Abstract
Osteoarthritis (OA) is a common disease affecting patients at different ages regardless of gender or ethnicity. As with many chronic diseases, OA is thought to have a multifactorial aetiology, which is not fully understood. Whereas the pathophysiological process of OA can be analysed at a cellular and molecular level, the interaction between genes and lifestyle remains an important factor in the development of this disease. The expanding awareness of different genes that may play a role in OA, together with many chemical mediators thought to be associated with the progression of the disease, will help in better management of this condition. Some of the chemical mediators recently implicated in this condition are the adipokines (leptin, adiponectin and resistin). Few but consistent studies suggest that leptin in association with obesity could be an important factor in OA aetiology. Hence, this could establish a strong and direct molecular link between patient life style (nurture) and the pathological process of OA (nature). However, neither a clear mechanism nor a direct clinical association linking leptin to OA has yet been established. In this article, we explore some of the genetic and environmental factors in OA aetiology. We discuss leptin in obesity and assess its possible association with OA aetiology. This should emphasise the important role of health professionals in treating obesity in order to control OA symptoms and possibly progression.
Keywords: Osteoarthritis, Gene, Homeostasis, Leptin, Environment
Introduction
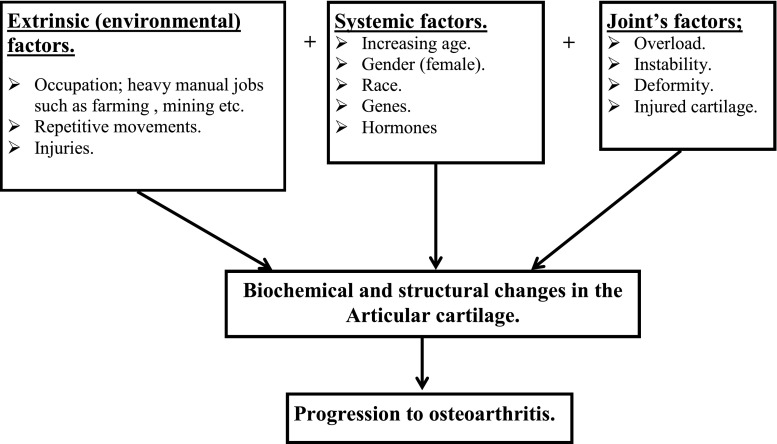
Osteoarthritis (OA) is a common progressive disease with a complicated aetiology affecting synovial joints and results in fibrillation of the extracellular matrix (ECM) and ultimately ulceration of the articular cartilage, leading to reduction in joint space [1, 2]. It effects 15 % of the world’s population and is strongly associated with aging [3]. In the UK, approximately eight million people suffer from OA, and one million are undergoing some form of treatment [4]. All ethnic groups are affected [5], but the prevalence differs from one race to another. For example, it is more prevalent in the white Europeans and less so in black Africans, Indians and Chinese [5, 6]. OA is also more common in women [7] and strongly associated with obesity (knee) [8, 9] and manual jobs (hip and knee) [7, 10, 11], reflecting the complex interaction between genetics and environmental factors in its aetiological process (Fig. 1).
Fig. 1.
Factors influencing the onset of osteoarthritis (OA). Many factors in isolation or combination can influence onset and progression of the disease
Gradual erosion of the articular cartilage in OA is preceded by compositional and mechanical changes in the ECM mediated by an imbalanced regulation of a plethora of complex molecules, such as metalloproteinases and multiple inflammatory factors [12–14]. This overwhelms the homeostatic processes of the articular cartilage, leading to its structural failure [15].
Genetic and environmental influences on the development of the disease
As with other chronic conditions, such as hypertension and asthma, OA has been classified as a common, multifactorial disorder with environmental and genetic causes [16, 17]. Its possible genetic basis was first recognised in the 1940s. Subsequent studies revealed that a twin or sibling of someone with OA was more likely to develop the disease than the general population [16, 18]. Since then, the genetic influence on OA pathogenesis has consistently been shown by many epidemiological, twin-pair and family-clustering studies [18–21]. Genome-wide association and linkage-scan studies have uncovered a plethora of genes that may be implicated in OA aetiology [22], and now it is thought that the genetic influence could explain >50 % of the variations in susceptibility to this condition [16, 18]. It is evident that OA may have a polygenic inheritance mode transmitted in a non-Mendelian manner, whereby the interaction of many genetic defects may be responsible [19]. The severity of the disease is determined by the amount and the severity of gene defects.
