Abstract
Purpose
Polycystic ovary syndrome (PCOS) is a most common endocrine disorder of reproductive age women. Interleukin-6 is involved in the pathophysiological characteristics associated with polycystic ovary syndrome (PCOS). The-174 G/CIL-6 gene promoter region single nucleotide polymorphism (SNP) may influence or modulate gene function and/or transcriptional efficiency. The current study was aimed to evaluate the association between IL-6 gene −174 G/C promoter polymorphism and Polycystic Ovary Syndrome in South Indian women.
Methods
In the present study, we examined the genotypic and allele distribution among the PCOS patients (n = 104) and controls (n = 156). The genotypes of IL-6−174 G/C SNP were analyzed by polymerase chain reaction (PCR) and sequencing analysis. The allele frequency and genotype distributions of cases and controls were analyzed using Fisher’s exact test.
Results
The genotype frequencies observed among the 104 cases and 156 controls were G/G 66.3 % and 49.4 %, G/C 29.8 % and 46.8 %, and C/C 3.8 % and 3.8 % (OR: 1.6226, CI: 1.0574–2.4899). The G and C allele frequencies were 81.25 % and 72.8 %, and 18.75 % and 27.2 %, respectively. The genotype and allele distribution revealed significant differences between PCOS patients and controls (all P values < 0.05).
Conclusion
Our findings showed a significant statistical association between IL-6−174 G/C SNP and PCOS risk in South Indian women. The ‘G’ allele frequency influences significantly higher in PCOS patients than controls. However, the exact mechanism by which ‘G’ allele frequency influence PCOS patients is yet to be determined.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-013-0111-1) contains supplementary material, which is available to authorized users.
Keywords: Interleukin-6 promoter, Polycystic ovary syndrome, Polymorphism
Introduction
Polycystic ovary syndrome (PCOS) is a highly prevalent endocrine disorder that affects approximately 6–10 % of women of reproductive age [1]. It is characterized by chronic anovulation, clinical and/or biochemical hyperandrogenism, and polycystic ovary, commonly leading to infertility [2]. It is also associated with hirsutism, obesity, dyslipidemia, atherosclerosis, insulin resistance and type 2 diabetes mellitus [3–6]. The observation of familial aggregation indicates heritable tendency of the PCOS, but the etiology and pathogenesis remains elusive.
Cytokines are cell-signaling protein molecules that are secreted by numerous cells, play a key role in intercellular communication. Interleukin-6 (IL-6) is a pleiotropic cytokine, secreted as a T-cell-derived factor by a variety of cell types including lymphocytes, monocytes, and endothelial cells [7]. It has endocrine as well as paracrine and autocrine actions implicated in several physiologic and pathologic processes including immunity and inflammation, activation of fibroblasts, mast cells, endothelial cells, monocytes and keratinocytes [8, 9]. Furthermore, it plays a pivotal role in reproductive physiology including regulation of ovarian steroid production, follicular maturation and the processes of fertilization and implantation [10]. IL-6 has also been shown to modulate ovarian development and function [11]. It can mediate the angiogenic process associated with follicle development which leads to ovarian carcinogenesis [12].
The human IL-6 gene is located at 7p21–24 locus with an upstream promoter containing 303 bp [12]. A common G/C single nucleotide polymorphism (SNP) in the IL-6 promoter at the np −174 influences its transcription rate. Previous studies have been reported association of IL-6 –174 SNP with the development and progression of various human diseases, including hyperandrogonism, multiple myeloma, colorectal Cancer, type-2 diabetes mellitus, breast cancer, endometriosis, chronic periodontitis, alzheimer’s disease, acute coronary syndrome and coronary heart disease [13–23]. Few scientific groups have also been investigated the prevalence of IL-6 –174G/C SNP in PCOS in Turkish and Caucasian population, however no reports are documented in Indian population [24, 25]. In the present study, for the first time we report the association between IL-6 –174G/C SNP and risk of developing PCOS in South Indian women.
