Abstract
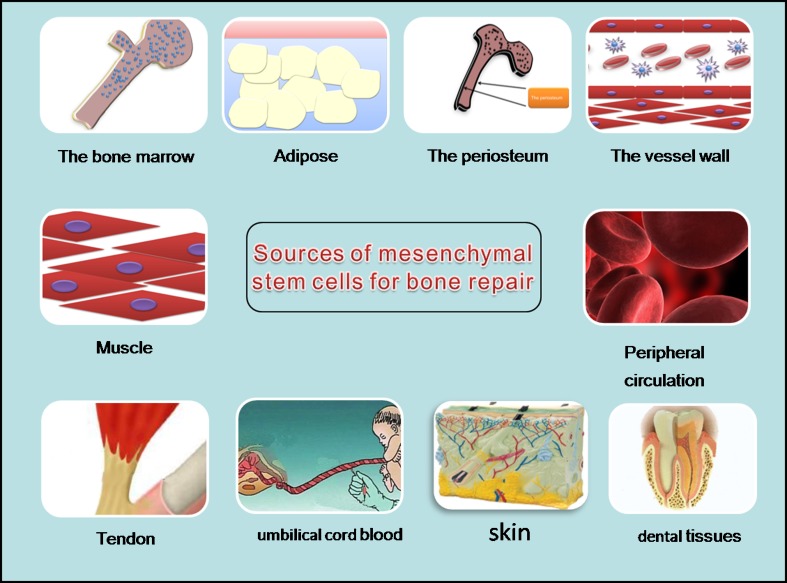
Mesenchymal stem cells (MSCs) are non-haematopoietic stromal stem cells that have many sources, such as bone marrow, periosteum, vessel walls, adipose, muscle, tendon, peripheral circulation, umbilical cord blood, skin and dental tissues. They are capable of self-replication and of differentiating into, and contributing to the regeneration of, mesenchymal tissues, such as bone, cartilage, ligament, tendon, muscle and adipose tissue. The homing of MSCs may play an important role in the repair of bone fractures. As a composite material, the formation and growth of bone tissue is a complex process, including molecular, cell and biochemical metabolic changes. The recruitment of factors with an adequate number of MSCs and the micro-environment around the fracture are effective for fracture repair. Several studies have investigated the functional expression of various chemokine receptors, trophic factors and adhesion molecules in human MSCs. Many external factors affect MSC homing. MSCs have been used as seed cells in building tissue-engineered bone grafts. Scaffolds seeded with MSCs are most often used in tissue engineering and include biotic and abiotic materials. This knowledge provides a platform for the development of novel therapies for bone regeneration with endogenous MSCs.
Keywords: Mesenchymal stem cells, Homing, Chemotaxis, Differentiation, Bone fracture
Introduction
Mesenchymal stem cells (MSCs) have been used widely in stem cell transplantation, tissue engineering and immunotherapy. They can migrate to sites of injury in animals, but the underlying mechanisms remain unclear. Chemokine receptors with their ligands and adhesion molecules play an important role in the tissue-specific homing of leukocytes and take part in transporting haematopoietic precursors into and through tissues. It is very likely that similar mechanisms govern the migration of MSCs.
This review covers the sources of MSCs and cytokines and external factors that affect MSC homing in bone fractures, as well as bone tissue engineering with MSCs. It hopes to suggest ways to make better use of MSCs in bone fractures.
Sources of MSCs for fracture repair
MSC niches, as defined by Scadden, enable homeostasis and maintain MSC populations [60]. Niches for MSCs of different origins are located adjacent to vessel walls, on the endosteal surfaces of trabecular bone, within the interfibrillary spaces, or perivascular [15]. MSCs have also been found in umbilical cord blood [44], dental tissues [56] and synovial fluid [35]. The bone marrow, endosteum and perivascular cells are perhaps more important in skeletal repair.
Bone marrow is still the most important source of stem cells [10]. For many years, bone marrow extracts have been used for treating non-union gaps in orthopaedic surgery. The periosteum is another source of MSCs [12, 50]. Bone marrow mesenchymal stem cells (BMSCs) were identified within the endosteal callus in areas of new bone formation in a mouse fracture model [25]. Nevertheless, harvesting MSCs from periosteum is difficult. In comparison, vessel walls are present throughout the body. Angiogenesis is important for bone fracture repair. Some studies have investigated the roles of mesenchymal pluripotent cells from vessel walls (pericytes) in bone healing [18, 69]. Pericytes can be induced to express chondrogenic and adipogenic markers when cultured under defined conditions. Muscle has been one of the most feasible sources of cells for bone repair [73], but harvesting is difficult and the cells may have limited capacity for differentiation. The role of circulating MSCs in bone healing remains controversial. Other sources of MSCs include adipose tissue, tendon, umbilical cord blood, dental tissues and skin [5, 37, 43, 63, 87].
The sources of MSCs are widespread (Fig. 1). The ideal stem cell source for fracture repair should be easily accessible, harvesting should be non-invasive and cells should be rapidly expandable by in vitro culture. Survival and integration within the host bone tissue should be qualified, and the cell source should show no tumourigenicity.
Fig. 1.
