Abstract
Purpose
The aim of this study was to evaluate the long-term results of arthroscopic excision of pigmented villonodular synovitis (PVNS) of the knee joint.
Methods
We retrospectively assessed the results of arthroscopic excision of PVNS done in 40 patients from 1987 to 2012 by the senior author (JVS). No radiotherapy was given to any patient. All patients were followed for a mean of seven years. At follow-up functional assessment was done using the Lysholm score. Recurrence-free survival and recurrence-free survival probability were calculated.
Results
No recurrence was noted in the localised variety. In the diffuse variety the five year recurrence-free survival probability was 57 %. Twelve patients developed recurrences between three months and two years. No recurrence was noted after two years. The mean recurrence interval was 6.25 months.
Conclusions
We concluded from this series that arthroscopic excision is an effective treatment for localised as well as diffuse PVNS. Recurrences can also be successfully dealt with by arthroscopic excision with excellent functional outcome.
Introduction
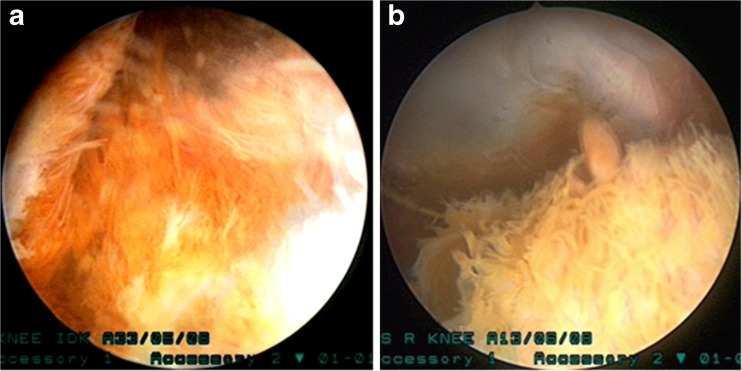
Pigmented villonodular synovitis (PVNS) is a rare benign disease of the synovial membrane which is characterised by hypervascular neoplastic proliferation of the synovium with deposition of macrophages, multinucleated giant cells and hemosiderin. Haemosiderin deposition gives the typical brown appearance of the synovium seen at arthroscopy (Fig. 1a, b). The term PVNS was coined by Jaffe et al. [1] who described it as a disease affecting joints, tendon sheath and bursae. Depending on the extent of synovial involvement two growth patterns of PVNS have been described, a localised form affecting a specific region of the joint and a diffuse form which affects a large portion of the synovial membrane [2]. Usually it is a monoarticular disease of people in their third and fourth decade. The knee joint is the most commonly involved joint followed by hip and ankle joints [3]. A yearly incidence of 1.8 cases per million population with arthroscopic incidence of one in 250 cases has been reported [4].
Fig. 1.
a, b Villous hypertrophy with brown pigmentation is characteristic of diffuse PVNS
The aetiology of this disease is uncertain but the presence of aneuploidy and trisomy 7 in many diseased synovial cells indicates a neoplastic origin. Centrifugal growth patterns and ability to erode surroundings and DNA flow cytometry also support this view [5]. Around 20 cases of malignant transformation and metastases have been reported so far in the English literature [6]. Extensive involvement of surrounding structures including muscles, bone and skin and a multifocal variety involving several joints [7] have also been reported. Perka et al. [8] have suggested localised PVNS as reactive granuloma and generalised PVNS as neoplastic disease.
Patients usually present with swelling and decreased range of motion. A mechanical symptom like locking and catching mimicking a meniscal tear are common in the localised variety. Pain is not usually a complaint. Patients may not seek treatment for years due to vague symptoms. X-ray of the knee frequently shows features of advanced osteoarthritis with soft tissue swelling about the joint. Erosion and subchondral cysts are more common in PVNS of hip and ankle joints in comparison to the more spacious knee joint. Magnetic resonance imaging (MRI) is helpful in differentiating PVNS from other synovial diseases. On MRI it is typically seen as a synovial mass that displays low signal intensity on T1- and T2-weighted pulse sequences. Foci of brighter T1 and T2 signals indicate a relatively low concentration of hemosiderin [11].
