Abstract
Purpose
We investigated the effect of electrical stimulation on rabbit oocyte activation using intracytoplasmic sperm injection (ICSI) to determine whether viable offspring can be produced from deceased rabbit sperm using ICSI.
Methods
Sperm were collected from a heterozygote GFP male rabbit 5 h after sacrifice and cryopreserved in liquid nitrogen. Mature oocytes were fertilized using ICSI. A series of electrical pulse procedures were used to activate oocytes before and/or after ICSI. Following ICSI, zygotes were cultured in B2 medium for 4 days or transferred into the oviducts of recipient rabbits at the 2- or 4-cell stage.
Results
The blastocyst formation rate was significantly greater in oocytes that received one or two pulses prior to ICSI compared to controls and other electrically stimulated groups. In the single pulse before ICSI group, 23 % of the blastocysts expressed GFP, which was significantly greater than all other groups. However, those that received treatment before and after, or just following ICSI, showed a significant decrease in embryo survival. Finally, embryos from the single pulse before ICSI group were transferred into recipient female rabbits and a full-term kit was successfully delivered.
Conclusions
One pulse of electrical stimulation prior to sperm injection was an effective method to activate rabbit oocytes for fertilization. Sperm collected from a deceased rabbit is able to produce viable embryos through ISCI that are capable of normal fetal and kit development.
Keywords: Rabbit, Oocytes, ICSI, Electric stimulation, Embryo development
Introduction
The development of intracytoplasmic sperm injection (ICSI) has provided an important tool for the study of sperm-oocyte interaction during fertilization. Healthy offspring derived from ICSI have been reported in several mammalian species, including rabbits [1], cattle [2], humans [3], mice [4], horses [5], sheep [6], pigs [7], and monkeys [8]. In humans, ICSI is widely used as a means to address severe male factor infertility. This technique provides an option for males with semen parameters that are well below reference values as described by the World Health Organization [9]. Successful fertilization with ICSI is possible as long as the complete genome can participate in embryonic development and produce offspring [4].
Many laboratories have attempted to use ICSI in rabbits [10–13]. However, successful techniques have yet to be well established, primarily due to the low efficiency of oocyte activation following injection. A chemical or physical stimulus can activate oocytes by increasing intracellular calcium concentrations and inactivating M-phase-promoting factor (MPF). Ionomycin and N-6 dimethylaminopurine (6DMAP), which induce calcium release and enhance the inactivation of MPF respectively, have previously been used to activate oocytes and increase cleavage rates [14]. Electrical stimulation has also been used as a successful method to activate oocytes in rabbits [15, 16]. However, the impact of electrical stimulation on rabbit oocyte activation in combination with ICSI has not been previously reported.
The objective of the present study was to evaluate the effect of electrical stimulation on rabbit oocyte activation and fertilization before and/or after ICSI. In addition, the current investigation determined whether sperm taken from an already deceased male rabbit can produce viable embryos and offspring.
Materials and methods
All experiments were carried out in accordance with the procedures and principles outlined by the Ethics Committee of Shanghai Jiao Tong University, School of Medicine. Unless otherwise stated, reagents were purchased from Gibco Co. (Grand island NY. USA).
Animals
Mature New Zealand white rabbits (over 6 months) were used as donors for oocytes and recipients for embryo transfer. The rabbits were housed in an environmentally controlled room, with a 10-h/14-h dark/light cycle at a temperature of 20 ± 3 °C and humidity of 60 % ± 15 %. Free access to food and water was provided.
Media
HEPES-balanced RD medium (hRD, a 1:1 mixture of RPMI-1640 and DMEM, supplemented with 10 % fetal bovine serum (FBS), 2 mM/L HEPES, 2 mM/L L-glutamine and 100 μM/L NEAA) was used for oocyte collection. and transfer. The electric stimulation solution (ESS), a 0.3 M sorbitol/L solution, contained 0.5 mM magnesium acetate, 0.1 mM calcium acetate, 10 mM HEPES and 1 mg/ml bovine serum albumin (BSA). B2 medium and tris-yolk dimethyl sulphoxide (TYD) were prepared as previously reported [17, 18].