Material and methods
Subjects
One hundred and four women of reproductive age with PCOS and one hundred and fifty six healthy women were recruited at the infertility institute and research centre (IIRC), Secundrabad, India. Patients were selected as per the Rotterdam consensus criteria to diagnose PCOS [26]. All subjects (PCOS and controls) were nonpregnant, non-smokers, normotensive. The body mass index (BMI) was below 25 in control subjects and was up to 26 in the cases. BMI was calculated as body weight (kg) divided by body height squared (m2). The characteristics of PCOS women and controls were summarized in Table 1. All the participants included in study were of South Indian origin (Dravidian linguistic group).
Table 1.
Clinical characteristics of PCOS and control group
| Variable | Total Controls (n = 156) | Total PCOS (n = 104) |
|---|---|---|
| Age (years) | 30.00 ± 5.17 | 26.35 ± 3.88 |
| Weight (kg) | 57.02 ± 9.87 | 59.37 ± 12.19 |
| BMI (kg/m2) | 23.87 ± 2.88 | 24.07 ± 4.22 |
| FSH (μU/ml) | 6.21 ± 1.74 | 5.92 ± 2.0 |
| LH (μU/ml) | 5.61 ± 1.63 | 7.73 ± 2.47 |
| LH:FSH | 0.88 ± 0.21 | 1.45 ± 0.89 |
| Presence of overweight and obesity | 10(6.41) | 31 (29.08) |
Data are given as mean ± S.D or n (%)
Cases
Criteria for the diagnosis of PCOS included oligoovulation (cycles longer than 35 days or less than 26 days1, elevated free testosterone levels (0.5 ng/dl; the cut-off level for free testosterone level was the mean ±2 SD according to normal levels in controls), oligomenorrhea or amenorrhea. Ferriman- Gallway (FG) score of ≥7 was used to determine hirsutism. In accordance with the above criteria poly cystic ovary (PCO) morphology was determined by transvaginal ultrasonography (TVS), which defines PCOS as the presence of 12 or more small (2 to 9 mm) follicles in each ovary. Women with other causes of hyperandrogonism such as hyperprolactinemia, androgen-secreting tumors, Cushing syndrome and non classic congenital hyperplasia, because of causes other than PCOS were excluded from this study.
Controls
Control subjects no signs of menstrual dysfunction had androgen levels within normal range, normal glucose tolerance, and no family history of type 2 diabetis mellitus, hirsutism, and infertility. Blood samples were collected, and plasma was removed followed by storage at −20 °C until analysis was performed.
Informed written consent form was obtained from all subjects prior to participation in this study. The study was approved by ethical committee and review Board of centre for cellular and molecular biology (CCMB), Hyderabad.
DNA extraction
Genomic DNA was extracted from 1 mL of EDTA anticoagulated whole blood by the method described earlier [27]. Both cases and controls were genotyped in a randomized, blinded fashion.
Determination of the IL-6 genotype
The genotypes of IL-6 –174 G/C SNP (NCBI SNP CLUSTER ID: rs1800795) were analyzed by polymerase chain reaction (PCR) and sequencing analysis as per the protocols described earlier [28]. PCRs were performed in a total volume of 25 μl containing 50 ng genomic DNA, 2 to 6 pmol of each primer, 1X Taq polymerase buffer (1.5 mM MgCl2), and 0.25 U of Amplitaq DNA polymerase (Perkin Elmer, Foster City, CA). Primers were designed by using primer3plus software (http://primer3plus.com/cgi-bin/dev/primer3plus.cgi). Primer characteristics were included in the supplementary Table 1. The primers used were 5′-TGACTTCAGCTTTACTCTTGT-3′ (forward), and 5′-CTGATTGGAAACCTTATTAAG-3′ (reverse). PCR amplification was performed in a programmable thermal cycler gradient PCR system (Eppendorf AG, Hamburg, Germany). The PCR amplification was performed for 35 cycles (denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min, extension at 72 °C for 1 min, and final extension for 10 min at 72 °C). Beta-2 micro globulin gene was used as a housekeeping gene in PCR amplification. The PCR product of 198 bp was analyzed by 1.5 % agarose gel stained with ethidium bromide and then sequenced with a Taq-Dye deoxy-terminator cycle sequencing kit (Applied BioSystems, Foster City, USA) using an automated ABI 3770 DNA sequencer (Applied BioSystems, Foster City, USA). Genotype calling was performed using Chromas V.2 software (Technelysium Ltd., Australia).