Mercedes et al., in their review, listed more than 26 possibly important genes thought to harbour susceptibility to OA, with a variable level of involvement [18]. These include genes coding for structural proteins of the ECM, such as collagen type 2, and cytokines involved in the inflammatory process associated with this condition. Some genes discovered thus far do not pose a consistent increased susceptibility to OA development [18, 20]. However, the involvement of the interleukin 1 (IL-1) gene and the asporin gene (ASPN) have been the most compelling, showing a significantly strong statistical association [22]. Other genes thought to be of importance include that encoding vitamin D and its receptor, the oestrogen receptor α gene (Er-α) and the secreted frizzled-related protein 3 gene (FRZB) [16, 18, 19].
Following the announcement of the sequencing of the human genome in April 2003 [23], there has been considerable emphasis on the genetic role in the pathogenesis of diseases, including OA. This project created a base for further research into the mode of inheritance of genetic diseases based on isolating and cloning mutated genes [24]. The next important step in genetics is the human proteome project, which aims to identify all proteins present in tissues and cells and their alternations in relation to health and illness [25]. Researchers in other projects have diverted their attention into the possible involvement of mitochondria and mitochondrial DNA in the pathogeneses of OA [26]. Some studies demonstrate the association of certain mitochondrial DNA polymorphism with the severity of OA in the European population [18].
The role of the environment in OA aetiology has been demonstrated by directly linking OA to some jobs and activities undertaken by individuals [10, 27] (Table 1). In a cross-sectional study involving more than 10,000 people, Rossignol et al. [7] reported an increase in the prevalence of hip and knee OA in people involved in more physically demanding occupations, with an earlier incidence in women than in men. Activities involving kneeling, squatting and heavy lifting are associated with knee OA [30, 31], particularly in patients with a high body mass index (BMI) [11]. Framing and other heavy manual jobs are associated with hip OA [10, 27], whereas textile workers have a higher incidence of hand OA [7]. Despite the consistency in these studies, some authors report that the association between occupation and OA is weak [27], suggesting that another and more potent factor (or factors) may play a significant role in the aetiology of the disease.
Table 1.
Occupation and osteoarthritis. Some occupational activities have been associated with osteoarthritis of the main joints
| Occupational hazard | Reference citations |
|---|---|
| Hip | |
| Heavy manual work | [27, 28]. |
| Tasks involving uncomfortable joint position | [7]. |
| Female cleaners | [7]. |
| Female postal workers | [29]. |
| Male farmers | [7, 27, 29]. |
| Builders | [7, 29]. |
| Fire fighters and food-processing workers | [29]. |
| Knee | |
| Tasks involving uncomfortable joint position | [7]. |
| Repetitive bending, kneeling, squatting | [28, 30, 31]. |
| Carrying loads | [32]. |
| Carpenters and miners | [33]. |
| Female cleaners | [7, 29]. |
| Fire fighters | [29]. |
| Construction workers | [29, 31]. |
| Hand and wrist | |
| Movements repetition, such as bending and twisting | [7, 28, 34] |
| Work at a pace set by a machine | [7, 34] |
| Textile workers | [7] |
| Female cleaners | [7] |
| Technicians and assemblers | [35] |
| Elbow | |
| Heavy physical work | [36] |
| Foundry workers | [37] |
OA and role of obesity and leptin
Many mechanisms are proposed for the degenerative process in the articular cartilage [13]. OA progression is broadly divided into three stages [1]: stage I involves breakdown of the cartilage matrix by proteolytic enzymes; stage II involves further damage to the articular cartilage, fibrillation and erosion, together with the release of products into the joint space; stage III is characterised by inflammatory responses as a result of ingestion of products and synthesis of proinflammatory cytokines and proteases. Some inflammatory components include tumour necrosis factor alpha (TNF-α) and interleukin-1, (IL-1), which lead to the slowing of ECM synthesis and increased catabolic activities of metalloproteinases [14, 38].
