0.50 | 0.482 |
| Egg mass | .058 | 4 | 0.014 | 34.89 | 0.0001 |
| Egg mass * Species | .035 | 4 | 0.009 | 21.19 | 0.0001 |
| Egg mass * Sex | .001 | 4 | 0.001 | 0.49 | 0.739 |
| Egg mass * Species * Sex | .003 | 4 | 0.001 | 1.80 | 0.129 |
Figure 1. Mass evolution was measured in 28 eggs and hatchlings of the grass snake N. natrix (open circles) and 51 eggs and hatchlings Viperine snake N. maura (grey triangles).
A repeated measure ANOVA with species as factor and egg/hatchling mass as the repeated measure over time yielded F7, 469 = 7.38; P < 0.001. Egg masses were measured every week. Snake masses were measured at birth and 3 weeks old. Snakes were unfed. Means ± SE are plotted.
Heart rates
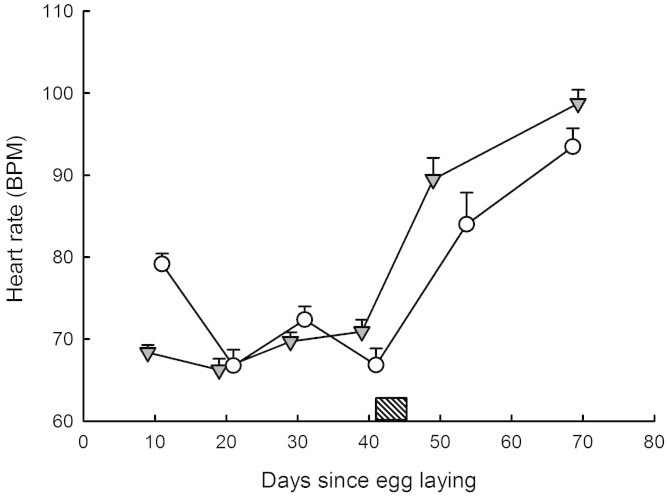
A two factor repeated measures ANOVA with species and sex as factors and the five successive heart rate readings as repeated measures yielded F4, 284 = 0.47, P = 0.76 (global interaction term; see Table 2). There was no difference in heart rates between sexes. Heart rates changed throughout the incubation period and after hatching (P < 0.001). Heart rate trajectories were comparable between species (see Fig. 2). There was, however, an interaction between heart rate readings across time and species (P < 0.001; see Fig. 2), possibly due to an initial difference in heart rates between N. natrix and N. maura (incubation time = 10 days). Post-hoc analysis (Tukey HSD) confirmed that heart rates only differed between species at egg age = 10 days (P < 0.001). I calculated mean embryo heart rates, averaged across the incubation period, for each individual within each species.
Table 2. Statistical results of a repeated measure ANOVA with species and sex as factors and heart rates as repeated variable.
| Effect | SS | Df | MS | F | P |
|---|---|---|---|---|---|
| Species | 0.006 | 1 | 0.006 | 1.27 | 0.263 |
| Sex | 0.001 | 1 | 0.001 | 0.27 | 0.603 |
| Species * Sex | 0.008 | 1 | 0.008 | 1.84 | 0.179 |
| Heart rate | 1.143 | 4 | 0.286 | 93.36 | 0.0001 |
| Heart rate * Sex | 0.001 | 4 | 0.001 | 0.04 | 0.997 |
| Heart rate * Species | 0.089 | 4 | 0.022 | 7.23 | 0.0001 |
| Heart rate * Sex * Species | 0.006 | 4 | 0.001 | 0.47 | 0.761 |
Figure 2. Heart rates were measured in 28 eggs and hatchlings N. natrix (open circles) and 51 eggs and hatchlings N. maura (grey triangles).
A repeated measure ANOVA with species as factor and heart beats as the repeated measure over time (every 10 days from laying and in hatchlings aged 24.35 ± 3.74 days) yielded: effect of species F1, 76 = 0.80, P = 0.38; effect of time F4, 304 = 101.66, P < 0.001; interaction term F4, 304 = 8.40, P < 0.001. Hatching occurred between the 41st and 45th days post laying (dash box). All measurements were made at a constant 28°C. Means ± SE are plotted.