Statistical analysis
The odds ratio and 95 % confidence interval (CI) values were calculated accordingly. For statistical analysis, we carried out the statistical package for social sciences version 11.0 (SPSS, Inc, Chicago, IL). ‘p’ values < 0.05 were considered as statistically significant. Differences between genotype distribution and allele frequency were tested by Fisher’s exact test. The genotype distribution among subjects was tested for Hardy–Weinberg equilibrium (HWE) using Fisher’s exact test.
Results
In the present study, IL-6 polymorphisms were analyzed in 104 women with PCOS and 156 unrelated non pregnant healthy controls. The mean age of PCOS women was 26.35 ± 3.8. Patient and control groups were matched for age 20–38. Table 1 summarizes the clinical and laboratory characteristics of controls and PCOS women.
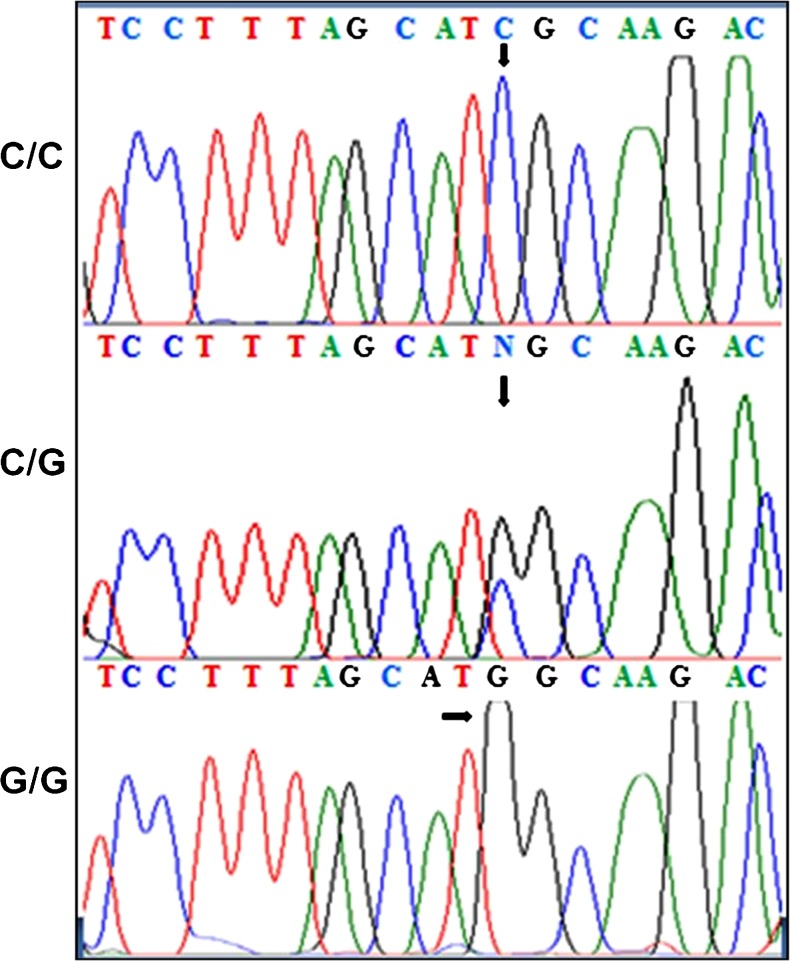
All subjects (n = 260) were successfully genotyped. Amongst both cases and controls, the genotype distributions as well as allele frequencies were in Hardy–Weinberg equilibrium (p > 0.05). Sequence analysis of the 198 bp product of the IL-6 –174G/C promoter region SNP were shown in Fig. 1. The G/G and C/C homozygotes manifested as a single peak, whereas the heterozygote G/C as a double peak.
Fig. 1.