The diagnosis of PVNS is confirmed by histopathology. On the basis of common histopathological features of the localised nodular and diffuse varieties, Rao and Vigorita [9] have proposed a common histogenesis. However, invasiveness, aneuploidy, high recurrence rate, chromosomal abnormalities, malignant transformation and metastases suggest generalised PVNS as a distinct variety [8]. In this paper we present a retrospective review of 40 cases of PVNS of the knee joint to assess the result of arthroscopic excision as a sole treatment of this rare disease.
Materials and methods
This study included 40 histopathology-proven cases of PVNS (Table 1), arthroscopically treated by the senior author (JVS) during 25 years from June 1987 to August 2012. In 15,000 cases of knee arthroscopy done by him for various causes, PVNS was found in only 40 cases amounting to an incidence of 0.26 %; 31 patients were male and nine were female. The average age of the patients was 44 years (range 21–76 years); 18 cases involved the right knee and 22 the left, and 11 cases were of the localised variety and 29 cases were diffuse. The average follow-up was seven years (range two to ten years). One case was lost to follow-up. In all patients after clinical examination X-ray and MRI of the involved joint were done to rule out more common diagnoses. Laboratory investigations including synovial fluid examination were not conclusive. In almost all patients synovial fluid was blood stained. Routine haematological and biochemical investigations were normal.
Table 1.
Patients’ data
| Patient no. | Age (years) | Sex | Intraoperative findings | Follow-up (years) | Recurrence | Final joint function | Variety |
|---|---|---|---|---|---|---|---|
| 1 | 42 | M | – | 5 | 2 recurrences | Good | Diffuse |
| 2 | 40 | M | – | 5 | 1 recurrence | Excellent | Diffuse |
| 3 | 42 | M | – | 3 | No | Excellent | Diffuse |
| 4 | 46 | F | – | 5 | 1 recurrence | Excellent | Diffuse |
| 5 | 48 | F | Patellar chondropathy grade 2 | 3 | No | Excellent | Diffuse |
| 6 | 52 | F | Discoid lateral meniscus | 4 | No | Excellent | Diffuse |
| Diffuse villous hypertrophy | |||||||
| 7 | 40 | M | Cartilage damage medial femoral condyle grade 2, patellar chondropathy | 4 | No | Excellent | Localised to suprapatellar pouch |
| 8 | 42 | M | Cartilage damage medial femoral condyle grade 2 | 5 | 1 recurrence | Good | Diffuse |
| 9 | 47 | M | Diffuse synovial hypertrophy, brown pigmentation | Lost to follow-up | – | – | Diffuse |
| 10 | 50 | M | Patellar chondromalacia | 5 | No | Excellent | Diffuse |
| Villous hypertrophy | |||||||
| 11 | 50 | M | Medial meniscus tear with grade 2 cartilage damage medial femoral condyle | 6 | 1 recurrence | Excellent | Diffuse |
| Diffuse synovial hypertrophy, brown pigmentation | |||||||
| 12 | 52 | M | Cartilage damage lateral femoral condyle grade 2, patellar chondropathy | 8 | No | Excellent | Localised to suprapatellar pouch |
| 13 | 48 | M | Localised embedded mass | 9 | No | Excellent | Localised to lateral gutter |
| 14 | 36 | M | Cartilage damage medial compartment with medial meniscal tear | 5 | 1 recurrence | Excellent | Diffuse |
| 15 | 38 | F | Villous hypertrophy, brown pigmentation | 4 | 1 recurrence | Excellent | Diffuse |
| 16 | 28 | F | Villous hypertrophy, pigmentation | 4 | 1 recurrence | Good | Diffuse |
| 17 | 34 | M | Erosive changes, loss of articular cartilage both compartments | 10 | 3 recurrences | Good | Diffuse |
| 18 | 48 | M | Localised pedunculated mass | 5 | No | Excellent | Localised to medial gutter |
| 19 | 48 | M | Osteoarthritic changes with diffuse synovial hypertrophy | 3 | No | Excellent | Diffuse |
| 20 | 46 | M | Osteoarthritic changes patella | 3 | No | Excellent | Localised to suprapatellar pouch |