The biomechanical effect of weight is thought to exacerbate the degenerative process and symptoms of OA directly by excessive loading of the joints and indirectly by stimulating mechanoreceptors on the surface of chondrocytes [39, 40]. Activation of these receptors is thought to induce production of inflammatory mediators and metalloproteinases responsible for cartilage destruction [41]. Nevertheless, whereas excessive weight is considered an aetiological factor, it does neither explains the association of hand OA with obesity [15, 42] nor the high risk of developing OA in more than one joint in the same individual [43]. Some authors argue that the only role of biomechanical load is to determine which joint manifests the first symptoms [15], and that OA is driven by systemic and metabolic processes other than the biomechanical influence of weight [44, 45], hence the presence of OA in non-weight-bearing joints.
Using the example of knee OA, Clockaerts et al. [46] suggested that the accumulation of immune cells, adipocytes and nerve fibres (peptidergic C fibres) in the infrapatellar fat pad (IFFP) influences cartilage metabolism, which provides an insight into other possible mechanisms that may be implicated in OA, especially in non-weight-bearing joints [44].
Adipose tissues are known as excess-fat-storage tissues but are now recognised as a form of connective tissue containing adipocytes, fibroblasts, leucocytes and macrophages involved in the process of inflammation [47, 48]. They produce ILs, growth factors including TNF-α, which are known to have an influence on OA [46]. Furthermore, adipose tissue secretes adipokines such as leptin and resistin [49]. These are found at increased concentration levels in obese individuals, hence their possible implication in cartilage metabolism and OA [50, 51]. Lack of exercise and a diet high in fat content are associated with increased levels of leptin within the serum [52]; conversely, reduction in BMI is associated with reduction in leptin serum levels [53]. This may suggest that the body’s natural homeostatic control system for leptin within the joint fails to restore the normal homeostatic balance of leptin within the joint in obese individuals.
Leptin is a 16-kDa, nonglycosylated peptide hormone encoded by the obese (LEP) gene [54]. Leptin enters the circulation, crossing the blood–brain barrier to act on the hypothalamus to control food intake and energy expenditure [55]. It is involved in regulating other physiological processes, including lipid homeostasis, thermogenesis [56], insulin secretion [57], reproductive functions [58], angiogenesis and immune function [59, 60]. It is also shown to regulate bone growth directly by inducing osteoblast proliferation, collagen synthesis and bone mineralisation or indirectly by releasing antiosteogenic factor [17]. It is thought that leptin has an anabolic effect on cartilage by stimulating TGF-β and insulin-like growth factor (IGF) [44, 61]. Furthermore, leptin is classified as an adipocytokine because it shares common structural properties with IL-6 cytokines [54] and therefore is thought to play a role in the immune response [62].
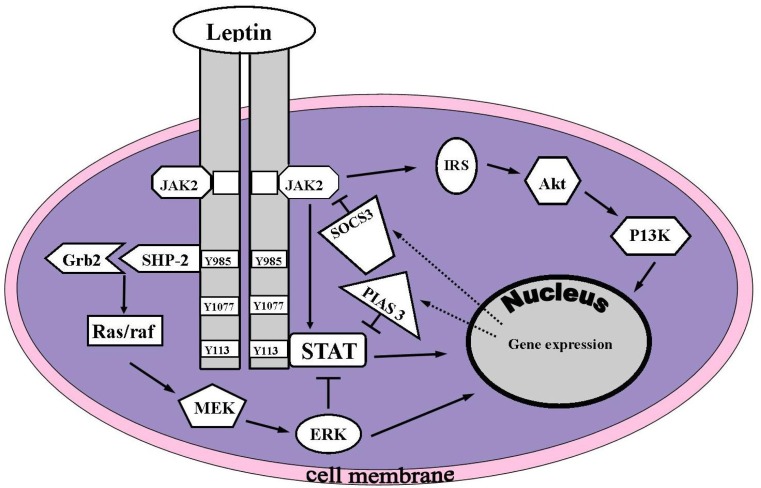
An increased level of freely circulating leptin in blood plasma is thought to diffuse into the joint space [61]. The high levels of leptin in the synovial fluid are detected by sensory receptors for leptin on chondrocytes [61, 63]. It induces its effects by stimulating the leptin receptor, which is encoded by the LEPR gene with at least five isoforms existing in humans. The leptin receptor (OB-Rb) activates the Janus kinase/signal transducer, an activator of the transcription signal transduction (JAK/STAT) pathway [54] (Fig. 2).