Sources of MSCs for bone repair
MSC homing depends on the conditions
As a composite material, the formation and growth of bone tissue is a complex process, including molecular, cell and biochemical metabolic changes. Bone tissue has a dense structure; its stress and biological environment are relevant to its structure and function [58]. The immediate response to fracture is haematoma formation and inflammation. After a bone fracture, the formation of a haematoma prevents more bleeding and the loss of factors. The inflammatory response provides the initial stabilisation between the two bone ends, which initiate signalling cascades that aid in healing. After the haematoma has formed, precursor cells aggregate and form new blood vessels, fibroblasts and other supporting cells; granulation tissue forms between the fracture ends.
Transplanted MSCs were found to have specific systemic anti-inflammatory effects on cytokines released after tibia fracture [25]. They had no effect on interleukin 13 (IL-13) or IL-10 at any time, but had a significant effect on reducing IL-6 levels at day three and tumour necrosis factor α (TNF-α) and IL-1β levels one and three days after fracture. This process may limit tissue injury and prevent the development of fibrosis to promote rapid regeneration.
Progenitors are recruited to the fracture site in the initial stages of healing (day one) and proliferate at around day three [17]. At this time, the fracture environment is complex: vascular disruption during the initial stage of fracture creates a localised hypoxic environment [6] that acts as a useful regulatory stimulus for many cells, including MSCs and bone cells [22, 55]. New bone formation is thought to occur under low oxygen tension [6]. Hypoxic tissues express genes that increase cell survival under hypoxic conditions and re-establish the vasculature for oxygen delivery [64]. In addition, hypoxia induces the production of chemotactic factors implicated in cell migration, differentiation and new bone formation. The platelets, inflammatory cells and macrophages arriving at the site of injury secrete cytokines and growth factors, including IL-1 to IL-6, platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and bone morphogenetic protein (BMP) [59]. This cellular response leads to the invasion of MSCs, which differentiate into osteoblasts and chondrocytes to complete the repair [61]. Numerous studies have confirmed that MSCs form bone by differentiating into osteoblasts [7, 27, 85]. In addition, many other studies have confirmed that the recruitment of factors with an adequate amount of MSCs and the micro-environment around the fracture are effective for fracture repair [71, 84, 88].
Process of fracture healing is affected by associated molecular mechanisms
During the fracture process, the recruitment of circulating progenitor cells to the site of injury occurs as a normal biological process [42, 47]. The mechanisms for MSC migration to the bone injury site are not completely known. Cytokines and chemokines may play vital roles in these processes, and many of these factors are chemo-attractants. MSCs express many chemokine receptors [29] and chemokine-mediated MSC migration occurs in vitro and in vivo.
Not all factors promote the migration of MSCs. Ode et al. [53] suggested that MSC migration is also under the control of CD73/CD29 in cells stimulated mechanically. Collectively, CD73 and CD29 mediate the mechanical stimulation to reduce MSC migration.
One of the best-investigated factors is stromal cell-derived factor 1 (SDF-1)/pre-B cell growth-stimulating factor/CXC ligand 12 (CXCL12), considered a master regulator of CXC receptor 4 (CXCR4)-positive stem and progenitor cells. When transfected at sites of ischaemic injury, this factor modulates cell differentiation into mature reparative cells [57, 76]. Toupadakis et al. [70] investigated the role of SDF-1/CXCR4 signalling in fracture healing by injecting AMD3100, a CXCR4 antagonist. Although CXCR4 is expressed in only a small part of the MSC surface [76], it still plays an important role in stem cell migration and is a key factor in bone marrow stromal cell migration [25, 38]. Therefore, improving the CXCR4 ligand in bone marrow stromal cells should promote their proliferation and migration [23].
SDF-1 and other cytokines associate and interact. Shinohara et al. [65] demonstrated that SDF-1 and monocyte chemo-attractant protein 3 (MCP-3) jointly regulate the homing of MSCs from the systemic circulation in fracture repair: the proportion of cells expressing SDF-1 and MCP-3 was significantly greater in transduced than non-transduced MSCs. Therefore, the homing of MSCs from the systemic circulation is involved in fracture repair via an SDF-1–MCP-3 pathway. The two factors act synergistically. Another study found that by promoting the expression of BMP-2, SDF-1 increased MSC migration and differentiation in promoting fracture healing [25]. SDF-1 promoted autocrine and other factors, such as VEGF and basic fibroblast growth factor (bFGF) paracrine factor. All of these cytokines inhibit cellular apoptosis [23]. The homing pathways may be modulated to enhance the contribution of recruitment and migration of MSCs in the systemic circulation at sites of skeletal injury.