Semen collection and preparation
Semen was collected from 5-month-old heterozygote green fluorescent protein (GFP) male rabbits 5 h following sacrifice. Sperm from the cauda epididymis was collected in B2 medium, diluted 1:4 with TYD and loaded into straws. Straws were frozen and stored in liquid nitrogen. Frozen sperm were then thawed at room temperature for 10 s and then plunged into a 37 °C water bath for 1 min at the time of the experiment. Sperm were washed in phosphate-buffered saline and centrifuged at 700 × g for 5 min. The supernatant was discarded and the final pellet was gently re-suspended in 1 mL B2 medium.
Oocytes collection
Mature female rabbits were superovulated by injection of 100 IU pregnant mare stimulation gonadotropin (PMSG, Tianjin Huafu Biotechnology Corporation, Tianjin, China) followed by 100 IU human chorionic gonadotropin (hCG, Ningbo Hormone Factory, Ningbo, China) 96 h later. At 14–15 h after hCG injection, the cumulus-oocyte complexes were collected by flushing the oviduct with hRD medium. Oocytes were freed from cumulus cells by gentle pipetting in hRD medium containing 0.1 % hyaluronidase (Sigma-Aldrich), washed three times with hRD medium and then incubated in the same medium at 38 °C with 5 % CO2.
Intracytoplasmic sperm injection
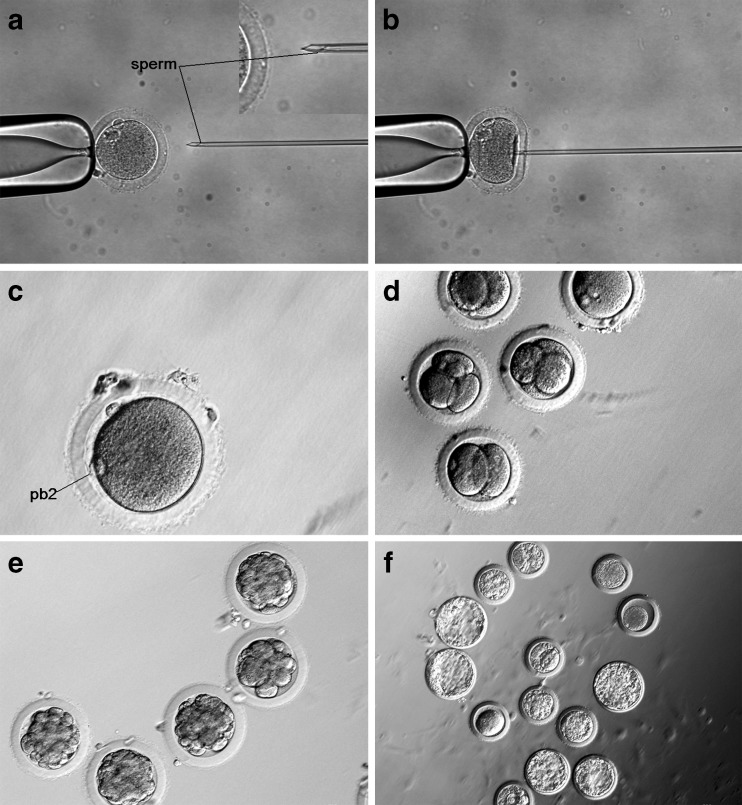
Sperm injection pipettes were prepared as reported [19]. Approximately 1 μl of the incubated sperm suspension was mixed with a 30 μl drop of B2 containing 10 % polyvinylpyrrolidone (PVP, Sigma-Aldrich, USA) (w/v) on the lid of a 60-mm Falcon dish covered with mineral oil (Sigma-Aldrich, USA). Between 10 and 15 oocytes were transferred to 50-μl B2-HEPES medium. The plasma membrane of each individual sperm was damaged by exerting pressure with the tip of the injection pipette. From all the sperm, a randomly selected single sperm was then aspirated, tail first, into the injection pipette. The oocytes were held by the holding pipette at the 3 o’clock position with the 1st polar body at the 12 o’clock position (Fig. 1a). The oocyte membrane was broken by cytoplasm aspiration. when the injection pipette at was at the 9 o’clock position. Sperm were injected into the oocytes cytoplasm (Fig. 1b), and the injection of PVP into the ooplasm was avoided if at all possible.
Fig. 1.
