Abstract
Purpose
Primary bone lymphoma is a rare disease. Little is reported about surgical procedures in these patients. We evaluated a single-centre consecutive series of 21 patients for results, prognostic factors and surgical treatment.
Methods
Patient ages ranged from 19 to 82 years. The most frequently affected site was the spine (six cases), followed by the ileum, femur and mandible (three cases each). Four patients were treated with chemotherapy and 17 with chemotherapy and radiation therapy. Six patients were affected by a pathological fracture. Surgery was performed in four patients (19 %), in two cases before chemotherapy, in one case during chemotherapy and in one case after chemotherapy and radiotherapy. Five patients died within the range of three to 36 months after diagnosis. Average follow-up of the remaining patients was 62.8 (19–145) months.
Results
Overall survival was 74.2 % at five years. The only positive prognostic factor was complete remission after chemotherapy. A trend for better survival was present for International Prognostic Index (IPI) score (P = 0.051), under 40 years of age (P = 0.10) and abnormal lactate dehydrogenase (LDH) values (P = 0.10), but it did not reach statistical significance.
Conclusions
Surgical treatment should be aimed at restoring function and eliminating pain while minimising delays in the chemotherapy schedule. When feasible, postponing surgery until after chemotherapy is advisable.
Keywords: Primary lymphoma of bone, Chemotherapy, Timing of surgery, Prognostic factors
Introduction
Primary bone lymphoma is a rare disease, representing only 2 % of all bone tumours and 5 % of all extranodal lymphomas [1, 2]. The definition itself is still somewhat controversial, but there is now a prevalent agreement to consider lymphomas as primary lymphomas of bone when the disease affects one or more bones with or without involvement of local lymph nodes but with no evidence of disease in distant nodes or other extraosseous sites, i.e. Ann Arbor stage I and stage II disease [3]; a lymphoma with the above-mentioned pattern can be considered a primary lymphoma of bone, as well as when involvement of bone marrow is present at diagnosis, i.e. Ann Arbor stage IV [4, 5]. Due to the rarity of the disease, few series are reported in the literature, and only retrospective multicentre reviews involve high numbers of patients [6–8].
Chemotherapy and radiotherapy are the cornerstones of treatment for all lymphomas, and most papers primarily address these issues. Little is reported about surgical procedures, even if surgery does, in some cases, become necessary for fractures or impending fractures. An incidence of orthopaedic surgical procedures other than biopsy, ranging from 13.8 % to 26 % [7–10] or even 47 % [5], is reported, but most papers either do not mention incidence or type of surgery [4, 6, 11–15] or report incomplete data about the surgery performed [16, 17]. Therefore, we decided to review our series in a single-centre, consecutive series of patients affected by primary bone lymphoma with the aim of: (1) evaluating incidence of surgery , (2) examining type of surgery and its combination with adjuvant treatment and (3) evaluating whether surgery should precede or follow radiotherapy and chemotherapy treatment.
Material and methods
Among patients treated for lymphoma at our haematological department from 1999 to February 2011, 21 adults fitted the above-mentioned criteria for primary lymphoma of bone. There were 13 men and eight women, with age at diagnosis ranging from 19 to 82 years (average 51). Affected bone, staging and different treatment regimens are presented in Table 1. The most commonly affected site was the spine (six cases), followed by the ileum, femur and mandible (three cases each).
Table 1.