Transforming growth factor β (TGF-β) exists in normal tissue cells and is the most abundant in transformed cells in animal bone tissue and platelets. TGF-β is released by platelets in the initial inflammatory phase of fracture healing and may be involved in the initiation of callus formation. TGF-β may also be expressed and secreted by chondrocytes, osteoblasts, macrophages and other inflammatory cells stored in the bone matrix. TGF-β is an effective chemotactic factor for bone marrow-derived MSCs. It promotes the proliferation of MSCs, preosteoblasts, chondrocytes and osteoblasts and induces collagen, osteopontin, osteonectin, proteoglycans, alkaline phosphatase and other extracellular proteins [48]. Self-designed bioscaffolds infused spatially with TGF-β3-adsorbed collagen hydrogel have been used to repair the defect in a rabbit model of a humeral head defect [8]. TGF-β3 recruited 130 % more cells to the regenerated articular cartilage than did spontaneous cell migration without TGF-β3, which promoted both avascular cartilage and vascularised bone formation. Since TGF-β may play a major role in cartilage formation and endochondral bone formation [13], it could promote BMP signalling to enhance osteogenesis and inhibit the activation of osteoclasts by promoting their apoptosis. Chondrogenic and osteogenic cells have many receptors, and TGF-β plays roles in each phase of fracture healing. Although TGF-β may promote cell proliferation, its osteoinductive effect is limited. Repeat or large-dose medication has a more significant induction effect, but is difficult to achieve in the clinic and may have side effects.
In the process of fracture healing, FGF is synthesised by monocytes, mesenchymal cells, macrophages, osteoblasts and chondrocytes. It can regulate cell migration [4], differentiation and proliferation and take part in angiogenesis and wound healing. Its effect is achieved by bonding to receptor tyrosine kinases. FGF plays an important role in vascular morphogenesis and mesenchymal cell mitosis in early fracture healing. The use of exogenous bFGF can accelerate bone repair and stimulate callus remodelling to restore the biomechanical properties of bone rapidly [11]. FGF can improve new bone volume and mineral content. Its effect is dose dependent. A single injection can promote the proliferation of chondrogenic precursors in the callus and form more cartilage, but cannot promote chondrocyte maturation or accelerate cartilage bone tissue replacement [72].
NEL-like molecule-1 (NELL-1) is a craniosynostosis-associated molecule that controls osteoblast differentiation [2]. NELL-1 has potent osteoinductive capacity for bone regeneration in several animal models [2, 46, 66, 81]. However, its capacity for promoting the repair of long bone defects remains unknown. Xue et al. [78] used adenoviral transfection and a variety of in vivo analyses to investigate the effect of NELL-1 on femoral distraction osteogenesis. Osteocalcin and osteopontin expression were increased with adenoviral NELL-1 transfection. A rat model of femoral distraction osteogenesis showed improved regeneration of good-quality bones and accelerated bone union at a high rate via sustained delivery of adenoviral transfection of NELL-1 protein into the local area of distraction. BMSCs with modified BMP-2 and NELL-1 genes promoted new bone formation and maturation in a rabbit maxillary sinus model and rapid distraction osteogenesis in a rabbit tibial defect model [77, 86]. The BMP-2 and NELL-1 genes may have a synergistic effect on the osteogenic differentiation of BMSCs.
Blood PDGF is strongly chemotactic for inflammatory cells and has a strong stimulatory effect on MSCs and osteoblast proliferation and migration [40]. From the early to middle stages of bone healing, PDGF promotes the effects of mesenchymal cells in cartilage and bone formation in development. The combined application of PDGF and BMP accelerates bone defect repair, but it is still uncertain whether PDGF can be used for the clinical treatment of fractures.
In addition, insulin-like growth factor plus MSCs can improve fracture healing, mostly through endochondral ossification [49], and parathyroid hormone probably plays a role in healing bone defects by increasing the proliferation of MSCs with reduced senescence and apoptosis [16].
Fracture healing is not an isolated process; various factors act synergistically to affect stem cell homing and promote differentiation, and a low dose can provide better therapeutic effects. Bai et al. [3] found that BMP-2, VEGF and bFGF dose- and time-dependently had strong synergistic effects on the osteogenic differentiation of MSCs, with lower concentrations of each factor sufficient to show the synergistic effect. More study is needed of the right time to use the appropriate cytokines at reduced dosages.
Since MSCs at the fracture site need mechanical stimulation for sustained therapeutic success in vivo, the mechanical micro-environment of MSCs needs to be considered during the regeneration process. Many studies have investigated the cellular characteristics and functional behaviour of MSCs in response to mechanical loading and suggest that mechanical embedding promotes bone regeneration when stem cells are transplanted into the fracture site.
External factors affecting MSC homing
In addition to the impact of the internal environmental on MSC homing, some external factors also have effects. One study introduced axial displacement stimulation after systemic stem cell transplantation to investigate how fracture healing affects the characteristics of the callus [74]. Experimental Sprague–Dawley rats with right femoral fractures were injected with MSCs in the tail vein. With mechanical stimulation, the mineral content increased and cartilage content decreased by ten days after the fracture. After 48 days, the mineral content peaked and the cartilage content decreased, and MSC populations were still found. Therefore, the timing of mechanical stimulation affected the physical and chemical properties of the callus and MSC migration to the fracture site.
Griffin et al. [26] showed that degenerate wave (DW) and capacitive coupling (CC) reduced cytotoxicity, while increasing human MSC invasion and proliferation in vivo. They used DW, CC, pulsed electromagnetic fields and direct current [69] to stimulate human bone marrow-derived MSCs. They analysed cell activity, including proliferation, cell kinetics, cytotoxicity and apoptosis. DW had the greatest proliferative and least apoptotic and cytotoxic effects, compared to the other waveforms. Stimulation affected cell intrusion and proliferation, especially with DW and CC. DW or CC applications improved the bone fracture healing rate, perhaps by increasing MSC recruitment to the fracture site.