Three genotypes for the –174G/C IL-6 gene promoter polymorphism: DNA sequencing analysis of different PCR amplified product using a reverse primer containing –174G/C IL-6 gene promoter locus
Genotyping of IL-6 –174G/C Promoter polymorphism
The genotype distribution and allele frequencies are summarized in Table 3. The genotype frequencies among the 104 cases and 156 controls were G/G 66.3 % and 49.4 %, G/C 29.8 % and 46.8 %, and C/C 3.8 % and 3.8 %. The ‘G’ and ‘C’ allele frequencies were 81.25 % and 72.8 %, and 18.75 % and 27.2 %, respectively. The genotype (p = 0.02) and allele (p = 0.0259) distribution revealed significant differences between patients and controls (all ‘p’ values < 0.05). There was significant elevation of G/G genotype frequency in patients as compared to controls (Table 3). The allele frequency also showed similar trend indicating that ‘G’ allele might confer risk to PCOS and ‘C’ allele offer protection against the disease.
Table 3.
Genotype and allele frequencies of IL-6 -174G/C polymorphism in PCOS patients and controlsa
| Genotypes/Alleles | Cases n =104 | Controls n =156 | P-value (%) | Odds ratio | 95%CI |
|---|---|---|---|---|---|
| Genotypes | |||||
| G/G | 69 (66.3) | 77 (49.4) | 0.02b | ||
| G/C | 31 (29.8) | 73 (46.8) | |||
| C/C | 4 (3.8) | 6 (3.8) | |||
| Alleles | |||||
| C | 169 (81.25) | 227 (72.8) | 0.0259c | 1.6226 | 1.0574–2.4899 |
| C | 39(18.75) | 85 (27.2) | |||
a Percentage values were used for statistical analysis
b Fisher’s exact test (3 × 2 table at 2 df), P < 0.05
c Fisher’s exact test (2 × 2 table at 1 df), P < 0.05
CI confidence interval
Genotyping of other SNPs of IL-6 gene promoter
In addition to the IL-6 –174G/C promoter polymorphism, we also genotyped five other SNPs present in IL-6 gene promoter (Table 2). However, we could not observe any of them.
Table 2.
IL-6 gene promoter SNPs genotyped in the present study
| S.No | Primers | ds SNP id | IUPAC code | NTa | SNP status |
|---|---|---|---|---|---|
| 1 | F-5′TGACTTCAGCTTTACTCTTGT3′ R-5′CTGATTGGAAACCTTATTAAG3′ |
rs184229712 | R | A/G | Absent |
| 2 | ″ | rs36215459 | W | A/T | Absent |
| 3 | ″ | rs35178191 | TC | -/C | Absent |
| 4 | ″ | rs2069829 | Y | C/T | Absent |
| 5 | ″ | rs2234683 | S | G/C | Absent |
| 6 | ″ | rs1800795 | C | G/C | Present |
a Nucleotide change
SNP single nucleotide polymorphism
Discussion
SNPs are common DNA sequence variations among individuals that play a significant role in development of several human diseases. SNPs particularly in gene promoters and protein coding regions may modulate gene function and/or transcriptional efficiency. The association between IL-6 –174G/C polymorphism and the risk of developing various diseases has been investigated by different scientific groups with inconsistent results. Some studies showed association and some no association [8, 19, 29, 30].
In the present study, we found that the frequency of ‘G’ allele was significantly higher in PCOS patients than unaffected controls from the same population (Table 3). In addition, genotype frequency of the G/G was also significantly higher in PCOS patients (p = 0.02). Thus, our results indicate IL-6 as a candidate gene for PCOS. However, earlier studies showed inconsistent results (Table 4). One of the previous study have demonstrated no statistical significant association between IL-6 –174G/C polymorphism and PCOS risk which is not in agreement with the present result [25]. This disagreement may be due to differences in ethnicity, genetic variants and epigenetic environmental factors among populations (Table 4).
Table 4.