Fig. 2.
Mechanism of leptin Intercellular signaling. Upon leptin binding to the leptin receptor (OB-Rb), the Janus kinase/signal transducer and activator of transcription (JAK/STAT), mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and phosphatidylinositol 3-kinase (PI3K) pathways are activated. Akt protein kinase B; Grb-2 growth-receptor-bound 2; IRS insulin receptor substrate; MEK mitogen-activated protein kinase kinase; PIAS3 protein inhibitor of activated signal transducer and activator of transcription 3 (STAT3); Raf MEK-kinase; Ras G-protein; SHP2 domain-containing protein-tyrosine phosphatase; PTPN11, SOCS3 suppressor of cytokine signalling-3. Reproduced with kind permission from [60]
In obese individuals, there is an increase in leptin serum and synovial fluid levels [50, 61]. In OA articular cartilage, an increased level of leptin messenger RNA (mRNA) is found in chondrocytes [63] in association with an increased level in synovial fluid [50, 61, 63]. Demund et al. demonstrated a correlation between the level of leptin expression by chondrocytes and the increased grade of cartilage destruction [61]. Even when comparing OA and normal cartilage in the same joint, Simopoulou et al. found an increased expression of leptin and its receptors on chondrocytes inhabiting the OA cartilage zones [63].
In laboratory studies on mice, leptin induces formation of proteoglycans, IGF and TGF-β1 in a dose-dependant response [54, 61], as well as IL1B, gelatinase B (MMP-9) and human procollagenase 3 (MMP-13) expression [63], which confer detrimental effects on chondrocyte proliferation at high concentration levels [54, 63]. These studies suggest that leptin may be involved in regulating chondrocyte metabolism and the ECM composition in OA. In degenerative vertebral spine discs, leptin and its receptor have been detected at high concentration levels promoting proliferation of nucleus cells, which also suggests an important role for leptin in pathogenesis of intervertebral disc degeneration [64].
Laboratory findings provide convincing evidence of a possible role for leptin in OA pathogenesis. However, a few authors failed to establish a direct association between serum leptin (and other adipokines) and arthritis in the hand. In a study involving 44 patients, Massengale et al. [65] could not demonstrate any correlation between the grade of hand OA and the levels of serum adipokinase. This has been supported by a larger study involving more than 1,000 patients, in which the authors could not demonstrate a significant difference in serum leptin concentration levels in patients with OA (symptomatic and asymptomatic) and controls [66]. However, leptin was correlated with the visual analogue scale (VAS) for pain, which may yield support for its inflammatory role in OA [65, 67, 68]. The contradictory results between laboratory studies and recent clinical studies cast a shadow on the exact role of leptin in OA pathogenesis; however, the lack of correlation between serum levels of leptin and OA severity could be explained by the following:
Leptin may not have any role in OA initiation but could be a secondary response involved in the subsequent inflammatory phase following articular cartilage degradation. Hence the high levels of leptin found in zones affected by OA and low levels in normal cartilage within the same joint [63]. If the high concentration level of leptin in obese individuals plays a major role in OA initiation, then leptin expression would be distributed uniformly in all joint cartilage and would also result in uniform cartilage destruction. However, the existence of leptin as an inflammatory mediator in OA cartilage may still potentiate the degradation process [67, 68].
Leptin at a low concentration levels stimulates chondrocyte proliferation but inhibits proliferation at high concentration levels [63], which are only found in association with high BMI [61]. Therefore, its inhibitory role may manifest only in obese individuals. Hence the high serum leptin levels correlated with knee OA in patients with high BMI [67].
In OA, leptin concentration in synovial fluid is greater than that of serum levels [50, 67]. Hence, serum levels may not reflect true leptin synovial levels, which have a direct influence on articular cartilage. This could be the reason that studies by Massengale et al. [65, 66] failed to establish an association between serum leptin and hand OA.
