Abstract
The dicentric Y chromosomes are the most commonly found in the structural aberration of Y chromosome. If the dicentric chromosome has completely symmetric arms, it is considered an isodicentric chromosome. The sites of breakage and fusion at Yp and Yq are variable, but breakage and fusion at the pseudo-autosomal region has never been reported. Herein we reported identification de novo isodicentric (Yq12) in a fetus. The fusion occurred at Yq pseudo-autosomal region very close the telomere and resulted in duplication of Y chromosome. The baby was grossly normal at birth. In conclusion, isodicentric Y chromosome could result from breakage and fusion at the Yq pseudo-autosomal region.
Keywords: Y chromosome, Isodicentric chromosome, Pseudo-autosomal region
Introduction
The structural abnormalities affecting the human Y chromosome include deletions, rings, Y- autosomal or Y- X translocations, isochromosomes, and dicentrics [1]. Among these structural abnormalities, dicentric Y chromosomes are the most commonly found [2, 3]. These dicentric Y chromosomes correspond to two different types: dic (Yq), resulting from the fusion between the short arms of two Y chromosomes in which some Yp material is maintained, and dic (Yp) arising from fusion at the Yq arms. If the dicentric chromosome has completely symmetric arms, it is considered an isodicentric chromosome. The sites of breakage and fusion at Yp and Yq are highly variable [4].
Most (approximately 90 %) of dicentric Y chromosomes are present in mosaic form. The mosaicism may be more or less complex depending on its meiotic or post—zygotic origin and on its mitotic stability, and it usually includes of a 45, X cell line. Patients carrying a dicentric Y chromosome have a wide range of somatic, genital, and gonadal phenotypic manifestations, depending on the structure of the dicentric Y chromosome, the Yp and Yq breakpoints, and the types of mosaicism [5].
Here we report a case of de novo isodicentric (Yq12) in the fetus. The fusion occurred at Yq pseudo-autosomal region very close the telomere and resulted in duplication of Y chromosomes. The baby was grossly normal at birth.
Case report
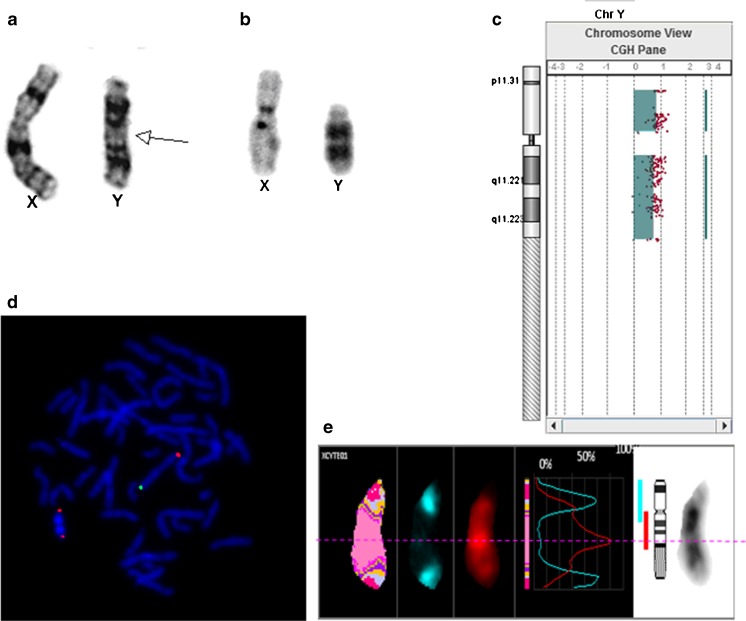
A 36-year-old Taiwanese woman, gravida 1 para 0, underwent amniocentesis at the 18th week of gestation due to advanced maternal age. Chromosome analysis of the amniocytes showed an abnormal Y chromosome in all metaphase cells analyzed. It was tentatively designated as 46, X, idic (Y)(q12)(Fig. 1a). She was conceived spontaneously and had not experienced miscarriage. Karyotype of the couple was normal male (46, XY) and normal female (46, XX), respectively. She decided to continue the pregnancy and delivered a gross normal male baby with a body weight of 3,020 g and body length of 50 cm. The external genital organ of the newborn baby was normal. The C-banding showed the Y chromosome contained two centromeres and two heterochromatin blocks (Fig. 1b). The Array-based comparative genomic hybridization (aCGH) using a high density CytoScan V5 was applied to detect genomic aberrations. The CytoScan V5 chip encompasses 60,000 DNA probes with a resolution of about 50 kb (Agilent Technologies, USA). The aCGH result (Fig. 1c) showed the baby had duplication of the entire Y chromosome. Deletion mapping analysis of the Y chromosome by screening 10 genes present in the three AZF regions and 5 genes outside the AZF regions, as described in our previous publications, did not detect any gene deletions [6, 7]. FISH was used to characterize the abnormal Y chromosome by using the Cytocell Subtelomere Specific Probes Kits (Cytocell, Inc.) which contained X-Yp Subtelomere Specific Probe corresponding to the loci DXYS129 labeled with Texas Red fluorophore and X-Yq Subtelomere Specific Probe corresponding to the loci DXYS61 labeled with FITC fluorophore. Multicolor banding was performed using the Xcyte Y mBAND probe kit (MetaSystem, Inc.). The result of mBAND and FISH analysis show the abnormal Y chromosome was composed of the two intact Y chromosomes fused at the second pseudo-autosomal region of Yq (Fig. 1d). The karyotype was designated as ish idic (Y)(q11.23)(mBAND Y+, DXYS129++).