Comparison of previous case–control studies with the present study
| Polymorphism | Present study | Walch et al. | Erdogan et al. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| −174 G/C | PCOS n = 104 (%) | Controls n = 156 (%) | p-value/odds ratio | PCOS n = 62 (%) | Controls n = 94(%) | p-value/odds ratio | PCOS n =88 (%) | Controls n = 119 (%) | p-value/odds ratio |
| Genotypes | |||||||||
| C/C | 4 (3.8) | 6 (3.8) | 0.02 | 8 (38.7) | 16 (45.7) | 0.48 | 5 (5.7) | 12(10.1) | < 0.001 |
| G/C | 31 (29.8) | 73 (46.8) | 30 (48.4) | 35 (37.2) | 26 (29.5) | 75(63.0) | |||
| G/G | 69 (66.3) | 77 (49.4) | 24 (36.2) | 43 (28.6) | 57 (64.8) | 32 (26.9) | |||
| Alleles | |||||||||
| C | 39 (18.75) | 85 (27.2) | 0.0259/1.6226 | 46 (37.1) | 67 (35.6) | 0.89/1.1 | 30 (20.5) | 99(41.6) | < 0.001 |
| G | 169 (81.25) | 227 (72.8) | 78 (62.9) | 121 (64.4) | 146 (79.5) | 139(58.4) | |||
The –174G/C SNP is located in the IL-6 promoter which is known to influence expression of the gene. Previous transient transfection studies revealed stronger expression for ‘G’ allele compared to ‘C’ in response to stimuli such as lipopolysaccaride and IL-1 [31, 32]. Several transcription factors, including activator protein-1 (AP-1), nuclear factor kappaB (NF-κB) and cyclic adenosine monophosphate (cAMP) response element binding protein (CREB), contribute to the regulation of IL-6 in vivo [33]. The –174G/C SNP maps to a negative regulatory domain (−225 bp to −164 bp) which is closer to the cAMP-responsive element (CRE). Furthermore, this SNP is contained within a sequence, bearing partial nucleotide homology with the Smad4 binding element and the presence of the ‘C’ allele may bind Smad4 more effectively and thereby inhibit IL-6 transcription [34]. Furthermore, elevated levels of IL-6 have been reported in the serum of PCOS patients [35].
IL-6 is produced by various types of lymphoid and nonlymphoid cells, such as T cells, B cells, monocytes, fibroblasts, keratinocytes, endothelial cells, mesangium cells and several tumor cells [36]. In addition, IL-6 is also produced by granulosa and thecal cells of ovary, with a potential role in the autocrine and/or paracrine regulation of ovarian function [37]. IL-6 is thought to be capable of directly influencing follicle stimulating hormone (FSH) stimulated progesterone production by granulosa cells in vitro [38]. Moreover, IL-6 appears to mediate the angiogenic process associated with follicle development [12].
PCOS is considered to be a multifactorial disease with a characteristic of chronic inflammation [39]. However, contradictory observations have been reported regarding concentrations of pro-inflammatory cytokines such as IL-6 in serum of PCOS patients [40]. Although, we did not measure the levels of IL-6 in the present study, it is noteworthy that earlier investigations have reported association of the homozygous ‘CC’ genotype with higher serum IL-6 levels in contrast to studies indicating higher levels for the ‘GG’ genotype [31, 32, 41, 42]. It appears that transcriptional control of IL-6 is multifaceted and it is determined by a combination of base alterations at several sites rather than by an isolated SNP [43]. Furthermore, it is unclear whether the variation in cytokine levels is a cause or a consequence of the disease.
Our results draw preliminary conclusions due to relatively small sample size. However, there is a necessitate for further larger-scale study including other loci of the IL-6 gene to confirm our findings, and to fully examine the possible relationship between IL-6 gene polymorphisms.
In summary, based on our results, the present study concludes significant association between IL-6 –174G/C polymorphism and PCOS in South Indian women. There was significant increase of G/G genotype frequency in patients as compared to controls and the allele frequency also showed similar trend representing that ‘G’ allele might confer risk and ‘C’ allele offer protection against the disease. We propose that IL-6 may be regarded as a candidate gene for PCOS. To the best of our knowledge, this is the first study regarding the association between IL-6 –174G/C polymorphism and PCOS susceptibility in Indian women.