Naturally occurring electric fields may guide cell migration to fracture sites during healing. Owing to the low oxygen tension with electrolysis and high pH of the extracellular micro-environment, the resulting electrical stimulation facilitates chondrocyte and osteoblast differentiation and matrix mineralisation. Electrical stimulation promotes the maturation of callus to accelerate fracture healing [83].
Low-level laser irradiation can increase the proliferative potential of MSCs [23]. The laser activates bone formation by promoting the proliferation and generation of chondrocytes and osteoblasts. Subsequently, local capillary formation and the deposition of calcium salts increase at the fracture site. Therefore, the use of diode lasers may be a valid approach for preconditioning MSCs in vitro before transplantation.
Low-intensity pulsed ultrasound (LIPUS) can enhance MSC-calcium phosphate composites. LIPUS increases the expression of some bone proteins, increases the bone mineral content and density, and improves the biomechanical properties of bone. By improving cartilage formation, LIPUS accelerates the process of endochondral bone formation [32].
Seed cells in tissue-engineered bone
MSCs lack immunogenicity in vitro. They do not express co-stimulatory molecules or major histocompatibility class II factors, such as CD40, CD80 and CD86 [34]. Most importantly, MSCs do not induce lymphocyte proliferation [39]. Transplanted allogeneic MSCs can still be detected in recipients some time after transplantation. This implies a lack of immune recognition and clearance. The interactions between allogeneic MSCs and immune cells and the mechanisms involved in the tolerance of MSC-mediated induction in vivo reduce the incidence of graft-versus-host disease and modulate inflammation [1]. However, memory CD4+ T cells and specific antibodies have been detected in hosts after infusion of allogeneic MSCs [80]. Allogeneic MSC differentiation also affects the immune characteristics of cells [31, 41]. MSCs may be immunosuppressive, but this does not confer sufficient immune privilege. Therefore, studies should examine the role of allogeneic MSCs in tissue engineering.
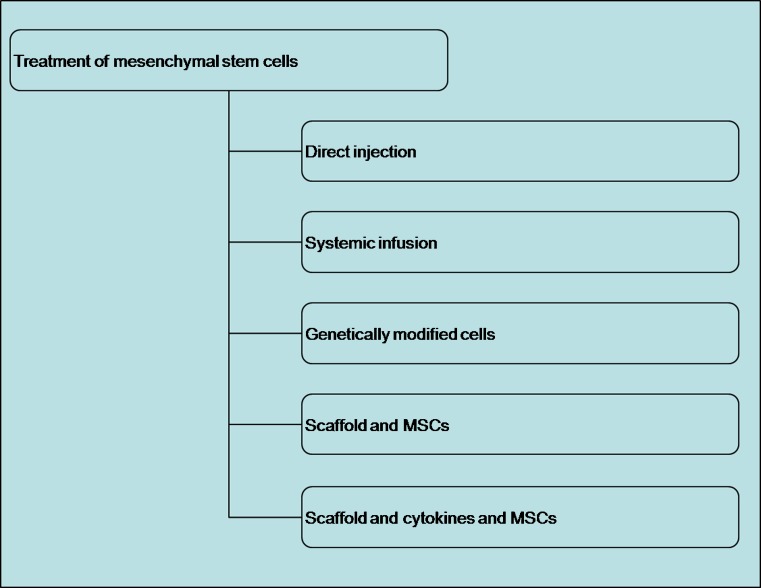
In vivo, only 0.001∼0.01 % of mononuclear cells from bone marrow are MSCs, so an efficient isolation method is required [14]. Density gradient centrifugation is the method most commonly used for harvesting, purifying and expanding MSCs quickly [52]. MSC treatments include direct injection, systemic infusion, genetically modified cells, composite scaffolds with MSCs and composite scaffolds with cytokines (Fig. 2). Most infused MSCs are trapped in the lungs; only a few migrate to the site of injury [21, 62]. Therefore, direct infusion may be better than systemic infusion of MSCs. However, Horwitz et al. [30] treated osteogenesis imperfecta successfully by infusing MSCs systemically to promote bone regeneration. Many experiments have confirmed that injection of MSCs at the site of an injury has therapeutic effects [28]. The genetic modification of MSCs involves transfection with viral or non-viral vectors. Both can express growth factors over the long term. However, the optimal growth factors and vectors need to be identified to ensure effective, safe and consistent treatment.
Fig. 2.
MSCs for treatment
Scaffolds seeded with MSCs are most often used in tissue engineering and include biotic and abiotic materials [33, 51, 79, 82]. MSCs include differentiated and undifferentiated cells. Kawate et al. [36] used MSC/β-tricalcium phosphate composite granules to treat steroid-induced osteonecrosis of the femoral head. An anatomical bioscaffold composite of poly-epsilon-caprolactone and hydroxyapatite infused with TGF-β3 was fabricated to repair injured rabbit shoulder joints [8]. Electrospinning technology can be used to produce bone mimetics and scaffolds of polycaprolactone (PCL), collagen I and nanohydroxyapatite (PCL/col/HA), which adsorb and release PDGF and support good MSC adhesion and proliferation [40]. The activation of integrin-related signalling cascades is better than with scaffolds composed of PCL or collagen I alone.