Heart rates, egg size and body size
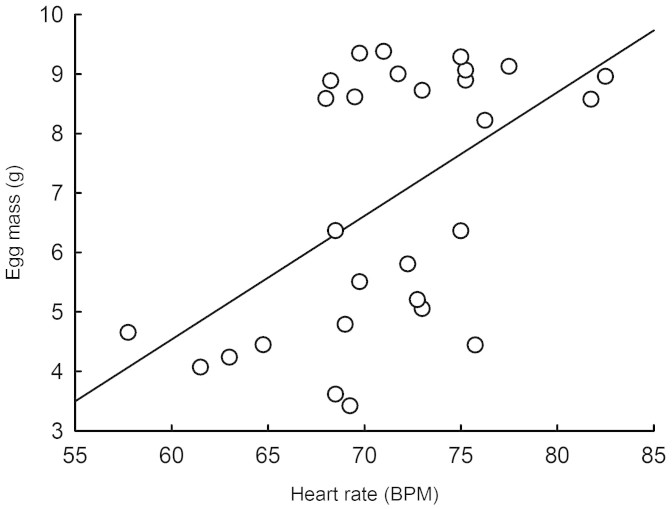
N. maura egg mass at oviposition largely explained hatchling body size (body mass, snout-vent length and BCI (Body Condition Index; see methods for details); Linear Regressions; all R > 0.47; all P < 0.006), while N. natrix egg mass at oviposition positively correlated with hatchling body mass and snout-vent length (both R > 0.88; both P < 0.001), but not with BCI (R = 0.36; P = 0.057). Embryo mean heart rates were not correlated with incubation duration in either species (both R < 0.17; both P > 0.25). Embryo mean heart rates were, however, positively correlated to mean egg mass in N. natrix (R = 0.54; P = 0.003; Fig. 3) but not in N. maura (R = 0.01; P = 0.99). Post-hatching heart rates were unrelated to hatchling body size in N. natrix (all R < 0.24; all P > 0.21). In N. maura however, post-hatching heart rates were positively correlated with SVL (R = 0.37; P < 0.009), negatively correlated with BCI (R = 0.31 P < 0.034), but unrelated to body mass (R = 0.01 P = 0.93).
Figure 3. Linear regression of mean embryo heart rates against mean egg mass in 28 N. natrix eggs (R = 0.54; F1, 26 = 10.66; P < 0.003).
Shift in heart rates and growth rates
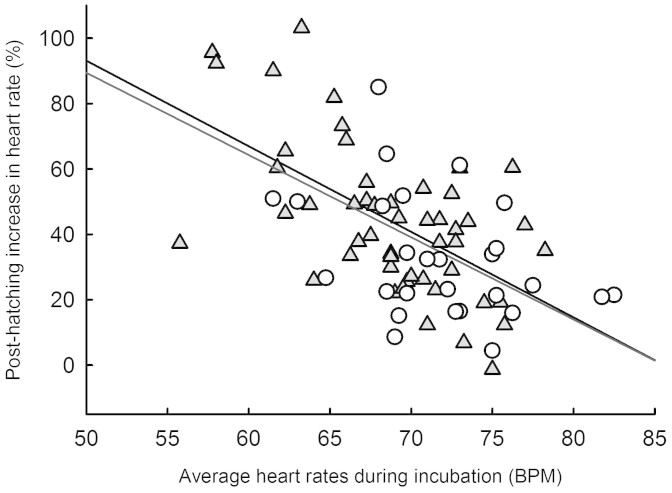
In N. maura, the over-birth shift in heart rates was not related to concurrent growth rates in body mass (R = 0.21; P = 0.15), but negatively correlated with growth rate in snout-vent length (R = 0.34; P < 0.017). In N. natrix however, the shift in heart rates was not correlated with any of the body size traits or growth rates recorded (all R < 0.31; all P > 0.11). Surprisingly, there was a negative correlation between the relative amplitude of the shift in heart rates (% of increase) and the mean heart rates across the incubation period (Fig. 4), both in N. maura (R = 0.58; P < 0.001) and N. natrix (R = 0.57; P < 0.002).