ICSI on rabbit oocytes and embryo development in the one pulse before ICSI group. a Injection pipette containing a sperm. b Injection pipette penetrating the zona pellucida and oolemma. c Second polar body. d Embryos at the cleavage stage. e Embryos at the morula stage. f Embryos at the blastocyst stage
Activation of oocytes
Denuded oocytes were activated using five different electrical stimulation conditions, including a single pulse before ICSI, two pulses before ICSI, one pulse before and after ICSI, one pulse after ICSI and a control (no electrical stimulation). Sham injection groups were also included in each of the three conditions. For electrical stimulation, the oocytes were placed in a chamber filled with ESS for 3 min to allow for equilibration. Ten to fifteen oocytes were then moved into an electrode wire chamber where a pulse of 2.0 kV/cm was administered for 25 μs (BTX830, USA). All activated oocytes were washed three times and transferred into B2 medium.
In vitro culture and evaluation of embryo development
After ICSI and electrical stimulation, zygotes were cultured in B2 medium at 38 °C in 5 % CO2. Both the ICSI and sham-injected oocytes were cultured continuously for 4 days in B2 medium for the in vitro developmental potential evaluation. Pronuclear formation was examined between 3 and 7 h after sperm or sham injection. Oocytes with a second polar body and two pronuclei were considered to be fertilized. The rate of GFP-positive blastocysts was determined after the embryos were cultured for 4 days.
Embryo transfer
Cycles of female recipients were synchronized with oocytes donors by injecting 100 IU hCG to induce ovulation. Only embryos that developed to the 2-cell or 4-cell stages were selected for transfer. Eight to fifteen embryos were surgically transferred to both oviducts of the recipients. Pregnancy was determined by palpation 12–15 days after embryo transfer. Kits were delivered by cesarean section on gestational day 31.
PCR analyses
Genomic DNA was isolated from the dead transgenic rabbit and the offspring from ICSI with a MiniBEST Universal Genomic DNA Extraction Kit (TaKaRa, Dalian, China). PCR amplification was performed with Premix rTaq Version 2.0 (TaKaRa) and the primers (5′-GAGAGGCTATTCGGCTATG-3′) and (5′-CAAGGTGAGATGACAGGAG-3′). PCR conditions were: 10 min at 98 °C; 35 cycles of 98 °C for 10 s, 56 °C for 30 s, and 72 °C for 30s; and 72 °C for 10 min. DNA fragment size was 274 bp. DNA isolated from wild-type rabbits was used as a negative control.
Statistical analysis
The rates of pronuclei formation, embryonic cleavage and blastocyst formation were analyzed by chi-square tests with the level of significance set at p < 0.05.
Results
Prior to cryopreservation, 20 % of sperm collected from the cauda were motile. After thawing, we found a decrease in motility, with only 1 % of sperm being motile. As shown in Table 1, electrical stimulation did not significantly alter oocyte survival when compared to controls. Untreated oocytes had a survival rate of 87 % compared to the single (80 %) and double pulse (90 %) electrical stimulation conditions. However, when one pulse before and after ICSI and one pulse after ICSI were applied, we observed a significant decrease in oocyte survival rates (48 % and 47 %, respectively) when compared to both the controls and groups that received electrical treatment before ICSI.
Table 1.
Comparison of development of ICSI oocytes and embryos after different electrical stimuli
| Treatment | No. of oocytes | No. of oocytes survived (%) | No. of pronuclei formation (%)* | No. of embryos cleaved (%)** | No. of embryos blastocysts (%)+ | No. of EGFP-positive blastocyst (%)++ |
|---|---|---|---|---|---|---|
| Without activation | ||||||
| ICSI | 235 | 205 (87)a | 67 (33)b | 60 (29)d | 11 (5)c | 1 (10)b |
| Sham | 40 | 40 (100)a | 0 (0)d | 0 (0)f | 0 (0)d | NA |
| One pulse before ICSI | ||||||
| ICSI | 139 | 126 (90)a | 88 (70)a | 75 (60)b | 43 (34)a | 10 (23)a |
| Sham | 45 | 42 (93)a | 6 (14)c | 5 (12)e | 0 (0)d | NA |
| Two pulses before ICSI | ||||||
| ICSI | 86 | 69 (80)a | 46 (67)a | 43 (62)b | 26 (37)a | 3 (12)b |
| Sham | 48 | 40 (83)a | 29 (73)a | 28 (70)a | 16 (40)a | NA |
| One pulse after ICSI | ||||||
| ICSI | 46 | 22 (48)b | 15 (68)a | 13 (59)b | 4 (18)b | 0 (0)c |
| One pulse before and after ICSI | ||||||
| ICSI | 38 | 18 (47)b | 10 (56)a | 8 (44)c | 2 (11)b | 0 (0)c |
Different letter superscripts in the same column indicate a significant difference (p < 0.05)
NA not available
* Percentage of embryos with pronuclei/surviving oocytes
** Percentage cleaved/surviving oocytes
+ Percentage of blastocysts/surviving oocytes
++ Percentage of EGFP-positive embryos/blastocyst embryos
In the control group, second polar body extrusion and pronuclei formation (Fig. 1c) occurred 5 to 7 h after ICSI, compared to just 3–4 h after ICSI in the electrically activated groups. The pronuclei formation rate was found to be significantly greater in groups that received electrical stimulation when compared to controls. The pronuclear formation rate in the one pulse before ICSI group was similar to that of the two pulses before ICSI group (70 % and 67 % respectively). Pronuclei formation rate was similar in the sham injection group that received two pulses before ICSI (73 %).