Patient characteristics
| Patient no. | Diagnosis | Age | Sex | Site | Ann Arbor stage | Remission | Recurrence | Follow-up (months) | Status at follow-up | Chemotherapy | Radiotherapy | Surgery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | FL | 62 | F | Spine (multiple sites) | IV | Y | N | 125 | NED | Firenze 2 | Y | N |
| 2 | DLBCL | 67 | F | L3-L4 | IV | N | / | 12 | DOD | MiCEP | Y | N |
| 3 | FL | 72 | M | Parietal | I | Y | N | 57 | DOC/NED | R-MiCEP | Y | N |
| 4 | SLL | 74 | F | D11-L2 | IV | N | N | 5 | DOD | CHlorambucil | Y | N |
| 5 | DLBCL | 56 | M | Iliac bone | I | Y | Y | 36 | DOD | R-CHOP | Y | N |
| 6 | DLBCL | 64 | F | Iliac bone | I | N | / | 6 | DOD | R | Y | N |
| 7 | DLBCL | 37 | M | Scapula | I | Y | N | 67 | NED | R-CHOP | Y | N |
| 8 | DLBCL | 40 | M | Dorsal + lumbar spine | IV | Y | N | 95 | NED | MACOP-B + ABMT | N | Y |
| 9 | DLBCL | 41 | M | Pelvis (multiple sites) | IV | N | / | 3 | DOD | MACOP-B | N | N |
| 10 | DLBCL | 34 | M | Mandible | I | Y | N | 94 | NED | R-CHOP | Y | N |
| 11 | DLBCL | 23 | F | Humerus | IV | Y | N | 88 | NED | Mega-CHOP + ABMT | Y | Y |
| 12 | DLBCL | 55 | M | Sacrum | I | Y | N | 79 | NED | R-CHOP | Y | N |
| 13 | DLBCL | 54 | M | Tibia | I | Y | N | 78 | NED | R-CHOP | Y | Y |
| 14 | DLBCL | 54 | M | D11-L2 | I | Y | N | 77 | NED | R-CHOP | Y | Y |
| 15 | DLBCL | 35 | M | Mandible | I | Y | N | 44 | NED | R-CHOP | Y | N |
| 16 | DLBCL | 40 | F | Scapula | IV | Y | N | 43 | NED | R-CHOP + ABMT | N | N |
| 17 | DLBCL | 82 | M | Ethmoid | I | Y | N | 33 | NED | R--MiCEP | Y | N |
| 18 | DLBCL | 70 | M | Sacrum | II | Y | N | 31 | NED | R-CHOP | Y | N |
| 19 | DLBCL | 19 | M | Femur | I | Y | N | 34 | NED | R-CHOP | Y | N |
| 20 | DLBCL | 45 | F | Sacrum | IV | Y | N | 22 | NED | R-CHOP | N | N |
| 21 | DLBCL | 57 | M | Scapula | II | Y | N | 19 | NED | R-CHOP | Y | N |
DLBCL diffuse large B-cell lymphoma, FL follicular lymphoma, SLL small lymphocytic lymphoma, NED no evidence of disease, DOD dead of disease, DOC/NED dead of concomitant disease with no evidence of lymphoma, R-MiCEP rituximab, mitoxantrone, cyclophosphamide, etoposide, prednisone, R-CHOP rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate (Oncovin), prednisone, MACOP-B methotrexate A-doxorubicin, cyclophosphamide, O-vincristine, prednisone, and bleomycin, ABMT autologous bone marrow transplant
The most common histological type was diffuse large B-cell lymphoma (DLBCL), accounting for 85.7 % of cases (18 patients); two patients were affected by follicular lymphoma and one by small lymphocytic lymphoma. Ann Arbor stage was stage I in 11 patients, stage II in two and stage IV in eight. Six patients presented with a pathological fracture (tibia in one, humerus in one, spine in four). In all cases, diagnosis was obtained by a percutaneous or open biopsy. Staging at presentation was accomplished by a computed tomography (CT) scan of the neck, chest, abdomen and pelvis with contrast medium, a total body positron emission tomography (PET) scan (starting from 2003), a bone marrow biopsy, evaluation of blood parameters including lactate dehydrogenase (LDH) and beta-2 microglobulin.
Four patients were treated with chemotherapy alone; one patient underwent radiation treatment and rituximab; the remaining 16 underwent combined treatment of chemotherapy and radiation therapy; two patients underwent bone marrow transplantation (BMT).
Chemotherapy regimens used were: CHOP (cyclophosphamide, H-doxorubicin, O-vincristine, prednisone) [18] in 13 patients; a CHOP-like regimen with addition of bleomycin (Firenze-2) in one patient; MiCEP (mitoxantrone, cyclophosphamide, etoposide, prednisone) [19] in three; MACOP-B (methotrexate, A-doxorubicin, cyclophosphamide, O-vincristine, prednisone, bleomycin) [20] in two; chlorambucil in one. In 16 patients treated after 2003, rituximab was added to the chemotherapy regimen. Radiation therapy was administered with a median dose of 37.5 (range 30–50) Gy.