Figure 4. Relative amplitude of the shift in heart rates (% of increase) against heart rates averaged across the incubation period in N. natrix (open circles; R = 0.57; F1, 26 = 12.42; P < 0.002) and N. maura (grey triangles; R = 0.58; F1, 48 = 24.75; P < 0.001).
Discussion
This experimental study demonstrated that an ontogenetic shift in heart rates from embryo to hatchling stage may occur in Natricine snakes, and, as in emydid turtles, rather late in embryogenesis (i.e. in the final quarter of the incubation period4). Further, the direction of the shift was opposite to that of turtles: heart rates sharply increased immediately after hatching with similar amplitude in both N. maura and N. natrix, respectively by 43.92 ± 22.84% and 35.92 ± 24.52%. As such, these results are not only contrary to expectations based on previous studies of squamates reptiles (i.e. little or no shift in heart rates from embryo to hatchling5,6) but suggest that snake heart rates behaved more like birds (i.e. increase in heart rates) rather than turtles or lizards.
Given this unexpected result, one cannot exclude that some of the discrepancies found across relevant studies4,5,6 may stem from inconstant recording methods of metabolic rates in both embryos and hatchlings. For instance, while metabolic rates were in all cases recorded at similar temperatures in this study (between 28 and 30°C), Angilletta et al.5 estimated metabolic rates in the lizard Sceloporus undulatus using a flow-through system rather than via heart rate readings. Moreover, Radder & Shine6 used, as in the current study, the Buddy digital egg monitor, but anaesthetized week-old lizards, Bassiana duperreyi, with gaseous (Fluothane) inhalation prior to recording heart rates. On the other hand, the way Du et al.4 collected their data on emydid turtles was fully consistent with the method applied to the current study. There is also a possibility that Natricine snakes are simply unusual in this regard and an exception amongst squamates. Further studies using consistent methods on a wider range of species will easily address these two possibilities. Nevertheless, this study provides new and challenging data to our current understanding, within and across amniotic groups, of the ontogenetic shift in heart rates from embryo to hatchling.
The bird ontogenetic shift in heart rates is explained by the development of endothermic metabolism, and hence an elevation in metabolic rate7. Thus, a sharp increase in heart rates may also presumably be related to a sudden shift in metabolic rates in young snakes. Yet, this was not supported by the data: the increase in heart rates was not related to growth rates in body mass recorded during the first 3 weeks after birth in both N. natrix and N. maura unfed snakes. In fact, the increase in heart rates was surprisingly negatively related to growth rates in snout-vent length: snakes that increased their heart rates the most sharply grew the least after birth. One striking result may provide some clues towards the explanation of this counter-intuitive finding: in both species and with similar amplitude, the shift in heart rates from embryo to hatchling was negatively correlated with heart rates throughout the incubation period. In other words, embryos that displayed slow heart rates during the incubation period strongly increased heart rates after birth (perhaps in a compensatory manner), while embryos that displayed already relatively high heart rates hardly shifted at all after birth (see Fig. 4). Further, as in turtles4, post-hatching heart rates in Natrix appear to be maintained through into adult life. Mean post-hatching heart rates were 98.74 ± 12.15 BPM in N. maura and 93.46 ± 11.02 BPM in N. natrix. Such values are directly comparable with heart rates measured in sub-adult N. maura that maintained heart rates between 90 and 100 BPM at 28°C body temperature13. This strongly suggests that high post-hatching heart rates in Natricine snakes are in fact “the norm”. Some eggs seemingly experienced lower than normal heart rate values while incubating, and at birth, recovered normal heart rate values.