Although the combination of growth factors and scaffolds remains a promising approach, the long-term release of growth factors for promoting the proliferation and maintenance of MSCs is limited. BMP-2-transfected MSCs promote bone formation in mouse hind limbs and in the bony union of critical-sized mouse radial defects [19]. Therefore, the genetic modification of MSCs so that they express growth factors, which involves transfecting MSCs using viral or non-viral vectors, is a suitable alternative [20]. Numerous osteoinductive growth factors have been used to modify MSCs and provide successful bone induction in vivo. However, studies have shown that human MSCs enhance some tumour formation and growth in vivo [68, 75]. The transfection approach and long-term cell culture can increase the tumourigenic potential of MSC therapy [45, 67]. Consequently, it is necessary to minimise the risk of malignant transformation with stricter control of cell handling procedures and cautious use of MSCs.
Clinical consequences
Promoting bone regeneration is a unique challenge for both clinicians and scientists. The mechanisms of MSC homing related to bone fracture repair, including recruitment, migration, differentiation and proliferation, are still not entirely clear. Much is known about the role of molecular mediators, cell populations and the overall cascade of events in the bone repair process. Studies have confirmed the role of MSCs in fracture healing: the extraction of bone marrow and in vitro amplification and replantation can accelerate healing. However, the specific roles of MSCs in fracture healing are still not fully clear. By modulating chemokine–chemokine receptor interactions, MSCs may increase their ability to correct inherited disorders of mesenchymal tissues or facilitate tissue repair in vivo. Much research needs to be done. The effect of stem cells is clear, but the focus is on how to use MSCs clinically, such as when and in what way, and how to use relevant cytokines and other favourable factors to better stimulate the role of MSCs. The “diamond concept” [24] for biological enhancement supports the implantation of MSCs, scaffolds and growth factors. The ideal biological environment is equally important [9]. However, MSCs also suppress some T-cell functions in transplanted hosts, which could facilitate tumour growth, so caution is needed [54]. Even so, we still need to find a simple, effective way to promote the homing of MSCs to facilitate fracture repair and treat bone defects and non-union.
Acknowledgments
This study was supported by a Grant from the National Technology Research and Development Program of China (2012 AA020502,2012CB518106, BWS11J025), NSFC (8107458,31240048,30930092). We thank Xiaolong Xu for technical support with the imaging work.
Conflict of interest
None.
References
- 1.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 2.Aghaloo T, Jiang X, Soo C, et al. A study of the role of nell-1 gene modified goat bone marrow stromal cells in promoting new bone formation. Mol Ther. 2007;15:1872–1880. doi: 10.1038/sj.mt.6300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai Y, Li P, Yin G, et al. BMP-2, VEGF and bFGF synergistically promote the osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. Biotechnol Lett. 2013;35:301–308. doi: 10.1007/s10529-012-1084-3. [DOI] [PubMed] [Google Scholar]
- 4.Battula VL, Bareiss PM, Treml S, et al. Human placenta and bone marrow derived MSC cultured in serum-free, b-FGF-containing medium express cell surface frizzled-9 and SSEA-4 and give rise to multilineage differentiation. Differentiation. 2007;75:279–291. doi: 10.1111/j.1432-0436.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 5.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 6.Brighton CT, Krebs AG. Oxygen tension of healing fractures in the rabbit. J Bone Joint Surg Am. 1972;54:323–332. [PubMed] [Google Scholar]
- 7.Bruder SP, Kurth AA, Shea M, et al. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998;16:155–162. doi: 10.1002/jor.1100160202. [DOI] [PubMed] [Google Scholar]
- 8.Buijs JT, Henriquez NV, van Overveld PG, et al. TGF-beta and BMP7 interactions in tumour progression and bone metastasis. Clin Exp Metastasis. 2007;24:609–617. doi: 10.1007/s10585-007-9118-2. [DOI] [PubMed] [Google Scholar]
- 9.Calori GM, Giannoudis PV. Enhancement of fracture healing with the diamond concept: the role of the biological chamber. Injury. 2011;42:1191–1193. doi: 10.1016/j.injury.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 11.Chen WJ, Jingushi S, Aoyama I, et al. Effects of FGF-2 on metaphyseal fracture repair in rabbit tibiae. J Bone Miner Metab. 2004;22:303–309. doi: 10.1007/s00774-003-0487-6. [DOI] [PubMed] [Google Scholar]
- 12.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24:274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Amelio P, Cristofaro MA, Grimaldi A, et al. The role of circulating bone cell precursors in fracture healing. Calcif Tissue Int. 2010;86:463–469. doi: 10.1007/s00223-010-9362-3. [DOI] [PubMed] [Google Scholar]