Follow-up was accomplished by clinical examination combined with blood tests every three months in the first year after the end of therapy, with alternating total body CT and MRI of the involved bone every follow-up visit. In the second and third year after therapy, clinical and instrumental examinations were performed every six months. [18F]-fluorodeoxyglucose positron emission tomography (FDG-PET) scan was used at the end of therapy to confirm complete remission was obtained and only in the case of suspected recurrence thereafter. Any surgical procedure was recorded for type of surgery and timing in respect of chemotherapy and radiotherapy treatment. Survival was determined according to the method of Kaplan–Meyer. Evaluation of factors affecting survival was accomplished according to log-rank test. Statistical analysis was performed using IBM SPSS Statistics v20. The study was performed in accordance with the Declaration of Helsinki of 1975. The study was retrospective, and no objection/exception was formulated by the local internal review board.
Results
At the end of therapy, 17 of our patients (81 %) reached complete disease remission. Persisting radiological abnormalities after treatment is a common finding in bone lymphomas and it does not prevent assessment of a remission state, which is evaluated upon the disappearance of local and systemic signs of lymphoma [5, 11]. One patient achieved partial remission. Three patients achieved no remission, and disease progression led to early death. Patients with partial or no remission died, respectively, at three, five, six and 12 months from diagnosis. In one patient, recurrence occurred 25 months after primary diagnosis; the patient died 11 months thereafter.
Average follow-up of the remaining patients was 62.8 (range 19–145) months. One of these patients died of concomitant disease 57 months after diagnosis of lymphoma; there was complete lymphoma remission at the time of death. Fifteen patients were alive at the time of this study, and all of them showed no evidence of disease. Kaplan–Meyer overall disease-related survival was 74.2 % at five years.