Are there any clues as to why some eggs may have incubated under sub-optimal conditions? Because the most drastic change between embryo and hatchling is a shift in respiration mode (i.e. through the egg shell versus lungs), one possibility is that some eggs experienced sub-optimal conditions during embryogenesis, perhaps due to impaired gas exchange due to egg positioning (for instance: embryo facing the substrate rather than the open air). Alternatively, it is striking that despite large differences in mean embryo heart rates amongst individual eggs (up to 40% in N. maura and 43% in N. natrix), incubation duration was unrelated to mean embryo heart rates but more so to egg mass at oviposition (i.e. because small eggs tended to have slower heart rates). This result is contrary to that found in other non-avian reptiles14 as well as in birds15 where embryonic heart rate (1) predicts incubation period and (2) is higher in species with smaller egg sizes and shorter incubation periods16.
In the current experiment, the data suggest that small eggs developed at slower rates than large eggs, presumably resulting in hatching synchrony: despite massive differences in egg size (up to 44% in N. maura and 195% in N. natrix), all eggs hatched within 45.38 ± 1.55 days for N. maura and 43.57 ± 0.99 days for N. natrix. Synchronized hatching is wide-spread amongst organisms17, including squamates18. Synchronized hatching may enhance offspring survival by diluting an individual's risk of predation or by simply swamping predators upon emergence19,20. Further studies are necessary to test whether or not, and via which mechanism, egg metabolism may be adjusted to relative egg size in order to promote hatching synchrony in snakes.
Methods
Study animals and study site
The viperine water snake (N. maura) occurs in France, Spain, Portugal, south-west Switzerland, north-west Italy and on a few Mediterranean islands21. This species is largely aquatic and individuals are always found in the vicinity of water22. Its close relative, the grass snake N. Natrix, is one of the most widely distributed snakes in Europe, ranging from southern England and Scandinavia to northern Africa and from the Atlantic Ocean to western Russia22. Study animals were caught along the banks of the Lez River and surrounding pasture and woodland in South-west Ariège, France. The study site was a twenty kilometre stretch of river including tributaries, between the localities of Moulis (42° 57′ 43″ N; 1° 05′ 30″ E) and Le pont (42° 52′ 32″ N 0° 57′ 19″ E).
Animals used
A total of 16 gravid female viperine water snakes (hereafter N. maura) and 7 grass snakes (hereafter N. natrix) were captured in May and June 2011 and brought back to the laboratory (Station d'Ecologie Expérimentale à Moulis). Gravid females were housed individually in plastic containers (60 cm × 40 cm × 15 cm). A 40 W lamp fitted above each cage and set on a 12/12 h day and night timer provided heating opportunities. Each enclosure contained a 3 cm layer of moist peat, a clean water bowl, a shelter (terracotta roof tile) and an egg laying box. The egg laying box was a black plastic container (15 cm × 10 cm × 5 cm), with a 4 cm diameter side opening to allow the snake in and out, filled with a 2 cm layer of moist vermiculite. Laying dates spanned from the 17th of July to the 12th of August 2012 (N. maura) and from the 7th of July to the 24th of July 2012 (N. natrix). A total of 231 N. maura eggs (mean clutch size = 13.3 ± 5.7) and 156 N. natrix eggs (mean clutch size = 22.3 ± 6.8) were obtained. For the purpose of the current experiment, we randomly selected a number of eggs from each N. maura clutch (50 eggs in total; 3.1 ± 2.4 eggs per clutch) and each N. natrix clutch (28 eggs in total; N = 4.0 ± 4.5 per clutch). All females were returned to their exact site of capture within two weeks of egg-laying. From capture to release, females were offered small dead trouts once a week (frozen trouts were purchased from a local fish farm: Pisciculture des Chutes d'Aston, Les cabannes 09310).