In conclusion, biomechanical causes of OA in obesity play a vital role in OA progression, but the associated high levels of adipokines (such as leptin) in chondrocytes suggest a possible implication of leptin in OA pathogenesis. Studying the multifactorial aetiology and involvement of many complex processes in OA will continue to reveal different chemical mediators that may appear to have an association with OA pathogenesis. The difficulty will be distinguishing between molecules that play a role in OA initiation and progression phases and those that emerge as a localised secondary response to the condition. This may also explain why target therapy against some of the chemical components of OA has not yielded any clinical benefit [15].
Whatever the mechanisms linking obesity to OA—be it biomechanical or chemical—there should be a concerted effort to counteract the effect of obesity on joints. In fact, this provides a venue for health-care workers to help reduce progression and symptoms of OA associated with obesity. Obesity is a modifiable factor [69] that could be reduced or eliminated by education and support/guidance given by health-care professionals. This is important because the increasing epidemic of obesity worldwide could potentially lead to an increase in the incidence of OA.
References
- 1.Martel-Pelletier J. Pathophysiology of osteoarthritis. Osteoarthritis Cartilage. 2004;12(Suppl A):S31–S33. doi: 10.1016/j.joca.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Walker J. Effective management strategies for osteoarthritis. Br J Nurs. 2011;20:81–85. doi: 10.12968/bjon.2011.20.2.81. [DOI] [PubMed] [Google Scholar]
- 3.Fajardo M, Di Cesare PE. Disease-modifying therapies for osteoarthritis: current status. Drugs Aging. 2005;22:141–161. doi: 10.2165/00002512-200522020-00005. [DOI] [PubMed] [Google Scholar]
- 4.Arthritis Care (2004) OA Nation. In: The most comprehensive UK report of people with osteoarthritis. http://www.arthritiscare.org.uk/PublicationsandResources/Forhealthprofessionals/OANation. Accessed 09 Sept 2013
- 5.Croft P. The occurrence of osteoarthritis outside Europe. Ann Rheum Dis. 1996;55:661–664. doi: 10.1136/ard.55.9.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koopman WJ, Moreland LW. Arthritis and allied conditions: A textbook of rheumatology (Arthritis & Allied Conditions) Philadelphia: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 7.Rossignol M, Leclerc A, Allaert FA, Rozenberg S, Valat JP, Avouac B, Coste P, Litvak E, Hilliquin P. Primary osteoarthritis of hip, knee, and hand in relation to occupational exposure. Occup Environ Med. 2005;62:772–777. doi: 10.1136/oem.2005.020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marks R, Allegrante JP. Body mass indices in patients with disabling hip osteoarthritis. Arthritis Res. 2002;4:112–116. doi: 10.1186/ar387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther. 2009;11:229. doi: 10.1186/ar2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen KD, Chen JC, Callahan LF, Golightly YM, Helmick CG, Renner JB, Jordan JM. Associations of occupational tasks with knee and hip osteoarthritis: the Johnston County Osteoarthritis Project. J Rheumatol. 2010;37:842–850. doi: 10.3899/jrheum.090302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coggon D, Croft P, Kellingray S, Barrett D, McLaren M, Cooper C. Occupational physical activities and osteoarthritis of the knee. Arthritis Rheum. 2000;43:1443–1449. doi: 10.1002/1529-0131(200007)43:7<1443::AID-ANR5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Hedbom E, Hauselmann HJ. Molecular aspects of pathogenesis in osteoarthritis: the role of inflammation. Cell Mol Life Sci. 2002;59:45–53. doi: 10.1007/s00018-002-8404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroeppel JP, Crist JD, Anderson HC, Wang J. Molecular regulation of articular chondrocyte function and its significance in osteoarthritis. Histol Histopathol. 2011;26:377–394. doi: 10.14670/HH-26.377. [DOI] [PubMed] [Google Scholar]