Six patients were affected by a pathological fracture (28.6 %). A surgical procedure was performed in four cases (19 %): two patients with pathological fracture of the sacrum or lumbar spine underwent conservative treatment; in three patients, an open procedure was performed; in one patient, a percutaneous vertebroplasty was done. Surgical intervention at the spine level was performed for fracture with myelopathy or disabling pain. In one case, acute myelopathy was present due to pathological fracture of the sixth dorsal vertebra. The patient underwent an open procedure with curettage of the lesion, vertebroplasty and posterior fixation (in the Neurosurgery Unit, University of Firenze). The diagnosis of lymphoma was made on specimens collected during surgery, and subsequent chemotherapy was started. Percutaneous vertebroplasty was performed in one patient (at two levels); the procedure was performed early after beginning chemotherapy treatment due to disabling pain. In the limbs, choice of surgery was determined by the presence of a pathological fracture. In one patient, surgery was performed after completion of chemotherapy and radiotherapy. The patient was affected by lymphoma of the proximal humerus, with severe bone loss and involvement of surrounding soft tissues. After completion of medical and radiation treatment, resection and reconstruction with a megaprosthesis were performed (Fig. 1). In the second case, a fracture of the tibia occurred a few days after diagnostic biopsy. Surgery was performed as the first-line treatment and followed by chemotherapy and radiotherapy. In this case, fixation with a plate was performed, without curettage or resection of the affected bone (Fig. 2).
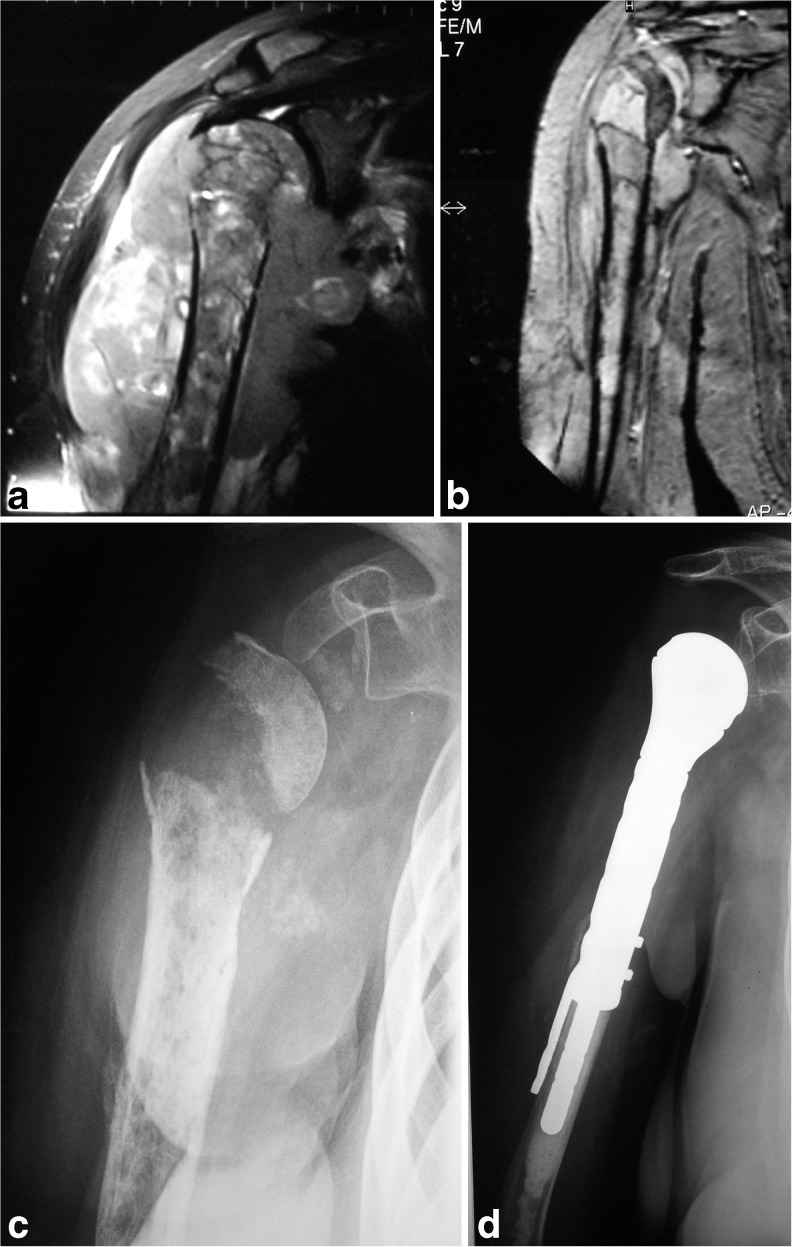
Fig. 1.
A 23-year-old woman with primary bone lymphoma affecting the right proximal humerus: a coronal magnetic resonance imaging (MRI) view at presentation; b coronal MRI after chemotherapy and radiotherapy; c X-ray after chemotherapy and radiotherapy; d resection and reconstruction with prosthesis were performed
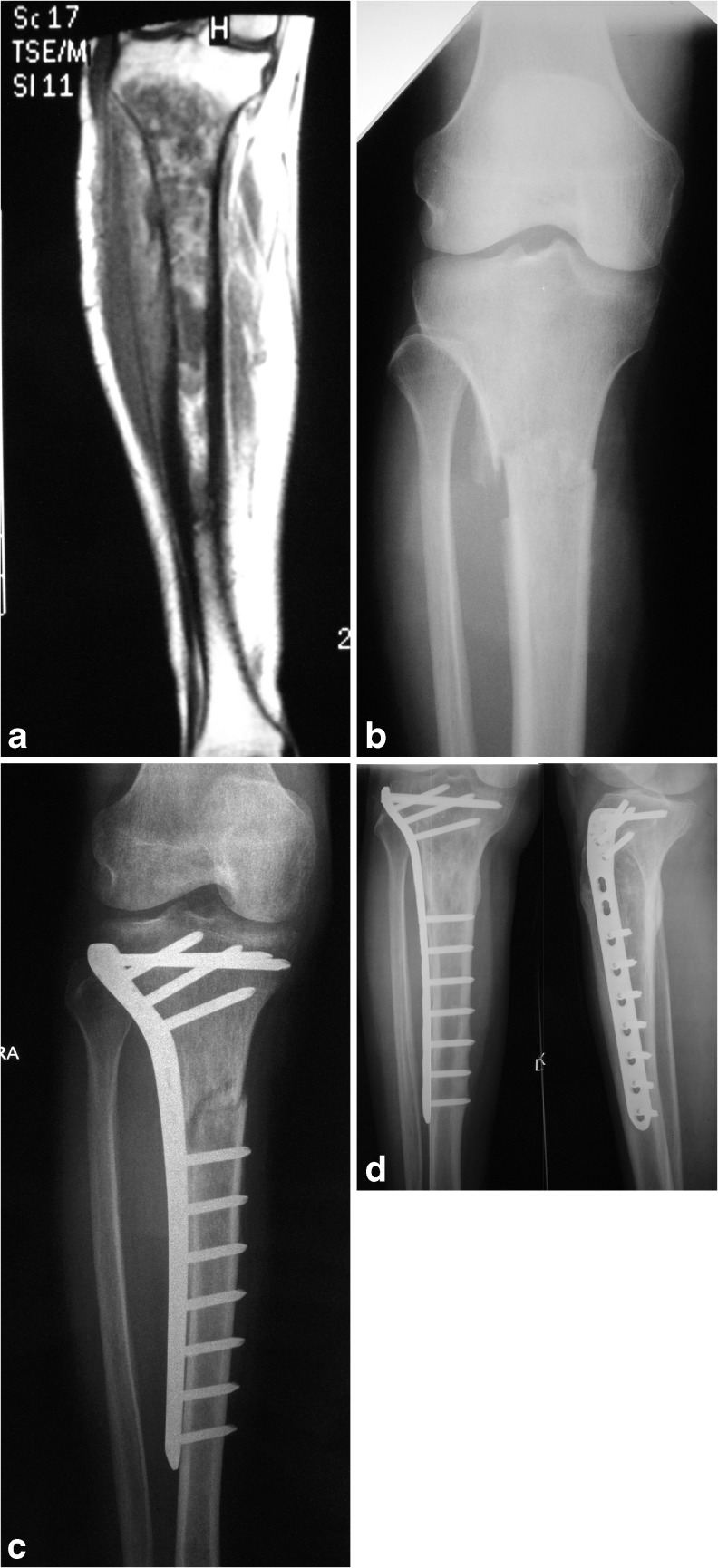
Fig. 2.