Data collection
Eggs were collected within 12 hours of laying, placed on a 2 cm layer of wet vermiculite and transferred into an Aqualytic incubation chamber set at a constant 28°C. Eggs were individually marked with a letter (coding for litter of origin) and a number (egg number within each litter) for identification purposes. Eggs were measured in mass to the nearest 0.01 g using a digital scale within 12 hours of laying, and then every 7 days throughout the incubation period. Hatching occurred between the 19th of August and the 6th of September 2012 (N. natrix), and between the 21st of August and the 25th of September 2012 (N. maura). Hatchlings were measured within 12 hours of hatching in body mass (±0.01 g), and snout-vent length to the nearest 0.1 mm. Hatchlings were sexed by eversion of the hemipenes and marked by scale-clipping for identification. Siblings were housed together in plastic boxes (15 cm × 10 cm × 5 cm), with a water dish, shelter and paper towel as substrate. Snakes were remeasured in body mass, snout-vent length and total length at 3 weeks old, after which they were given their first meal (small dead minnows ranging from 0.5 g to 1 g; supplied by Armorvif ). After completion of the measurements, all young snakes were released at the maternal capture site.
Within each species, a body condition index23 (BCI) was calculated for each snake, using the residual values of the linear least-squares regression of Log (body mass) against Log (snout-vent length).
Capture, breeding, experimentation, release and ethics permits for N. maura and N. natrix were delivered by the Préfecture de l'Ariège (Arrété #2012-11). All experiments were performed in accordance with the relevant guidelines and regulations.
Heart rate measurements
We estimated resting metabolic rate by measuring snakes' heart rates, owing to the accurate physiological relationship existing between heart rate and VO224. We measured embryo heart rates as well as hatchling heart rates using the Buddy digital egg monitor (MK2, Avitronics) under the protocol described for eggs14 and neonate snakes13. The Buddy system works by shining an infrared beam onto the surface of the egg, detecting minute distortions caused by embryonic heart beats. Embryo heart rates were measured first at incubation day 10, and then every 10 days until hatching. Hatchling heart rates were measured at age 6.8 ± 4.2 days in a sub-sample of 22 juveniles snakes (15 N. maura and 7 N. natrix) and at age = 24.3 ± 3.7 days (N = 79). Snakes were placed inside individual testing bags (made of cotton material) fitted to their size, and into temperature controlled chambers for 30 min in order to reach testing temperature (28°C) and ensure minimum stress levels at the time of testing13. Each bag was then placed onto the sensor pad of a Buddy digital egg monitor (MK2, Avitronics) for heart rate reading (a stable reading was obtained after approximately 30 seconds).
Statistical analysis
All numerical data were Log-transformed prior to analysis. Data were checked for normality using Shapiro-Wilk W tests. Data analysis was performed using two factor repeated measures ANOVAs, post-hoc Tukey HSD and Linear regressions. Means ± standard deviations are given unless otherwise stated.
Author Contributions
F.A. collected, analysed the data, and wrote the article.
Acknowledgments
I wish to thank numerous field and laboratory helpers: Mélodie Tort, Tom Sarraude, Thomas Achkar, Alice Thiney and Gaëlle Blanvillain. I also wish to thank the CNRS, the Université Paul Sabatier for funding (Project EXUVIE), and the Agence Nationale pour la Recherche. This work is part of the ANR INDHET. There is no conflict of interest associated with this manuscript. This work is part of the “Laboratoire d'Excellence” (LABEX) entitled TULIP (ANR -10-LABX-41).