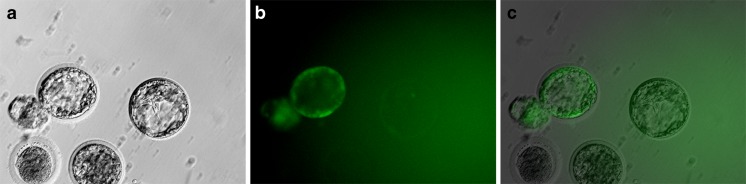
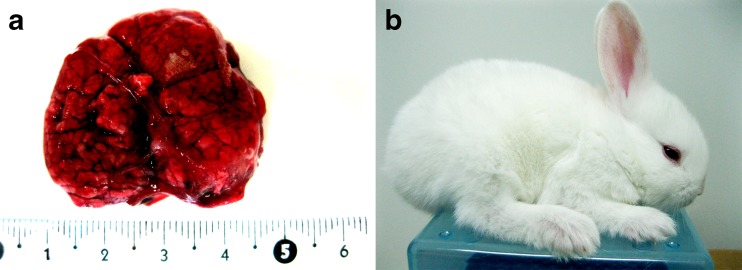
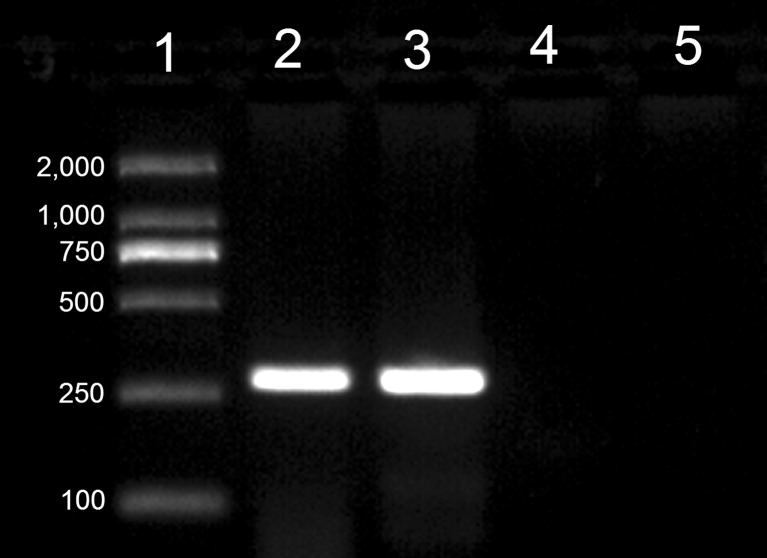
On the second morning after ICSI, both 2- and 4-cell embryos were detected (Fig. 1d). In all groups, the cleavage rate was found to be similar to that of the pronuclear formation rate. Of the cleaved embryos, only a few developed into morulae 3 days later (Fig. 1e). Four days following ICSI treatment, the embryos entered the blastocyst stage (Fig. 1f). There was no significant difference found in the rate of blastocyst formation between the one and two pulse before ICSI group (34 % vs. 37 %), but these two groups had a significantly greater formation rate when compared to the other ICSI groups. A significant increase in GFP activation was observed in the group that received a single electrical pulse before ICSI compared to that observed in the other groups, with 23 % of the blastocysts expressing GFP (Fig. 2). A total of 93 embryos from the group that received one pulse before ICSI were transferred to four recipients. Of the four recipients, pregnancy was established in one rabbit which was maintained for the full term. At the time of the cesarean section, it was noted that there was an abnormally placenta four time larger than normal (Fig. 3a). Following birth, the kit appeared healthy and continued to develop normally (Fig. 3b). The result of genotype analysis by PCR showed that the target band could be amplified from the kit and the dead transgenic rabbit (Fig. 4). It means that the kit is derived from the dead transgenic rabbit by ICSI.