- 14.Hoff P, Buttgereit F, Burmester GR, Jakstadt M, Gaber T, Andreas K, Matziolis G, Perka C, Rohner E. Osteoarthritis synovial fluid activates pro-inflammatory cytokines in primary human chondrocytes. Int Orthop. 2013;37:145–151. doi: 10.1007/s00264-012-1724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aspden RM. Osteoarthritis: a problem of growth not decay? Rheumatology. 2008;47:1452–1460. doi: 10.1093/rheumatology/ken199. [DOI] [PubMed] [Google Scholar]
- 16.Spector TD, MacGregor AJ. Risk factors for osteoarthritis: genetics. Osteoarthritis Cartilage. 2004;12(Suppl A):S39–S44. doi: 10.1016/j.joca.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Mutabaruka MS, Aoulad Aissa M, Delalandre A, Lavigne M, Lajeunesse D. Local leptin production in osteoarthritis subchondral osteoblasts may be responsible for their abnormal phenotypic expression. Arthritis Res Ther. 2010;12:R20. doi: 10.1186/ar2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez-Moreno M, Rego I, Carreira-Garcia V, Blanco FJ. Genetics in osteoarthritis. Curr Genomics. 2008;9:542–547. doi: 10.2174/138920208786847953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loughlin J. Genetic epidemiology of primary osteoarthritis. Curr Opin Rheumatol. 2001;13:111–116. doi: 10.1097/00002281-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Loughlin J, Irven C, Fergusson C, Sykes B. Sibling pair analysis shows no linkage of generalized osteoarthritis to the loci encoding type II collagen, cartilage link protein or cartilage matrix protein. Br J Rheumatol. 1994;33:1103–1106. doi: 10.1093/rheumatology/33.12.1103. [DOI] [PubMed] [Google Scholar]
- 21.Doherty M. Genetics of hand osteoarthritis. Osteoarthritis Cartilage. 2000;8(Suppl A):S8–S10. doi: 10.1053/joca.2000.0327. [DOI] [PubMed] [Google Scholar]
- 22.Loughlin J. The genetic epidemiology of human primary osteoarthritis: current status. Expert Rev Mol Med. 2005;7:1–12. doi: 10.1017/S1462399405009257. [DOI] [PubMed] [Google Scholar]
- 23.[No authors listed] (2010) A gene-centric human proteome project: HUPO-the Human Proteome organization. Mol Cell Proteomics 9:427–429. doi:10.1074/mcp.H900001-MCP200 [DOI] [PMC free article] [PubMed]
- 24.Gottesman MM, Collins FS. The role of the human genome project in disease prevention. Prev Med. 1994;23:591–594. doi: 10.1006/pmed.1994.1094. [DOI] [PubMed] [Google Scholar]
- 25.Legrain P, Aebersold R, Archakov A, Bairoch A, Bala K, Beretta L, Bergeron J, Borchers CH, Corthals GL, Costello CE, Deutsch EW, Domon B, Hancock W, He F, Hochstrasser D, Marko-Varga G, Salekdeh GH, Sechi S, Snyder M, Srivastava S, Uhlen M, Wu CH, Yamamoto T, Paik YK, Omenn GS. The human proteome project: current state and future direction. Mol Cell Proteomics. 2011;10(M111):009993. doi: 10.1074/mcp.M111.009993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maneiro E, Martin MA, de Andres MC, Lopez-Armada MJ, Fernandez-Sueiro JL, del Hoyo P, Galdo F, Arenas J, Blanco FJ. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:700–708. doi: 10.1002/art.10837. [DOI] [PubMed] [Google Scholar]
- 27.Maetzel A, Makela M, Hawker G, Bombardier C. Osteoarthritis of the hip and knee and mechanical occupational exposure–a systematic overview of the evidence. J Rheumatol. 1997;24:1599–1607. [PubMed] [Google Scholar]
- 28.Sowers M, Karvonen-Gutierrez CA, Palmieri-Smith R, Jacobson JA, Jiang Y, Ashton-Miller JA. Knee osteoarthritis in obese women with cardiometabolic clustering. Arthritis Rheum. 2009;61:1328–1336. doi: 10.1002/art.24739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.da Costa BR, Vieira ER. Risk factors for work-related musculoskeletal disorders: a systematic review of recent longitudinal studies. Am J Ind Med. 2010;53:285–323. doi: 10.1002/ajim.20750. [DOI] [PubMed] [Google Scholar]