| Localised pedunculated mass | |||||||
| 21 | 58 | M | Diffuse villous hypertrophy with pigmentation, patellar chondropathy | 7 | No | Excellent | Diffuse |
| 22 | 59 | M | Diffuse villous hypertrophy with pigmentation grade 4 osteoarthritic changes lateral femoral condyle | 5 | No | Excellent | Diffuse |
| 23 | 62 | M | Diffuse villous hypertrophy with pigmentation, cartilage damage medial compartment grade 4 | 9 | No | Excellent | Diffuse |
| 24 | 76 | M | Diffuse villous hypertrophy with pigmentation, grade 4 patellofemoral osteoarthritic changes | 5 | 2 recurrences | Good | Diffuse |
| 25 | 58 | F | Diffuse villous hypertrophy with pigmentation, grade 4 patellofemoral osteoarthritic changes | 5 | 2 recurrences | Good | Diffuse |
| 26 | 58 | F | Diffuse villous hypertrophy with pigmentation, grade 4 patellofemoral osteoarthritic changes | 7 | 3 recurrences | Excellent | Diffuse |
| 27 | 56 | F | Diffuse villous hypertrophy with pigmentation, cartilage damage medial femoral condyle grade 4 | 2 | No | Excellent | Diffuse |
| 28 | 48 | M | Diffuse villous hypertrophy with pigmentation, cartilage damage lateral femoral condyle grade 2 | 2 | No | Excellent | Diffuse |
| 29 | 30 | M | Diffuse villous hypertrophy with pigmentation | 2 | No | Excellent | Diffuse |
| 30 | 31 | M | Diffuse villous hypertrophy with pigmentation | 3 | No | Excellent | Diffuse |
| 31 | 25 | M | Localised pedunculated | 6 | No | Excellent | Localised to lateral gutter |
| 32 | 22 | M | Diffuse villous hypertrophy with pigmentation | 10 | No | Excellent | Diffuse |
| 33 | 21 | M | Diffuse villous hypertrophy with pigmentation | 3 | No | Excellent | Diffuse |
| 34 | 26 | M | Localised embedded | 7 | No | Excellent | Localised to suprapatellar pouch |
| 35 | 36 | M | Localised embedded | 7 | No | Good | Localised to suprapatellar pouch |
| 36 | 42 | M | Diffuse villous hypertrophy with pigmentation, cartilage damage medial femoral condyle grade 2 | 6 | No | Good | Diffuse |
| 37 | 48 | M | Localised embedded | 4 | No | Excellent | Localised medial plica |
| 38 | 46 | M | Diffuse villous hypertrophy with pigmentation | 4 | No | Excellent | Diffuse |
| 39 | 28 | F | Localised pedunculated | 4 | No | Excellent | Localised to suprapatellar pouch |
| 40 | 32 | M | Localised pedunculated | 3 | No | Excellent | Localised to suprapatellar pouch |
The diagnosis was confirmed after arthroscopy and histopathological examination of the shaved synovium. We routinely performed arthroscopic synovectomy in the diffuse variety with five portals, anteromedial, anterolateral, high anteromedial, high anterolateral and posteromedial. Rarely a posterolateral portal was needed to complete the synovectomy. Localised cases were amenable to excision by routine anteromedial and anterolateral portals. No macroscopic tumour was left after synovectomy in all cases. In the post-operative period our rehabilitation protocol included early full weight-bearing walking, quadriceps and hamstring building exercises and cycling. Post-operative radiotherapy was not given to any patient. Patients were followed up at monthly intervals for six months and then three and six monthly. At every visit patients were examined for pain, swelling due to effusion and range of motion. Recurrences were diagnosed on the basis of clinical and MRI findings. Functional scoring was done using the Lysholm knee score. It is a simple scoring system consisting of limp, use of cane/crutches, locking sensation in the knee, giving way sensation of the knee, pain, swelling, ability to squat and climb stairs. Kaplan-Meier life table analysis was done to determine local recurrence-free survival. We received Ethics Committee approval for the publication of the study.