Fig. 2.
GFP detection in the embryos following 72 h of culture. a Blastocysts under bright light. b Blastocysts under UV light (major at 488 nm). c Merge image
Fig. 3.
The rabbit derived from the sperm of a deceased rabbit. a An abnormally large placenta found on gestational day 31. b One-month-old rabbit
Fig. 4.
The PCR analysis of the rabbits. Lane 1, DMA marker. Lane 2, the deceased transgenic rabbit. Lane 3, the rabbit produced by ICSI. Lane 4, the wild rabbit. Lane 5, Water
Discussion
Here, we showed that electrical stimulation of oocytes prior to ICSI had an impact on markers of fertilization. We observed a significant increase of pronuclei formation, embryo cleavage, blastocyst formation and GFP expression. It has been previously reported that activation of oocytes was not necessary for ICSI when mature and fresh rabbit sperm were used [12, 19]. However, with the use of frozen or freeze-dried sperm, oocyte activation significantly increased fertilization rates [14, 20].
In vivo, oocyte activation is required for fertilization and proper embryonic development. The calcium ion concentration plays an important role in activation of the oocytes [21]. A rapid increase in calcium concentration results in the decline of MPF [22, 23]. In the current investigation, oocytes that were activated via electrical stimulation showed increased cleavage and blastocyst formation rates. This suggests that electric stimulation improved the overall efficiency of the ICSI procedure.
Consistent with previous reports, the second polar body extrusion and the formation of pronuclei occurred 5–7 h following ICSI in non-activated oocytes groups [19]. In this report, we found that two pronuclei formation occurred after just 3–4 h in oocytes that were activated by electrical stimulation. These data suggest electrical pulse treatment accelerates pronuclei formation after ICSI. We also found that the pronuclei formation rate, cleaved rate and blastocyst rate were significantly improved in the single pulse before ICSI group compared to previous reports [10, 11, 24]. Others have also reported similar improvements [14].
The litter rate in the present study is lower than previously reported [13, 19]. This finding may be related to the quality of the sperm. In the present investigation, sperm were collected from male rabbits that had been deceased for 5 h. In addition, sperm vitality and motility were found to be significant reduced following cryopreservation.
In the present investigation, the two pulse sham injection group showed a higher blastocyst rate than both the one and two pulse before ICSI groups, which indicated that the sham condition had greater levels of parthenogenetic activation. In a previous report, sham-injected rabbit oocytes treated with CHX/DMAP were able to cleave and develop into blastocysts [25]. Further investigation into ploidy analysis is required, as parthenogenetically cleaved embryos were found in the present study.
Electric pulses have been used to improve the efficiency of ICSI in bovines. Double electric stimulation, before and after injection, has been shown to be an effective method of activating bovine oocytes and sustaining embryo development to the morula and blastocyst stages [24]. In our experiments, when the electric treatment was applied after ICSI, the oocyte survival rate was less than 50 % that of the other electrically stimulated groups. The high mortality rate following electrical stimulation before and after ICSI may be the result of damage induced by injection itself on rabbit oocytes. Damage to the plasma membrane following the injection may weaken it and lead to breakage during the electrical stimulation treatment. This would explain the significant decrease of blastocyst formation rate and the lack of fluorescence expression in blastocysts in groups electrically stimulated following ICSI.
An abnormally large placenta was found with the live birth rabbit. This finding has been rarely reported in previous investigations of ICSI. Increased placental size has been previously reported in cloned animals [26–29]. The reason for the abnormal placenta in the ICSI-treated rabbit was not clear and requires further investigation.
In conclusion, the present study is the first to use electrical stimulation to activate rabbit oocytes for ICSI and to produce offspring from sperm collected from a deceased rabbit. It is clear from our results that electrical stimulation prior to ICSI enhances oocyte activation. Finally, the sperm from a deceased rabbit was shown to be viable and can be used for ICSI.