Electronic supplementary material
(DOC 29 kb)
Acknowledgments
We are most grateful to all of the patients who participated in the present study and for providing a Project Assistant fellowship in OU-DST PURSE Programme (Lr No: A-07/PURSE/Coord/2011) to BM.
Author Disclosure Statement
No competing financial interests exist
Funding
This study was supported in part by grants from the Department of Science and Technology (DST), India (Lr No: SR/FT/LS-188/2009) to BM.
Footnotes
Capsule
IL-6 −174 G/C promoter polymorphism is associated with PCOS in south Indian women. ‘G’ allele might confer risk and ‘C’ allele offer protection against the disease.
Contributor Information
Venkat Reddy Tumu, Email: venkatreddytm@gmail.com.
Suresh Govatati, Email: sureshgovatati@gmail.com.
Praveen Guruvaiah, Email: renapraveen@gmail.com.
Mamata Deenadayal, Email: iircoxegene@rediffmail.com.
Sisinthy Shivaji, Email: shivas@ccmb.res.in.
Manjula Bhanoori, Phone: +91-9989661469, FAX: +91-40-27097044, Email: bhanoorim@yahoo.co.in.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 3.Goodarzi MO, Azziz R. Diagnosis, epidemiology, and genetics of the polycystic ovary syndrome. Best Pract Res Clin Endocrinol Metab. 2006;20:193–205. doi: 10.1016/j.beem.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Carmina E. Cardiovascular risk and events in polycystic ovary syndrome. Climacteric. 2009;12:22–25. doi: 10.1080/13697130903003842. [DOI] [PubMed] [Google Scholar]
- 5.Sarbani M, Annapurna M. Molecular and genetic factors contributing to insulin resistance in polycystic ovarian syndrome. Indian J Med Res. 2010;131:743–760. [PubMed] [Google Scholar]
- 6.Kandaraki E, Christakou C, Diamanti KE. Metabolic syndrome and polycystic ovary syndrome…; and vice versa. Arq Bras Endocrinol Metabol. 2009;53:227–237. doi: 10.1590/S0004-27302009000200014. [DOI] [PubMed] [Google Scholar]
- 7.Tseng JF, Ryan IP, Milam TD, Murai JT, Schriock ED, Landers DV, et al. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab. 1996;81(3):1118–1122. doi: 10.1210/jc.81.3.1118. [DOI] [PubMed] [Google Scholar]
- 8.Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL-6 and related molecules (IL-1 and TNF) FASEB J. 1990;4:2860–2867. [PubMed] [Google Scholar]
- 10.Sukhikh GT, Vanko LV. Interrelationship between immune and reproductive system in humans. Russ J Immunol. 1999;4:312–314. [PubMed] [Google Scholar]
- 11.Eddie SL, Childs AJ, Jabbour HN, Anderson RA. Developmentally regulated IL6-type cytokines signal to germ cells in the human fetal ovary. Mol Hum Reprod. 2012;18(2):88–95. doi: 10.1093/molehr/gar061. [DOI] [PubMed] [Google Scholar]
- 12.Motro B, Itin A, Sachs L, Keshet E. Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci U S A. 1990;87(8):3092–3096. doi: 10.1073/pnas.87.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowcock AM, Kidd JR, Lathrop GM, Daneshvar L, May LT, Ray A, et al. The human “interferon beta2/hepatocyte stimulating factor/interleukin6” gene: DNA polymorphism studies and localization to chromosome 7p21. Genomics. 1988;3(1):8–16. doi: 10.1016/0888-7543(88)90152-8. [DOI] [PubMed] [Google Scholar]
- 14.Villuendas G, San Millán JL, Sancho J, Escobar-Morreale HF. The −597 G– > A and −174 G– > C polymorphisms in the promoter of the IL-6 gene are associated with hyperandrogenism. J Clin Endocrinol Metab. 2002;87(3):1134–1141. doi: 10.1210/jc.87.3.1134. [DOI] [PubMed] [Google Scholar]