Fig. 1.
The karyotype, array-based comparative genomic hybridization (aCGH) and fluorescence in situ hybridization pictures of the proband. a G-banding of the sex chromosomes. b C-banding of the sex chromosomes. c aCGH shows the duplication of the Y chromosome euchromatic region. d FISH analysis of the Y chromosome. The green signals represent Xq-Yq subtelomere, while red signals represent Xp-Yp subtelomere. e mBAND analysis of the Y chromosome
Discussion
Prenatal detection of an idic (Yp) or (Yq) often poses significant counseling dilemma given many cases are presented in the mosaic form and have highly variable clinical features. Only a few cases showing non-mosaic iso-dicentric Y chromosome have been previously described in humans. DesGroseilliers et al. [2006] [8] have described 2 male patients showing a non-mosaic idic (Y) with breakpoints at Yq11.21. Those patients showed the karyotype 46, X, idic (Y)(q11.21), therefore lacking most of Yq and were positive for the Y centromere (DYZ3) and SRY but negative for the Yq heterochromatin (DYZ1). The long arm of chromosome Y is enriched with palindromes that were shown to mediate recombination between arms of sister chromatids to generate the idic (Yp), explaining their higher prevalence as compared to idic (Yq) [9]. Our case is unique given the non-mosaic feature. It would be desirable to investigate presence of 45, X cells in different tissues by FISH analysis. Another unique feature for our case is that the breakpoint occurred at Yq11.23 pseudo-autosomal region 2 (PAR-2) very close to the telomere. Pseudo-autosomal regions are also hot-spots for genetic recombination and gene conversion [10]. The aCGH data showed the duplication of entire Y chromosome. Concerning the amount of chromosomal DNA content per cell, this case was comparable to XYY males. The XYY condition was usually asymptomatic with only mild phenotypic abnormalities [11]. Two non-mosaic male patients were reported by DesGroseillier et al. [2006] [8] who postulated that the isodicentric Y chromosome was stabilized early during gametogenesis in the father. The stability of dicentrics may result from inactivation of one of the centromeres [2], or due to presence of a very small distance between two active centromeres and thus behaving as monocentric [12]. To the best of our knowledge, there have been very few eports on formation of dicentric Y chromosome with breakage and fusion at the pseudo-autosomal regions. In 2005, Heinritz reported an adult male with Klinefelter phenotype and an isodicentric Y chromosome (47, XX, + idic (Y)(q12)). Although it is highly likely that that case also had breakage and fusion at the PAR-2 [13], the gene dosage and break points were not confirmed by other methods. Our case should be the first documented case of idic(Yp) with breakage and fusion at the PAR-2.
Sex-chromosome abnormalities occur in approximately 0.2 % of live births [14]. Common sex-chromosome anomalies such as 45, X; 47, XXY; 47, XXX and 47, XYY have been well described and these common sex chromosome aneuploides are associated variable phenotypes [15]. This case actually represents a unique type of 47, XYY. The phenotypes of 47,XYY individuals commonly include tall stature, macrocephaly, macroorchidism, hypotonia, hypertelorism, and tremor, but they are usually presented with normal fertility [16]. Significant raised mortality has been observed for disease of various organ systems (nervous system, circulatory system, respiratory system, genitourinary system, and congenital anomalies) in men with an extra Y chromosome. However, the cancer incidence and mortalities are not increased [17]. Emerging evidence also suggested higher rates of problem behaviors and hyperactive/impulsive symptoms [18]. Considering important role of pseudo-autosomal region of meiotic sex chromosome pairing and intact pseudo-autosomal regions (PARs) in this case, it is anticipated the meiotic paring would not be compromised [19].
Footnotes
Capsule
Isodicentric Y chromosome could result from breakage and fusion at the Yq pseudo-autosomal region.