Results
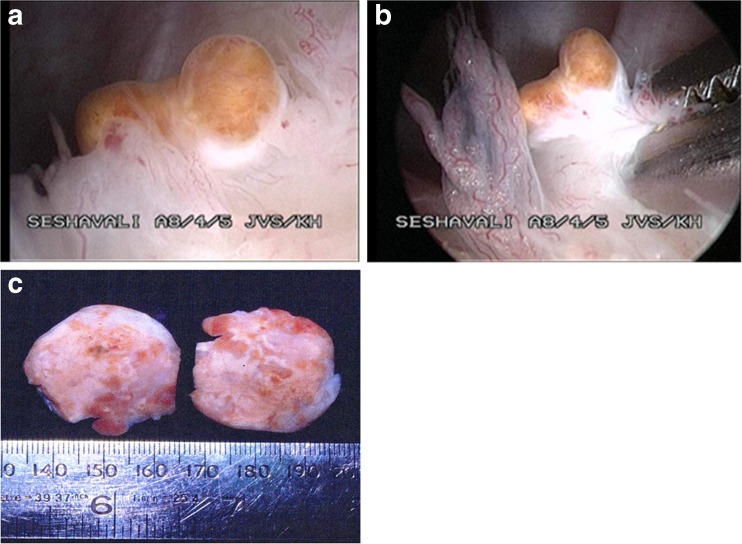
Initially most of the patients were diagnosed as having traumatic or tubercular synovitis, osteoarthritis and meniscal tear. The duration of symptoms was three months to five years. MRI was convincing in most patients with the diffuse variety about neoplastic pathology especially with gadolinium enhancement. In the localised variety it was helpful to rule out other pathological conditions like meniscal tear. Effusion due to blood in the synovial fluid was invariably present in all cases. There were no complications of post-operative infection, neurovascular damage, deep venous thrombosis (DVT) or wound healing. Twelve patients developed recurrences in the 1st year after surgery between three and 12 months. Five patients developed their second recurrence between 14 and 18 months and two patients had their third recurrence in the 24th month. No recurrence was noted after two years and all recurrences were treated by arthroscopic resynovectomy. No apparent progression of osteoarthritic changes was noted on imaging in follow-ups. Localised masses were either pedunculated or embedded but distinct from the surrounding synovium (Fig. 2a, b). Small pedunculated masses were free to move in the joint causing symptoms of locking and catching mimicking a meniscal tear. In the diffuse variety brownish pigmentation with freely movable finger-like filiform villi without any evidence of coalescence were classic for PVNS. The smallest size tumour was an embedded 3 × 2 cm mass (Fig. 2c).
Fig. 2.
a–c Localised villonodular synovitis, seen distinct from the surrounding synovium (a, b). Cut section of small localised PVNS measuring 3 × 2 cm (c)
In our series around 64 % of nodular PVNS were localised to the suprapatellar pouch (Table 2). The diagnosis was confirmed by histopathological features in all cases. Both diffuse and localised varieties had similar histological features. Villous hypertrophy of the synovial lining and subsynovial foci of multinucleated giant cells with intermixed foam cells, round cells and pearls positive pigment were characteristic features.
Table 2.