- 15.Hulkkonen J, Vilpo J, Vilpo L, Koski T, Hurme M. Interleukin-1 beta, interleukin-1 receptor antagonist and interleukin-6 plasma levels and cytokine genepolymorphisms in chronic lymphocytic leukemia: correlation with prognostic parameters. Haematologica. 2000;85(6):600–606. [PubMed] [Google Scholar]
- 16.Belluco C, Olivieri F, Bonafè M, Giovagnetti S, Mammano E, Scalerta R, et al. 174 G > C polymorphism of interleukin 6 gene promoter affects interleukin 6 serum level in patients with colorectal cancer. Clin Cancer Res. 2003;9(6):2173–2176. [PubMed] [Google Scholar]
- 17.Vozarova B, Fernández-Real JM, Knowler WC, Gallart L, Hanson RL, Gruber JD, et al. The interleukin-6 (−174) G/C promoter polymorphism is associated with type-2 diabetes mellitus in Native Americans and Caucasians. Hum Genet. 2003;112(4):409–413. doi: 10.1007/s00439-003-0912-x. [DOI] [PubMed] [Google Scholar]
- 18.Lukas AH, Christoph G, Tilmann L. Interleukin-1 and Interleukin-6 Gene Polymorphisms and the Risk of Breast Cancer in Caucasian Women. Clin Cancer Res. 2005;11:5718–5721. doi: 10.1158/1078-0432.CCR-05-0001. [DOI] [PubMed] [Google Scholar]
- 19.Bhanoori M, Babu KA, Deenadayal M, Kennedy S, Shivaji S. The interleukin-6–174G/C promoter polymorphism is not associated with endometriosis in South Indian women. J Soc Gynecol Investig. 2005;12(5):365–369. doi: 10.1016/j.jsgi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Qi HP, Qu ZY, Duan SR, Wei SQ, Wen SR, Bi S. IL-6-174 G/C and −572 C/G polymorphisms and risk of Alzheimer’s disease. PLoS One. 2012;7(6):e37858. doi: 10.1371/journal.pone.0037858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalburgi NB, Bhatia A, Bilichodmath S, Patil SR, Mangalekar SB, Bhat K. Interleukin-6 promoter polymorphism (−174 G/C) in Indian patients with chronic periodontitis. J Oral Sci. 2010;52(3):431–437. doi: 10.2334/josnusd.52.431. [DOI] [PubMed] [Google Scholar]
- 22.Babu BM, Reddy BP, Priya VH, Munshi A, Rani HS, Latha GS, et al. Cytokine gene polymorphisms in the susceptibility to acute coronary syndrome. Genet Test Mol Biomarkers. 2012;16(5):359–365. doi: 10.1089/gtmb.2011.0182. [DOI] [PubMed] [Google Scholar]
- 23.Guo-hua Z, Hai-ying C, Shang QX. Polymorphisms of -174G.C and -572G.C in the Interleukin 6 (IL-6) Gene and Coronary Heart Disease Risk: a Meta-Analysis of 27 Research Studies. PLoS ONE. 2012;7:e34839. doi: 10.1371/journal.pone.0034839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erdogan M, Karadeniz M, Berdeli A, Tamsel S, Yilmaz C. The relationship of the interleukin-6–174 G > C gene polymorphism with cardiovascular risk factors in Turkish polycystic ovary syndrome patients. Int J Immunogenet. 2009;36(5):283–288. doi: 10.1111/j.1744-313X.2009.00867.x. [DOI] [PubMed] [Google Scholar]
- 25.Walch K, Grimm C, Zeillinger R, Huber JC, Nagele F, Hefler LA. A common interleukin 6 gene promoter polymorphism influences the clinical characteristics of women with polycystic ovary syndrome. Fertil Steril. 2004;81(6):1638–1641. doi: 10.1016/j.fertnstert.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Govatati S, Chakravarty B, Deenadayal M, Kodati VL, Manolla ML, Sisinthy S, et al. p53 and risk of endometriosis in Indian women. Genet Test Mol Biomarkers. 2012;16(8):865–873. doi: 10.1089/gtmb.2011.0295. [DOI] [PubMed] [Google Scholar]
- 28.Govatati S, Tangudu NK, Deenadayal M, Chakravarty B, Shivaji S, Bhanoori M. Association of E-cadherin single nucleotide polymorphisms with the increased risk of endometriosis in Indian women. Mol Hum Reprod. 2012;18(5):280–287. doi: 10.1093/molehr/gar079. [DOI] [PubMed] [Google Scholar]