Acknowledgments
The study was supported by grants from the Natural Science Foundation of China (Nos. 81170756, 31101048), the Shanghai Natural Science Funding (Nos. 10140901700, 11140901600, 11ZR1418800) and the National Transgenic Foundation of China (2011ZX08008-003).
Footnotes
Capsule Suitable electrical stimulation on rabbit oocytes could increase the fertilization and embryo development when the sperm from deceased rabbit were used for ICSI, and sperm from deceased rabbit are capable of full term and kit development.
Contributor Information
Shangang Li, Phone: +86-21-63846590, Email: lis101@163.com.
Xuejin Chen, Phone: +86-21-63846590, Email: chenxuejin@shsmu.edu.cn.
References
- 1.Iritani A, Hosoi Y. Microfertilization by various methods in mammalian species. Prog Clin Biol Res. 1989;294:145–149. [PubMed] [Google Scholar]
- 2.Goto K, Kinoshita A, Takuma Y, Ogawa K. Fertilisation of bovine oocytes by the injection of immobilised, killed spermatozoa. Vet Rec. 1990;127(21):517–520. [PubMed] [Google Scholar]
- 3.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–18. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 4.Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod. 1995;52(4):709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 5.Cochran R, Meintjes M, Reggio B, Hylan D, Carter J, Pinto C, et al. Production of live foals from sperm-injected oocytes harvested from pregnant mares. J Reprod Fertil Suppl. 2000;56:503–512. [PubMed] [Google Scholar]
- 6.Catt SL, Catt JW, Gomez MC, Maxwell WM, Evans G. Birth of a male lamb derived from an in vitro matured oocyte fertilised by intracytoplasmic injection of a single presumptive male sperm. Vet Rec. 1996;139(20):494–495. doi: 10.1136/vr.139.20.494. [DOI] [PubMed] [Google Scholar]
- 7.Kolbe T, Holtz W. Birth of a piglet derived from an oocyte fertilized by intracytoplasmic sperm injection (ICSI) Anim Reprod Sci. 2000;64(1–2):97–101. doi: 10.1016/S0378-4320(00)00204-9. [DOI] [PubMed] [Google Scholar]
- 8.Chan AW, Luetjens CM, Dominko T, Ramalho-Santos J, Simerly CR, Hewitson L, et al. Foreign DNA transmission by ICSI: injection of spermatozoa bound with exogenous DNA results in embryonic GFP expression and live rhesus monkey births. Mol Hum Reprod. 2000;6(1):26–33. doi: 10.1093/molehr/6.1.26. [DOI] [PubMed] [Google Scholar]
- 9.Jones J, Horne G, Fitzgerald C. Who needs ICSI? A nationwide UK survey on ICSI use. Hum Fertil (Camb) 2012;15(3):144–149. doi: 10.3109/14647273.2012.720051. [DOI] [PubMed] [Google Scholar]
- 10.Keefer CL. Fertilization by sperm injection in the rabbit. Gamete Res. 1989;22(1):59–69. doi: 10.1002/mrd.1120220107. [DOI] [PubMed] [Google Scholar]
- 11.Hoshi K, Yazawa H, Yanagida K, Sato A. Microinsemination of rabbit oocytes with heat-treated sperm: embryonic development. Arch Androl. 1992;29(3):233–237. doi: 10.3109/01485019208987730. [DOI] [PubMed] [Google Scholar]
- 12.Zheng YL, Jiang MX, Zhang YL, Sun QY, Chen DY. Effects of oocyte age, cumulus cells and injection methods on in vitro development of intracytoplasmic sperm injection rabbit embryos. Zygote. 2004;12(1):75–80. doi: 10.1017/S0967199404002643. [DOI] [PubMed] [Google Scholar]
- 13.Ogonuki N, Inoue K, Miki H, Mochida K, Hatori M, Okada H, et al. Differential development of rabbit embryos following microinsemination with sperm and spermatids. Mol Reprod Dev. 2005;72(3):411–417. doi: 10.1002/mrd.20363. [DOI] [PubMed] [Google Scholar]