- 14.D’Ippolito G, Schiller PC, Ricordi C, et al. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 15.da Silva ML, Sand TT, Harman RJ, et al. MSC frequency correlates with blood vessel density in equine adipose tissue. Tissue Eng Part A. 2009;15:221–229. doi: 10.1089/ten.tea.2007.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Bernardo G, Galderisi U, Fiorito C, et al. Dual role of parathyroid hormone in endothelial progenitor cells and marrow stromal mesenchymal stem cells. J Cell Physiol. 2010;222:474–480. doi: 10.1002/jcp.21976. [DOI] [PubMed] [Google Scholar]
- 17.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Farrington-Rock C, Crofts NJ, Doherty MJ, et al. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 19.Gamradt SC, Abe N, Bahamonde ME, et al. Tracking expression of virally mediated BMP-2 in gene therapy for bone repair. Clin Orthop Relat Res. 2006;450:238–245. doi: 10.1097/01.blo.0000223989.49400.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamradt SC, Lieberman JR. Genetic modification of stem cells to enhance bone repair. Ann Biomed Eng. 2004;32:136–147. doi: 10.1023/B:ABME.0000007798.78548.b8. [DOI] [PubMed] [Google Scholar]
- 21.Gao J, Dennis JE, Muzic RF, et al. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 22.Genetos DC, Toupadakis CA, Raheja LF, et al. Hypoxia decreases sclerostin expression and increases Wnt signaling in osteoblasts. J Cell Biochem. 2010;110:457–467. doi: 10.1002/jcb.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannelli M, Chellini F, Sassoli C, et al. Photoactivation of bone marrow mesenchymal stromal cells with diode laser: effects and mechanisms of action. J Cell Physiol. 2013;228:172–181. doi: 10.1002/jcp.24119. [DOI] [PubMed] [Google Scholar]
- 24.Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: the diamond concept. Injury. 2007;38(Suppl 4):S3–S6. doi: 10.1016/S0020-1383(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 25.Granero-Moltó F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27:1887–1898. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin M, Iqbal SA, Sebastian A, et al. Degenerate wave and capacitive coupling increase human MSC invasion and proliferation while reducing cytotoxicity in an in vitro wound healing model. PLoS One. 2011;6:e23404. doi: 10.1371/journal.pone.0023404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu Q, Cai Y, Huang C, et al. Curcumin increases rat mesenchymal stem cell osteoblast differentiation but inhibits adipocyte differentiation. Pharmacogn Mag. 2012;8:202–208. doi: 10.4103/0973-1296.99285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann A, Gross G. Tendon and ligament engineering in the adult organism: mesenchymal stem cells and gene-therapeutic approaches. Int Orthop. 2007;31:791–797. doi: 10.1007/s00264-007-0395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honczarenko M, Le Y, Swierkowski M, et al. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 30.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang XP, Sun Z, Miyagi Y, et al. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419–2429. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- 32.Hui CF, Chan CW, Yeung HY et al (2011) Low-intensity pulsed ultrasound enhances posterior spinal fusion implanted with mesenchymal stem cells-calcium phosphate composite without bone grafting. Spine (Phila Pa 1976) 36:1010–1016 [DOI] [PubMed]
- 33.Ivkovic A, Marijanovic I, Hudetz D, et al. Regenerative medicine and tissue engineering in orthopaedic surgery. Front Biosci (Elite Ed) 2011;3:923–944. doi: 10.2741/e299. [DOI] [PubMed] [Google Scholar]
- 34.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Jones EA, English A, Henshaw K, et al. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum. 2004;50:817–827. doi: 10.1002/art.20203. [DOI] [PubMed] [Google Scholar]
- 36.Kawate K, Yajima H, Ohgushi H, et al. Tissue-engineered approach for the treatment of steroid-induced osteonecrosis of the femoral head: transplantation of autologous mesenchymal stem cells cultured with beta-tricalcium phosphate ceramics and free vascularized fibula. Artif Organs. 2006;30:960–962. doi: 10.1111/j.1525-1594.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim SH, Kim YS, Lee SY, et al. Gene expression profile in mesenchymal stem cells derived from dental tissues and bone marrow. J Periodontal Implant Sci. 2011;41:192–200. doi: 10.5051/jpis.2011.41.4.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitaori T, Ito H, Schwarz EM, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60:813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 39.Klyushnenkova E, Mosca JD, Zernetkina V, et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 40.Kodama A, Kamei N, Kamei G, et al. In vivo bioluminescence imaging of transplanted bone marrow mesenchymal stromal cells using a magnetic delivery system in a rat fracture model. J Bone Joint Surg Br. 2012;94:998–1006. doi: 10.1302/0301-620X.94B7.28521. [DOI] [PubMed] [Google Scholar]
- 41.Kotobuki N, Katsube Y, Katou Y, et al. In vivo survival and osteogenic differentiation of allogeneic rat bone marrow mesenchymal stem cells (MSCs) Cell Transplant. 2008;17:705–712. doi: 10.3727/096368908786092793. [DOI] [PubMed] [Google Scholar]