A 54-year-old man with primary bone lymphoma affecting the right tibia: a magnetic resonance imaging (MRI); b a few days after biopsy, a pathological fracture occurred; c before beginning chemotherapy and radiotherapy, the fracture was fixed with a plate without curettage of the involved bone; d fracture completely healed and the patient was disease free at 78 months
Log-rank test for comparison of factors potentially affecting survival was accomplished for sex, age (under 40 or under 60 years), lesion site (appendicular skeleton, spine, cranium), stage, International Prognostic Index (IPI) score (over 2), abnormal LDH levels, achievement of complete remission after primary treatment and presence of a pathological fracture. Due to the high prevalence in our series of patients treated with radiotherapy and patients who received rituximab in their treatment schedule, these two factors were not available for statistical evaluation of their role on survival. Also, different chemotherapeutic regimens could hardly be compared because of the small number of patients. A statistically significant difference in survival was present for complete remission after primary treatment only (P < 0.001). A trend for better survival was present for IPI score (P = 0.051), age under 40 years (P = 0.10) and abnormal LDH values (P = 0.10), but it did not reach statistical significance. No prognostic value was detectable for sex, lesion site or stage and presence of a pathological fracture.
Discussion
Overall survival of patients affected by primary bone lymphomas was 74.2 % at five years. Different values were found by different authors in previous papers, ranging from 58 to 90 % [6, 7, 10–13, 16, 17], as showed in Table 2. An overall survival of 95 % at eight years in a homogeneous cohort of patients affected by diffuse large B-cell lymphomas treated with anthracycline-containing chemotherapeutic regimens with the addition of rituximab was also reported [14].
Table 2.
Data from series reported in the literature
| Article | Patient no. | DLBCL % | Stage I | Stage II | Stage IV | Pathological fracture % | Surgery | OS (years) | Identified prognostic factors (years) |
|---|---|---|---|---|---|---|---|---|---|
| Barbieri 2004 [11] | 77 | 96 | 56 | 44 | 0 | NA | NA | 88 (15) | Age (40) |
| Beal 2006 [12] | 82 | 80 | 78 | 4 | 19 | 12 | NA | 88 (5) | CHT + RT, age (40) |
| Ramadan 2007 [6] | 131 | 79 | 26 | 20 | 54 | 22 % long bones 58 % spinal chord compression |
NA | 62 (5) 41 (10) in DLBCL |
IPI, age (60) |
| Catlett 2008 [13] | 30 | 87 | 60 | 10 | 30 | NA | NA | 73 | IPI CHT + RT |
| Jawad 2010 [7] | 1,500 | 66 | 58 | 11 | 31 | NA | 26.3 | 58 (5) 45 (10) |
Stage, age |
| Alencar 2010 [5] | 53 | 83 | 66 | 11 | 23 | 21 | 47.2 | 100 (4) | / |
| Horsman 2006 [16] | 37 | 73 | 70 | 30 | 0 | 16 | NA | 64.5 (5) 49.6 (10) |
CR, age (60) |
| Ford 2007 [10] | 22 | 91 | 77 | 0 | 23 | 4.5 | 22.7 | 74 (10) | / |
| Marshall 2002 [9] | 28 | NA | 100 | 0 | 0 | 43 | 18 | 60 (5) 48 (10) |
PF, age (60) |
| Maruyama 2007 [25] | 28 | 68 | 14 | 18 | 68 | NA | NA | 84 (3) | Histological subtypes |
| Pellegrini 2011 [14] | 21 | 100 | 0 | 9.5 | 90.5 | NA | NA | 95 (8) | / |
| Christie 2011 [17] | 31 | 97 | 68 | NA | NA | 9.7 | NA | 90 (5) | / |
| Cai 2012 [8] | 116 | 78 | 80 | 20 | 0 | 17 | 13.8 | 76 (5) | IPI CR RT dose CHT |
| Our series | 21 | 86 | 52.4 | 9.5 | 38.1 | 28.6 | 19 | 74 (5) | CR |
DLBCL diffuse large B-cell lymphoma, CHT chemotherapy, RT radiation therapy, IPI International Prognostic Index, CR complete response, PF pathological fracture, NA not available