- 14.Li QY, Hou J, Chen YF, An XR. Full-term development of rabbit embryos produced by ICSI with sperm frozen in liquid nitrogen without cryoprotectants. Reprod Domest Anim. 2010;45(4):717–722. doi: 10.1111/j.1439-0531.2009.01340.x. [DOI] [PubMed] [Google Scholar]
- 15.Collas P, Robl JM. Factors affecting the efficiency of nuclear transplantation in the rabbit embryo. Biol Reprod. 1990;43(5):877–884. doi: 10.1095/biolreprod43.5.877. [DOI] [PubMed] [Google Scholar]
- 16.Ozil JP. The parthenogenetic development of rabbit oocytes after repetitive pulsatile electrical stimulation. Development. 1990;109(1):117–127. doi: 10.1242/dev.109.1.117. [DOI] [PubMed] [Google Scholar]
- 17.Staessen C, Janssenswillen C, De Clerck E, Van Steirteghem A. Controlled comparison of commercial media for human in-vitro fertilization: Menezo B2 medium versus Medi-Cult universal and BM1 medium. Hum Reprod. 1998;13(9):2548–2554. doi: 10.1093/humrep/13.9.2548. [DOI] [PubMed] [Google Scholar]
- 18.Stranzinger GF, Maurer RR, Paufler SK. Fertility of frozen rabbit semen. J Reprod Fertil. 1971;24(1):111–113. doi: 10.1530/jrf.0.0240111. [DOI] [PubMed] [Google Scholar]
- 19.Deng M, Yang XJ. Full term development of rabbit oocytes fertilized by intracytoplasmic sperm injection. Mol Reprod Dev. 2001;59(1):38–43. doi: 10.1002/mrd.1005. [DOI] [PubMed] [Google Scholar]
- 20.Chen H. Fertilization by intractoplasmic sperm injection in rabbit oocytes. Acta Laboratorium Animal is Scientia Sinica. 2008;16(4):251–253. [Google Scholar]
- 21.Ozil JP, Huneau D. Activation of rabbit oocytes: the impact of the Ca2+ signal regime on development. Development. 2001;128(6):917–928. doi: 10.1242/dev.128.6.917. [DOI] [PubMed] [Google Scholar]
- 22.Tesarik J. Calcium in oocyte maturation. How the spermatozoon awakens the oocyte: lessons from intracytoplasmic sperm injection. Hum Reprod. 1994;9(6):977–978. doi: 10.1093/oxfordjournals.humrep.a138670. [DOI] [PubMed] [Google Scholar]
- 23.Kline D, Kline JT. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev Biol. 1992;149(1):80–89. doi: 10.1016/0012-1606(92)90265-I. [DOI] [PubMed] [Google Scholar]
- 24.Hwang S, Lee E, Yoon J, Yoon BK, Lee JH, Choi D. Effects of electric stimulation on bovine oocyte activation and embryo development in intracytoplasmic sperm injection procedure. J Assist Reprod Genet. 2000;17(6):310–314. doi: 10.1023/A:1009496726343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu JL, Kusakabe H, Chang CC, Suzuki H, Schmidt DW, Julian M, et al. Freeze-dried sperm fertilization leads to full-term development in rabbits. Biol Reprod. 2004;70(6):1776–1781. doi: 10.1095/biolreprod.103.025957. [DOI] [PubMed] [Google Scholar]
- 26.Zheng Y. Placental abnormalities in animal cloning. Chem Life. 2011;31(2):317–320. [Google Scholar]
- 27.Tanaka S, Oda M, Toyoshima Y, Wakayama T, Tanaka M, Yoshida N, et al. Placentomegaly in cloned mouse concepti caused by expansion of the spongiotrophoblast layer. Biol Reprod. 2001;65(6):1813–1821. doi: 10.1095/biolreprod65.6.1813. [DOI] [PubMed] [Google Scholar]
- 28.Ravelich SR, Breier BH, Reddy S, Keelan JA, Wells DN, Peterson AJ, et al. Insulin-like growth factor-I and binding proteins 1, 2, and 3 in bovine nuclear transfer pregnancies. Biol Reprod. 2004;70(2):430–438. doi: 10.1095/biolreprod.103.021139. [DOI] [PubMed] [Google Scholar]
- 29.Inoue K, Wakao H, Ogonuki N, Miki H, Seino K, Nambu-Wakao R, et al. Generation of cloned mice by direct nuclear transfer from natural killer T cells. Curr Biol. 2005;15(12):1114–1118. doi: 10.1016/j.cub.2005.05.021. [DOI] [PubMed] [Google Scholar]