References
- Birchard G. F. & Reiber C. L. Heart rate during development in the turtle embryo: Effect of temperature. J. Comp. Physiol. B 166, 461–466 (1996). [DOI] [PubMed] [Google Scholar]
- Birchard G. F. An ontogenetic shift in the response of heart rates to temperature in the developing snapping turtle (Chelydra serpentina). J. Thermal Biol. 25, 287–291 (2000). [DOI] [PubMed] [Google Scholar]
- Nechaeva M. V., Vladimirova I. G. & Alekseeva T. A. Oxygen consumption as related to the development of the extraembryonic membranes and cardiovascular system in the European pond turtle (Emys orbicularis) embryogenesis. Comp. Biochem. Physiol. A 148, 599–610 (2007). [DOI] [PubMed] [Google Scholar]
- Du W. G., Zhao B. & Shine R. Embryos in the fast lane: high-temperature heart rates of turtles decline after hatching. PLoS ONE 5, e9557 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angilletta M. J., Lee V. & Silva A. C. Energetics of lizard embryos are not canalized by thermal acclimation. Physiol. Biochem. Zool. 79, 573–580 (2006). [DOI] [PubMed] [Google Scholar]
- Radder R. & Shine R. Thermally induced torpor in fullterm lizard embryos synchronizes hatching with ambient conditions. Biol. Letters 2, 415–416 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. T. & Tazawa H. Ontogeny of heart rate in embryonic and nestling crows (Corvus corone and Corvus macrorhynchos). J. Comp. Phys. B 169, 256–262 (1999). [Google Scholar]
- Pough E. H. et al. Herpetology ((Englewood Cliffs, Prentice-HallEnglewood Cliffs, Prentice-Hall, 1998). [Google Scholar]
- Bobyn M. L. & Brooks R. J. Incubation conditions as potential factors limiting the northern distribution of snapping turtles,. Chelydra serpentina. Can. J. Zool. 72, 28–37 (1994). [Google Scholar]
- Warner D. A. & Shine R. Fitness of juvenile lizards depends on seasonal timing of hatching, not offspring body size. Oecologia 154, 65–73 (2007). [DOI] [PubMed] [Google Scholar]
- Pike D. A., Pizzatto L., Pike B. A. & Shine R. Estimating survival rates of uncatchable animals: the myth of high juvenile mortality in reptiles. Ecology 89, 607–611 (2008). [DOI] [PubMed] [Google Scholar]
- Olsson M. & Shine R. Growth to death in lizards. Evolution 56, 1867–1870 (2002). [DOI] [PubMed] [Google Scholar]
- Aubret F., Tort M. & Blanvillain G. A non-invasive method of measuring heart rates in small reptiles and amphibians. Herp. Review 44, 421–423 (2013). [Google Scholar]
- Du W. G., Radder R. S., Sun B. & Shine R. Determinants of incubation period: do reptilian embryos hatch after a fixed total number of heart beats? J. Exp. Biol. 212, 1302–1306 (2009). [DOI] [PubMed] [Google Scholar]
- Ar A. & Tazawa H. Analysis of heart rate in developing bird embryos: effects of developmental mode and mass. Comp. Biochem. Physiol. A 124, 491–500 (1999). [DOI] [PubMed] [Google Scholar]
- Du W. G., Ye H., Zhao B., Pizzatto L., Ji X. & Shine R. Patterns of interspecific variation in the heart rates of embryonic reptiles. PloS ONE 6, e29027 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donoghue M. & Boutin S. Does reproductive synchrony affect juvenile survival rates of northern mammals? Oikos 74, 115–121 (1995). [Google Scholar]
- Vitt L. J. Ecology and life history of the scansorial arboreal lizard Plica plica (Iguanidae) in Amazonian Brazil. Can. J. Zool. 69, 504–511 (1991). [Google Scholar]
- Arnold S. J. & Wassersug R. J. Differential predation on metamorphic anurans by garter snakes (Thamnophis): social behavior as a possible defense. Ecology 59, 1014–1022 (1978). [Google Scholar]
- Dehn M. M. Vigilance for predators: detection and dilution effects. Behav. Ecol. Sociobiol. 26, 337–342 (1990). [Google Scholar]
- Gasc J. P. et al. Atlas of Amphibians and Reptiles in Europe (Muséum National d'Histoire Naturelle & Service du Patrimoine Naturel, Paris, 1997). [Google Scholar]
- Hailey S., Davies P. M. C. & Pulford E. Lifestyle and thermal ecology of natricine snakes. Brit. J. Herp. 6, 261–268 (1982). [Google Scholar]
- Bonnet X. & Naulleau G. Utilisation d'un indice de condition corporelle (BCI) pour l'étude de la reproduction chez les serpents. Comptes rendus de l'Académie des sciences. Série 3, Sciences de la vie 317, 34–41 (1994). [PubMed] [Google Scholar]
- Butler P. J., Green J. A., Boyd I. L. & Speakman J. R. Measuring metabolic rate in the field: the pros and cons of the doubly labelled water and heart rate methods. Funct. Ecol. 18, 168–183 (2004). [Google Scholar]