- 29.Smith PC, Hobisch A, Lin DL, Culig Z, Keller ET. Interleukin-6 and prostate cancer progression. Cytokine Growth Factor Rev. 2001;12(1):33–40. doi: 10.1016/S1359-6101(00)00021-6. [DOI] [PubMed] [Google Scholar]
- 30.Nishimoto N. Interleukin-6 in rheumatoid arthritis. Curr Opin Rheumatol. 2006;18:277–281. doi: 10.1097/01.bor.0000218949.19860.d1. [DOI] [PubMed] [Google Scholar]
- 31.Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102(7):1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonafè M, Olivieri F, Cavallone L, Giovagnetti S, Mayegiani F, Cardelli M, et al. A gender–dependent genetic predisposition to produce high levels of IL-6 is detrimental for longevity. Eur J Immunol. 2001;31(8):2357–2361. doi: 10.1002/1521-4141(200108)31:8<2357::AID-IMMU2357>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 33.Dendorfer U. Molecular biology of cytokines. Artif Organs. 1996;20:437–444. doi: 10.1111/j.1525-1594.1996.tb04529.x. [DOI] [PubMed] [Google Scholar]
- 34.Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, et al. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell. 1998;1(4):611–617. doi: 10.1016/S1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- 35.Fulghesu AM, Sanna F, Uda S, Magnini R, Portoghese E, Batetta B. IL-6 serum levels and production is related to an altered immune response in polycystic ovary syndrome girls with insulin resistance. Mediators Inflamm. 2011;389317. [DOI] [PMC free article] [PubMed]
- 36.Horii Y, Muraguchi A, Suematsu S, Matsuda T, Yoshizaki K, Hirano T, et al. Regulation of BSF-2/IL-6 production by human mononuclear cells. Macrophage-dependent synthesis of BSF-2/IL-6 by T cells. J Immunol. 1988;141(5):1529–1535. [PubMed] [Google Scholar]
- 37.Gorospe WC, Hughes FM, Jr, Spangelo BL. Interleukin-6: effects on and production by rat granulosa cells in vitro. Endocrinology. 1992;130:1750–1752. doi: 10.1210/en.130.3.1750. [DOI] [PubMed] [Google Scholar]
- 38.Machelon V, Emilie D, Lefevre A, Nome F, Durand-Gasselin I, Testart J. Interleukin-6 biosynthesis in human preovulatory follicles: some of its potential roles at ovulation. J Clin Endocrinol Metab. 1994;79(2):633–642. doi: 10.1210/jc.79.2.633. [DOI] [PubMed] [Google Scholar]
- 39.Olszanecka-Glinianowicz M, Banaś M, Zahorska-Markiewicz B, Janowska J, Kocełak P, Madej P, et al. Is the polycystic ovary syndrome associated with chronic inflammation per se? Eur J Obstet Gynecol Reprod Biol. 2007;133(2):197–202. doi: 10.1016/j.ejogrb.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 40.Escobar-Morreale HF, Luque-Ramírez M, González F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril. 2011;95:1048–1058. doi: 10.1016/j.fertnstert.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones KG, Brull DJ, Brown LC. Interleukin-6 (IL-6) and the prognosis of abdominal aortic aneurysms. Circulation. 2001;103:2260–2265. doi: 10.1161/01.CIR.103.18.2260. [DOI] [PubMed] [Google Scholar]
- 42.Jerrard-Dunne P, Sitzer M, Risley P, Steckel DA, Buehler A, Von Kegler S, et al. Interleukin-6 promoter polymorphism modulates the effects of heavy alcohol consumption on early carotid artery atherosclerosis: the Carotid Atherosclerosis Progression Study (CAPS) Stroke. 2003;34(2):402–407. doi: 10.1161/01.STR.0000053849.09308.B2. [DOI] [PubMed] [Google Scholar]
- 43.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275:18138–18144. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 29 kb)