Location of nodular (localised) PVNS
| Localised PVNS (11 cases) | |
|---|---|
| Suprapatellar pouch | 7 (64 %) |
| Lateral gutter | 2 |
| Medial gutter | 1 |
| Medial plica | 1 |
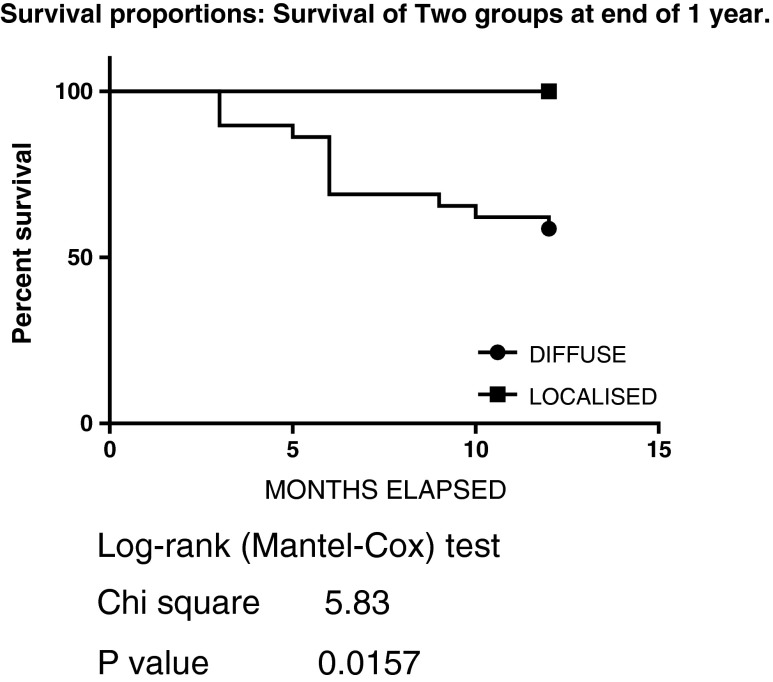
Recurrence-free survival probability was 69 % at the end of one year and 82 % between 12 and 24 months. Overall five year recurrence-free survival probability was 57 % (0.69 × 0.82). No recurrence was seen in the localised variety up to the latest follow-up. This finding was also consistent with results reported by other authors [2, 20]. Kaplan-Meier life table analysis (Fig. 3) was done to determine local recurrence-free survival.
Fig. 3.
Survival proportions: survival of two groups at the end of 1 year
Discussion
Cases of PVNS are presented because of their rarity. Most of the published studies are on PVNS of the knee joint as it is the most commonly involved joint and also recurrences are more common in the knee as compared to other joints [25]. The majority of patients have vague signs and symptoms so arthroscopy is both diagnostic and therapeutic. Diagnosis based on synovial fluid examination has been proposed by Zimmermann-Górska et al. [10] but we could not find a positive correlation in all cases. In this series we present 40 cases but we suspected it before arthroscopy only in seven cases, based on MRI and clinical findings. MRI has the highest sensitivity but other synovial pathologies like amyloid arthropathy, haemophilic arthropathy, synovial chondromatosis and haemangioma cannot be ruled out. [11]. Osteoarthritic changes are more common in PVNS patients. Even middle-aged patients have advanced cartilage damage (Table 1). Cytokines [interleukin (IL)-1, IL-6, tumour necrosis factor (TNF) alpha] and matrix metalloproteinases (MMPs) (MMP-9) may mediate bone and cartilage destruction [12].
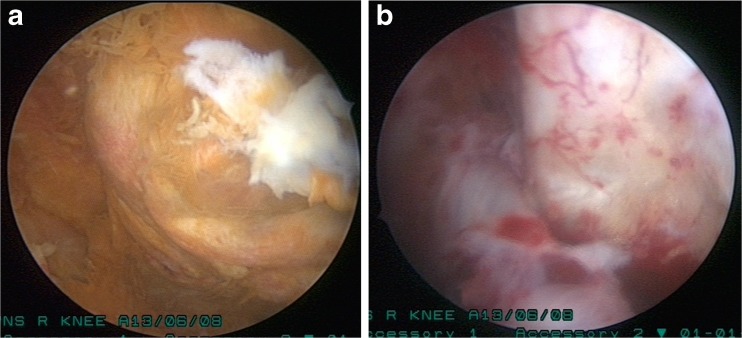
Many treatment modalities have been tried with variable success rates for treatment of PVNS including arthroscopic and open excision [13], synovectomy and arthroplasty [14], low-dose external beam radiotherapy [15] and imatinib therapy [16] etc. Synovectomy is the mainstay of the treatment. External beam radiotherapy or intra-articular yttrium-90 is usually combined with surgical excision to prevent recurrences [17, 18, 19]. Many authors have proved the efficacy of arthroscopic synovectomy in both localised and diffuse PVNS [20, 21]. To reduce the recurrence rate, excision of diseased synovium from the posterior compartment is important. We routinely made a posteromedial portal to address it. Synovial shavers and radiofrequency ablation are very useful in removal of diseased synovium (Fig. 4a, b). The main benefit of arthroscopic synovectomy over open synovectomy is low post-operative morbidity. Open synovectomy produces pain and stiffness. Long-term side effects of radiotherapy for soft tissue tumours including the secondary cancer are well known.