Such a wide range of results among different series could be explained by the different therapeutic regimens adopted, but it warrants further investigation because the analysis of currently available data is complicated by the heterogeneity of the series reported, often characterised by the use of a wide range of different treatments even by the same author. In our series, different chemotherapeutic regimens were adopted, and the limited number of patients makes it difficult to perform an adequate comparison of the results obtained. Considering this limitation, the main aim of our work was to analyse the prognosis for primary bone lymphomas, and particularly the role of surgery as treatment, in a consecutive series patients, as little and often incomplete data are reported in the literature regarding this issue. Hystotype distribution in our series confirms the prevalence of DLBCL in primary lymphomas of bone: 18 of 21 patients (85.7 %) presented with this diagnosis. Other authors report a similar prevalence, with DLBCL accounting for 73 % to >90 % [4–6, 12, 13, 16, 17]. Pathological fractures are reported in 10–21 % of patients [4, 5, 12, 16], but a rate of 43 % was found in one series [9]. In our series, 28.6 % of patients suffered a pathological fracture. The presence of a pathological fracture was found to be a negative prognostic factor by some authors [9, 21], but this finding was not confirmed by other series [5, 11]. Our data supported the latter finding.
Age seems to be an important factor in our series, as outlined previously by different authors [6, 7, 9, 11, 12, 16], with younger age accounting for a better prognosis; however, in our series, there was only a trend for a better survival in younger patients, which did not reach statistical significance. The only positive prognostic factor found in our series was achievement of complete remission after primary treatment.
In our patients, a surgical procedure other than biopsy was performed only in patients affected by a pathological fracture: four of six patients (28.6 %) with pathological fracture underwent surgery (19 % of the complete series) ; two patients affected by a pathological fracture in the spine with no evident risk for developing myelopathy were conservatively treated. A similar rate of surgery was reported by Jawad et al. [7], who reviewed a wide multicentre database of 1,500 primary bone lymphomas. They reported a surgical procedure other than biopsy occurring in 26.3 % of patients. Marshall et al. [9] report five surgical procedures in 28 patients (17.8 %). Alencar et al. [5] are the only authors reporting a higher rate of initial surgery, with internal fixation or resection performed in 47 % of patients.
Marshall et al. [9] state that surgery in patients with primary lymphoma of bone is indicated for biopsy, prophylactic fixation of impending fractures, treatment of fractures before or after radiotherapy and systemic therapy, and theoretically in patients with disease unresponsive to conventional therapy (radiotherapy and chemotherapy). A recent paper by Mavrogenis et al. [22] emphasises data that could contradict this statement: they report better survival in patients affected by primary bone lymphoma who were surgically treated with a wide-margin excision in comparison with intralesional surgery, but among patients affected by this disease, the authors’s review addressed only those who underwent surgery and not the entire population. Nonetheless, their report is to be considered and warrants further investigation.
In our opinion surgery should be limited to a very select subset of patients; also, lesions at fracture risk can often successfully heal with chemotherapy and radiotherapy, without surgical treatment, with rest and no weight bearing. When long bones are affected, surgery is usually indicated when a pathological fracture is present, as occurred in one patient in our series (tibial fracture): Early surgical treatment, before starting of chemotherapy, allowed a much higher quality of life for the patient during subsequent treatment. Complete disease remission and fracture healing were accomplished at the end of treatment.