- 42.Kumagai K, Vasanji A, Drazba JA, et al. Circulating cells with osteogenic potential are physiologically mobilized into the fracture healing site in the parabiotic mice model. J Orthop Res. 2008;26:165–175. doi: 10.1002/jor.20477. [DOI] [PubMed] [Google Scholar]
- 43.Lavoie JF, Biernaskie JA, Chen Y, et al. Skin-derived precursors differentiate into skeletogenic cell types and contribute to bone repair. Stem Cells Dev. 2009;18:893–906. doi: 10.1089/scd.2008.0260. [DOI] [PubMed] [Google Scholar]
- 44.Lee OK, Kuo TK, Chen WM, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 45.Liu C, Chen Z, Zhang T, et al. Multiple tumor types may originate from bone marrow-derived cells. Neoplasia. 2006;8:716–724. doi: 10.1593/neo.06253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu SS, Zhang X, Soo C, et al. The osteoinductive properties of Nell-1 in a rat spinal fusion model. Spine J. 2007;7:50–60. doi: 10.1016/j.spinee.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 47.Ma X, Zhang X, Jia Y, et al. Dexamethasone induces osteogenesis via regulation of hedgehog signalling molecules in rat mesenchymal stem cells. Int Orthop. 2013;37:1399–1404. doi: 10.1007/s00264-013-1902-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mendelson A, Frank E, Allred C, et al. Chondrogenesis by chemotactic homing of synovium, bone marrow, and adipose stem cells in vitro. FASEB J. 2011;25:3496–3504. doi: 10.1096/fj.10-176305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myers TJ, Yan Y, Granero-Molto F, et al. Systemically delivered insulin-like growth factor-I enhances mesenchymal stem cell-dependent fracture healing. Growth Factors. 2012;30:230–241. doi: 10.3109/08977194.2012.683188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakahara H, Goldberg VM, Caplan AI. Culture-expanded human periosteal-derived cells exhibit osteochondral potential in vivo. J Orthop Res. 1991;9:465–476. doi: 10.1002/jor.1100090402. [DOI] [PubMed] [Google Scholar]
- 51.Nohmi S, Yamamoto Y, Mizukami H, et al. Post injury changes in the properties of mesenchymal stem cells derived from human anterior cruciate ligaments. Int Orthop. 2012;36:1515–1522. doi: 10.1007/s00264-012-1484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nunes SP, Galembeck F. Percoll and Ficoll self-generated density gradients by low-speed osmocentrifugation. Anal Biochem. 1985;146:48–51. doi: 10.1016/0003-2697(85)90393-8. [DOI] [PubMed] [Google Scholar]
- 53.Ode A, Kopf J, Kurtz A, et al. CD73 and CD29 concurrently mediate the mechanically induced decrease of migratory capacity of mesenchymal stromal cells. Eur Cell Mater. 2011;22:26–42. doi: 10.22203/ecm.v022a03. [DOI] [PubMed] [Google Scholar]
- 54.Otto WR, Rao J. Tomorrow’s skeleton staff: mesenchymal stem cells and the repair of bone and cartilage. Cell Prolif. 2004;37:97–110. doi: 10.1111/j.1365-2184.2004.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raheja LF, Genetos DC, Yellowley CE. The effect of oxygen tension on the long-term osteogenic differentiation and MMP/TIMP expression of human mesenchymal stem cells. Cells Tissues Organs. 2010;191:175–184. doi: 10.1159/000235679. [DOI] [PubMed] [Google Scholar]
- 56.Rodríguez-Lozano FJ, Bueno C, Insausti CL, et al. Mesenchymal stem cells derived from dental tissues. Int Endod J. 2011;44:800–806. doi: 10.1111/j.1365-2591.2011.01877.x. [DOI] [PubMed] [Google Scholar]
- 57.Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160–170. doi: 10.1038/sj.leu.2402763. [DOI] [PubMed] [Google Scholar]
- 58.Rubin C, Turner AS, Bain S, et al. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412:603–604. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 59.Rui YF, Lui PP, Lee YW, et al. Higher BMP receptor expression and BMP-2-induced osteogenic differentiation in tendon-derived stem cells compared with bone-marrow-derived mesenchymal stem cells. Int Orthop. 2012;36:1099–1107. doi: 10.1007/s00264-011-1417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 61.Schindeler A, McDonald MM, Bokko P, et al. Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol. 2008;19:459–466. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 62.Schrepfer S, Deuse T, Reichenspurner H, et al. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 63.Schuh EM, Friedman MS, Carrade DD, et al. Identification of variables that optimize isolation and culture of multipotent mesenchymal stem cells from equine umbilical-cord blood. Am J Vet Res. 2009;70:1526–1535. doi: 10.2460/ajvr.70.12.1526. [DOI] [PubMed] [Google Scholar]
- 64.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 65.Shinohara K, Greenfield S, Pan H, et al. Stromal cell-derived factor-1 and monocyte chemotactic protein-3 improve recruitment of osteogenic cells into sites of musculoskeletal repair. J Orthop Res. 2011;29:1064–1069. doi: 10.1002/jor.21374. [DOI] [PubMed] [Google Scholar]