Fig. 4.
a, b Diffuse PVNS before and after shaving. Synovial shavers produce effective excision if used through different anterior and posterior portals
Although factors that can predict recurrences have not been established [17] the diffuse variety and residual disease due to subtotal synovectomy are the well-known factors responsible for recurrences. In this study we found recurrences can be dealt with by arthroscopic synovectomy as successfully as the index surgery and also with low morbidity. In many published studies recurrence rates of arthroscopic synovectomy were as high as 50–60 % [22, 23]. Our 1st year survival rate was 69 %. A long follow-up is required to study the recurrences as they have been reported as long as 17 years after surgery [24]. Schwartz et al. reported a median recurrence interval of five years after index surgery [25]. In contrast to these studies the mean recurrence interval in our series is 6.25 months. The strength and distinguishing features of our study are adequate sample size and only one modality of treatment (arthroscopic excision for both primary disease and recurrences) for this rare disease with seven year follow-up.
The conclusion derived from this series is that in the hands of an experienced arthroscopic surgeon, arthroscopic excision gives results as good as open synovectomy and recurrences can also be managed with a good success rate with low morbidity. Recurrences do not affect the functional outcome. Similar results have also been reported by other authors [21].
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Jaffe HL, Lichtenstein L, Suro CJ. Pigmented villonodular synovitis, bursitis and tenosynovitis. Arch Pathol. 1941;31:731–765. [Google Scholar]
- 2.Granowitz SP, D’Antonio J, Mankin HL. The pathogenesis and long-term end results of pigmented villonodular synovitis. Clin Orthop. 1976;114:335–351. [PubMed] [Google Scholar]
- 3.Dorwart RH, Genant HK, Johnston WH, et al. Pigmented villonodular synovitis of synovial joints: clinical, pathologic, and radiologic features. AJR Am J Roentgenol. 1984;143:877–885. doi: 10.2214/ajr.143.4.877. [DOI] [PubMed] [Google Scholar]
- 4.Myers BW, Masi AT, Feigenbaum SL. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59:223–238. doi: 10.1097/00005792-198005000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Flandry FC, Hughston JC. Pigmented villonodular synovitis. J Bone Joint Surg Am. 1987;69:942–949. [PubMed] [Google Scholar]
- 6.Imakiire N, Fujino T, Morii T, et al. Malignant pigmented villonodular synovitis in the knee - report of a case with rapid clinical progression. Open Orthop J. 2011;5:13–16. doi: 10.2174/1874325001105010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botez P, Sirbu PD, Grierosu C, et al. Adult multifocal pigmented villonodular synovitis–clinical review. Int Orthop. 2013;37(4):729–733. doi: 10.1007/s00264-013-1789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perka C, Labs K, Zippel H, et al. Localized pigmented villonodular synovitis of the knee joint: neoplasm or reactive granuloma? A review of 18 cases. Rheumatology. 2000;39:172–178. doi: 10.1093/rheumatology/39.2.172. [DOI] [PubMed] [Google Scholar]
- 9.Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66(1):76–94. [PubMed] [Google Scholar]
- 10.Zimmermann-Górska I, Puszczewicz M, Bialkowska-Puszczewicz G. Diagnostic value of synovial fluid analysis in pigmented villonodular synovitis (PVS)- a proposal of diagnostic criteria. Arthritis Res. 2001;3(Suppl A):P117. doi: 10.1186/ar286. [DOI] [Google Scholar]