In the upper limb, considering the minor disability achievable with the use of a brace, delayed surgical treatment can be adopted, allowing immediate chemotherapy and radiotherapy treatment. In our series, in a patient affected by lymphoma of the proximal humerus with extension to the surrounding soft tissues, this choice allowed to achieve a significant shrinking of the lesion after preoperative chemotherapy and radiation treatment, and this made surgery easier and safer (Fig. 1). Resection of the proximal humerus and reconstruction with a megaprosthesis was performed.
Specific considerations should be devoted to lymphomas involving the spine for the risk of neurological deficits due to a vertebral collapse. In this case, a more extended indication for surgery can be adopted, also allowing the advantage of actual availability of mini-invasive techniques, such as vertebroplasty.
Timing of surgery is another controversial issue. On the basis of our experience, we believe that surgery should be postponed as long as possible in order to prevent delaying medical treatment. In this concept, treating pathological fractures can also be delayed after chemotherapy and radiotherapy if fracture location and patient conditions make it possible, as when dealing with fractures of the upper limb, where immobilisation with a sling or bandage does not cause a major impairment in quality of daily living. Some authors report an increased incidence of fractures after radiotherapy [23, 24], and this could lead to a preference for early stabilisation of the affected bones. In our series, no fracture occurred after radiation treatment, a result similar to those reported by other authors who explored this subject [10, 17].
In conclusion, surgical treatment in primary bone lymphomas should aim to restore function and eliminate pain while minimising potential delays in chemotherapy initiation. Immediate surgical treatment is warranted in myelopathy or risk of myelopathy from spinal disease and fractures of the lower limb to avoid long periods of confinement to bed.
Acknowledgments
Conflicts of interest
None.
References
- 1.Freeman C. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252–260. doi: 10.1002/1097-0142(197201)29:1<252::AID-CNCR2820290138>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 2.Rudders RA. Primary extranodal lymphoma: response to treatment and factors influencing prognosis. Cancer. 1978;42:406–416. doi: 10.1002/1097-0142(197808)42:2<406::AID-CNCR2820420205>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 3.Ostrowski ML, Unni KK, Banks PM, et al. Malignant lymphoma of bone. Cancer. 1986;58:2646–2655. doi: 10.1002/1097-0142(19861215)58:12<2646::AID-CNCR2820581217>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 4.Gianelli U, Patriarca C, Moro A, et al. Lymphomas of the bone: a pathological and clinical study of 54 cases. Int J Surg Pathol. 2002;10:257–266. doi: 10.1177/106689690201000403. [DOI] [PubMed] [Google Scholar]
- 5.Alencar A, Pitcher D, Byrne G, Lossos IS. Primary bone lymphoma – the University of Miami experience. Leuk Lymphoma. 2010;51:39–49. doi: 10.3109/10428190903308007. [DOI] [PubMed] [Google Scholar]
- 6.Ramadan KM, Shenkier T, Sehn LH, et al. A clinicopathological retrospective study of 131 patients with primary bone lymphoma: a population-based study of successively treated cohorts from the British Columbia Cancer Agency. Ann Oncol. 2007;18:129–135. doi: 10.1093/annonc/mdl329. [DOI] [PubMed] [Google Scholar]
- 7.Jawad MU, Min ES, Cheung MC, Koniaris LG, Scully SP. Primary Lymphoma of Bone in Adult Patients. Cancer. 2010;116:871–879. doi: 10.1002/cncr.24828. [DOI] [PubMed] [Google Scholar]
- 8.Cai L, Stauder MC, Zhang Y, et al. Early-stage Primary Bone Lymphoma: A retrospective, multicenter Rare Cancer Network (RCN) Study. Int J Rad Oncol Biol Phys. 2012;83:294–291. doi: 10.1016/j.ijrobp.2011.06.1976. [DOI] [PubMed] [Google Scholar]