References
- 1.Codina-Pascual M, Oliver-Bonet M, Navarro J, Starke H, Liehr T, Gutiérrez-Mateo C, et al. FISH characterization of a dicentric Yq (p11.32) isochromosome in an azoospermic male. Am J Med Genet. 2004;127A:302–306. doi: 10.1002/ajmg.a.30027. [DOI] [PubMed] [Google Scholar]
- 2.Hsu LYF. Phenotype/ karyotype correlations of Y chromosome aneuploidy with emphasis on structural aberrations in postnatally diagnosed cases. Am J Med Genet. 1994;53:108–140. doi: 10.1002/ajmg.1320530204. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida A, Nakahori Y, Kuroki Y, Motoyama M, Araki Y, Miura K, et al. Dicentric Y chromosome in an azoospermic male. Mol Hum Reprod. 1997;3:709–712. doi: 10.1093/molehr/3.8.709. [DOI] [PubMed] [Google Scholar]
- 4.Robinson DO, Dalton P, Jacobs PA, Mosse K, Power MM, Skuse DH, et al. A molecular and FISH analysis of structurally abnormal Y chromosome in patients with Turner syndrome. J Med Genet. 1999;36:279–284. [PMC free article] [PubMed] [Google Scholar]
- 5.Tuck-Muller CM, Chen H, Martínez JE, Shen CC, Li S, Kusyk C, et al. Isodicentric Y chromosome: cytogenetic, molecular, and clinical studies and review of the literature. Hum Genet. 1995;96:119–129. doi: 10.1007/BF00214200. [DOI] [PubMed] [Google Scholar]
- 6.Kuo PL, Wu RC, Lin SJ, Tzeng CC, Liu HS, Huang KE. Detection of Y-chromosome sequences in patients with X-chromosome abnormalities. J Formos Med Assoc. 1995;94:529–534. [PubMed] [Google Scholar]
- 7.Lin YM, Lin YH, Teng YN, Hsu CC, Lin JSN, Kuo PL. Gene-based screening for Y chromosome deletions in Taiwanese men presenting with spermatogenic failure. Fertil Steril. 2002;77:897–903. doi: 10.1016/S0015-0282(02)03059-5. [DOI] [PubMed] [Google Scholar]
- 8.DesGroseilliers M, Beaulieu Bergeron M, Brochu P, Lemyre E, Lemieux N. Phenotypic variability in isodicentric Y patients: study of nine cases. Clin Genet. 2006;70:145–150. doi: 10.1111/j.1399-0004.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 9.Lange J, Skaletsky H, van Daalen SKM, Embry SL, Korver CM, Brown LG, et al. Isodicentric Y chromosomes and sex disorders as byproducts of homologous recombination that maintains palindromes. Cell. 2009;138:855–869. doi: 10.1016/j.cell.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarbajna S, Denniff M, Jeffreys AJ, Neumann R, Artigas MS, Veselis A, et al. A major recombination hotspot in the XqYq pseudoautosomal region gives new insight into processing of human gene conversion events. Hum Mol Genet. 2012;21:2029–2038. doi: 10.1093/hmg/dds019. [DOI] [PubMed] [Google Scholar]
- 11.Lalatta F, Folliero E, Cavallari U, Segni MD, Gentilin B, Fogliani R, et al. Early manifestations in a cohort of children prenatally diagnosed with 47. XYY. Role of multidisciplinary counseling for parental guidance and prevention of aggressive behavior. Ital J Pediatr. 2012;38:52. doi: 10.1186/1824-7288-38-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemyre E, der Kaloustian VM, Duncan AM. Stable non-Robertsonian dicentric chromosomes: four new cases and a review. J Med Genet. 2001;38:76–79. doi: 10.1136/jmg.38.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinritz W, Kotzot D, Heinze S, Kujat A, Kleemann WJ, Froster UG. Molecular and cytogenetic characterization of a non-mosaic isodicentric Y chromosome in a patient with Klinefelter syndrome. Am J Med Genet A. 2005;132A:198–201. doi: 10.1002/ajmg.a.30446. [DOI] [PubMed] [Google Scholar]
- 14.Robinson A, Linden MG, Bender BG. Prenatal diagnosis of sex chromosome abnormalities. In: Milunsky A, editor. Genetic disorders and the fetus: diagnosis, prevention and treatment. 4. Baltimore and London: Johns Hopkins University Press; 1998. pp. 249–285. [Google Scholar]
- 15.Bruyère H, Speevak MD, Winsor EJT, de Fréminville B, Farrell SA, McGowan-Jordan J, et al. Isodicentric Yp: prenatal diagnosis and outcome in 12 cases. Prenat Diagn. 2006;26:324–329. doi: 10.1002/pd.1406. [DOI] [PubMed] [Google Scholar]
- 16.Bardsley MZ, Kowal K, Levy C, Gosek A, Ayari N, Tartaglia N, et al. 47, XYY Syndrome: clinical phenotype and timing of ascertainment. J Pediatr. 2013;163:1085–1094. doi: 10.1016/j.jpeds.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins CD, Swerdlow AJ, Schoemaker MJ, Wright AF, Jacobs PA. UK clinical cytogenetics group. Mortality and cancer incidence in males with Y polysomy in Britain: a cohort study. Hum Genet. 2007;121:691–696. doi: 10.1007/s00439-007-0365-8. [DOI] [PubMed] [Google Scholar]
- 18.Ross JL, Roeltgen DP, Kushner H, Zinn AR, Reiss A, Bardsley MZ, et al. Behavioral and social phenotypes in boys with 47, XYY syndrome or 47. XXY Klinefelter Syndr Pediatr. 2012;129:769–778. doi: 10.1542/peds.2011-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kauppi L, Barchi M, Baudat F, Romanienko PJ, Keeney S, Jasin M. Distinct properties of the XY pseudoautosomal region crucial for male meiosis. Science. 2011;331:916–920. doi: 10.1126/science.1195774. [DOI] [PMC free article] [PubMed] [Google Scholar]