- 11.Garnes HW, Ortiguera CJ, Nakhleh RE. Pigmented villonodular synovitis. Radiographics. 2008;28:1519–1523. doi: 10.1148/rg.285075190. [DOI] [PubMed] [Google Scholar]
- 12.O’Keefe RJ, Rosier RN, Teot LA, et al. Cytokine and matrix metalloproteinase expression in pigmented villonodular synovitis may mediate bone and cartilage destruction. Iowa Orthop J. 1998;18:26–34. [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma V, Cheng EY. Outcomes after excision of pigmented villonodular synovitis of the knee. Clin Orthop Relat Res. 2009;467:2852–2858. doi: 10.1007/s11999-009-0922-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma X, Shi G, Xia C, et al. Pigmented villonodular synovitis: a retrospective study of seventy five cases (eighty one joints) Int Orthop. 2013;37(6):1165–1170. doi: 10.1007/s00264-013-1858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park G, Kim YS, Kim JH, et al. Low-dose external beam radiotherapy as a postoperative treatment for patients with diffuse pigmented villonodular synovitis of the knee: 4 recurrences in 23 patients followed for mean 9 years. Acta Orthop. 2012;83(3):256–260. doi: 10.3109/17453674.2012.678803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blay JY, El Sayadi H, Thiesse P, Garret J, Ray-Coquard I. Complete response to imatinib in relapsing pigmented villonodular synovitis/tenosynovial giant cell tumor (PVNS/TGCT) Ann Oncol. 2008;19(4):821–822. doi: 10.1093/annonc/mdn033. [DOI] [PubMed] [Google Scholar]
- 17.Adem C, Sebo TJ, Riehle DL, et al. Recurrent and non-recurrent pigmented villonodular synovitis. Ann Pathol. 2002;22(6):448–452. [PubMed] [Google Scholar]
- 18.Franssen MJ, Boerbooms AM, Karthaus RP, Buijs WC, et al. Treatment of pigmented villonodular synovitis of the knee with yttrium-90 silicate: prospective evaluations by arthroscopy, histology, and 99mTc pertechnetate uptake measurements. Ann Rheum Dis. 1989;48:1007–1013. doi: 10.1136/ard.48.12.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enneking WF, Dunham W, Gebhardt MC, et al. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop. 1993;286:241–246. [PubMed] [Google Scholar]
- 20.Moskowitz R, Parisien JS. Localized pigmented villonodular synovitis of the knee. Arthroscopic treatment. Clin Orthop. 1991;271:218–224. [PubMed] [Google Scholar]
- 21.Zvijac JE, Lau AC, Hechtman KS, Uribe JW, Tjin-A-Tsoi EW. Arthroscopic treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 1999;15:613–617. doi: 10.1053/ar.1999.v15.015061. [DOI] [PubMed] [Google Scholar]
- 22.Chin KR, Brick GW. Extraarticular pigmented villonodular synovitis: a cause for failed knee arthroscopy. Clin Orthop Relat Res. 2002;404:330–338. doi: 10.1097/00003086-200211000-00049. [DOI] [PubMed] [Google Scholar]
- 23.Ogilvie-Harris DJ, McLean J, Zarnett ME. Pigmented villonodular synovitis of the knee. The results of total arthroscopic synovectomy, partial arthroscopic synovectomy, and arthroscopic local excision. J Bone Joint Surg Am. 1992;74:119–123. [PubMed] [Google Scholar]
- 24.Panagiotopoulos E, Tyllianakis M, Lambiris E, Siablis D. Recurrence of pigmented villonodular synovitis of the knee 17 years after the initial treatment. A case report. Clin Orthop. 1993;295:179–182. [PubMed] [Google Scholar]
- 25.Schwartz HS, Unni KK, Pritchard DJ. Pigmented villonodular synovitis. A retrospective review of affected large joints. Clin Orthop. 1989;247:243–255. [PubMed] [Google Scholar]