- 66.Siu RK, Lu SS, Li W, et al. Nell-1 protein promotes bone formation in a sheep spinal fusion model. Tissue Eng Part A. 2011;17:1123–1135. doi: 10.1089/ten.tea.2010.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takeuchi M, Takeuchi K, Kohara A, et al. Chromosomal instability in human mesenchymal stem cells immortalized with human papilloma virus E6, E7, and hTERT genes. In Vitro Cell Dev Biol Anim. 2007;43:129–138. doi: 10.1007/s11626-007-9021-9. [DOI] [PubMed] [Google Scholar]
- 68.Tian LL, Yue W, Zhu F, et al. Human mesenchymal stem cells play a dual role on tumor cell growth in vitro and in vivo. J Cell Physiol. 2011;226:1860–1867. doi: 10.1002/jcp.22700. [DOI] [PubMed] [Google Scholar]
- 69.Tintut Y, Alfonso Z, Saini T, et al. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–2510. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- 70.Toupadakis CA, Wong A, Genetos DC, et al. Long-term administration of AMD3100, an antagonist of SDF-1/CXCR4 signaling, alters fracture repair. J Orthop Res. 2012;30:1853–1859. doi: 10.1002/jor.22145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsai MT, Lin DJ, Huang S, et al. Osteogenic differentiation is synergistically influenced by osteoinductive treatment and direct cell-cell contact between murine osteoblasts and mesenchymal stem cells. Int Orthop. 2012;36:199–205. doi: 10.1007/s00264-011-1259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ueno M, Urabe K, Naruse K, et al. Influence of internal fixator stiffness on murine fracture healing: two types of fracture healing lead to two distinct cellular events and FGF-2 expressions. Exp Anim. 2011;60:79–87. doi: 10.1538/expanim.60.79. [DOI] [PubMed] [Google Scholar]
- 73.Usas A, Huard J. Muscle-derived stem cells for tissue engineering and regenerative therapy. Biomaterials. 2007;28:5401–5406. doi: 10.1016/j.biomaterials.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weaver AS, Su YP, Begun DL, et al. The effects of axial displacement on fracture callus morphology and MSC homing depend on the timing of application. Bone. 2010;47:41–48. doi: 10.1016/j.bone.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wislet-Gendebien S, Poulet C, Neirinckx V, et al. In vivo tumorigenesis was observed after injection of in vitro expanded neural crest stem cells isolated from adult bone marrow. PLoS One. 2012;7:e46425. doi: 10.1371/journal.pone.0046425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wynn RF, Hart CA, Corradi-Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 77.Xia L, Xu Y, Chang Q, et al. Maxillary sinus floor elevation using BMP-2 and Nell-1 gene-modified bone marrow stromal cells and TCP in rabbits. Calcif Tissue Int. 2011;89:53–64. doi: 10.1007/s00223-011-9493-1. [DOI] [PubMed] [Google Scholar]
- 78.Xue J, Peng J, Yuan M, et al. NELL1 promotes high-quality bone regeneration in rat femoral distraction osteogenesis model. Bone. 2011;48:485–495. doi: 10.1016/j.bone.2010.10.166. [DOI] [PubMed] [Google Scholar]
- 79.Yu Z, Zhu T, Li C, et al. Improvement of intertrochanteric bone quality in osteoporotic female rats after injection of polylactic acid-polyglycolic acid copolymer/collagen type I microspheres combined with bone mesenchymal stem cells. Int Orthop. 2012;36:2163–2171. doi: 10.1007/s00264-012-1543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zangi L, Margalit R, Reich-Zeliger S, et al. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells. 2009;27:2865–2874. doi: 10.1002/stem.217. [DOI] [PubMed] [Google Scholar]
- 81.Zhang X, Zara J, Siu RK, et al. The role of NELL-1, a growth factor associated with craniosynostosis, in promoting bone regeneration. J Dent Res. 2010;89:865–878. doi: 10.1177/0022034510376401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y, Wang F, Chen J, et al. Bone marrow-derived mesenchymal stem cells versus bone marrow nucleated cells in the treatment of chondral defects. Int Orthop. 2012;36:1079–1086. doi: 10.1007/s00264-011-1362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao Z, Watt C, Karystinou A, et al. Directed migration of human bone marrow mesenchymal stem cells in a physiological direct current electric field. Eur Cell Mater. 2011;22:344–358. doi: 10.22203/ecm.v022a26. [DOI] [PubMed] [Google Scholar]
- 84.Zhi L, Chen C, Pang X, et al. Synergistic effect of recombinant human bone morphogenic protein-7 and osteogenic differentiation medium on human bone-marrow-derived mesenchymal stem cells in vitro. Int Orthop. 2011;35:1889–1895. doi: 10.1007/s00264-011-1247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu H, Liu YL, Chen JD, et al. Effect of osteogenically and adipogenically differentiated bone mesenchymal stem cells from mouse on osteoclast formation. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012;20:1187–1190. [PubMed] [Google Scholar]
- 86.Zhu S, Song D, Jiang X, et al. Combined effects of recombinant human BMP-2 and Nell-1 on bone regeneration in rapid distraction osteogenesis of rabbit tibia. Injury. 2011;42:1467–1473. doi: 10.1016/j.injury.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 87.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zuo Q, Cui W, Liu F, et al. Co-cultivated mesenchymal stem cells support chondrocytic differentiation of articular chondrocytes. Int Orthop. 2013;37:747–752. doi: 10.1007/s00264-013-1782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]