- 30.Amin S, Goggins J, Niu J, Guermazi A, Grigoryan M, Hunter DJ, Genant HK, Felson DT. Occupation-related squatting, kneeling, and heavy lifting and the knee joint: a magnetic resonance imaging-based study in men. J Rheumatol. 2008;35:1645–1649. [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen LK. Knee osteoarthritis: influence of work involving heavy lifting, kneeling, climbing stairs or ladders, or kneeling/squatting combined with heavy lifting. Occup Environ Med. 2008;65:72–89. doi: 10.1136/oem.2007.032466. [DOI] [PubMed] [Google Scholar]
- 32.Vingard E, Alfredsson L, Goldie I, Hogstedt C. Occupation and osteoarthrosis of the hip and knee: a register-based cohort study. Int J Epidemiol. 1991;20:1025–1031. doi: 10.1093/ije/20.4.1025. [DOI] [PubMed] [Google Scholar]
- 33.Rossignol M. Primary osteoarthritis and occupation in the Quebec national health and social survey. Occup Environ Med. 2004;61:729–735. doi: 10.1136/oem.2003.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Reilly SC, Muir KR, Doherty M. Occupation and knee pain: a community study. Osteoarthritis Cartilage. 2000;8:78–81. doi: 10.1053/joca.1999.0274. [DOI] [PubMed] [Google Scholar]
- 35.Dillon C, Petersen M, Tanaka S. Self-reported hand and wrist arthritis and occupation: data from the U.S. National Health Interview Survey-Occupational Health Supplement. Am J Ind Med. 2002;42:318–327. doi: 10.1002/ajim.10117. [DOI] [PubMed] [Google Scholar]
- 36.Stanley D. Prevalence and etiology of symptomatic elbow osteoarthritis. J Shoulder Elbow Surg. 1994;3:386–389. doi: 10.1016/S1058-2746(09)80024-4. [DOI] [PubMed] [Google Scholar]
- 37.Mintz G, Fraga A. Severe osteoarthritis of the elbow in foundry workers. Arch Environ Health. 1973;27:78–80. doi: 10.1080/00039896.1973.10666322. [DOI] [PubMed] [Google Scholar]
- 38.Woessner JF, Jr, Gunja-Smith Z. Role of metalloproteinases in human osteoarthritis. J Rheumatol Suppl. 1991;27:99–101. [PubMed] [Google Scholar]
- 39.Millward-Sadler SJ, Salter DM. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann Biomed Eng. 2004;32:435–446. doi: 10.1023/B:ABME.0000017538.72511.48. [DOI] [PubMed] [Google Scholar]
- 40.Pottie P, Presle N, Terlain B, Netter P, Mainard D, Berenbaum F. Obesity and osteoarthritis: more complex than predicted! Ann Rheum Dis. 2006;65:1403–1405. doi: 10.1136/ard.2006.061994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, Setton LA, Weinberg JB. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004;423:17–26. doi: 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- 42.Cicuttini FM, Baker JR, Spector TD. The association of obesity with osteoarthritis of the hand and knee in women: a twin study. J Rheumatol. 1996;23:1221–1226. [PubMed] [Google Scholar]
- 43.Hirsch R, Lethbridge-Cejku M, Scott WW, Jr, Reichle R, Plato CC, Tobin J, Hochberg MC. Association of hand and knee osteoarthritis: evidence for a polyarticular disease subset. Ann Rheum Dis. 1996;55:25–29. doi: 10.1136/ard.55.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffin TM, Fermor B, Huebner JL, Kraus VB, Rodriguiz RM, Wetsel WC, Cao L, Setton LA, Guilak F. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis Res Ther. 2010;12:R130. doi: 10.1186/ar3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heep H, Hilken G, Hofmeister S, Wedemeyer C. Osteoarthitis of leptin-deficient ob/ob mice in response to biomechanical loading in micro-CT. Int J Biol Sci. 2009;5:265–275. doi: 10.7150/ijbs.5.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clockaerts S, Bastiaansen-Jenniskens YM, Runhaar J, Van Osch GJ, Van Offel JF, Verhaar JA, De Clerck LS, Somville J. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthritis Cartilage. 2010;18:876–882. doi: 10.1016/j.joca.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Suhaimi EA, Shehzad A. leptin, resistin and visfatin: the missing link between endocrine metabolic disorders and immunity. Eur J Med Res. 2013;18:12. doi: 10.1186/2047-783X-18-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Presle N, Pottie P, Dumond H, Guillaume C, Lapicque F, Pallu S, Mainard D, Netter P, Terlain B. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthritis Cartilage. 2006;14:690–695. doi: 10.1016/j.joca.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/BJN20041213. [DOI] [PubMed] [Google Scholar]