- 9.Marshall DT, Amdur RJ, Scarborough MT, et al. Stage IE primary non-Hodgkin’s lymphoma of bone. Clin Orthop Relat Res. 2002;405:216–222. doi: 10.1097/00003086-200212000-00028. [DOI] [PubMed] [Google Scholar]
- 10.Ford DR, Wilson D, Sothi S, Grimer R, Spooner D. Primary Bone Lymphoma d Treatment and Outcome. Clin Oncol. 2007;19:50–55. doi: 10.1016/j.clon.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Barbieri E, Cammelli S, Mauro F, et al. Primary nonHodgkin’s lymphoma of the bone: treatment and analysis of prognostic factors for stage I and stage II. Int J Radiat Oncol Biol Phys. 2004;59:760–764. doi: 10.1016/j.ijrobp.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Beal K, Allen L, Yahalom J. Primary Bone Lymphoma: Treatment Results and Prognostic Factors with Long-Term Follow-up of 82 Patients. Cancer. 2006;106:2652–2656. doi: 10.1002/cncr.21930. [DOI] [PubMed] [Google Scholar]
- 13.Catlett JP, Williams SA, O’Connor SC, et al. Primary lymphoma of bone: an institutional experience. Leuk Lymphoma. 2008;49:2125–2132. doi: 10.1080/10428190802404030. [DOI] [PubMed] [Google Scholar]
- 14.Pellegrini C, Gandolfi L, Quirini F, Ruggieri P, et al. Primary Bone Lymphoma: Evaluation of chemoimmunotherapy as front-line treatment in 21 patients. Clin Lymph Myeloma Leuk. 2011;11:321–325. doi: 10.1016/j.clml.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Ghavam Nasiri MR, Varshoee F, Mohtashami S, et al. Primary bone lymphoma: a clinicopathological retrospective study of 28 patients in a single institution. J Res Med Sci. 2011;16:814–820. [PMC free article] [PubMed] [Google Scholar]
- 16.Horsman JM, Thomas J, Hough R, et al. Primary bone lymphoma: a retrospective analysis. Int J Oncol. 2006;28:1571–1575. doi: 10.3892/ijo.28.6.1571. [DOI] [PubMed] [Google Scholar]
- 17.Christie D, Dear K, Lei T, et al. Limited chemotherapy and shrinking field radiotherapy for osteolymphoma (primary bone lymphoma): results from the trans-tasman radiation oncology group 99.04 and Australasian leukaemia and lymphoma group ly02 prospective trial. Int J Radiat Oncol Biol Phys. 2011;80:1164–1170. doi: 10.1016/j.ijrobp.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 18.McKelvey EM, Gittlieb JA, Wilson HE, et al. Hydroxyldaunomycin (Adriamycin) combination in malignant lymphoma. Cancer. 1976;38:1484–1493. doi: 10.1002/1097-0142(197610)38:4<1484::AID-CNCR2820380407>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 19.Rigacci L, Carpaneto A, Alterini R, et al. Treatment of large cell lymphoma in elderly patients with a mitoxantrone, cyclophosphamide, etoposide, and prednisone regimen: long-term follow-up results. Cancer. 2003;97:97–104. doi: 10.1002/cncr.11032. [DOI] [PubMed] [Google Scholar]
- 20.Klimo P, Connors JM. MACOP-B chemotherapy for the treatment of diffuse large-cell lymphoma. Ann Int Med. 1985;102:596–602. doi: 10.7326/0003-4819-102-5-596. [DOI] [PubMed] [Google Scholar]
- 21.Fidias P, Spiro I, Sobczak ML, et al. Long-term results of combined modality therapy in primary bone lymphomas. Int J Radiat Oncol Biol Phys. 1999;45:1213–1218. doi: 10.1016/S0360-3016(99)00305-3. [DOI] [PubMed] [Google Scholar]
- 22.Mavrogenis AF, Angelini A, Pala E, Zinzani P, Ruggieri P. The role of surgery for haematologic neoplasms of bone. Acta Orthop Belg. 2012;78:382–392. [PubMed] [Google Scholar]
- 23.Stokes SH, Walz BJ. Pathological fracture after radiation therapy for primary non-Hodgkin’s malignant lymphoma of bone. Int J Radiat Oncol Biol Phys. 1983;9:1153–1159. doi: 10.1016/0360-3016(83)90173-6. [DOI] [PubMed] [Google Scholar]
- 24.Lucraft HH. Primary lymphoma of bone: A review of 13 cases emphasising orthopaedic problems. Clin Oncol. (1991;3:265–269. doi: 10.1016/S0936-6555(05)80879-9. [DOI] [PubMed] [Google Scholar]
- 25.Maruyama D, Watanabe T, Beppu Y, et al. Primary Bone Lymphoma: a new and detailed characterization of 28 patients in a single-institution study. Jpn J Clin Oncol. 2007;v37:216–223. doi: 10.1093/jjco/hym007. [DOI] [PubMed] [Google Scholar]