- 52.Kratz M, von Eckardstein A, Fobker M, Buyken A, Posny N, Schulte H, Assmann G, Wahrburg U. The impact of dietary fat composition on serum leptin concentrations in healthy nonobese men and women. J Clin Endocrinol Metab. 2002;87:5008–5014. doi: 10.1210/jc.2002-020496. [DOI] [PubMed] [Google Scholar]
- 53.Monti V, Carlson JJ, Hunt SC, Adams TD. Relationship of ghrelin and leptin hormones with body mass index and waist circumference in a random sample of adults. J Am Diet Assoc. 2006;106:822–828. doi: 10.1016/j.jada.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 54.Malizos KN, Aspasia T. The role of leptin in osteoarthritis and cartilage metabolism. Eur Muscoskel Rev. 2008;3:84–86. [Google Scholar]
- 55.Sandell LJ. Obesity and osteoarthritis: is leptin the link? Arthritis Rheum. 2009;60:2858–2860. doi: 10.1002/art.24862. [DOI] [PubMed] [Google Scholar]
- 56.Hausberg M, Morgan DA, Mitchell JL, Sivitz WI, Mark AL, Haynes WG. leptin potentiates thermogenic sympathetic responses to hypothermia: a receptor-mediated effect. Diabetes. 2002;51:2434–2440. doi: 10.2337/diabetes.51.8.2434. [DOI] [PubMed] [Google Scholar]
- 57.Zhao AZ, Bornfeldt KE, Beavo JA. leptin inhibits insulin secretion by activation of phosphodiesterase 3B. J Clin Invest. 1998;102:869–873. doi: 10.1172/JCI3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hileman SM, Pierroz DD, Flier JS. leptin, nutrition, and reproduction: timing is everything. J Clin Endocrinol Metab. 2000;85:804–807. doi: 10.1210/jc.85.2.804. [DOI] [PubMed] [Google Scholar]
- 59.Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol. 2007;4:1–13. [PubMed] [Google Scholar]
- 60.Bernotiene E, Palmer G, Gabay C. The role of leptin in innate and adaptive immune responses. Arthritis Res Ther. 2006;8:217. doi: 10.1186/ar2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P, Pottie P. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003;48:3118–3129. doi: 10.1002/art.11303. [DOI] [PubMed] [Google Scholar]
- 62.Faggioni R, Feingold KR, Grunfeld C. leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J. 2001;15:2565–2571. doi: 10.1096/fj.01-0431rev. [DOI] [PubMed] [Google Scholar]
- 63.Simopoulou T, Malizos KN, Iliopoulos D, Stefanou N, Papatheodorou L, Ioannou M, Tsezou A. Differential expression of leptin and leptin’s receptor isoform (Ob-Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism. Osteoarthritis Cartilage. 2007;15:872–883. doi: 10.1016/j.joca.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 64.Li Z, Shen J, Wu WK, Yu X, Liang J, Qiu G, Liu J. The role of leptin on the organization and expression of cytoskeleton elements in nucleus pulposus cells. J Orthop Res. 2013 doi: 10.1002/jor.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Massengale M, Lu B, Pan JJ, Katz JN, Solomon DH. Adipokine hormones and hand osteoarthritis: radiographic severity and pain. PLoS One. 2012;7:e47860. doi: 10.1371/journal.pone.0047860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Massengale M, Reichmann WM, Losina E, Solomon DH, Katz JN. The relationship between hand osteoarthritis and serum leptin concentration in participants of the Third National Health and Nutrition Examination Survey. Arthritis Res Ther. 2012;14:R132. doi: 10.1186/ar3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Boer TN, van Spil WE, Huisman AM, Polak AA, Bijlsma JW, Lafeber FP, Mastbergen SC. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis Cartilage. 2012;20:846–853. doi: 10.1016/j.joca.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 68.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 69.Lubbeke A, Finckh A, Puskas GJ, Suva D, Ladermann A, Bas S, Fritschy D, Gabay C, Hoffmeyer P. Do synovial leptin levels correlate with pain in end stage arthritis? Int Orthop. 2013 doi: 10.1007/s00264-013-1982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]